Abstract
Thyroid dysfunction is common in individuals with diabetes mellitus (DM) and may contribute to the associated cardiac dysfunction. However, little is known about the extent and pathophysiological consequences of low thyroid conditions on the heart in DM. DM was induced in adult female Sprague Dawley (SD) rats by injection of nicotinamide (N; 200 mg/kg) followed by streptozotocin (STZ; 65 mg/kg). One month after STZ/N, rats were randomized to the following groups (N = 10/group): STZ/N or STZ/N + 0.03 μg/mL T3; age-matched vehicle-treated rats served as nondiabetic controls (C). After 2 months of T3 treatment (3 months post-DM induction), left ventricular (LV) function was assessed by echocardiography and LV pressure measurements. Despite normal serum thyroid hormone (TH) levels, STZ/N treatment resulted in reductions in myocardial tissue content of THs (T3 and T4: 39% and 17% reduction versus C, respectively). Tissue hypothyroidism in the DM hearts was associated with increased DIO3 deiodinase (which converts THs to inactive metabolites) altered TH transporter expression, reexpression of the fetal gene phenotype, reduced arteriolar resistance vessel density, and diminished cardiac function. Low-dose T3 replacement largely restored cardiac tissue TH levels (T3 and T4: 43% and 10% increase versus STZ/N, respectively), improved cardiac function, reversed fetal gene expression and preserved the arteriolar resistance vessel network without causing overt symptoms of hyperthyroidism. We conclude that cardiac dysfunction in chronic DM may be associated with tissue hypothyroidism despite normal serum TH levels. Low-dose T3 replacement appears to be a safe and effective adjunct therapy to attenuate and/or reverse cardiac remodeling and dysfunction induced by experimental DM.
INTRODUCTION
Cardiac complications are the leading cause of morbidity and mortality in individuals with diabetes mellitus (DM). DM increases the risk of morbid events in individuals with coronary artery disease (CAD), hypertension (HTN), hypercholesterolemia, and postmyocardial infarction (MI) (1,2). Moreover, DM can lead to cardiac dysfunction, microvascular impairment, structural remodeling and eventually heart failure (HF) in the absence of a known underlying pathology (for example, CAD or HTN) (3,4). The pathophysiology of diabetic cardiomyopathy has been reviewed recently (3,5,6).
Thyroid hormones (THs) are important regulators of the cardiovascular system and peripheral vasculature (7,8). THs influence myocardial contractility, total peripheral resistance and ultimately cardiac output (7,8). Overt and subclinical hypothyroidism are associated with cardiovascular dysfunction including impaired contractility and decreased cardiac output, increased peripheral vascular resistance, impaired coronary blood flow and relaxation abnormalities, which results in an increased clinical risk for all-cause and cardiovascular mortality (9–12).
In rodent models, we demonstrated previously that chronic hypothyroidism by itself can lead to dilated HF characterized by severe left ventricular (LV) dysfunction, microvascular impairment, and maladaptive chamber remodeling (13,14). In addition, MI, pressure overload, pathological hypertrophy and HF have been shown to cause local cardiac tissue hypothyroidism by augmenting the expression and activity of cardiac TH hormone-degrading enzymes (15–18).
DM patients have a higher frequency of clinical and subclinical hypothyroidism than non-DM individuals (19–21), which is a likely contributor to increased cardiovascular risk and poor outcomes in this population (22). DM and hypothyroidism produce similar negative effects on cardiac function and are associated with impaired contractility (3,7,8,23), α to β myosin heavy chain (MHC) isoform shift (7,8,13,24), microvessel/blood flow abnormalities (4,13,25–27), and dys-regulation of Ca2+ handling (23,28–31). Cumulatively, these observations suggest that individuals with DM are at increased risk for cardiac dysfunction associated with both systemic and/or local thyroid hormone imbalance.
A study by Davidson et al. showed improved heart function with T3 treatment of STZ-SHR rats (32). However, induction of diabetes in SHR resulted in severe body mass reduction and worsening cachexia from the administered T3 dose. While encouraging, the potential benefits from a therapeutic dose of T3 in DM with stable body mass has not been determined. In addition, the relationship between DM and cardiac tissue hypothyroidism has not been explored in great depth. Although low tissue TH levels have been speculated in the setting of diabetes, to our knowledge, this observation has never been confirmed. If, in fact, DM leads to low tissue TH levels, restoration of TH homeostasis by physiologic TH supplementation is likely to help attenuate and/or reverse cardiac dysfunction in this high risk population. In the current investigation, we show that chronic DM, without superimposed pathological cardiac stimuli, can lead to local cardiac tissue hypothyroidism irrespective of serum TH levels. Our results demonstrate the important relationship between local TH dysfunction and cardiac dysfunction in DM hearts.
MATERIALS AND METHODS
Detailed materials and methods are available in the Supplemental Data.
Animal Model and Experimental Design
Five-month-old female SD rats were obtained from Harlan (Indianapolis, IN, USA). To replicate a mild, noninsulin-dependent diabetic phenotype, nicotinamide (N; 200 mg/kg) pretreatment was given prior to streptozotocin (STZ; 65 mg/kg). Eight days after STZ/N, fasting blood glucose (BG) was used to confirm DM (Supplementary Figure 1; control: 6.5 mmol/L; STZ/N: 14.1 mmol/L). One month after STZ/N injections, diabetic rats were randomly assigned to one of the following groups (N = 10/group): STZ/N or STZ/N + 0.03 μg/mL T3 (STZ/N-T3); Age matched rats receiving vehicle injections only served as nondiabetic controls (control; C). T3 was administered noninvasively as solution (T3: ethanol/glycerol) dissolved in drinking water (final concentration in water: 0.03 μg/mL T3). After 2 months of T3 treatment (3 months post-DM induction), terminal cardiac function was assessed by echocardiography and LV hemodynamics. All animals were kept on a 12-h light–dark cycle and food and water were provided ad libitum. All experiments and protocols were performed in accordance with the Guide for the Care and Use of Laboratory Animals (33), and approved by the University of South Dakota Animal Care and Use Committee.
Measurement of Serum TH and Cardiac TH Levels and of Blood Glucose
Serum TH levels were measured using commercial enzyme-linked immunosorbent assay (ELISA) kits (TSH: ALPCO, Salem, NH, USA; T3, FT3, T4, FT4: Monobind, Lake Forest, CA, USA) according to manufacturer specifications. Cardiac TH extracts from pooled LV tissues homogenates were measured using tandem HPLC-mass spectrometry as described previously (15). Blood glucose (BG) levels were measured using an Ascensia Contour blood glucose meter (Bayer, Pittsburgh, PA, USA).
Real-Time PCR
Gene expression was evaluated using commercially available primers (SABio-sciences, Qiagen Inc., Valencia, CA, USA) for deiodinase induction pathways, cardiac genes, TH transporters, cytosolic TH-binding proteins, vascular growth regulators, LV collagen and custom generated primers for DIO3 deiodinase (Sigma-Aldrich, St. Louis, MO, USA) (Supplementary Table 1). Gene expression was normalized using housekeeping genes cyclophilin A and Rplp1.
Immunohistochemistry and Immunofluorescence
Formalin-fixed, paraffin-embedded tissue sections from the middle of the LV (5–7 μm) were used to evaluate changes in LV terminal resistance arterioles, capillaries, LV fibrosis, and DIO3 expression.
Arteriole and capillary staining
FITC-Isolectin-B4 (IB4; Vector, Burlingame, CA, USA) and Cy3-α-smooth muscle actin (α-SMA; Sigma-Aldrich) were used in combination to label endothelial cells and vascular smooth muscle cells respectively. Arteriolar numeric density (ND), arteriolar length density (LD), capillary ND density, and total capillary length were quantified using previously described methods (34,35).
