Abstract
IMPORTANCE
Autism spectrum disorder (ASD) is a brain-based pervasive developmental disorder, which—by growing consensus—is associated with abnormal organization of functional networks. Several previous studies of ASD have indicated atypical hemispheric asymmetries for language.
OBJECTIVE
To examine the asymmetry of functional networks using a data-driven approach for a comprehensive investigation of hemispheric asymmetry in ASD.
DESIGN, SETTING, AND PARTICIPANTS
This cross-sectional study involved 24 children with ASD and 26 matched typically developing children at San Diego State University and the University of California, San Diego. Data from 10 children had to be excluded for excessive motion, resulting in final samples of 20 participants per group.
MAIN OUTCOMES AND MEASURES
Asymmetry indices of functional networks identified from independent component analysis of resting-state functional magnetic resonance imaging data.
RESULTS
Temporal concatenation independent component analysis, performed separately in each group, showed significant group differences in asymmetry indices for 10 out of 17 functional networks. Without exception, these networks (visual, auditory, motor, executive, language, and attentional) showed atypical rightward asymmetry shifts in the ASD group.
CONCLUSIONS AND RELEVANCE
Atypical rightward asymmetrymay be a pervasive feature of functional brain organization in ASD, affecting sensorimotor, as well as higher cognitive, domains.
Autism spectrum disorder (ASD) is characterized by sociocommunicative impairments, repetitive behaviors, and restricted interests.1 While viewed as a neurological disorder, its precise neural bases are not well understood. Growing evidence suggests that sociocommunicative, cognitive, and sensorimotor impairments are related to abnormalities of distributed networks, rather than of single brain loci.2-4 Reports of atypical hemispheric asymmetries in ASD come from anatomical5-7 and functional imaging studies.8-12 Eyler and colleagues13 observed atypical rightward asymmetry of receptive language activations during natural sleep in infants down to age 12.5 months, indicating that abnormal lateralization may predate language acquisition. However, all of the studies cited above focused on brain regions or stimuli specifically related to language. Arecent study on gene expression in post mortem frontal cortex showed abnormalities in patterning pathways affecting lateralization in ASD,14 possibly suggesting a broader impact on asymmetries even outside the language domain.
While far from conclusive, these intriguing findings prompt the question whether atypical asymmetry could be a fundamental feature of brain organization in ASD. Indeed, theoretical considerations about possible left-hemisphere dysfunction15,16 or, alternatively, predominant right-emisphere impairment17,18 have a long history in autism research. However, these conjectures were largely based on limited behavioral findings, and conclusive imaging evidence beyond the language domain is lacking. Functional asymmetries related to nonverbal processing in ASD have received little attention, with only 2 studies reporting atypical left-hemisphere processing for motor tasks.19,20 Moreover, functional studies have typically applied a priori models of task-driven activation effects. Since ASD is a pervasive developmental disorder whose neurofunctional bases are not well understood, hypothesis-driven approaches targeting single domains may, however, fail to provide comprehensive evidence because unexpected abnormalities may be missed.
Therefore, we used the data-driven approach of independent component analysis (ICA). Applied to functional magnetic resonance imaging (fMRI) data, ICA identifies brain regions that are temporally coherent (ie, characterized by correlated time series) and can be interpreted as spatially distributed networks.21 It does not require a priori selection of regions of interest or expected temporal signal responses, making it an ideal tool for comprehensive network analyses.22 Functional MRI components detected by ICA are associated with known functional networks22,23 and are stable within and across participants.24 Resting-state fMRI is frequently used for assessing intrinsic blood oxygen level–dependent fluctuations, providing a method for identifying multiple functional networks in a task-free setting.25-27 Patterns of functional connectivity in resting-state fMRI studies are highly consistent across participants and sessions,28,29 and detected functional networks correspond to those from conventional activation fMRI.22
To our knowledge, the present study is the first to use resting- state fMRI and ICA to investigate the asymmetry of functional networks and links with diagnostic and cognitive profiles in ASD. Considering the limited available evidence, we hypothesized that atypical asymmetry would be detected for a multitude of functional networks beyond those identified in previous single-domain studies and that atypical asymmetries would be related to diagnostic and cognitive measures.
Methods
Data were collected from a total of 50 participants, including 24 high-functioning children and adolescents with ASD and 26 typically developing (TD) participants. No participants with medical or psychiatric conditions (other than ASD) were included. Four participants with ASD and 2 TD participants were excluded because of excessive head motion (>1.5 mm), and 4 additional TD participants (3 girls, 1 left-handed boy)were excluded to restore group matching for sex and handedness. The resulting sample included 20 participants with ASD and 20TD participants (Table). Informed consent was collected from all participants in accordance with the institutional review boards of the University of California at San Diego and San Diego State University. For participants younger than the age of 18 years (all except 1 TD participant), written parental consent was also obtained. The 2 groups were matched on age, handedness, and nonverbal IQ. Autism spectrum disorder diagnoses were confirmed using the Autism Diagnostic Interview–Revised,30 the Autism Diagnostic Observation Schedule,31 and expert clinical opinion. All participants had verbal and nonverbal IQ scores greater than 70 on the Wechsler Abbreviated Scale of Intelligence.32 Hand preference was assessed with the Edinburgh Handedness Inventory.33
Table.
