Abstract
Background
Hormonal status influences hemostatic factors including fibrinogen, factor VII and plasminogen activator inhibitor (PAI-1), and concentrations differ among men, premenopausal and postmenopausal women. This study examines how phases of the menstrual cycle influence variability of fibrinogen, factor VII and PAI-1.
Design
We studied 103 subjects (39 premenopausal women, 18 postmenopausal women, and 46 men) during three, randomized, 8-week energy and nutrient-controlled experimental diets in the DELTA (Dietary Effects on Lipids and Thrombotic Activity) Study. Fasting blood samples were collected weekly during the last four weeks of each diet period and hemostatic factors were quantified. Two linear mixed-effects models were used for fibrinogen, factor VII and PAI-1: one to estimate and compare group-specific components of variance, the other to estimate additional fixed effects representing cyclical functions of day of menstrual cycle in premenopausal women.
Results
Systematic cyclical variation with day of menstrual cycle was observed for fibrinogen (p<0.0001), factor VII (p=0.0012), and PAI-1 (p=0.0024) in premenopausal women. However, the amplitude of cycling was small relative to the total magnitude of intra-individual variability. In addition, the intra-individual variance and corresponding coefficient of variation observed in premenopausal women did not differ from postmenopausal women and men.
Conclusions
The variability in hemostatic factors in premenopausal women is no greater than for postmenopausal women or men. Consequently, premenopausal women can be included in studies investigating hemostatic factor responses without controlling for stage of menstrual cycle.
Keywords: menstrual cycle, fibrinogen, factor VII, plasminogen activator inhibitor, controlled dietary intervention
INTRODUCTION
There are many established and novel biomarkers which are predictors of cardiovascular disease (CVD) events (reviewed in [1]). They span multiple biological processes including lipid deposition (atherogenesis), inflammation, hemostasis, thrombosis, and oxidative stress. The utility of these biomarkers for determining CVD risk and improvements in risk status following clinical intervention is affected by their biological variability.
Hemostatic factors such as fibrinogen, factor VII and plasminogen activator inhibitor (PAI-1) are affected by hormonal status [2]. While the observed differences are mixed with respect to apparent pro- and anti-coagulant function, it is likely these differences have clinical significance since exogenous estrogen is associated with increased risk of thrombosis [3].
Much of what we know about sex hormone status and coagulation status comes from studies of exogenous estrogen, and is reasonably consistent. Various studies have reported that postmenopausal women taking hormone therapy (HT; estrogen in combination with progestin) had significantly lower plasma levels of fibrinogen, factor VII, tissue plasminogen activator (t-PA), E-selectin, and PAI-1 than postmenopausal women not taking HT [4].. However, exogenous hormone therapy raises estrogen levels beyond those observed during normal menstrual cycles. Therefore, it is unclear whether hormonal changes during the normal menstrual cycle would influence hemostatic factors to the same degree as HT. However, the few studies that have investigated the effects of hormone fluctuations during the menstrual cycle on hemostatic factors have been inconsistent [5].
To assess whether cyclical changes in fibrinogen, factor VII and PAI-1 occur during the menstrual cycle, we analyzed these hemostatic factors at multiple time-points during three different menstrual cycles (to reduce within subject variance) in subjects who participated in a well-controlled, multicenter feeding study. This design mitigated confounding effects induced by self-selected diet variations that often occur over the menstrual cycle [6]. We hypothesized that fluctuations in hemostatic factors observed across the menstrual cycle in premenopausal women would not differ from post-menopausal women and men.
MATERIALS AND METHODS
Study Design
This study was part of the multicenter DELTA (Dietary Effects on Lipids and Thrombotic Activity) Study that was carried out at Columbia University, Pennington Biomedical Research Center, The Pennsylvania State University, and the University of Minnesota; the Coordinating Center was at the University of North Carolina, Chapel Hill and the Central Hemostasis Laboratory at the University of Vermont between September 1993 and December 1995. In brief, subjects were studied over three 8-week periods during which time they were fed three different diets that contained varying amounts of total fat and saturated fat (26, 29, and 35% of calories from fat with 6, 9 and 15% from saturated fat, respectively), and 300 mg/day of cholesterol. Trans fatty acids contributed less than 1.5% of calories. The nutrient composition of the test diets was verified by chemical assay. Diets were assigned in a randomized crossover design and were adjusted in calories to assure that weight did not change during the study. All meals were provided during the 8-week feeding periods. During weekdays, participants were required to eat two meals each day on site and were provided with a third packaged meal. On weekends, all meals except Saturday dinner were provided; this meal was self-chosen with guidance from study staff. Compliance was confirmed via on site tray checks and self-reported dietary compliance forms. The study design and methodologies used have been described elsewhere [7–11]. Reporting of the study conforms to STROBE statement along with references to STROBE statement and the broader EQUATOR guidelines [12].
Subjects
103 healthy normolipidemic adults, aged 22–67 years were enrolled: 39 premenopausal women (22–51 years), 18 postmenopausal women (43–67 years), and 46 men (22–65 years) [8]. Because circulating concentrations of fibrinogen and PAI-1 increase with age, we divided men into 2 groups; < 40 years, (n=30) and ≥ 40 years (n=16) to provide comparison groups for premenopausal and postmenopausal women, respectively. 25% of the subjects were black and 75% were white. Subjects were recruited in a multi-step process that included an initial phone screening (age, ethnicity, review of major exclusion criteria), and 2 center visits. Visit 1 gathered detailed medical, social and diet history, and excluded individuals who had a BMI>32 kg/m2 and total cholesterol <25th percentile or >90th percentile. If subjects were not excluded at this visit, they returned for a fasting blood sample (complete blood count, chemistry screen and lipid profile). Plasma triglycerides and HDL cholesterol, measured at the last screening visit, had to be below the 90th and above the 10th percentile, respectively. Subjects also were required to be free of chronic disease (including documented cardiovascular, renal, gastrointestinal disease, hypertension and diabetes, and cancer within the previous 5 years) and taking no medications known to modify lipids or thrombotic factors. At time of recruitment, premenopausal women were free of known menstrual cycle abnormalities, and had self-reported menstrual cycles of ~28-days. This was validated by menstrual calendars identifying days of menses during each 8-week feeding period. Premenopausal women were not permitted to take oral contraceptives from the levonorgestrel category due to their potential to influence lipid and lipoprotein metabolism [13]. A small number of women (n=6) taking other forms of oral contraceptives were included. Postmenopausal women were not permitted to be on HT. All subjects signed a consent form to participate, and protocols were approved by the Institutional Review Boards at each Research Center.