LV DIO3 deiodinase staining
LV tissue sections were stained using a commercially available IHC/DAB detection kit (Epitomics, Burlingame, CA, USA) and a previously validated rabbit anti-DIO3 antibody (36–38) (Novus, Littleton, CO, USA). Specificity was confirmed using an equivalent concentration of rabbit IgG in place of primary antibody (Supplementary Figure 2).
Myocardial fibrosis
LV tissue sections were stained with Masson trichrome for visualization of myocardial fibrosis. Values are represented as the proportion of collagen normalized to the total tissue area.
Data Analysis
All data are expressed as means (SD), unless otherwise noted. Statistical analysis was performed by paired Student t test (pre- versus post-BW), one-way analysis of variance (ANOVA) followed by Holm-Sidak post hoc analysis, or nonparametric one-way ANOVA (Kruskal-Wallis) ranked measurements with Dunn post hoc analysis. Logarithmic transformations were performed when necessary. Statistical analysis was performed using SigmaPlot 11 (Systat Software, San Jose, CA, USA). Gene expression was analyzed by the ΔΔCt method using online software from SABiosciences. Values of p < 0.05 were considered statistically significant.
All supplementary materials are available online at www.molmed.org.
RESULTS
Physical Data
The effectiveness of the STZ/N treatment was confirmed by the two-fold increase in serum glucose levels versus control (C, STZ/N, STZ/N-T3: 13, 26, 24 mmol/L, respectively). Changes in body weight, LV weight and heart weight in the STZ/N- and STZ/N-T3-treated animals were similar to the control group over the course of the study (Supplementary Table 2). Notably, Nicotinamide pre-treatment prevented any reduction in body weight and heart weight commonly observed with STZ treatment alone (39). Furthermore, the T3 dose used to treat the STZ animals had no effect on body temperature, heart rate or blood pressure measurements (Supplementary Table 2).
Diabetic Hearts Have Reduced Cardiac Tissue TH Levels despite Normal Serum TH Levels
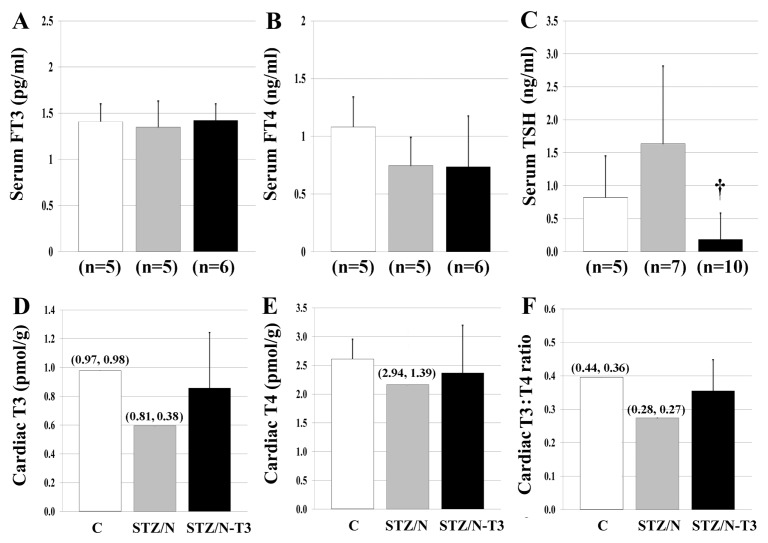
Terminal TH concentrations in serum and cardiac tissue are shown in Figure 1 and Supplementary Figure 3. STZ/N was not associated with changes in serum T4, T3, FT3 or FT4 levels; however, TSH values were two-fold higher compared with control. Notably, the T3 dose used to treat the STZ/N animals caused a significant reduction in serum TSH levels despite the lack of increase in serum T3, FT3 or suppression of serum T4 or FT4.
Figure 1.
Serum and tissue THs. (A) Serum FT3, free triiodothyronine in serum. (B) Serum FT4, free thyroxine in serum. (C) TSH, thyroid stimulating hormone in serum. (D) Cardiac T3, triiodothyronine in cardiac tissue. (E) Cardiac T4, thyroxine in cardiac tissue. (F) Cardiac T3:T4 ratio, ratio of intracardiac T3 to T4. Values represented as means (SD) for groups with ≥3 samples and means (raw values) for groups with two samples; n = 2–3 pooled samples/ group for D–F. Each pooled LV sample within an experimental group consisted of pooled LV tissue from 2 to 4 hearts (6 to 8 hearts/group); †p < 0.05 versus STZ/N. Statistical analysis was only performed on A–C.
In contrast to the normal serum TH levels in STZ/N animals, cardiac tissue T3 and T4 content was reduced substantially (39% and 17% reduction, respectively), indicating a 33% decrease in the T3:T4 ratio (Figure 1D–F). The apparent physiologic replacement dosage of T3 appeared to prevent the decline in cardiac tissue T3 levels and normalized the T3:T4 ratio without elevating serum TH levels or producing overt signs of hyperthyroidism (Supplementary Table 2).
T3 Treatment Attenuates DM-Induced Hemodynamic Dysfunction and Significantly Improves Cardiac Relaxation
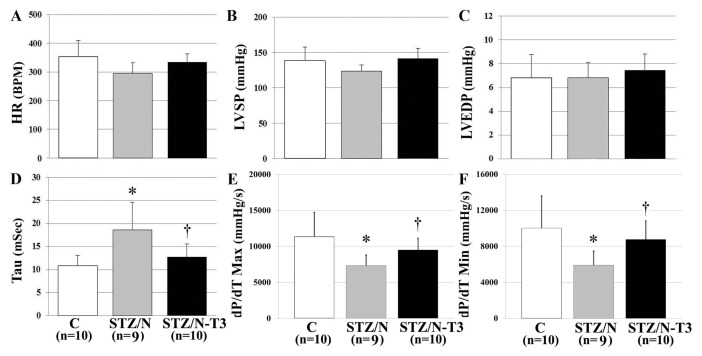
Hemodynamic data are summarized in Figure 2 and Supplementary Table 2. STZ/N did not produce a significant effect on LV systolic pressure or heart rate. However, STZ/N led to cardiac dysfunction as evidenced by significant reductions in the maximal rate of pressure development (dP/dT Max; 36% reduction versus C, p < 0.01) and maximal rate of pressure decline (dP/dT Min; 41% reduction versus C, p < 0.01), with a significantly increased time constant of isovolumic relaxation (tau; 72% increase versus C, p < 0.01). T3 treatment of the STZ/N animals normalized measurements of contractile function including dP/dT Max, dP/dT Min and tau. These findings may be explained by an enhancement of the β adrenergic system as evidenced by the tendency for increased β1 adrenergic receptor expression in TH-treated hearts (Supplementary Figure 4).
Figure 2.
T3 treatment attenuates DM-induced hemodynamic dysfunction and significantly improves parameters of cardiac relaxation. Values are means (SD). (A) HR, heart rate. (B) LVSP, LV systolic pressure. (C) LVEDP, LV end-diastolic pressure. (D) Tau, time constant of LV isovolumic relaxation. (E) dP/dT Max, maximal rate of pressure development. (F) dP/dT, Maximal rate of pressure decline. n, Number of animals per group. *p < 0.05 versus control; †p < 0.05 versus STZ/N.
DM Is Associated with Adverse Cardiac Chamber Remodeling Which Is Attenuated by T3 Treatment
Echocardiographic data are summarized in Table 1. STZ/N-treated rats had significantly increased LV chamber internal diameter (LVID; p < 0.01) in systole, while anterior (AW; p < 0.01) and posterior (LVPW; p < 0.01) wall thickness was decreased in systole. This adverse remodeling was associated with an elevation in the anatomical parameters of systolic wall stress as indicated by a 39% increase in LVID/LVPW ratio when compared with controls. T3 treatment tended to cause normalization of these STZ/N-induced changes in systolic parameters.