Participant Characteristics
| Autism Spectrum Disorder (n = 20), Mean (SD) [Range]a | Typically Developing (n = 20), Mean (SD) [Range] | P Value (Between-Group t Test) | |
|---|---|---|---|
| Sex, No. | |||
| Male | 19 | 18 | |
| Female | 1 | 2 | |
| Handedness, No. | |||
| Left | 3 | 3 | |
| Right | 17 | 17 | |
| Age, y | 14.6 (2.2) [9.2-18.7] | 14.7 (1.6) [12.1-17.9] | .89 |
| IQ | |||
| Nonverbal | 112 (16) [70-140] | 109 (12) [77-129] | .53 |
| Verbal | 112 (15) [87-147] | 105 (11) [83-126] | .15 |
| Full scale | 114 (14) [96-141] | 108 (11) [78-126] | .15 |
| ADOS | |||
| Social | 7.2 (2.9) [3-13] | ||
| Communication | 3.1 (2.0) [0-9] | ||
| Repetition | 1.9 (1.2) [0-4] | ||
| ADI | |||
| Social | 15.6 (6.6) [0-24] | ||
| Communication | 11.5 (7.0) [0-25] | ||
| Repetition | 5.2 (2.6) [0-11] |
Abbreviations: ADI, Autism Diagnostic Interview; ADOS, Autism Diagnostic Observation Schedule.
Participants had autistic disorder (n = 6), Asperger’s disorder (n = 12), or pervasive developmental disorder not otherwise specified (n = 1). Diagnostic subclassification was unavailable for 1 participant. Medication information for 4 participants with autism spectrum disorder is provided in the Supplement.
Magnetic Resonance Imaging
Imaging data were acquired at the Center for Functional Magnetic Resonance Imaging at the University of California at San Diego using aGEMR7503T system with an 8-channel head coil. High-resolution 3-dimensional anatomical images were acquired using a standard T1-weighted fast spoiled-gradientre-called- echo sequence (repetition time = 614 milliseconds; echo time = 6.5 milliseconds; matrix = 172 × 256; flip angle = 45°; 256 axial slices/resolution = 1 mm3). T2*- weighted echo planar imaging data were collected for 185 points (repetition time = 2 seconds; echo time = 30 milliseconds; bandwidth = 128 kHz; matrix = 64 × 64; field of view = 220 mm; flip angle = 90°; in-plane resolution = 3.438 × 3.438mm; slice thickness = 3.40 mm; 42 axial slices covering the whole brain). The first 5 frames were excluded to allow for steady-state magnetization, resulting in 180 total whole-brain volumes. Throughout the scan, a white fixation cross on a black background was displayed in the center of a screen. Participants were instructed to simply keep their eyes on the fixation cross.
Data Preprocessing
Field map correction for functional images was performed to remove distortions resulting from magnetic field inhomogeneity. Preprocessing was then conducted using Analysis of Functional NeuroImages.34 Functional data were slice-time corrected, motion corrected by aligning to the first time point, coregistered to the anatomical image, and standardized to the N27Colin brain template in Talairach space.35 Head motion was estimated by first computing the sum of squared differences between successive time points, as detected in spatial realignment of functional images. Then, the root mean square of this displacement was obtained to describe the total motion. The average total motion was 0.078 mm for the TD group and 0.104 mm for the ASD group, and this difference was not significant (t1,38 = 0.79, P = .27). Blood oxygen level–dependent signal spikes (SD >2.5) were replaced with an average of the time points before and after using 3dDespike in Analysis of Functional Neuro Images. There was no group difference in the number of signal spikes and replaced time points. The resulting images were smoothed with an isotropic Gaussian smoothing kernel at 10 mm full-width at half-maximum, slightly below the 12 mm full-width at half-maximum implemented in previous ICA studies22,23 (eAppendix in Supplement provides further technical details).
Independent Component Analyses
Temporal concatenation–group ICA was performed with voxelwise variance normalization using Melodic version 3.0,25 implemented in the Functional MRI of the Brain Software Library.36,37 We used 2 ICA approaches: (1) fixed dimensionality estimation (FDE) with model order set at 20 components and (2) automated dimensionality estimation (ADE). Fixed dimensionality estimation (limited to 20 components) was first performed for the TD group to cross-validate our ICA results with those reported in previous studies inTDadults,22,23 which had equally set model order at 20 components. Following this first pass analysis, ICA was performed for 20 iterations separately for each group and the spatial components estimated from all iterations within the group were concatenated. Then, to extract the most consistent spatial components across all iterations within the group,22 ICA was executed (without variance normalization) on the concatenated results using the same model order (ie, 20; eAppendix in Supplement provides further technical explanations).
Data-driven methods for automated selection of model order—such as the Laplace approximation to the model evidence, the Bayesian information criterion, or the Akaike information criterion—have been shown to be reliable in estimating the latent dimensionality and could thus be superior to arbitrarily chosen model orders.38,39 Therefore, temporal concatenation–group ICA was performed using the same 20-iteration procedure as previously described to obtain consistent spatial components, but with ADE using the Laplace approximation to the Bayesian evidence, implemented in Melodic.39 The ADE procedure reduced the data to 86 dimensions for the TD group and 55 dimensions for the ASD group.