Measurements
Blood samples were collected once per week during the last four weeks of each 8-week diet period (there were three 8-week diet periods in the study). Thus, for each subject 12 samples were analyzed for hemostatic factors (four samples per diet period). Subjects fasted for at least 12 hours, and samples were collected in the morning to minimize the effect of diurnal variation in PAI-1. Subjects were seated for ten minutes before tourniquets were applied. One serum tube (clot activated) and two citrate tubes were processed with a tourniquet time of less than 2 minutes to avoid stasis. Citrated blood for fibrinogen and PAI-1 was placed on ice immediately. Citrated blood for factor VII was maintained at room temperature to avoid cold-activation of factor VII [14]. Samples were centrifuged for 30,000 g-minutes to remove platelets. The platelet-poor plasma and serum samples were then frozen at −80°C within 1–3 hours of collection. The blood collection and processing protocol was standardized within and across participating institutions.
Citrated plasma samples were analyzed for fibrinogen, factor VII and PAI-1. Fibrinogen was measured using the clot-rate method of Clauss [15]. Factor VII was assessed using a one-stage clot-rate assay based upon prothrombin time, using immunodeficient plasma and placental thromboplastin (standardized to World Health Organization reference plasma) [16]. PAI-1 antigen was measured using an ELISA method originally developed by Collen and colleagues [17]. This method is sensitive to free and latent forms of PAI-I antigen but not complexed forms; however, it exhibits a high degree of correlation with an assay of total PAI-1 (Asserachrome PAI-1 antigen, Diagnostica Stago; R = 0.82), and with PAI-1 activity (R = 0.77 [18]). Given the relatively stringent requirements for blood collection for a PAI-1 activity assay, coupled with the multi-center nature of DELTA and the fact that much of epidemiological data linking PAI-1 to diabetes and cardiovascular risk was assembled with assays for either uncomplexed PAI-1 or total PAI-1 [19], we chose to use the optimal PAI-1 ELISA available at that time. All samples from an individual participant were thawed and analyzed immediately, in duplicate, in a single batch on the same day. Coefficients of variation (CV) for the analytical methods were: fibrinogen (3.1 %), factor VII (5.3%) and PAI-1 (9.0%).
Serum samples from premenopausal women were analyzed for estradiol, luteinizing hormone (LH), and progesterone by radioimmunoassays (Diagnostics Products Corporation, Los Angeles, CA). These hormonal analyses were used along with the menstrual calendar to identify the phases of the menstrual cycle.
Statistical Analyses
The original study was powered on a a sample size of 96, which provided 90% power for detecting a 6.58-mg/dL change in total cholesterol when any two diets were compared via a test procedure of size α=0.01. Comparable values for LDL cholesterol, HDL cholesterol, triglycerides, and Lp(a) were 5.45, 2.54, 1.11, and 0.02 mg/dL [8]. Distributions of fibrinogen, factor VII and PAI-1 were examined and PAI-1 values were square root-transformed. For each variable two separate linear mixed-effects models [20] were fitted conditional on diet and subpopulation (premenopausal women, postmenopausal women, men< 40 years, men >40 years) to estimate and compare subpopulation-specific means and components of variance: model #1 did not include any predictor variables representing day of menstrual cycle, model #2 included additional fixed effects that represented the response for each premenopausal woman as cyclical functions of day of menstrual cycle. The cyclical functions were defined in terms of a constant term plus lower order sine and cosine functions as specified by the discrete Fourier transform: sin(2π Day/28), cos (2π Day/28), sin (4π Day/28), cos (4π Day/28)].
Both linear models included terms representing subpopulation, diet (A, B, C), calorie level (1500, 2000, 2500, 3000kcal.), period (1, 2, 3), race (W, B) and. research center. Both linear models assumed three components of variance: inter-subject variation around the overall subpopulation mean, inter-subject variation in diet-specific mean levels, and residual intraindividual variation. The magnitudes of these components of variance were assumed to be subpopulation specific.
To evaluate the robustness of the primary results to reasonable perturbations of the statistical methods used, auxiliary models were also fitted; e.g., models which included additional cyclical-function random effects, models which allowed variable-length menstrual cycles, and models with categorical clustering of the days within each cycle.
Among the premenopausal women, the index day (day 1) of the menstrual cycle was defined as the first day of menses as recorded in self-reported menstrual calendars. As an aid to interpretation of the primary analyses, linear mixed-effects models similar to model #2 were fitted on log10 scale and linear scale for estradiol, LH, and progesterone as a function of day of menstrual cycle.
Four phases of the menstrual cycle were defined by classifying days 3–9 as the follicular phase, days 10–16 as the ovulatory phase (based on time of elevation of LH), days 17–23 as a luteal phase, and days 24-2 as late luteal/menses phase (based on a fall to minima for all three hormones). This classification is consistent with that described in a previous publication from the same investigators, reporting on cyclic variations in lipids and lipoproteins [21].