Table 1.
LV echocardiography.a
| Control | STZ/N | STZ/N-T3 | |
|---|---|---|---|
| nb | 10 | 10 | 10 |
| LVIDdc (mm) | 7.39 (0.4) | 7.93 (0.8) | 7.96 (0.7) |
| AWd (mm)d | 1.33 (0.1) | 1.28 (0.2) | 1.25 (0.1) |
| LVPWd (mm)e | 1.36 (0.1) | 1.31 (0.2) | 1.29 (0.1) |
| LVIDs (mm)c | 4.19 (0.5) | 5.10 (0.8)f | 4.80 (0.8) |
| AWs (mm)d | 2.46 (0.2) | 2.15 (0.2)f | 2.34 (0.3) |
| LVPWs (mm)e | 2.47 (0.1) | 2.21 (0.2)f | 2.35 (0.2) |
| LVIDs/LVPWsg | 1.72 (0.3) | 2.38 (0.4)f | 2.10 (0.5) |
Values are means (SD).
n, Number per group.
LVID, LV internal diameter in diastole (d) and systole (s).
AW, anterior wall thickness in diastole (d) and systole (s).
LVPW, LV posterior wall thickness in diastole (d) and systole (s).
p < 0.05 versus control.
LVID/LVPW, ratio of LV internal diameter to posterior wall thickness in systole.
Loss of Small Arteriolar Resistance Vessels in the Diabetic Myocardium Is Prevented with T3 Treatment
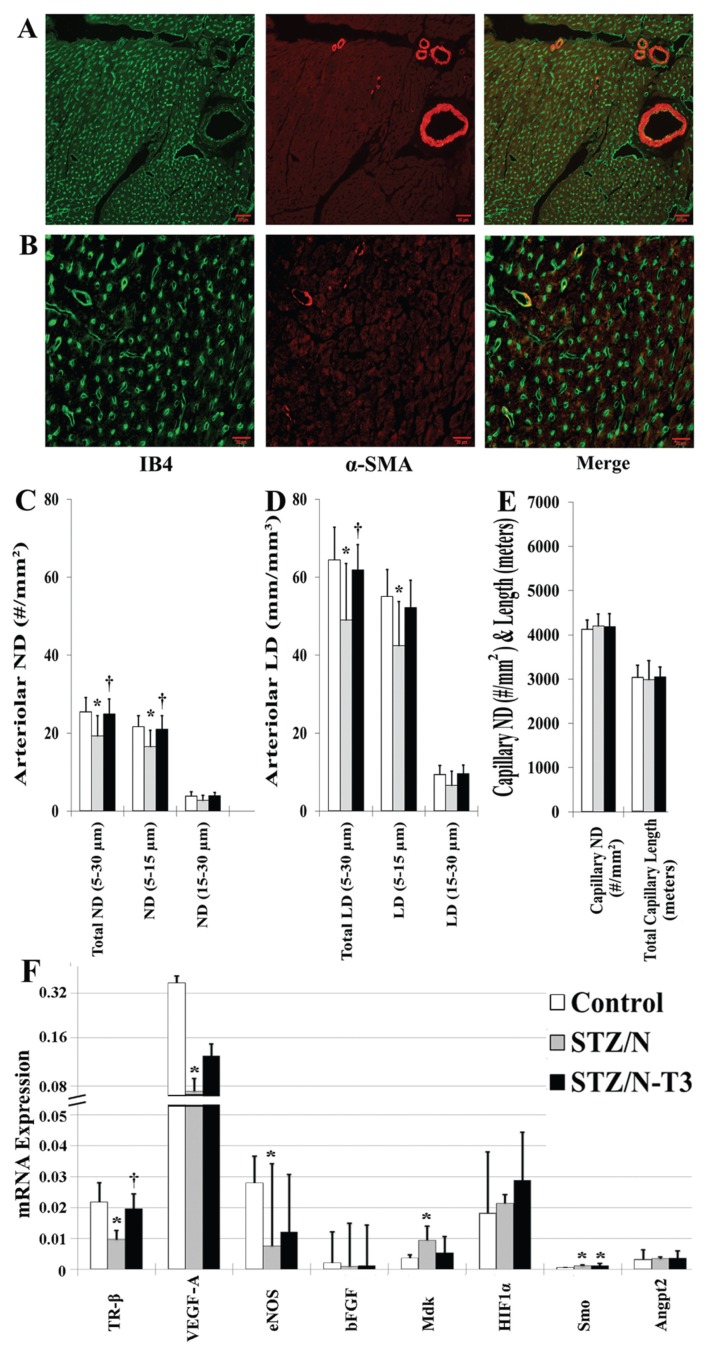
Arteriolar and capillary morphometric analysis is shown immunohistologically in Figures 3A, B and quantified in Figures 3C–E. Arteriolar numeric (ND) and length density (LD) were reduced significantly by ~25% in the STZ/N group compared with control (Figures 3C, D). The majority of this reduction came from losses in the smallest arterioles (<15 μm). Diabetic rats treated with T3 had arteriolar ND and LD values almost identical to nondiabetic controls. No differences were observed in total capillary density or total capillary length among groups (Figure 3E). Gene expression analysis of potential T3 responsive vascular growth regulators is shown in Figure 3F. STZ/N led to significant reductions in thyroid receptor β (TR-β, p < 0.01) and vascular growth modulators including vascular endothelial growth factor A (VEGF-A, p < 0.05), and endothelial nitric oxide synthase (eNOS, p < 0.05), and significant elevations in smoothened homolog (SMO, p < 0.01) and midkine (MdK, p < 0.05). T3 treatment tended to normalize vascular growth factor expression.
Figure 3.
Loss of small arteriolar resistance vessels in the diabetic myocardium is prevented with T3 treatment. Values represent means (SD). (A) Representative images of small arteriolar resistance vessels used for arteriolar numeric (ND) and length density (LD) quantification (scale bar = 50 μm). (B) Representative images of myocardial capillaries used for capillary numeric density and length quantification (scale bar = 20 μm). (C) Arteriolar ND, arteriolar numeric density quantification. (D) Arteriolar LD, arteriolar length density quantification. (E) Capillary ND & Length, capillary numeric density and length quantification. Arteriolar ND, arteriolar LD, capillary ND, capillary length were calculated using previously described methods (34). (F) Absolute gene expression of cardiac regulators of vascular function. n = 5–8/group; IB4, Isolectin-B4; α-SMA, α-smooth muscle actin; TR-β, thyroid receptor β; VEGF-A, vascular endothelial growth factor A; eNOS, endothelial nitric oxide synthase; bFGF; basic fibroblast growth factor; MdK, midkine; HIF1α, hypoxia inducible factor 1-α; SMO, smoothened homolog (Drosophila); Angpt2, angiopoietin-2; *p < 0.05 versus control; †p < 0.05 versus STZ/N.