For both FDE and ADE analyses, components were spatially correlated with those from previous ICA studies22,23 at a threshold of r > 0.3 (P < .001), which is more conservative than the threshold used by Smith and colleagues.22 Additional spatial comparisons were performed to assess whether any ADE components were subcomponents of those detected in the FDE analysis. Any such potential ADE components were combined and then spatially correlated with the corresponding FDE component.
The spatial and temporal aspects of the resultant components from each run (FDE and ADE) in each group (TD and ASD) were visually inspected, and artifactual components were excluded from further analyses, following procedures detailed by Kelly et al.40 Moreover, components with less than 80% of voxels located in graymatter were also considered noise and excluded (eAppendix in Supplement provides technical details). Each resulting component from each run (FDE and ADE) was matched between the TD and ASD groups using a highly conservative spatial correlation matching criterion of r > 0.5 for all voxels with positive intensities across the whole brain.
To determine group differences, the dual regression procedure was used to obtain single-subject–level spatial components from group-level spatial component.41,42 Dual regression involves 2 sequential steps: (1) an estimate of the time series corresponding to the group-level ICA maps for each participant and (2) regression of the estimated time series for each component on the individual’s resting-state data. Hemispheric masks were then applied to determine the average intensity of the component for each hemisphere. The cerebellum was excluded from this mask because of its mostly crossed connectivity with the cerebrum (eFigure 1 in Supplement provides illustration of the analysis pipeline).
An asymmetry index (right − left)/(right + left) was calculated for each participant and each component using the average beta scores extracted from the dual regression step for all voxels with positive intensities per hemisphere (eAppendix in Supplement). Permutation tests (10 000 iterations)were performed to test for differences between the TD and ASD groups, while controlling for age. Pearson correlation analyses were conducted in the ASD group for component-specific asymmetry indices and IQ and diagnostic scores (as listed in the Table).
Results
Independent component analysis was first performed on the data from the TD group with FDE set to 20 to determine whether results from the present study corresponded to the components reported by 2 previous studies in TD adults.22,23 Nine of the 10 components reported by Smith and colleagues22 were matched in the FDE analysis (r > 0.3, P < .001). Of the 20 components reported by Laird and colleagues,23 15 FDE components could be matched at the same threshold. A perfect correspondence was not expected since the present study included younger TD participants whose brain organization had not fully reached maturity.
The components from the FDE analyses were categorized as either true-signal or noise components, as just described. In the TD group, 16 true-signal components and 4 noise components were found. Fixed dimensionality estimation analysis in the ASD group yielded 15 true-signal components and 5 noise components. Thirteen true-signal components were spatially matched (r > 0.5) between groups. Of these, 7 components—which matched those described by Smith et al22 and Laird et al23 as frontoparietal, visual, executive control, auditory, and sensorimotor components—exhibited significant differences in hemispheric asymmetries (eFigure 2 in Supplement).
A subsequent ICA analysis was performed with automated dimensionality estimation, first on data from the TD group. To analyze the relationship between this ADE run and the FDE run, components from each run were matched through spatial correlations (r > 0.3,P < .001). When multiple ADE components matched one FDE component, they were combined to test whether they were subcomponents of the larger FDE component. For example, ADE components in panels C and K in the Figure were found to be subcomponents of the FDE component in panel B in eFigure 2 in Supplement. Similarly, ADE components in panels B and I in the Figure were subcomponents of the FDE component in panel D in eFigure 2 (Supplement). In each case, the composite ADE components could be matched to corresponding FDE components using a conservative spatial correlation threshold (r > 0.5, P < .001). Once good correspondence between ADE and FDE components was established for the TD group, an ADE analysis was performed on the ASD group.
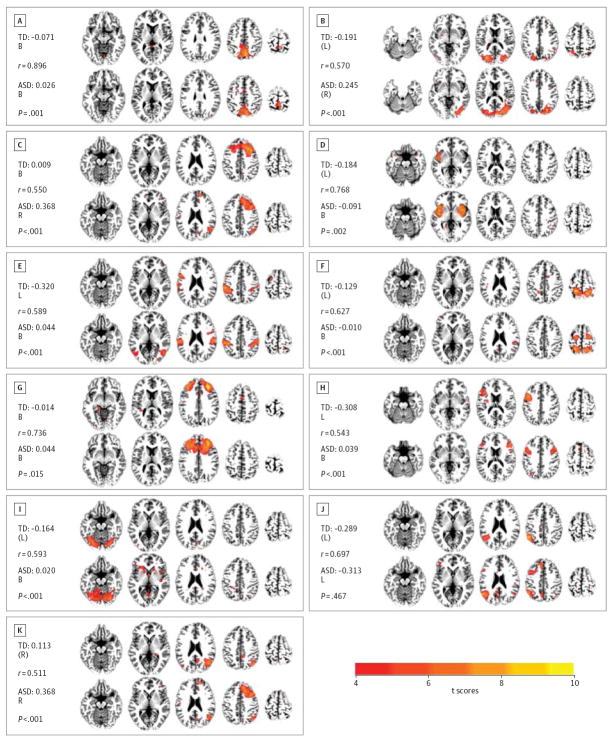
Figure. Components From Auto-dimensionality Analysis.