RESULTS
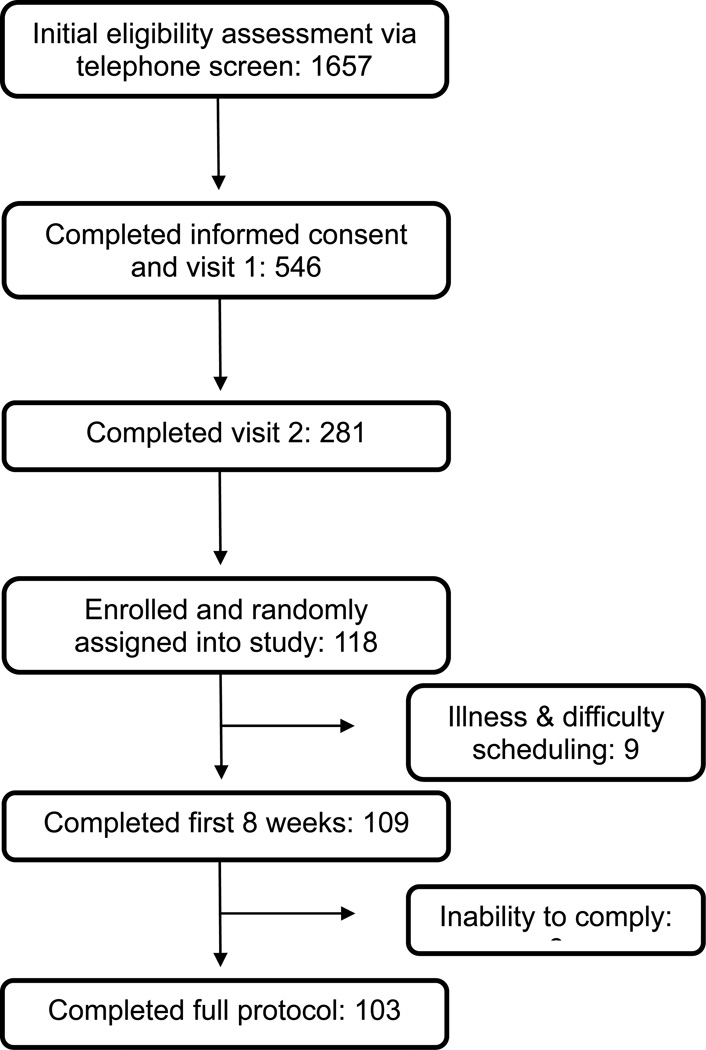
One-hundred and eighteen individuals were enrolled and randomized to a dietary treatment. During the first eight weeks of the study 9 subjects withdrew due to illness and scheduling difficulties. A further 6 subjects were unable to adhere to the study requirements, resulting in incomplete data. A total of 103 subjects with complete data were included in the final analysis (Figure 1).
Figure 1. Flow chart for selection of study participants.
All subjects in our study were healthy individuals. Their circulating concentrations of fibrinogen (200–400 mg/dL), factor VII (75–130%) and PAI-1 (0–40 ng/ml) were within the expected reference range for healthy adults (Table 1). Although not the focus of this paper, it is important to note that the effects of diet on these hemostatic factors were small and differed among the three diet treatments by ±10%; consequently, the inclusion of diet in the statistical model did not influence the main study outcomes. BMI (kg/m2) was not statistically different among groups. Age did not differ significantly between men in the <40 years group and pre-menopausal women. Post-menopausal women were older than men in the >40 years group (P=0.0034).
Table 1.
Intraindividual variability of hemostatic factors in men and women
| Women | Men | ||||
|---|---|---|---|---|---|
| Premenopausal (n=39) |
Postmenopausal (n=18) |
<40 years (n=30) |
> 40 years (n=16) |
||
| Age, y: Mean ± SD | 31.0 ± 8.2 | 57.5 ±7.8 | 28.5 ± 5.5 | 50.1 ± 6.8 | |
| Age, y: Range: | 22-51 | 43-67 | 22-39 | 41-65 | |
| BMI, kg/m2 Mean ± SD | 23.8 ± 3.4 | 25.7 ± 3.6 | 24.5 ± 2.8 | 25.2 ± 3.4 | |
| BMI, kg/m2 Range: | 17.3-29.8 | 18.8-32.1 | 18.5-30.8 | 18.8-30.9 | |
| Fibrinogen (mg/dl) |
mean a | 277 ± 16 | 326 ± 27 | 241 ± 16 | 284 ± 20 |
| variance b | 656 ± 88 | 827 ± 184 | 754 ± 130 | 459 ± 108 | |
| SD c | 25.6 | 28.8 | 27.5 | 21.4 | |
| CV (%) d | 9.2 | 8.8 | 11.4 | 7.6 | |
| Factor VII (%) | mean | 81 ± 6 | 97 ± 9 | 88 ± 7 | 91 ± 7 |
| variance | 37 ± 5 | 124 ± 27 | 31 ± 5 | 23 ± 5 | |
| SD | 6.04 | 11.1 | 5.57 | 4.78 | |
| CV (%) | 7.4 | 11.8 | 6.3 | 5.2 | |
| square root PAI-1 (ng/ml) |
mean | 2.4 ± 0.6 | 3.2 ± 0.8 | 2.7 ± 0.6 | 2.9 ± 0.8 |
| variance | 0.47 ± 0.06 | 0.55 ± 0.12 | 0.68 ± 0.12 | 0.29 ± 0.06 | |
| SD | 0.684 | 0.734 | 0.825 | 0.537 | |
| CV (%) | 28.5 | 23.4 | 30.1 | 18.4 | |
Estimate of the mean ± 1.96 standard errors (provides an approximate 95% confidence interval)
Estimate of residual intra-individual variance ± 1.96 standard errors (provides an approximate 95% confidence interval)
Standard deviation (SD) computed as the square root of the estimate of intra-individual variance.
Coefficient of variation (CV) computed as 100% × ( SD / mean ).
Table 2 summarizes the mean serum concentrations of estradiol, progesterone, and LH for four phases of the menstrual cycle in premenopausal women. These concentrations are within the expected range for each of the four phases.