DM is Associated with Reexpression of Fetal Genes in Cardiac Tissue
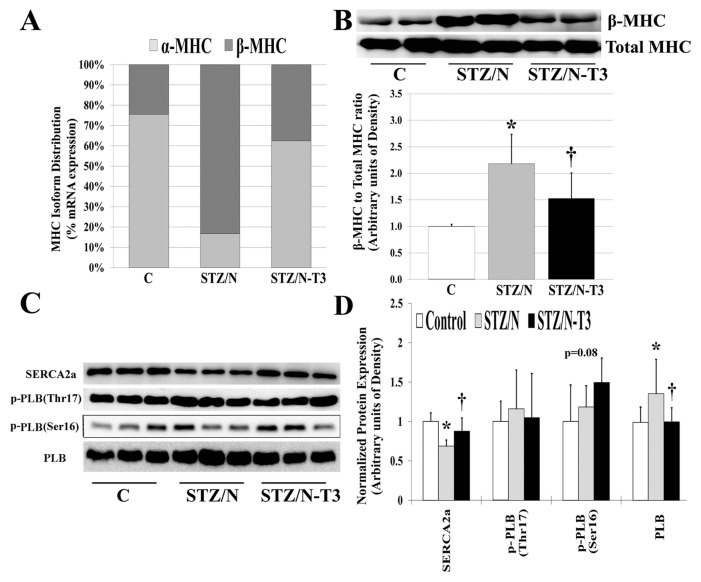
Protein and gene expression analyses of fetal genes are summarized in Figure 4 and Supplementary Figure 5. Untreated DM hearts showed reexpression of the fetal gene phenotype typically observed during cardiac hypertrophy, heart failure and tissue hypothyroidism (7,39). The myosin heavy chain (MHC) isoform changes observed in the STZ/N hearts were similar to those observed in hypothyroidism, and this was normalized with T3 treatment (Figure 4A). Protein analysis confirmed these MHC isoform gene expression changes (Figure 4B). The STZ/N-induced reduction in sarcoplasmic/endoplasmic reticulum calcium ATPase 2 (SERCA2a) protein and upregulation of PLB were reversed by T3 treatment (Figures 4C, D). Notably, Ser16 phosphorylation of PLB was increased (p = 0.08) with T3 treatment which, when expressed as a fraction of total PLB protein, indicates a reduced inhibitory effect of PLB on SERCA2a activity, which may explain the observed positive effect of T3 treatment on cardiac contractile function.
Figure 4.
Diabetes leads to reexpression of fetal genes in adult cardiac tissue. (A) MHC isoform mRNA distribution shown as % of total MHC mRNAs as measured by qPCR. (B) Protein expression of β-MHC isoform and total MHC detected by Western blot. Expression represented as ratio of β-MHC to total MHC expression. (C) Cardiac protein expression of sarcoplasmic reticulum proteins detected by Western blot. (D) Quantitative summary of normalized protein expression. MHC, myosin heavy chain; SERCA2a, sarcoplasmic/endoplasmic reticulum calcium ATPase 2; PLB, phospholamban; p-PLB, phosphorylated phospholamban (site of phosphorylation); n = 4–8/group. *p < 0.05 versus control; †p < 0.05 versus STZ/N.
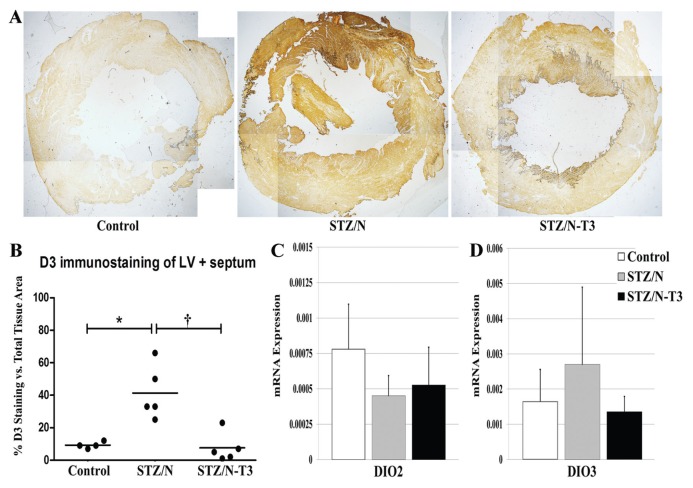
Deiodinase Expression in Diabetic Hearts
To explain the reduction in myocardial tissue content of T3, LV tissue sections were evaluated for the presence of DIO3 deiodinase by immunostaining and mRNA expression (Figure 5). Robust DIO3 protein expression was observed in untreated STZ/N hearts while the control and T3-treated hearts had low expression. DIO3 staining was observed in cardiac myocytes throughout the myocardium distributed in a heterogeneous manner similar to the pattern reported in post-MI rodents (15). To explore the role of cardiac deiodinase expression further, DIO1, DIO2 and DIO3 deiodinase mRNA expressions were evaluated. Both untreated and T3-treated diabetic hearts had a non-significant trend toward decreased DIO2 mRNA expression. Interestingly, only untreated diabetic hearts showed a tendency toward increased DIO3 mRNA expression. DIO3 deiodinase is the primary enzyme responsible for intracellular break-down and inactivation of both T4 (T4 to rT3) and T3 (T3 to T2) (41). This increased expression in untreated diabetic hearts may be responsible for the observed reductions in cardiac TH levels. Similarly, only untreated diabetic hearts showed a trend for increased DIO1 expression, although with variability (data not shown).
Figure 5.
LV Deiodinase Expression. (A) LV DIO3 staining in control and diabetic hearts at 2× magnification. (B) DIO3 IHC protein quantification. Values represented as the percentage of DIO3 staining versus the total tissue area. (C) DIO2, type 2 deiodinase mRNA expression. (D) DIO3, type 3 deiodinase mRNA expression. Gene expression values represented as means (SD). Gene expression was normalized using the housekeeping genes cyclophilin A and Rplp1; n = 3–5/group. *p < 0.05 versus control; †p < 0.05 versus STZ/N.
LV Collagen Expression
While significant collagen accumulation does not appear to be present at this early time point, STZ/N was associated with a 25% higher collagen content compared with control (1.27% versus 1.02%) and a 37% higher collagen content compared with STZ/N-T3 (1.27% versus 0.93%). Untreated diabetic rats had significant increases in the gene expression of transforming growth factor (TGF)β1 and TGFβ2. Treated and untreated diabetic rats had similar gene expression of collagen 1, collagen 3, lysyl oxidase and TGFβ3 (Supplementary Figure 7).
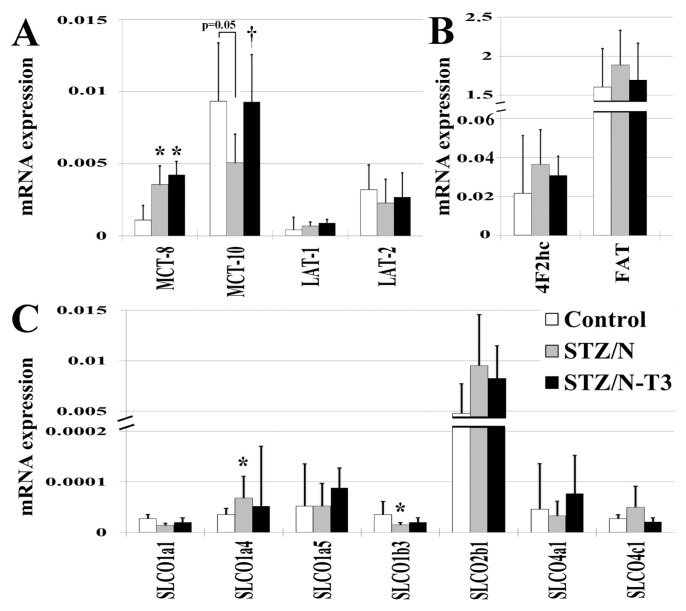
Expression of TH Transporters and Cytosolic TH-Binding Proteins in the Myocardium
To determine whether TH uptake into the myocardium might be reduced in diabetic hearts, we analyzed expression of known TH transporters (monocarboxylate transporter [MCT], solute carrier transporter family [SLC], solute carrier organic anion transporter family [SLCO], fatty acid translocase [FAT], large neutral amino acid transporter [LAT]) (Figure 6). The increased expression of the majority of TH transporters in the STZ/N rats was consistent with cardiac tissue hypothyroidism (35). T3 treatment tended to normalize mRNA expression of most TH transporters. The expression of several known cytosolic TH-binding proteins was decreased in diabetic hearts and T3 treatment tended to reverse this effect (Supplementary Figure 8).