In each panel, the typically developing component is shown at the top and the matched autism spectrum disorder (ASD) component at the bottom (with spatial correlation coefficient shown on the left). Next to the group labels are the group asymmetry indices. P values are derived from between-group permutation tests of asymmetry indices controlling for age. The matching of components to those from previous studies is abbreviated as: mtL, matched to Laird et al,22,23 and mtS, matched to Smith et al.22 A, Component involving visuospatial processing and reasoning (mtL); B, lateral visual area component (mtS); C, right frontoparietal component (mtL and mtS); D, auditory component (mtS); E and F, sensorimotor components (mtL and mtS); G, executive component (mtS); H, left frontoparietal component (mtL and mtS); I, lateral visual area component (mts); J, unmatched component (neither mtL nor mtS); and K, right frontoparietal component (mtL and mtS). Note that a single ASD component was matched to 2 separate TD components, shown in panels C and K. All images shown in neurological convention (left side is left hemisphere). Asymmetry index (AI) labels defined as follows: B indicates bilateral (AI: −0.1 to 0.1); L, left lateralized (AI < −0.3); (L), weakly left lateralized (AI: −0.3 to −0.1); R, right lateralized (AI > 0.3); and (R), weakly right lateralized (AI: 0.1 to 0.3).
Components from the ADE analyses were categorized as either true-signal or noise components. In the TD group, 41 true-signal components and 45 noise components were identified. In the ASD group, 30 true-signal components and 25 Noise components were found. Dual regression was used to obtain single-subject–level spatial maps,41,42 and asymmetry indices were calculated. Seventeen true-signal components were spatially matched between groups (r > 0.5, P < .001). Of these, 10 components exhibited significant differences in hemispheric asymmetry (Figure; eFigure 3 in Supplement provides additional components with no group differences). All of these 10 components were characterized by significantly higher asymmetry indices in the ASD group, reflecting rightward shifts of asymmetry. This pattern of consistent rightward shifts in ASD could also be confirmed for FDE components. As described above, 9 of 13 matched true-signal FDE components showed significant group differences in asymmetry indices. Each of these 9 had higher asymmetry indices in the ASD group, indicating greater rightward asymmetry (eFigure 2 in Supplement).
Finally, correlational analyses were performed for exploratory purposes between asymmetry indices and diagnostic and IQ scores in the ASD group. A large number of comparisons were performed and none of the correlations (shown in eFigure 4 in Supplement) achieved the significance level (P < .0005) that would have survived full correction. With the necessary extreme caution, we noted correlation coefficients r > 0.5 for rightward asymmetry of right frontoparietal22,23 component C (which was bilateral in the TD group) and lower symptom severity on Autism Diagnostic Interview social and communication scores, and for leftward asymmetry of component J (which was left dominant in both groups) and lower symptom severity on Autism Diagnostic Interview communication scores. Correlation coefficients at r > 0.5 were also seen for leftward asymmetry of left frontoparietal22,23 component H (which was left lateralized in the TD group, but bilateral in the ASD group) and higher verbal IQ scores, and for leftward asymmetry of component J (which was left dominant in both groups) and full-scale IQ scores.
Discussion
The present study used data-driven ICA of resting-state fMRI data for a comprehensive investigation of hemispheric asymmetry in adolescents with ASD. We detected a general trend toward rightward asymmetry of multiple functional brain systems in ASD. Thus, our findings go well beyond previously reported language-related functional and anatomical findings of increased rightward asymmetry6,8-10,12,43 and reduced leftward asymmetry44,45 in ASD compared with TD groups. In the present study, hemispheric a symmetries were detected in components thought to be implicated in auditory, visual, sensorimotor, executive, attentional, and visuospatial processing.
Studies of hemispheric asymmetries in ASD have typically focused on language processing,10,46 and many of these studies have reported aberrant right dominance of activations in auditory cortex.9,47-50 The present study detected an ICA component (Figure, D) considered to represent an auditory network.22 This component, which incorporated auditory cortices, was characterized by leftward asymmetry in the TD group but significantly greater participation of the right hemisphere in the ASD group, consistent with previous reports. These abnormal asymmetry findings may be of interest in the context of impaired auditory processing in ASD,51 which may in turn contribute to language and sociocommunicative impairments.
However, aside from atypical language-related asymmetries, which had been previously reported, we also detected abnormal asymmetries for many components related to other functional domains. The differences in hemispheric asymmetries we detected for sensorimotor components suggest that atypically enhanced engagement of the right hemisphere in ASD is not limited to domains of higher complex cognition. One component that included motor and somatosensory cortices (Figure, E) was mildly left dominant in the TD group, whereas asymmetry indices showed significantly greater participation of the right hemisphere in the ASD group. Another component (Figure, F), which included premotor and superior parietal regions, was also weakly left dominant in the TD group, again with significantly higher asymmetry scores in the ASD group, reflecting greater right hemisphere involvement. Such atypical rightward shifts of asymmetry for sensorimotor components is notable in the context of findings from behavioral and neuroscientific studies showing atypical sensory52 and motor53 processing in ASD.
Group differences in hemispheric specialization were also detected for 3 components (Figure, A, B, and I) that incorporated primary, as well as extrastriate, visual cortices and were, therefore, attributed to visual and visuospatial processing.22,23 Components Band I were both weakly left dominant in the TD group, but bilateral to weakly right dominant in the ASD group, with significant differences in asymmetry indices (Figure). Visuospatial23 component A was nominally bilateral in both groups (based on asymmetry thresholds applied in the Figure), but asymmetry indices were again significantly higher in the ASD group, reflecting greater right hemisphere involvement. While atypical visual functioning, including impairments and islands of superior abilities, has been observed in many ASD studies,54,55 few of these have examined hemispheric specialization for visual information processing in ASD (see review by Simmons et al56). Therefore, possible links between asymmetry and atypical visual processing in ASD remain an open question.