Table 2.
Hormone Concentrations in Premenopausal Women during Phases of the Menstrual Cycle*
| follicular phasea days 3–9 |
ovulatory phaseb days 10–16 |
luteal phase c days 17–23 |
late luteal/menses d days 24–28 |
|
|---|---|---|---|---|
| Estradiol (pg/mL) | 48 ±14 | 118 ±22 | 121 ±25 | 95 ±14 |
| Progesterone (ng/mL) | 1.3 ±1.2 | 2.0 ±1.1 | 8.5 ±1.7 | 6.1 ±1.3 |
| Luteinizing hormone (IU/mL) | 2.2 ±1.1 | 7.2 ±1.2 | 3.6 ±1.2 | 3.1 ±1.2 |
Estimate of the mean ±1.96 standard errors (provides an approximate 95% confidence interval). Estimates were obtained from a linear mixed-effects model for data from 39 premenopausal women.
Follicular phase was defined by low progesterone and estradiol and rising LH; estimate of the mean is for day 6.
Ovulatory phase was defined by low progesterone, high estradiol, high LH; estimate of the mean is for day 14.
Luteal phase was defined as high progesterone, high estradiol, and falling LH; estimate of the mean is for day 20.5.
Late luteal/menses phase was defined by falling progesterone and estradiol and minimum LH; estimate of the mean is for day 26.5.
Table 3 presents mean plasma concentrations of these hemostatic factors during the four phases of the menstrual cycle in premenopausal women. Cyclical variation with day of menstrual cycle was detected for fibrinogen (p<0.0001), factor VII (p=0.0012), and PAI-1 (p=0.0024). Fibrinogen was lowest during ovulation, while factor VII and PAI-1 were lowest during menses and the luteal phase, respectively.
Table 3.
Fibrinogen, Factor VII and PAI-1 Concentrations in Premenopausal Women during Phases of the Menstrual Cycle*
| follicular phasea days 3–9 |
ovulatory phaseb days 10–16 |
luteal phasec days 17–23 |
late luteal/menses d days 24–28 |
p-value† | |
|---|---|---|---|---|---|
| Fibrinogen (mg/dL) | 271 ±17 | 269 ±17 | 281 ±17 | 288 ±16 | < 0.0001 |
| Factor VII (%) | 81.6 ±6.0 | 82.4 ±6.0 | 81.6 ±6.0 | 79.2 ±6.0 | 0.0012 |
| square root PAI-1 (ng/dL) | 2.48 ±0.67 | 2.45 ±0.67 | 2.18 ±0.67 | 2.41 ±0.67 | 0.0024 |
Entry is the estimate of the mean ±1.96 standard errors (provides an approximate 95% confidence interval). Estimates were obtained from a linear mixed-effects model for data from 39 premenopausal women.
Follicular phase was defined by low progesterone and estradiol and rising LH; estimate of the mean is for day 6.
Ovulatory phase was defined by low progesterone, high estradiol, high LH; estimate of the mean is for day 14.
Luteal phase was defined as high progesterone, high estradiol, and falling LH; estimate of the mean is for day 20.5.
Late luteal/menses phase was defined by falling progesterone and estradiol and minimum LH; estimate of the mean is for day 26.5.
P-value for the F-test of the null hypothesis "concentration is not a function of day of menstrual cycle" (or equivalently, “the regression coefficients for the sine and cosine terms are all zero”)