Figure 6.
Expression of cardiac thyroid hormone transporters. Gene expression values represented as means (SD). Gene expression was normalized using the housekeeping genes cyclophilin A and Rplp1. (A) MCT, monocarboxylate transporters; LAT, large neutral amino acid transporters. (B) 4F2hc, 4F2 cell surface antigen heavy chain; FAT, fatty acid translocase; SLCO, solute carrier organic anion transporter family. n = 5/group; *p < 0.05 versus control; †p < 0.05 versus STZ/N.
DISCUSSION
This study evaluated the relationship between cardiac tissue hypothyroidism and cardiac dysfunction in the setting of DM. In addition, we sought to establish the efficacy and safety of low-dose T3 replacement as a potential adjunct therapy to attenuate and/or reverse early cardiac dysfunction in diabetic hearts. Very little is known regarding the influence of low thyroid function in the setting of DM-induced cardiac dysfunction. It has been hypothesized that decreased TH levels and/or TH signaling could play a protective role during cardiac pathology by reducing cardiac metabolism. Conversely, there is mounting evidence supporting the notion that cardiac tissue (myocardial) TH dysfunction is a common and deleterious feature of many disease states and cardiac pathologies (9,15–18). In accordance, we propose that low cardiac tissue TH levels represent a deleterious manifestation of tissue specific endocrine imbalance and restoration of tissue TH homeostasis is a logical and beneficial treatment option.
In the current study, DM led to cardiac fetal gene reexpression, contractile dysfunction, relaxation/diastolic abnormalities and microvascular remodeling. These responses appear to be present during the early stages of diabetic cardiac dysfunction and occur before signs of cardiac hypertrophy, LV dilation and fibrosis and systolic impairment as have been reported previously (3,4,29). Initiation of T3 treatment during this early period of dysfunction preserves cardiac function and prevents the loss of the arteriolar resistance vessel network. Importantly, this investigation demonstrates that physiologic T3 replacement can be safely administered over a long period of time and can positively influence the diabetic heart without causing symptoms of hyperthyroidism or elevating serum T3 and T4 levels. Since T3 treatment of STZ/N rats led to no changes in heart mass, body mass, body temperature, heart rate or blood pressure, it appears that the observed improvements occurred without significantly affecting metabolism.
Although it is well established that DM patients have a high frequency of clinical or subclinical hypothyroidism (19,20,22), the breadth and importance of cardiac tissue thyroid dysfunction is currently unknown. To our knowledge, this is the first investigation to show that DM alone, without concomitant pathological stimuli such as hypertension or myocardial infarction may lead to cardiac tissue hypothyroidism (see Figure 1). We also confirm that serum TH levels did not reflect the observed cardiac tissue hypothyroidism (normal THs in serum, reduced THs in cardiac tissue) in the setting of DM. Recently, our lab has published that in models of subclinical hypothyroidism, serum T3 and T4 levels can be within normal limits even when cardiac tissue T3 levels and LV function are significantly depressed (35,42). Findings from the current investigation support and extend these observations to the setting of DM. Clinical detection of subclinical thyroid dysfunction in the diabetic population is further complicated by several widely used antidiabetic drugs (for example, Metformin) which are known to artificially suppress serum TSH levels (43). Consequently, it appears that clinical assessment of serum TH levels alone is insufficient to accurately predict cardiac tissue TH levels and dysfunction in diabetic hearts with borderline hypothyroid conditions.
Myocardial TH inactivation by induction of DIO3 has been reported previously in several models of pathological ventricular hypertrophy/remodeling and HF (9,15–18). However, little is known regarding the influence of DIO3 expression in the diabetic heart. We hypothesized that the reduction in myocardial TH levels observed in diabetic hearts is the result of DIO3-mediated TH degradation. We observed a significant increase in DIO3 protein expression and a tendency for increased tissue DIO3 mRNA expression in diabetic hearts (see Figure 5) (18,44). In agreement with previous findings (15), we observed a heterogeneous DIO3 staining pattern throughout the myocardium with the vast majority of staining localized to cardiomyocytes. T3 treatment normalized DIO3 protein content in the tissue with a concomitant reduction in expression of DIO3 mRNA. Since DIO3 has been shown to be HIF-responsive (18), the correlation of changes in DIO3 expression, arteriolar vessel density and other reported parameters, suggest reversal of tissue hypoxia as a plausible mechanistic link between low-dose T3 treatment and correction of tissue T3 levels.
In addition to myocardial inactivation of THs, TH bioavailability within cardiac tissue is crucial to normal TH homeostasis. Although TH transport is a highly regulated process (45–48), TH transporter expression has not been explored in great depth in the heart. Therefore, we investigated the relationship between tissue TH status and the gene expression profile of TH transporters. We observed a general pattern of increased TH transporters within the untreated STZ/N group, which was normalized with TH treatment (see Figure 6). Of note, this expression pattern was not observed in one of the most important TH transporters, MCT-10. It is well established that MCT-8 and MCT-10 are the major TH transporters, and MCT-10 is arguably more active than MCT-8 (46). Interestingly, a similar expression pattern (increased MCT-8 and decreased MCT-10) has been reported in muscle from patients with prolonged critical illness with depressed serum TH levels and increased rT3 (possibly due to DIO3 induction). However, this expression pattern was not consistent in all organs or phases of critical illness (acute versus prolonged) (45). In the current study, attenuation of the decline in tissue THs by T3 treatment appeared to completely normalize MCT-10 expression while the expression of MCT-8 was further increased with T3. This observation, along with greater than a two-fold higher basal level of expression (see Figure 6), suggests that MCT-10 may play a crucial role in tissue TH bioavailability in the diabetic heart.
Due to its beneficial regulation of key Ca2+ handling proteins, myosin isoforms and antifibrotic activity, T3 represents an attractive adjunct therapy to improve relaxation/diastolic function, and prevent disease progression in the diabetic heart (7,8,49,50). LV stiffness, relaxation abnormalities and diastolic dysfunction are recognized as early and widespread pre-clinical manifestations of DM-related cardiac dysfunction and generally occur before the onset of severe systolic impairment (3,51,52). Abnormal Ca2+ handling is a cardinal feature of diabetic dysfunction and plays a causal role in relaxation abnormalities, diastolic dysfunction and fibrotic accumulation (3,28,29). Diabetic hearts are known to have increased dysfunction of Ca2+ regulatory proteins (for example, SERCA2a) and experimental normalization of calcium handling by the overexpression of key regulatory proteins (for example, SERCA2a) has been shown to improve myocardial function in diabetic cardiomyopathy (29). THs are known to augment myocardial relaxation and prevent calcium overload by stimulating the expression and activity of key Ca2+ regulators (for example, SERCA2a, Na+/Ca2+ exchanger, RYR2), while inhibiting the activity of PLB (7,8,13,30,31,53) and the opposite is true during hypothyroidism. In the current study, tissue hypothyroidism caused by STZ/N led to significant diastolic abnormalities and decreased expression of important Ca2+ handling genes. T3 replacement significantly improved functional parameters of diastolic function (tau, and dP/dT Min) (see Figure 2) and normalized the expression of SERCA2a, RyR2, and α/β-MHC (Figure 4 and Supplementary Figure 5). Interestingly, the dysfunction observed in the STZ/N group occurred before the onset of appreciable LV fibrosis (Supplementary Figure 7) and suggests that other abnormalities (for example, Ca2+ handling, blood flow, increased cardiomyocyte resting tension) are responsible for this early dysfunction. Our data indicate that detection of diastolic dysfunction before the occurrence of significant LV remodeling/hypertrophy, LV collagen accumulation and severe systolic impairment is critically important and represents a promising therapeutic window for T3 replacement therapy.