We further identified a component attributed to executive functions,22 which incorporated large portions of dorsolateral prefrontal and frontopolar cortices (Figure, G). While this component was overall bilateral in both groups, asymmetry indices were significantly higher in the ASD group, reflecting greater right hemisphere participation. This component also extended into medial prefrontal cortex in the ASD (but not the TD) group, consistent with findings of atypical activation in medial prefrontal cortex for executive tasks.57 In combination with our ICA finding, this is notable given that medial prefrontal cortex in the neurotypical brain is crucially involved in mentalizing abilities, known to be impaired in ASD.58,59 Of interest in the context of atypical asymmetry for component Gare also reports of deficits in executive functioning from behavioral studies of ASD.60
The frontoparietal components identified in our study (Figure, C, H, and K) provided further insight into atypical hemispheric asymmetries in ASD. Frontoparietal components were the only components that ICA consistently separated into distinct left and right lateralized networks in the FDE analysis (eFigure 2B and F in Supplement). This separation into distinct unilateral frontoparietal components is in agreement with findings in healthy adults22,23 and stands in contrast to other ICA components that were either bilateral or showed partial hemispheric asymmetry, without being accompanied by a separate component with mirrored asymmetry. Smith and colleagues22 consider the right frontoparietal network to be related to perception, somesthesis, and pain, whereas they attribute the left frontoparietal network to cognition and language. In our ADE analysis, ICA separated the right frontoparietal FDE component (eFigure 2B in Supplement) in the TD group into 2 separate components (Figure, C and K). Both components Cand K had strong representation in the right hemisphere in the TD group, but were significantly more right lateralizing in the ASD group. Component H—a strongly left lateralized frontoparietal component in the TD group—was matched to a frontoparietal component with extensive additional recruitment of the right hemisphere in the ASD group (Figure). Consistent with the overall pattern of results in our study, these frontoparietal components thus displayed significant rightward shifts of asymmetry in the ASD group.
Atypical asymmetries in frontoparietal networks may be of importance with respect to executive impairments (as discussed above), as well as attentional abnormalities, which have been observed in ASD.61,62 Our findings may also relate to a recent hypothesis of specific impairments in frontoparietal connectivity in ASD by Just and colleagues.63 As reviewed by these authors, a number of previous studies have reported such frontoparietal functional under connectivity in ASD associated with reduced activation in frontal regions, but enhanced activation in parietal regions, for a variety of tasks. While any possible links between frontoparietal connectivity and asymmetry findings remain to be further investigated, it is remarkable that in most of these studies, frontoparietal under connectivity was linked to smaller size of the corpus callosum, suggesting impaired inter hemispheric communication in ASD, which may relate to atypical asymmetries observed here.64
Notably, in all 10 of the networks with significant group differences in asymmetry, these differences were driven by rightward asymmetry shifts in the ASD group, reflecting reduced participation of the left hemisphere or increased participation of the right hemisphere (or both). This was observed for sensorimotor, as well as cognitive, networks, indicating that atypical functional rightward asymmetrymay be a pervasive feature of brain organization in ASD. Thus, our findings go far beyond existing evidence and show that atypical asymmetry is not exclusively tied to language-related functional networks (although our findings for networks D and H also support existing evidence). Furthermore, since our ASD group had a mean verbal IQ in the normal range, our findings suggest that atypical asymmetries are not solely found in children with ASD who have language impairment, as suggested in some previous studies.5,11
Hemispheric asymmetry is a fundamental feature of human brain organization. Differences in the columnar organization between hemispheres support an early-onset specialization of the left in fast temporal analysis of auditory and other sensory information.65 Although asymmetries in gene transcription in human embryonic brains have been identified,66 the molecular bases of hemispheric asymmetries are not fully understood.67,68 However, our current results may be related to gene expression findings from ASD post mortem brains, suggesting abnormalities in genes regulating the development of left-right asymmetries,14 which may indicate that atypical asymmetries reflect early-onset and possibly causative abnormalities in ASD. This is also supported by recent imaging findings showing atypical rightward asymmetry of receptive language activity in infants with ASD.69 It remains, however, unclear how our findings relate to atypical functional connectivity inASD,2,4 in particular, how impaired functional70,71 and anatomical72 interhemispheric connectivity may be linked to rightward asymmetry shifts.
We also explored potential links with diagnostic and IQ scores. Given the large number of comparisons performed, none reached significance after Bonferroni correction. While it may be noted that in a few cases asymmetry of frontoparietal components accounted for more than 25% of the variance of diagnostic and IQ scores, it is obvious that symptom severity is affected by numerous other developmental factors (biological, environmental, pharmaceutical, and treatment related).