Estimates of intra-individual variance for the hemostatic variables in each of the four subpopulations are presented in Table 1. These estimates were obtained from the linear mixed-effects model that did not include any predictor variables representing day of menstrual cycle (model #1). Compared to postmenopausal women and men under the age of 40, premenopausal women had a smaller intra-individual variance for fibrinogen and PAI-1. Intra-individual variance for factor VII was substantially less in premenopausal women and men compared to postmenopausal women. The four subpopulations did not differ markedly with respect to the corresponding CV estimates.
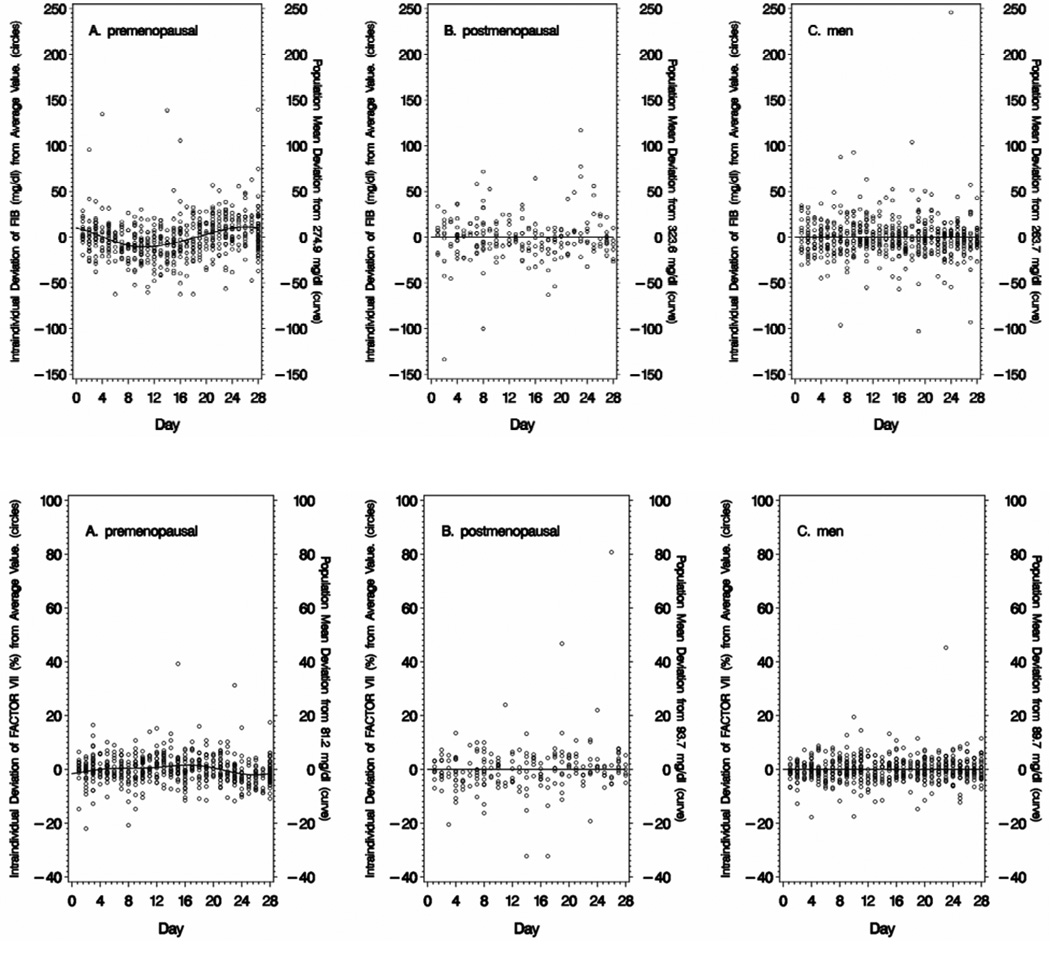
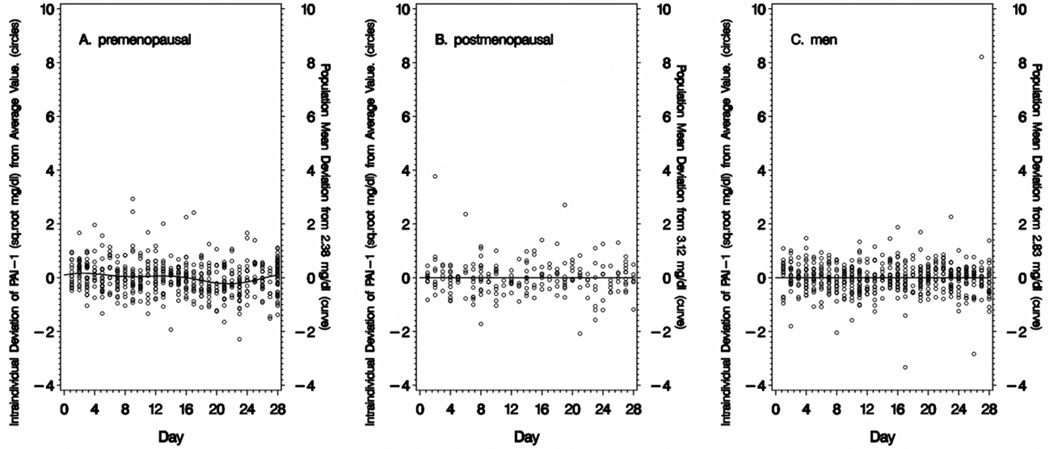
Intra-individual variation in fibrinogen, factor VII and square root PAI-1 is presented in Figure 2 for premenopausal women (Panel A), postmenopausal women (Panel B), and men (all ages combined) (Panel C). The line represents the deviation of the day-specific (time-varying) mean from the overall mean for the entire sample period (all 28 days). The time-varying means illustrate that distinct cyclical variation is present in premenopausal women but not in postmenopausal women or men.
Figure 2. Variability of Fibrinogen (FIB), Factor VII and Plasminogen Activator Inhibitor-1 (PAI-1) over 28-Days.
Data is displayed as deviation from a reference value. The curve or line represents the deviation of the sub-population mean for the analyte at that point in time compared with the overall population mean value for the entire time period (shown on the right y-axis). The circles represent the deviation of a single measurement of fibrinogen (FIB), factor VII or plasminogen activator inhibitor (PAI-1) for an individual from the overall mean value for that individual. Intraindividual variations are computed independently for each diet period. For premenopausal women (Panel A), the first day of menses was the index day - based on self-reported menstrual calendar and confirmed by hormone concentrations (estradiol, progesterone, luteinizing hormone). For postmenopausal women (Panel B) and men (Panel C) the index day was randomly selected for each subject.
The amplitude of the cycling in premenopausal women is small compared to the total magnitude of the intra-individual variation. For example, the amplitude of cycling in fibrinogen in premenopausal women is 18.8 mg/dL (from the minimum during the ovulation phase to the maximum at menses) (Table 3). This is considerably smaller than the intra-individual variation of 102.4 mg/dL (−2 SD to +2 SD) (Table 1).
DISCUSSION
Biologic variability is an important cause of uncertainty in interpreting values for clinical biomarkers. Whether a value is used to assign risk of disease (single measure) or to identify the effects of an intervention (serial measures), the uncertainty around the assayed value limits the validity of the interpretation. The continuing interest in identifying hemostatic factors that may contribute to thrombotic risk and to assess the effectiveness of interventions to modify those risk factors requires some basis for assuring that the change is more than a chance occurrence. For the three hemostatic factors evaluated, the current study characterized the contribution of systematic menstrual cycle variation to intra-individual variance.