Finally, we examined the effect of T3 replacement on the cardiac microvessel network in diabetic hearts since THs have been shown to be important proangiogenic regulators of the cardiac vascular network and influence vasoreactivity and blood flow mechanisms (7,14,27,34,54). We have previously shown that hypothyroidism caused by thyroidectomy leads to significant reductions in arteriolar length density and that TH treatment leads to robust proliferation of endothelial cells, pericytes, vascular smooth muscle cells and normalization of arteriolar LD (34,42). Moreover, we have reported that experimental hypothyroidism leads to arteriolar resistance vessel remodeling and impaired resting and maximum blood flow in a manner dependent on the duration of hypothyroidism (13). It is well established that diabetic hearts are at an increased risk for both macro- and microvascular pathology and although many factors likely contribute to diabetic vascular dysfunction (for example, hyperglycemia, reactive oxygen species), low cardiac tissue TH levels may contribute to or exacerbate microvascular dysfunction in diabetic hearts. Cardiac microvessel remodeling, endothelial dysfunction, increased arteriolar tone/vasoreactivity, decreased vascular growth factor expression, impaired myocardial blood flow and diminished blood flow reserve are common features of both DM and overt/subclinical hypothyroidism (4,13,25–27,54–56).
In the current study, DM was associated with reductions in both number and density (~25%) of small arteriole resistance vessels (Figure 3). In addition, untreated diabetic hearts had significantly reduced gene expression of key vascular regulators (TR-β, VEGF-A, eNOS) (see Figure 3) and a trend for reduced bFGF gene expression. It is not surprising that diminished expression of these important vascular regulators would lead to vascular remodeling, considering that aberrant expression or knockout models of these regulators exhibit severe microvascular impairment and abnormal angiogenesis (4,57–59). Interestingly, diabetic hearts had significant upregulation of the heparin-binding growth factor MdK, which has been shown to be negatively regulated by THs and is increased during experimental hypothyroidism (34). Increased MdK levels also have been shown to be a predictor of cardiac events and deteriorating cardiac function in patients with chronic heart failure (60).
T3-treated diabetic hearts showed preservation of small arteriole resistance vessels, with attenuation of the decline in vascular gene expression (see Figure 3). The beneficial effects of T3 treatment also may be due to preservation of coronary perfusion (preserved SBP and LV hemodynamics) or by direct regulation of ECs, pericytes and VSMCS. Our findings are consistent with reports noting that reversing cardiac hypothyroidism by THs or their analogues can preserve the small arteriole resistance vessel network (14,34,42,56,57). Moreover, we have previously shown that the TH analogue DITPA (3,5-diiodothyropropionic acid) can prevent arteriolar loss in hypothyroid rodents independent of cardiac function (61). The above findings suggest that the combined influence of THs on arteriolar vascular tone and density and the preserved gene expression of vascular regulators are likely to reverse the increased coronary vascular resistance and to improve blood flow in diabetic hearts.
Despite this early arteriolar remodeling, no differences were observed in capillary density or total capillary length among control or diabetic groups (see Figure 3), suggesting that arteriolar remodeling and potential blood flow abnormalities occur before the onset of capillary rarefaction. It has been reported that capillary rarefaction is not present during the early evolution of DM-induced cardiac dysfunction and occurs later in disease progression (4). It is not surprising that we did not observe changes in capillary density, considering that there were no notable signs of myocyte hypertrophy, myocardial degeneration or replacement fibrosis at this early stage.
Our study has several limitations. First, due to the high tissue requirement for tissue TH quantitation method (~350–500 mg), LV samples from animals within the same treatment groups were pooled together. Unfortunately, this does not allow for individual correlation between TH status (serum and tissue) and cardiac function, thus limiting statistical power. Second, although extensive morphological characterization of the cardiac microvasculature was undertaken, we did not measure coronary blood flow. Thus, we cannot definitively conclude that arteriolar remodeling and tissue hypothyroidism led to impaired coronary flow in this study. However, numerous previous reports have confirmed that coronary flow is impaired by diabetes, low TH conditions and/or arteriolar remodeling and is augmented by or preserved with thyroid hormone treatment (4,13,25–27,54–56). Third, we were unable to find reliable commercial antibodies needed to confirm protein expression changes for the reported TH transporters, cytosolic binding proteins. Finally, due to limited resources and tissue availability, we were unable to measure DIO3 enzymatic activity to confirm increased tissue DIO3 protein content.
CONCLUSION
This study shows that chronic DM, without superimposed pathological cardiac stimuli, led to cardiac tissue hypothyroidism despite normal serum TH levels, potentially as a result of increased myocardial DIO3 activity. Low-dose replacement with T3 restored cardiac tissue TH levels, attenuated cardiac dysfunction, improved cardiac relaxation, reversed fetal gene expression and preserved the arteriolar resistance vessel network without causing overt symptoms of hyperthyroidism. T3 replacement therapy appears to be an effective adjunct therapy to attenuate and/or reverse adverse cardiac remodeling and dysfunction induced by experimental DM. In previous experiments, we noted that excessive doses of T4 (42) or T3 (35) were needed to restore cardiac tissue T3 levels and LV function in rats with primary hypothyroidism. The results of the current experiment are exciting since a low, therapeutic dose of T3 led to dramatic cardiac improvements. This suggests that cardiac tissue hypothyroidism secondary to heart diseases, can be restored safely. Other ongoing animal experiments in our lab suggest this may also be true for other heart diseases leading to HF. Finally, it will be exciting to investigate whether physiologic T3 treatment is beneficial in type 2 DM, a global crisis desperately in need of new treatment options.