Some potential limitations should be noted. First, since detection of asymmetry differences between groups relied on accurate spatial matching of the ICA components, we applied highly conservative quantitative matching criteria. Nonetheless, the higher number of components for the TD group detected in the ADE analysis was remarkable. This was partly driven by a greater number of true-signal components in the TD group, which is consistent with recent findings of reduced cortical functional differentiation in ASD73,74 as specialization and differentiation will be associated with a greater number of independent components. Second, no fully quantitative method for removal of noise components in ICA is currently available. We applied established procedures40 with an added quantitative gray matter criterion. Inspection of all components from the FDE analysis (eFigure 2 in Supplement) indicates that the pattern of our findings did not reflect misclassification (eg, erroneous classification of strongly left lateralizing components in the ASD group as noise). Finally, only high-functioning children and adolescents with ASD were included in this imaging study because low-functioning children (with full-scale IQs below70) cannot usually hold still during MRI scanning. The specific findings from this study may therefore not fully apply to low-functioning segments of the ASD population.
In conclusion, while a number of neuro imaging studies have assessed atypical lateralization in ASD, they have almost exclusively focused on language. Using a data-driven ICA approach, we identified a large number of components with atypical a symmetry, including components related to sensorimotor, visual, executive, attention, and language functions. Without exception, for components in all of these domains, asymmetry was shifted toward the right hemisphere in the ASD group. The results of our study suggest that atypical rightward asymmetry may be a general feature of brain organization in ASD, affecting many different functional brain systems.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by the National Institutes of Health grant R01-MH081023, with additional funding from grants T34-MH65102 (Mr Cardinale), T32-MH020068 (Ms Shih), and K01-MH097972 (Dr Fishman).
Footnotes
Author Contributions: Dr Müller had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Mr Cardinale and Ms Shih contributed equally to this study.
Additional Contributions: Special thanks to the participants and their families.We also thank Kun Lu, PhD (University of California, San Diego), for technical assistance.
Conflict of Interest Disclosures: None reported.
Previous Presentation: This study was presented in part at the Annual Meeting of the Society for Neuroscience; November 17, 2010; San Diego, California.
Supplemental content at jamapsychiatry.com
References
- 1.Levy SE, Mandell DS, Schultz RT. Autism. Lancet. 2009;374(9701):1627–1638. doi: 10.1016/S0140-6736(09)61376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Müller RA, Shih P, Keehn B, Deyoe JR, Leyden KM, Shukla DK. Underconnected, but how? a survey of functional connectivity MRI studies in autism spectrum disorders. Cereb Cortex. 2011;21(10):2233–2243. doi: 10.1093/cercor/bhq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schipul SE, Keller TA, Just MA. Inter-regional brain communication and its disturbance in autism. Front Syst Neurosci. 2011;5:10. doi: 10.3389/fnsys.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vissers ME, Cohen MX, Geurts HM. Brain connectivity and high functioning autism: a promising path of research that needs refined models, methodological convergence, and stronger behavioral links. Neurosci Biobehav Rev. 2012;36(1):604–625. doi: 10.1016/j.neubiorev.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 5.De Fossé L, Hodge SM, Makris N, et al. Language-association cortex asymmetry in autism and specific language impairment. Ann Neurol. 2004;56(6):757–766. doi: 10.1002/ana.20275. [DOI] [PubMed] [Google Scholar]
- 6.Herbert MR, Ziegler DA, Deutsch CK, et al. Brain asymmetries in autism and developmental language disorder: a nested whole-brain analysis. Brain. 2005;128(pt 1):213–226. doi: 10.1093/brain/awh330. [DOI] [PubMed] [Google Scholar]
- 7.Wan CY, Marchina S, Norton A, Schlaug G. Atypical hemispheric asymmetry in the arcuate fasciculus of completely nonverbal children with autism. Ann N Y Acad Sci. 2012;1252(1):332–337. doi: 10.1111/j.1749-6632.2012.06446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boddaert N, Belin P, Chabane N, et al. Perception of complex sounds: abnormal pattern of cortical activation in autism. Am J Psychiatry. 2003;160(11):2057–2060. doi: 10.1176/appi.ajp.160.11.2057. [DOI] [PubMed] [Google Scholar]
- 9.Flagg EJ, Cardy JE, Roberts W, Roberts TP. Language lateralization development in children with autism: insights from the late field magnetoencephalogram. Neurosci Lett. 2005;386(2):82–87. doi: 10.1016/j.neulet.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 10.Kleinhans NM, Müller RA, Cohen DN, Courchesne E. Atypical functional lateralization of language in autism spectrum disorders. Brain Res. 2008;1221:115–125. doi: 10.1016/j.brainres.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knaus TA, Silver AM, Kennedy M, et al. Language laterality in autism spectrum disorder and typical controls: a functional, volumetric, and diffusion tensor MRI study. Brain Lang. 2010;112(2):113–120. doi: 10.1016/j.bandl.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Müller RA, Behen ME, Rothermel RD, et al. Brain mapping of language and auditory perception in high-functioning autistic adults: a PET study. J Autism Dev Disord. 1999;29(1):19–31. doi: 10.1023/a:1025914515203. [DOI] [PubMed] [Google Scholar]