Only a few studies have investigated the influence of hormone cycling on hemostasis during the menstrual cycle [22–26]. Giardina et al. [24] reported statistically significantly higher concentrations of PAI-1 and D-dimer, but not fibrinogen in the follicular compared with the luteal phase. Kapiotis et al. [25] also observed the greatest concentration of activated factor VII during the follicular phase. In comparison, Chung et al. [23] reported that PAI-1 levels were highest in the luteal phase (compared with the ovulatory phase), although the luteal phase was not significantly different from the follicular phase. However, PAI-1 levels were measured using a different PAI-1 assay than we used which prevents direct comparison with our study. In a small cohort, Andersson et al. [22] reported significant associations of progesterone and estrogen-maximum and minimum with fibrinogen, factor VII, and PAI-1. Koh et al. [26] did not detect differences in these hemostatic factors between phases of the menstrual cycle in healthy women. A recent systematic review [5] reported that few studies observed cyclical variation in hemostatic factors during the menstrual cycle. Of those that did, values were lowest during menstruation and the early follicular phase. Based on these observations, the authors suggest that sampling during these phases will provide maximal sensitivity for testing hemostatic variables. However, there are several limitations to this conclusion. Not all studies included in this review confirmed menstrual phases by measuring hormone levels, and sampling of hemostatic variables did not always occur during each of the four menstrual phases. Furthermore, the majority of studies quantified hemostatic factor endpoints during a single menstrual cycle, and therefore intraindividual variability could not be determined.
With respect to strengths, the present study, due to its rigorous design, is well positioned to provide definitive information about cyclic variations in the target population. This study includes the largest number of premenopausal women to date (n=39), in a well-controlled setting, where sampling was conducted over multiple menstrual cycles. Subjects were in a stable, healthy metabolic condition; they consumed a well-characterized diet and did not gain or lose weight during the eight-month trial. Blood samples were drawn by trained phlebotomists using a rigorous blood collection and processing protocol to minimize variation due to differences in phlebotomy and handling techniques. In addition, samples were assayed four times for each of three feeding periods, adding to the assessment of variability. Our study also compared premenopausal women with other populations, specifically postmenopausal women and men. Collectively, the results of our study and those in the literature indicate that there is some variance in hemostatic factors over the menstrual cycle, however, it is of small magnitude and comparable to that observed for postmenopausal women and men.
It also is important to note several weaknesses. First, our list of assays was limited to fibrinogen, factor VII and PAI-1. These were chosen as key measures related to coagulation and inflammation, however it might have proved interesting to include several other factors such as factor VIII and Thrombin –Associated Fibrinolysis Inhibitor (TAFI), which we hope to do in the future. While 39 premenopausal women is the largest study of this type of which we are aware, a larger sample size would have allowed exploration of additional topics such as possible interactions between menstrual cycle variation and hemostatic geneotypes such as Factor VLeiden.. Second, we included in our analysis a small number of premenopausal women (n=6) taking third generation oral contraceptives. A recent review describes substantial increased risk for venous thromboembolism (VTE) in users of second and third generation oral contraceptives compared to non-pregnant, non-users; third generation contraceptives carry greater risk of VTE than second-generation contraceptives [27]. While relative risk for VTE does not equate to the levels of the individual hemostatic markers we assessed, it is a good surrogate, and therefore the inclusion of premenopausal women taking third generation oral contraceptives may have influenced our results to a small degree. However, given that the intra-individual variance for fibrinogen, factor VII and PAI-1 was similar in men, pre- and postmenopausal women, this suggests minimal influence of this medication on the study outcomes. A more thorough comparison of the effect of oral contraceptives on hemostatic factors would have required recruitment of a substantially larger cohort of women, with equivalent numbers of users and nonusers of oral contraceptives.
In summary, our study characterized menstrual cycle variation in fibrinogen, factor VII and PAI-1, and evaluated the magnitude of intra-individual variance in these measures for four subpopulations. The ranges of intra-individual CVs reported in our study were similar to, or less than, the biologic variation reported in a 1-year monitoring study of 17 healthy young men with constant life-styles [28]. Importantly, premenopausal women, postmenopausal women, and men (all ages), were similar with respect to the magnitude of intra-individual variance for these hemostatic factors. In premenopausal women these results suggest that in spite of cyclical variations in fibrinogen, factor VII and PAI-1 due to the menstrual cycle, intra-individual variance is not inflated, and is not greater than postmenopausal women and men.
Our results indicate clearly that no special experimental design is needed for the evaluation of hemostatic factor responses to interventions in premenopausal women relative to postmenopausal women or men. This is important for investigators planning prospective studies of hemostatic factors who wish to choose a sample size that will provide adequate precision for estimators and adequate power for test procedures. Specifically, our results indicate that including premenopausal women in studies of fibrinogen, factor VII, and PAI-1 does not require an increase in sample size to control for stage of the menstrual cycle.
ACKNOWLEDGMENTS
This work was supported by NIH grants (5-U01-HL 049644, HL 049648, HL 049649, HL 049651, and HL 049659, and M01-NCRR 00645), and the NIH Office for Research on Women’s Health. The following companies made in-kind contributions of products: AARHUS, Bertoli USA, Best Foods, Campbell Soup Company, Del Monte Foods, General Mills, Hershey Foods Corporation, Institute of Shortening and Edible Oils, Kraft General Foods, Land O’Lakes, McCormick Incorporated, Nabisco Foods Group, Neomonde Baking Company, Palm Oil Research Institute, Park Corporation, Procter and Gamble, Quaker Oats, Ross Laboratories, Swift-Armour and Eckrich, Van Den Bergh Foods, Cholestech, and Lifelines Technology Incorporated.. The study sponsors did not have any role in the study design, in collection, analysis, and interpretation of data, or any other aspect of the manuscript.