Supplemental Data
ACKNOWLEDGMENTS
We would like to thank Alice O’Connor (New York Institute of Technology College of Osteopathic Medicine [NYIT-COM] Histology Core) for her assistance with slide processing and histological staining, Jie Li (University of South Dakota) for her assistant with LV echocardiography measurements, and Alan Joy (NYIT-COM) for his help with LV fibrosis quantification. We would also like to thank Timothy Gant and Kate Dudek for their generosity in providing the DIO3 sequences. MHC antibodies (S58 and F59) were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and maintained by The University of Iowa, Department of Biology, Iowa City, IA. This project was supported by Grant Numbers RO1HL093160-01A1 and RO1HL103671 (AM Gerdes) from the National Heart, Lung, and Blood Institute (NHLBI). This research was also supported by an American Diabetes Association (ADA) Clinical Scientist Training Award 7-10-CST-01 (NY Weltman and AM Gerdes). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH), the NHLBI or the ADA. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnotes
Online address: http://www.molmed.org
DISCLOSURES
The authors declare they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Kannel W. Lipids, diabetes, and coronary heart disease: insights from the Framingham study. Am Heart J. 1985;110:1110–7. doi: 10.1016/0002-8703(85)90224-8. [DOI] [PubMed] [Google Scholar]
- 2.Fuller J, Shipley M, Rose G, Jarrett R, Keen H. Mortality from coronary heart disease and stroke in relation to degree of glycaemia: the Whitehall study. Br. Med. J. 1983;287:867–70. doi: 10.1136/bmj.287.6396.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falcao-Pires I, Leite-Moreira A. Diabetic cardiomyopathy: understanding the molecular and cellular basis to progress in diagnosis and treatment. Heart Fail Rev. 2011;17:325–44. doi: 10.1007/s10741-011-9257-z. [DOI] [PubMed] [Google Scholar]
- 4.Yoon Y, et al. Progressive attenuation of myocardial vascular endothelial growth factor expression is a seminal event in diabetic cardiomyopathy - Restoration of microvascular homeostasis and recovery of cardiac function in diabetic cardiomyopathy after replenishment of local vascular endothelial growth factor. Circulation. 2005;111:2073–85. doi: 10.1161/01.CIR.0000162472.52990.36. [DOI] [PubMed] [Google Scholar]
- 5.Miki T, Yuda S, Kouzu H, Miura T. Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail Rev. 2013;18:149–66. doi: 10.1007/s10741-012-9313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battiprolu PK, Lopez-Crisosto C, Wang ZV, Nemchenko A, Lavandero S, Hill JA. Diabetic cardiomyopathy and metabolic remodeling of the heart. Life Sci. 2013;92:609–15. doi: 10.1016/j.lfs.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein I, Ojamaa K. Mechanisms of disease: Thyroid hormone and the cardiovascular system. N. Engl. J. Med. 2001;344:501–9. doi: 10.1056/NEJM200102153440707. [DOI] [PubMed] [Google Scholar]
- 8.Kahaly G, Dillmann W. Thyroid hormone action in the heart. Endocr Rev. 2005;26:704–28. doi: 10.1210/er.2003-0033. [DOI] [PubMed] [Google Scholar]
- 9.Gerdes A, Iervasi G. Thyroid replacement therapy and heart failure. Circulation. 2010;122:385–93. doi: 10.1161/CIRCULATIONAHA.109.917922. [DOI] [PubMed] [Google Scholar]
- 10.Rodondi N, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304:1365–74. doi: 10.1001/jama.2010.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tseng F, et al. Subclinical hypothyroidism is associated with increased risk for all-cause and cardiovascular mortality in adults. J. Am. Coll. Cardiol. 2012;60:730–7. doi: 10.1016/j.jacc.2012.03.047. [DOI] [PubMed] [Google Scholar]
- 12.McQuade C, Skugor M, Brennan D, Hoar B, Stevenson C, Hoogwerf B. Hypothyroidism and moderate subclinical hypothyroidism are associated with increased all-cause mortality independent of coronary heart disease risk factors: a preCIS database study. Thyroid. 2011;21:837–43. doi: 10.1089/thy.2010.0298. [DOI] [PubMed] [Google Scholar]
- 13.Tang Y, Kuzman J, Said S, Anderson B, Wang X, Gerdes A. Low thyroid function leads to cardiac atrophy with chamber dilatation, impaired myocardial blood flow, loss of arterioles, and severe systolic dysfunction. Circulation. 2005;112:3122–30. doi: 10.1161/CIRCULATIONAHA.105.572883. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, et al. Thyroid hormone induces sprouting angiogenesis in adult heart of hypothyroid mice through the PDGF-Akt pathway. J. Cell. Mol. Med. 2012;16:2726–35. doi: 10.1111/j.1582-4934.2012.01593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pol C, et al. Left-ventricular remodeling after myocardial infarction is associated with a cardiomyocyte-specific hypothyroid condition. Endocrinology. 2011;152:669–79. doi: 10.1210/en.2010-0431. [DOI] [PubMed] [Google Scholar]
- 16.Wassen F, et al. Induction of thyroid hormone-degrading deiodinase in cardiac hypertrophy and failure. Endocrinology. 2002;143:2812–5. doi: 10.1210/endo.143.7.8985. [DOI] [PubMed] [Google Scholar]
- 17.Olivares E, et al. Thyroid function disturbance and type 3 iodothyronine deiodinase induction after myocardial infarction in rats - a time course study. Endocrinology. 2007;148:4786–92. doi: 10.1210/en.2007-0043. [DOI] [PubMed] [Google Scholar]
- 18.Simonides W, et al. Hypoxia-inducible factor induces local thyroid hormone inactivation during hypoxic-ischemic disease in rats. J. Clin. Invest. 2008;118:975–83. doi: 10.1172/JCI32824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray R, Seth D, Herd R, Brown N, Clark B. Prevalence of subclinical thyroidal failure in insulin dependent diabetes. J. Clin. Endocrinol. Metab. 1980;50:1034–7. doi: 10.1210/jcem-50-6-1034. [DOI] [PubMed] [Google Scholar]
- 20.Pittman C, Suda A, Chambers J, Ray G. Impaired 3,5,3 T3 production in diabetic patients. Metabolism. 1979;28:333–8. doi: 10.1016/0026-0495(79)90103-3. [DOI] [PubMed] [Google Scholar]
- 21.Duntas LJO, Brabant G. The interface between thyroid and diabetes mellitus. Clin Endocrinol. 2011;75:1–9. doi: 10.1111/j.1365-2265.2011.04029.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen H, et al. Subclinical hypothyroidism is a risk factor for nephropathy and cardiovascular diseases in Type 2 diabetic patients. Diabetic Med. 2007;24:1336–44. doi: 10.1111/j.1464-5491.2007.02270.x. [DOI] [PubMed] [Google Scholar]
- 23.Sayen M, Rohrer D, Dillmann W. Thyroid hormone response of slow and fast sarcoplasmic reticulum Ca2+ ATPase mRNA in striated muscle. Mol. Cell. Endocrinol. 1992;87:87–93. doi: 10.1016/0303-7207(92)90236-y. [DOI] [PubMed] [Google Scholar]
- 24.Haddad F, Masatsugu M, Bodell P, Qin A, McCue S, Baldwin K. Role of thyroid hormone and insulin in control of cardiac isomyosin expression. J. Mol. Cell. Cardiol. 1997;29:559–69. doi: 10.1006/jmcc.1996.0299. [DOI] [PubMed] [Google Scholar]
- 25.Baycan S, et al. Coronary flow reserve is impaired in subclinical hypothyroidism. Clin Cardiol. 2007;30:562–6. doi: 10.1002/clc.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taddei S, et al. Impaired endothelium-dependent vasodilatation in subclinical hypothyroidism: Beneficial effect of levothyroxine therapy. J. Clin. Endocrinol. Metab. 2003;88:3731–7. doi: 10.1210/jc.2003-030039. [DOI] [PubMed] [Google Scholar]
- 27.Takiguchi Y, Satoh N, Hashimoto H, Nakashima M. Reversal effect of thyroxine on altered vascular reactivity in diabetic rats. J. Cardiovasc. Pharmacol. 1989;13:520–4. [PubMed] [Google Scholar]
- 28.Akella A, Ding X, Cheng R, Gulati J. Diminished Ca2+ sensitivity of skinned cardiac muscle contractility coincident with troponin T-band shifts in the diabetic rat. Circ. Res. 1995;76:600–6. doi: 10.1161/01.res.76.4.600. [DOI] [PubMed] [Google Scholar]
- 29.Trost S, Belke D, Bluhm W, Meyer M, Swanson E, Dillmann W. Overexpression of the sarcoplasmic reticulum Ca2+-ATPase improves myocardial contractility in diabetic cardiomyopathy. Diabetes. 2002;51:1166–71. doi: 10.2337/diabetes.51.4.1166. [DOI] [PubMed] [Google Scholar]