- 13.Eyler LT, Pierce K, Courchesne E. A failure of left temporal cortex to specialize for language is an early emerging and fundamental property of autism. Brain. 2012;135(pt 3):949–960. doi: 10.1093/brain/awr364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow ML, Pramparo T, Winn ME, et al. Age-dependent brain gene expression and copy number anomalies in autism suggest distinct pathological processes at young versus mature ages. PLoS Genet. 2012;8(3):e1002592. doi: 10.1371/journal.pgen.1002592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawson G. Lateralized brain dysfunction in autism: evidence from the Halstead-Reitan neuropsychological battery. J Autism Dev Disord. 1983;13(3):269–286. doi: 10.1007/BF01531566. [DOI] [PubMed] [Google Scholar]
- 16.Fein D, Humes M, Kaplan E, Lucci D, Waterhouse L. The question of left hemisphere dysfunction in infantile autism. Psychol Bull. 1984;95(2):258–281. doi: 10.1037//0033-2909.95.2.258. [DOI] [PubMed] [Google Scholar]
- 17.Happé F. Autism: cognitive deficit or cognitive style? Trends Cogn Sci. 1999;3(6):216–222. doi: 10.1016/s1364-6613(99)01318-2. [DOI] [PubMed] [Google Scholar]
- 18.Ozonoff S, Miller JN. An exploration of right-hemisphere contributions to the pragmatic impairments of autism. Brain Lang. 1996;52(3):411–434. doi: 10.1006/brln.1996.0022. [DOI] [PubMed] [Google Scholar]
- 19.D’Cruz AM, Mosconi MW, Steele S, et al. Lateralized response timing deficits in autism. Biol Psychiatry. 2009;66(4):393–397. doi: 10.1016/j.biopsych.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dawson G, Warrenburg S, Fuller P. Hemisphere functioning and motor imitation in autistic persons. Brain Cogn. 1983;2(4):346–354. doi: 10.1016/0278-2626(83)90018-0. [DOI] [PubMed] [Google Scholar]
- 21.Calhoun VD, Liu J, Adali T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage. 2009;45(1 suppl):S163–S172. doi: 10.1016/j.neuroimage.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laird AR, Fox PM, Eickhoff SB, et al. Behavioral interpretations of intrinsic connectivity networks. J Cogn Neurosci. 2011;23(12):4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP. Reliable intrinsic connectivity networks: test-retest evaluation using ICA and dual regression approach. Neuroimage. 2010;49(3):2163–2177. doi: 10.1016/j.neuroimage.2009.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103(1):297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 28.Damoiseaux JS, Rombouts SA, Barkhof F, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shehzad Z, Kelly AM, Reiss PT, et al. The resting brain: unconstrained yet reliable. Cereb Cortex. 2009;19(10):2209–2229. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 31.Lord C, Risi S, Lambrecht L, et al. The Autism Diagnostic Observation Schedule-Generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- 32.Wechsler D. Wechsler Abbreviated Scale of Intelligence. New York, NY: The Psychological Corporation; 1999. [Google Scholar]
- 33.Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 34.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 35.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Stuttgart, Germany: Georg Thieme; 1988. [Google Scholar]
- 36.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 37.Woolrich MW, Jbabdi S, Patenaude B, et al. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45(1suppl):S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- 38.Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23(2):137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- 39.Minka TP. Automatic choice of dimensionality for PCA. Adv Neural Inf Process Syst. 2000;13:598–604. [Google Scholar]
- 40.Kelly RE, Jr, Alexopoulos GS, Wang Z, et al. Visual inspection of independent components: defining a procedure for artifact removal from fMRI data. J Neurosci Methods. 2010;189(2):233–245. doi: 10.1016/j.jneumeth.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beckmann CF, Mackay CE, Filippini N, Smith SM. Group comparison of resting-state FMRI data using multi-subject ICA and dual regression. Neuroimage. 2009;47(suppl 1):S148. [Google Scholar]
- 42.Filippini N, MacIntosh BJ, Hough MG, et al. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A. 2009;106(17):7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Redcay E, Courchesne E. Deviant functional magnetic resonance imaging patterns of brain activity to speech in 2-3-year-old children with autism spectrum disorder. Biol Psychiatry. 2008;64(7):589–598. doi: 10.1016/j.biopsych.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaffrey MS, Kleinhans NM, Haist F, et al. Atypical [corrected] participation of visual cortex during word processing in autism: an fMRI study of semantic decision. Neuropsychologia. 2007;45(8):1672–1684. doi: 10.1016/j.neuropsychologia.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gendry Meresse I, Zilbovicius M, Boddaert N, et al. Autism severity and temporal lobe functional abnormalities. Ann Neurol. 2005;58(3):466–469. doi: 10.1002/ana.20597. [DOI] [PubMed] [Google Scholar]
- 46.Tesink CM, Buitelaar JK, Petersson KM, et al. Neural correlates of pragmatic language comprehension in autism spectrum disorders. Brain. 2009;132(pt 7):1941–1952. doi: 10.1093/brain/awp103. [DOI] [PubMed] [Google Scholar]
- 47.Gage NM, Juranek J, Filipek PA, et al. Rightward hemispheric asymmetries in auditory language cortex in children with autistic disorder: an MRI investigation. J Neurodev Disord. 2009;1(3):205–214. doi: 10.1007/s11689-009-9010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mason RA, Williams DL, Kana RK, Minshew N, Just MA. Theory ofmind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia. 2008;46(1):269–280. doi: 10.1016/j.neuropsychologia.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Müller RA, Courchesne E, Allen G. The cerebellum: so much more. Science. 1998;282(5390):879–880. doi: 10.1126/science.282.5390.879d. [DOI] [PubMed] [Google Scholar]