Appendix
The DELTA Research Group is as follows: Columbia University: Henry N. Ginsberg, MD (Principal Investigator), Rajasekhar Ramakrishnan, DSc, Wahida Karmally, MS, RD, Lars Berglund, MD, PhD, Maliha Siddiqui, MS, RD, Niem-Tzu Chen, MS, Steve Holleran, BS, Colleen Johnson, RD, Roberta Holeman, Karen Chirgwin, Kellye Stennett, Lencey Ganga, Tajsudeen T. Towolawai, MBA, Minnie Myers, BS, Colleen Ngai, BS, Nelson Fontenez, BS, Jeff Jones, BS, Carmen Rodriguez, and Norma Useche; Pennington Biomedical Research Center: Michael Lefevre, PhD, and Paul Roheim, MD (Co-Principal Investigators), Donna Ryan, MD, Marlene M. Windhauser, PhD, RD, Catherine M. Champagne, PhD, RD, Donald Williamson, PhD, Richard Tulley, PhD, Ricky Brock, RN, Deonne Bodin, BS, MT, Betty Kennedy, MPA, Michelle Barkate, MS, RD, Elizabeth Foust, BS, and Deshoin York, BS; Pennsylvania State University: Penny Kris Etherton, PhD (Principal Investigator), Satya S. Jonnalagadda, PhD, Janice Derr, PhD, Abir Farhat-Wood, MS, Vikkie A. Mustad, MS, Kate Meaker, MS, Edward Mills, PhD, Mary-Ann Tilley, MS, RD, Helen Smiciklas-Wright, PhD, Madeline Sigman-Grant, RD, Jean Xavier-Guinard, PhD, Pamela Sechevich, MS, C. Channa Reddy, PhD, Andrea M. Mastro, PhD, and Allen Cooper, MD; University of Minnesota: Patricia J. Elmer, PhD (Principal Investigator), Aaron R. Folsom, MD, Nancy M. Van Heel, MS, RD, A. ChristineWold, RD, Kay L. Fritz, MA, RD, Joanne L. Slavin, PhD, and David R. Jacobs Jr, PhD; University of North Carolina at Chapel Hill: Barbara H. Dennis, PhD, and Paul W. Stewart, PhD (Co-Principal Investigators), C.E. Davis, PhD, James Hosking, PhD, Nancy Anderson, MSPH, Susan E. Blackwell, BS, Lynn Martin, MS, Hope Bryan, MS, W. Brian Stewart, BS, Jeffrey Abolafia, MA, Malachy Foley, BS, Conroy Zien, BA, Szu-Yun Leu, MS, Marson Youngblood, MPH, Thomas Goodwin, MAT, Monica Miles, and Jennifer Wehbie; Mary Imogene Bassett Research Institute: Thomas A. Pearson, MD, PhD, and Roberta G. Reed, PhD; University of Vermont: Russell P. Tracy, PhD, and Elaine Cornell, BS; Virginia Polytechnic and State University: Kent K. Stewart, PhD, and Katherine M. Phillips, PhD; Southern University: Bernestine B. McGee, PhD, RN, and Brenda Williams, BS; Beltsville Agricultural Research Center: Gary R. Beecher, PhD, Joanne M. Holden, MS, and Carol Davis, BS; and National Heart, Lung, and Blood Institute: Abby G. Ershow, ScD, David J. Gordon, MD, PhD, Michael Proschan, PhD, and Basil Rifkind, MD, FRCP.
Footnotes
The authors have indicated that they have no conflicts of interest.
CONTRIBUTIONS
PWS, MKF, PMKE, HNG, RPT, TAP, ML, RGR, PJE, and AGE designed and conducted the study. PWS performed the statistical analysis. AMH interpreted the data, drafted and prepared the manuscript. PWS, MKF, PMKE, HNG, RPT and AGE helped draft the manuscript. All authors have read and approved the final version of the manuscript.
REFERENCES
- 1.Ridker PM, Brown NJ, Vaughan DE, Harrison DG, Mehta JL. Established and Emerging Plasma Biomarkers in the Prediction of First Atherothrombotic Events. Circulation. 2004;109:IV6–IV19. doi: 10.1161/01.CIR.0000133444.17867.56. [DOI] [PubMed] [Google Scholar]
- 2.Trigg DE, Wood MG, Kouides PA, Kadir RA. Hormonal Influences on Hemostasis in Women. Semin Thromb Hemost. 2011;37:77–86. doi: 10.1055/s-0030-1270074. [DOI] [PubMed] [Google Scholar]
- 3.Cushman M. Epidemiology and Risk Factors for Venous Thrombosis. Semin Hematol. 2007;44:62–69. doi: 10.1053/j.seminhematol.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borgfeldt C, Li C, Samsioe G. Low-dose oral combination of 17[beta]-estradiol and norethisterone acetate in postmenopausal women decreases factor VII, fibrinogen, antithrombin and plasminogen activator inhibitor-1. Climacteric. 2004;7:78–85. doi: 10.1080/13697130310001651508. [DOI] [PubMed] [Google Scholar]
- 5.Knol HM, Kemperman RF, Kluin-Nelemans HC, Mulder AB, Meijer K. Haemostatic variables during normal menstrual cycle. A systematic review. Thromb Haemost. 2012;107:22–29. doi: 10.1160/TH11-07-0481. [DOI] [PubMed] [Google Scholar]
- 6.Lissner L, Stevens J, Levitsky D, Rasmussen K, Strupp B. Variation in energy intake during the menstrual cycle: implications for food-intake research. Am J Clin Nutr. 1988;48:956–962. doi: 10.1093/ajcn/48.4.956. [DOI] [PubMed] [Google Scholar]
- 7.Ginsberg HN. New directions in dietary studies and heart disease: The National Heart, Lung and Blood Institute sponsored multicenter study of Dietary Effects on Lipoproteins and Thrombogenic Activity. In: Longenecker JB, Kritchevsky D, Dremer MK, editors. Nutrition and Biotechnology in Heart Disease and Cancer. New York: Plenum Press; 1995. pp. 241–247. [DOI] [PubMed] [Google Scholar]
- 8.Ginsberg HN, Kris-Etherton P, Dennis B, Elmer PJ, Ershow A, Lefevre M, et al. Effects of Reducing Dietary Saturated Fatty Acids on Plasma Lipids and Lipoproteins in Healthy Subjects: The Delta Study, Protocol 1. Arterioscler Thromb Vasc Biol. 1998;18:441–449. doi: 10.1161/01.atv.18.3.441. [DOI] [PubMed] [Google Scholar]