- 30.Shenoy R, Klein I, Ojamaa K. Differential regulation of SR calcium transporters by thyroid hormone in rat atria and ventricles. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H1690–6. doi: 10.1152/ajpheart.2001.281.4.H1690. [DOI] [PubMed] [Google Scholar]
- 31.Ojamaa K, Kenessey A, Klein I. Thyroid hormone regulation of phospholamban phosphorylation in the rat heart. Endocrinology. 2000;141:2139–44. doi: 10.1210/endo.141.6.7514. [DOI] [PubMed] [Google Scholar]
- 32.Davidoff A, Rodgers R. Insulin, thyroid hormone, and heart function of diabetic spontaneously hypertensive rat. Hypertension. 1990;15:633–42. doi: 10.1161/01.hyp.15.6.633. [DOI] [PubMed] [Google Scholar]
- 33.Institute of Laboratory Animal Resources (U.S.), Committee on Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. Bethesda (MD): NIH; 1985. p. 83. Rev. 1985. (NIH publication; no. 85-23) [Google Scholar]
- 34.Savinova O, et al. Thyroid hormone promotes remodeling of coronary resistance vessels. Plos One. 2011;6:e25054. doi: 10.1371/journal.pone.0025054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weltman N, et al. Restoration of cardiac tissue thyroid hormone status in experimental hypothyroidism: a dose-response study in female rats. Endocrinology. 2013;154:2542–52. doi: 10.1210/en.2012-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freitas B, et al. Paracrine signaling by glial cell-derived triiodothyronine activates neuronal gene expression in the rodent brain and human cells. J. Clin. Invest. 2010;120:2206–17. doi: 10.1172/JCI41977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medina M, et al. The thyroid hormone-inactivating type III deiodinase is expressed in mouse and human beta-cells and its targeted inactivation impairs insulin secretion. Endocrinology. 2011;152:3717–27. doi: 10.1210/en.2011-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shukla PK, Sittig LJ, Ullmann TM, Redei EE. Candidate placental biomarkers for intrauterine alcohol exposure. Alcohol. Clin. Exp. Res. 2011;35:559–65. doi: 10.1111/j.1530-0277.2010.01373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen H, Brahmbhatt S, Gupta A, Sharma AC. Duration of streptozotocin-induced diabetes differentially affects p38-mitogen-activated protein kinase (MAPK) phosphorylation in renal and vascular dysfunction. Cardiovasc. Diabetol. 2005;4:3. doi: 10.1186/1475-2840-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kinugawa K, et al. Signaling pathways responsible for fetal gene induction in the failing human heart - Evidence for altered thyroid hormone receptor gene expression. Circulation. 2001;103:1089–94. doi: 10.1161/01.cir.103.8.1089. [DOI] [PubMed] [Google Scholar]
- 41.Bianco AC, Larsen PR. Cellular and structural biology of the deiodinases. Thyroid. 2005;15:777–786. doi: 10.1089/thy.2005.15.777. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Redetzke R, Said S, Pottala J, de Escobar G, Gerdes A. Serum thyroid hormone levels may not accurately reflect thyroid tissue levels and cardiac function in mild hypothyroidism. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H2137–43. doi: 10.1152/ajpheart.01379.2007. [DOI] [PubMed] [Google Scholar]
- 43.Cappelli C, et al. TSH-Lowering Effect of Metformin in Type 2 Diabetic Patients Differences between euthyroid, untreated hypothyroid, and euthyroid on L-T4 therapy patients. Diabetes Care. 2009;32:1589–90. doi: 10.2337/dc09-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pol C, Muller A, Simonides W. Cardiomyocyte-specific inactivation of thyroid hormone in pathologic ventricular hypertrophy: an adaptative response or part of the problem? Heart Fail Rev. 2010;15:133–42. doi: 10.1007/s10741-008-9133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mebis L, et al. Expression of thyroid hormone transporters during critical illness. Eur. J. Endocrinol. 2009;161:243–250. doi: 10.1530/EJE-09-0290. [DOI] [PubMed] [Google Scholar]
- 46.Friesema ECH, Jansen J, Jachtenberg JW, Visser WE, Kester MHA, Visser TJ. Effective cellular uptake and efflux of thyroid hormone by human monocarboxylate transporter 10. Mol Endocrinol. 2008;22:1357–69. doi: 10.1210/me.2007-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friesema E, Ganguly S, Abdalla A, Fox J, Halestrap A, Visser T. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J. Biol. Chem. 2003;278:40128–35. doi: 10.1074/jbc.M300909200. [DOI] [PubMed] [Google Scholar]
- 48.Visser W, Friesema E, Visser T. Minireview: thyroid hormone transporters: the knowns and the unknowns. Mol Endocrinol. 2011;25:1–14. doi: 10.1210/me.2010-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roy S, Mishra S, Ghosh G, Bandyopadhyay A. Thyroid hormone induces myocardial matrix degradation by activating matrix metalloproteinase-1. Matrix Biol. 2007;26:269–79. doi: 10.1016/j.matbio.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 50.Yao J, Eghbali M. Decreased collagen gene expression and absence of fibrosis in thyroid hormone induced myocardial hypertrophy-response of cardiac fibroblasts to thyroid hormone in vitro. Circ Res. 1992;71:831–9. doi: 10.1161/01.res.71.4.831. [DOI] [PubMed] [Google Scholar]
- 51.van Heerebeek L, et al. Diastolic stiffness of the failing diabetic heart - Importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43–51. doi: 10.1161/CIRCULATIONAHA.107.728550. [DOI] [PubMed] [Google Scholar]
- 52.Raev D. Which left ventricular function is impaired earlier in the evolution of diabetic cardiomyopathy? An echocardiographic study of young type I diabetic patients. Diabetes Care. 1994;17:633–9. doi: 10.2337/diacare.17.7.633. [DOI] [PubMed] [Google Scholar]
- 53.Oshiro Y, Shimabukuro M, Takasu N, Asahi T, Komiya I, Yoshida H. Triiodothyronine concomitantly inhibits calcium overload and postischemic myocardial stunning in diabetic rats. Life Sciences. 2001;69:1907–18. doi: 10.1016/s0024-3205(01)01274-7. [DOI] [PubMed] [Google Scholar]
- 54.Luidens M, Mousa S, Davis F, Lin H, Davis P. Thyroid hormone and angiogenesis. Vasc Pharmacol. 2010;52:142–5. doi: 10.1016/j.vph.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 55.McAllister R, Albarracin I, Price E, Smith T, Turk J, Wyatt K. Thyroid status and nitric oxide in rat arterial vessels. J Endocrinol. 2005;185:111–9. doi: 10.1677/joe.1.06022. [DOI] [PubMed] [Google Scholar]
- 56.Khalife W, et al. Treatment of subclinical hypothyroidism reverses ischemia and prevents myocyte loss and progressive LV dysfunction in hamsters with dilated cardiomyopathy. Am. J. Physiol Heart Circ Physiol. 2005;289:H2409–15. doi: 10.1152/ajpheart.00483.2005. [DOI] [PubMed] [Google Scholar]
- 57.Makino A, Suarez J, Wang H, Belke D, Scott B, Dillmann W. Thyroid hormone receptor-beta is associated with coronary angiogenesis during pathological cardiac hypertrophy. Endocrinology. 2009;150:2008–15. doi: 10.1210/en.2008-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kubis N, Richer C, Domergue V, Giudicelli J, Levy B. Role of microvascular rarefaction in the increased arterial pressure in mice lacking for the endothelial nitric oxide synthase gene (eNOS−/−) J Hypertens. 2002;20:1581–7. doi: 10.1097/00004872-200208000-00021. [DOI] [PubMed] [Google Scholar]
- 59.Carmeliet P, et al. Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF(164) and VEGF(188) Nat Med. 1999;5:495–502. doi: 10.1038/8379. [DOI] [PubMed] [Google Scholar]
- 60.Kitahara T, et al. Serum midkine as a predictor of cardiac events in patients with chronic heart failure. J. Card. Fail. 2010;16:308–13. doi: 10.1016/j.cardfail.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 61.Liu Y, Wang D, Redetzke R, Sherer BA, Gerdes AM. Thyroid hormone analog 3,5-diiodothyropropionic acid promotes healthy vasculature in the adult myocardium independent of thyroid effects on cardiac function. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H1551–7. doi: 10.1152/ajpheart.01293.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.