- 50.Wang AT, Lee SS, Sigman M, Dapretto M. Neural basis of irony comprehension in children with autism: the role of prosody and context. Brain. 2006;129(pt 4):932–943. doi: 10.1093/brain/awl032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Connor K. Auditory processing in autism spectrum disorder: a review. Neurosci Biobehav Rev. 2012;36(2):836–854. doi: 10.1016/j.neubiorev.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 52.Marco EJ, Hinkley LB, Hill SS, Nagarajan SS. Sensory processing in autism: a review of neurophysiologic findings. Pediatr Res. 2011;69(5, pt 2):48R–54R. doi: 10.1203/PDR.0b013e3182130c54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gowen E, Hamilton A. Motor abilities in autism: a review using a computational context. J Autism Dev Disord. 2013;43(2):323–344. doi: 10.1007/s10803-012-1574-0. [DOI] [PubMed] [Google Scholar]
- 54.Dakin S, Frith U. Vagaries of visual perception in autism. Neuron. 2005;48(3):497–507. doi: 10.1016/j.neuron.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 55.Samson F, Mottron L, Soulieres I, Zeffiro TA. Enhanced visual functioning in autism: an ALE meta-analysis. Hum Brain Mapp. 2012;33(7):1553–1581. doi: 10.1002/hbm.21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simmons DR, Robertson AE, McKay LS, Toal E, McAleer P, Pollick FE. Vision in autism spectrum disorders. Vision Res. 2009;49(22):2705–2739. doi: 10.1016/j.visres.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 57.Gilbert SJ, Bird G, Brindley R, Frith CD, Burgess PW. Atypical recruitment of medial prefrontal cortex in autism spectrum disorders: an fMRI study of two executive function tasks. Neuropsychologia. 2008;46(9):2281–2291. doi: 10.1016/j.neuropsychologia.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Castelli F, Frith C, Happé F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125(pt 8):1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- 59.Senju A. Spontaneous theory ofmind and its absence in autism spectrum disorders. Neuroscientist. 2012;18(2):108–113. doi: 10.1177/1073858410397208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hill EL. Executive dysfunction in autism. Trends Cogn Sci. 2004;8(1):26–32. doi: 10.1016/j.tics.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 61.Allen G, Courchesne E. Attention function and dysfunction in autism. Front Biosci. 2001;6:D105–D119. doi: 10.2741/allen. [DOI] [PubMed] [Google Scholar]
- 62.Keehn B, Müller R-A, Townsend J. Atypical attentional networks and the emergence of autism. Neurosci Biobehav Rev. 2013;37(2):164–183. doi: 10.1016/j.neubiorev.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Just MA, Keller TA, Malave VL, Kana RK, Varma S. Autism as a neural systems disorder: a theory of frontal-posterior underconnectivity. Neurosci Biobehav Rev. 2012;36(4):1292–1313. doi: 10.1016/j.neubiorev.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lo YC, Soong WT, Gau SS, et al. The loss of asymmetry and reduced interhemispheric connectivity in adolescents with autism: a study using diffusion spectrum imaging tractography. Psychiatry Res. 2011;192(1):60–66. doi: 10.1016/j.pscychresns.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 65.Hutsler J, Galuske RA. Hemispheric asymmetries in cerebral cortical networks. Trends Neurosci. 2003;26(8):429–435. doi: 10.1016/S0166-2236(03)00198-X. [DOI] [PubMed] [Google Scholar]
- 66.Sun T, Patoine C, Abu-Khalil A, et al. Early asymmetry of gene transcription in embryonic human left and right cerebral cortex. Science. 2005;308(5729):1794–1798. doi: 10.1126/science.1110324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lambert N, Lambot MA, Bilheu A, et al. Genes expressed in specific areas of the human fetal cerebral cortex display distinct patterns of evolution. PLoS One. 2011;6(3):e17753. doi: 10.1371/journal.pone.0017753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun T, Walsh CA. Molecular approaches to brain asymmetry and handedness. Nat Rev Neurosci. 2006;7(8):655–662. doi: 10.1038/nrn1930. [DOI] [PubMed] [Google Scholar]
- 69.Eyler LT, Pierce K, Courchesne E. A failure of left temporal cortex to specialize for language is an early emerging and fundamental property of autism. Brain. 2012;135(pt 3):949–960. doi: 10.1093/brain/awr364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anderson JS, Druzgal TJ, Froehlich A, et al. Decreased interhemispheric functional connectivity in autism. Cereb Cortex. 2011;21(5):1134–1146. doi: 10.1093/cercor/bhq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dinstein I, Pierce K, Eyler L, et al. Disrupted neural synchronization in toddlers with autism. Neuron. 2011;70(6):1218–1225. doi: 10.1016/j.neuron.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alexander AL, Lee JE, Lazar M, et al. Diffusion tensor imaging of the corpus callosum in Autism. Neuroimage. 2007;34(1):61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 73.Rudie JD, Shehzad Z, Hernandez LM, et al. Reduced functional integration and segregation of distributed neural systems underlying social and emotional information processing in autism spectrum disorders. Cereb Cortex. 2012;22(5):1025–1037. doi: 10.1093/cercor/bhr171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shih P, Keehn B, Oram JK, Leyden KM, Keown CL, Müller R-A. Functional differentiation of posterior superior temporal sulcus in autism: a functional connectivity magnetic resonance imaging study. Biol Psychiatry. 2011;70(3):270–277. doi: 10.1016/j.biopsych.2011.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.