- 9.Lefevre M, Ginsberg HN, Kris-Etherton PM, Elmer PJ, Stewart PW, Ershow A, et al. ApoE Genotype Does Not Predict Lipid Response to Changes in Dietary Saturated Fatty Acids in a Heterogeneous Normolipidemic Population. Arterioscler Thromb Vasc Biol. 1997;17:2914–2923. doi: 10.1161/01.atv.17.11.2914. [DOI] [PubMed] [Google Scholar]
- 10.Phillips K. Composition validation and monitoring as part of a multicenter clinical feeding trial of Step 1. FASEB Journal. 1996;10:A462. [Google Scholar]
- 11.Stewart K. Diet composition documentation in a multicenter clinical feeding trial. FASEB Journal. 1995;9:A289. [Google Scholar]
- 12.Simera I, Moher DHJ, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40:35–53. doi: 10.1111/j.1365-2362.2009.02234.x. [DOI] [PubMed] [Google Scholar]
- 13.Godsland IF, Crook D, Simpson R, Proudler T, Felton C, Lees B, et al. The Effects of Different Formulations of Oral Contraceptive Agents on Lipid and Carbohydrate Metabolism. New England Journal of Medicine. 1990;323:1375–1381. doi: 10.1056/NEJM199011153232003. [DOI] [PubMed] [Google Scholar]
- 14.Seligsohn U, Østerud B, Griffin JH, Rapaport SI. Evidence for the participation of both activated factor XII and activated factor IX in cold-promoted activation of factor VII. Thromb Res. 1978;13:1049–1056. doi: 10.1016/0049-3848(78)90233-5. [DOI] [PubMed] [Google Scholar]
- 15.Clauss A. Rapid physiological coagulation method in determination of fibrinogen. Acta Haematol. 1957;17:237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- 16.Cushman M, Cornell E, Howard P, Bovill E, Tracy R. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41:264–270. [PubMed] [Google Scholar]
- 17.Declerck PJ, Alessi MC, Verstreken M, Kruithof EK, Juhan-Vague I, Collen D. Measurement of plasminogen activator inhibitor 1 in biologic fluids with a murine monoclonal antibody-based enzyme-linked immunosorbent assay. Blood. 1988;71:220–225. [PubMed] [Google Scholar]
- 18.Juhan-Vague In, Pyke SDM, Alessi MC, Jespersen J, Haverkate F, Thompson SG, et al. Fibrinolytic Factors and the Risk of Myocardial Infarction or Sudden Death in Patients With Angina Pectoris. Circulation. 1996;94:2057–2063. doi: 10.1161/01.cir.94.9.2057. [DOI] [PubMed] [Google Scholar]
- 19.Thögersen AM, Jansson J-H, Boman K, Nilsson TK, Weinehall L, Huhtasaari F, et al. High Plasminogen Activator Inhibitor and Tissue Plasminogen Activator Levels in Plasma Precede a First Acute Myocardial Infarction in Both Men and Women: Evidence for the Fibrinolytic System as an Independent Primary Risk Factor. Circulation. 1998;98:2241–2247. doi: 10.1161/01.cir.98.21.2241. [DOI] [PubMed] [Google Scholar]
- 20.Muller KE, Stewart PW. Linear Model Theory: Univariate, Multivariate, and Mixed Models. NY: Wiley Interscience; 2006. [Google Scholar]
- 21.Reed RG, Kris-Etherton P, Stewart PW, Pearson TA. Variation of lipids and lipoproteins in premenopausal women compared with men and postmenopausal women. Metabolism. 2000;49:1101–1105. doi: 10.1053/meta.2000.8603. [DOI] [PubMed] [Google Scholar]
- 22.Andersson O, Blombäck M, Bremme K, Wramsby H. Prediction of changes in levels of haemostatic variables during natural menstrual cycle and ovarian hyperstimulation. Thromb Haemost. 1997;77:901–904. [PubMed] [Google Scholar]
- 23.Chung H, Rha S, Park J, Yoo N, Kim J, Roh J, et al. Physiological and pathological changes of plasma urokinase-type plasminogen activator, plasminogen activator inhibitor-1, and urokinase-type plasminogen activator receptor levels in healthy females and breast cancer patients. Breast Cancer Res Treat. 1998;49:41–50. doi: 10.1023/a:1005997421733. [DOI] [PubMed] [Google Scholar]
- 24.Giardina E-GV, Chen HJ, Sciacca RR, Rabbani LE. Dynamic Variability of Hemostatic and Fibrinolytic Factors in Young Women. J Clin Endocrinol Metab. 2004;89:6179–6184. doi: 10.1210/jc.2004-0598. [DOI] [PubMed] [Google Scholar]
- 25.Kapiotis S, Jilma B, Pernerstorfer T, Stohlawetz P, Eichler HG, Speiser W. Plasma levels of activated factor VII decrease during the menstrual cycle. Thromb Haemost. 1998;80:588–591. [PubMed] [Google Scholar]
- 26.Koh SCL, Prasad RNV, Fong YF. Hemostatic Status and Fibrinolytic Response Potential at Different Phases of the Menstrual Cycle. Clin Appl Thromb Hemost. 2005;11:295–301. doi: 10.1177/107602960501100308. [DOI] [PubMed] [Google Scholar]
- 27.Lidegaard Ø, Milsom IAN, Geirsson RT, Skjeldestad FE. Hormonal contraception and venous thromboembolism. Acta Obstetricia et Gynecologica Scandinavica. 2012;91:769–778. doi: 10.1111/j.1600-0412.2012.01444.x. [DOI] [PubMed] [Google Scholar]
- 28.Marckmann P, Sandström B, Jespersen J. The variability of and associations between measures of blood coagulation, fibrinolysis and blood lipids. Atherosclerosis. 1992;96:235–244. doi: 10.1016/0021-9150(92)90070-w. [DOI] [PubMed] [Google Scholar]