Abstract
Objective:
To determine whether low vitamin D concentrations are associated with an increased risk of incident all-cause dementia and Alzheimer disease.
Methods:
One thousand six hundred fifty-eight elderly ambulatory adults free from dementia, cardiovascular disease, and stroke who participated in the US population–based Cardiovascular Health Study between 1992–1993 and 1999 were included. Serum 25-hydroxyvitamin D (25(OH)D) concentrations were determined by liquid chromatography-tandem mass spectrometry from blood samples collected in 1992–1993. Incident all-cause dementia and Alzheimer disease status were assessed during follow-up using National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer's Disease and Related Disorders Association criteria.
Results:
During a mean follow-up of 5.6 years, 171 participants developed all-cause dementia, including 102 cases of Alzheimer disease. Using Cox proportional hazards models, the multivariate adjusted hazard ratios (95% confidence interval [CI]) for incident all-cause dementia in participants who were severely 25(OH)D deficient (<25 nmol/L) and deficient (≥25 to <50 nmol/L) were 2.25 (95% CI: 1.23–4.13) and 1.53 (95% CI: 1.06–2.21) compared to participants with sufficient concentrations (≥50 nmol/L). The multivariate adjusted hazard ratios for incident Alzheimer disease in participants who were severely 25(OH)D deficient and deficient compared to participants with sufficient concentrations were 2.22 (95% CI: 1.02–4.83) and 1.69 (95% CI: 1.06–2.69). In multivariate adjusted penalized smoothing spline plots, the risk of all-cause dementia and Alzheimer disease markedly increased below a threshold of 50 nmol/L.
Conclusion:
Our results confirm that vitamin D deficiency is associated with a substantially increased risk of all-cause dementia and Alzheimer disease. This adds to the ongoing debate about the role of vitamin D in nonskeletal conditions.
Recent meta-analyses confirm that low serum vitamin D concentrations are associated with prevalent Alzheimer disease (AD) dementia and cognitive impairment.1–3 This is cause for concern given the high rates of vitamin D deficiency in older adults4 and continued uncertainty about the causes of AD and other forms of dementia.5 Both the 1,25-dihydroxyvitamin D3 receptor and 1α-hydroxylase, the enzyme responsible for synthesizing the bioactive form of vitamin D, are found throughout the human brain.6 In vitro, vitamin D increases the phagocytic clearance of amyloid plaques by stimulating macrophages7,8 and reduces amyloid-induced cytotoxicity and apoptosis in primary cortical neurons.9 Vitamin D deficiency has also been linked to vascular dysfunction and ischemic stroke risk10 as well as brain atrophy.11 However, reverse causation is also possible, as the onset of dementia may lead to dietary changes and reduced outdoor activity, which in turn result in lower vitamin D concentrations.12
Previous prospective studies have established that low vitamin D concentrations in elderly adults are associated with an increased risk of cognitive decline.3,13–15 Furthermore, it has been hypothesized that the risk of cognitive decline markedly increases below a threshold between 25 and 50 nmol/L.12 However, preliminary prospective studies of vitamin D and dementia risk have been discordant. In a small study of 40 high-functioning elderly women, severe vitamin D deficiency (<25 nmol/L) was associated with a higher risk of non-AD dementias but not AD over 7 years.16 In contrast, in 10,186 individuals, severe vitamin D deficiency was associated with medical records indicating AD but not vascular dementia over 30 years of follow-up.17 The discrepancy in these findings may be due to a lack of statistical power16 or use of unstandardized dementia diagnoses from medical records, which may result in considerable misclassification.17 We therefore conducted what is to our knowledge the first large, prospective, population-based study incorporating a comprehensive adjudicated assessment of dementia and AD to examine their relationship with vitamin D concentrations.
METHODS
Participants.
Participants were selected from the Cardiovascular Health Study (CHS), a large, prospective, population-based study in the United States designed to investigate the underlying causes of cardiovascular disease in older men and women.18 The CHS recruited participants from 4 communities: Forsyth county, NC (36.1° north, 80.3° west); Sacramento county, CA (38.5° north, 121.4° west); Washington county, MD (39.6° north, 77.8° west); and Pittsburgh, PA (40.4° north, 80.0° west). The cohort consisted of 5,201 adults recruited in 1989–1990 and an additional 687 African-American participants recruited in 1992–1993. Of these 5,888 participants, 4,692 ambulatory participants had complete exam data in 1992–1993 (the baseline assessment for the current study). Serum 25-hydroxyvitamin D (25(OH)D) concentrations were not measured in 1,424 participants who had prevalent cardiovascular disease or stroke (one or more of the following: coronary heart disease, congestive heart failure, claudication, atrial fibrillation, pacemaker, implantable cardioverter defibrillator, stroke, or TIA), determined by medical records, ECG findings, and self-report.19 Further exclusions were insufficient serum volumes for vitamin D assay to be performed (<500 µL; n = 945) and missing adjudicated dementia status (n = 596).20 Participants with prevalent dementia at the time of the vitamin D collection (n = 69) were excluded from the main analyses but included in secondary analyses of prevalent dementia. This resulted in a final sample of 1,658 participants for the main prospective analyses and 1,727 participants for the secondary baseline analyses. Those lost to follow-up (defined as participants with serum 25(OH)D measured but no diagnostic assessment of incident dementia) were older (mean [SD], 74.3 [5.4] years vs 73.8 [4.6] years, p = 0.03), were more likely to be nonwhite (20.0% vs 13.1%, p < 0.001), and had lower serum 25(OH)D concentrations (mean [SD], 61.2 [39.4] nmol/L vs 64.4 [26.5] nmol/L, p = 0.03), but they were no more likely to be female (71.4% vs 69.2%, p = 0.31) or less educated (27.4% vs 23.4% did not finish high school, 53% vs 54% finished high school/some college/vocational qualifications, and 19.6% vs 22.6% completed college or professional qualifications, p = 0.10).
Standard protocol approvals, registrations, and patient consents.
The institutional review boards at each participating institution approved the research protocols, and all participants provided written informed consent.
Serum 25(OH)D measurement.
Serum samples collected in 1992–1993 were stored at −70 °C at the Laboratory for Clinical Biochemistry Research at the University of Vermont, and measurements were performed by the University of Washington Clinical Nutrition Research Unit in 2008.21 Total 25(OH)D (the sum of 25(OH)D2 and 25(OH)D3) was measured using liquid chromatography-tandem mass spectrometry (LC-MS) on a Waters Quattro micro mass spectrometer (Waters, Milford, MA); the interassay coefficient of variation was <3.4%.19 Calibration of serum 25(OH)D concentrations was verified using SRM 972 from the National Institute of Standards and Technology.22
Diagnosis of all-cause dementia and AD.
Dementia and AD status was assessed in 1998–1999 by a committee of neurologists and psychiatrists on the basis of annual cognitive assessments, repeat MRI scans, medical records, questionnaires, and proxy interviews. Diagnosis of dementia was based on a progressive or static cognitive deficit with impairment in at least 2 cognitive domains and a history of normal cognitive function before the onset of abnormalities. Incident all-cause dementia and AD were diagnosed according to the National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria. Further details can be found elsewhere.23
Covariates.
We adjusted for covariates identified as potential confounders1–3,12: age in years, season of blood collection (December–February, March–May, June–August, September–November), education status (did not finish high school, finished high school/some college/vocational qualifications, completed college/professional qualifications), sex, body mass index (BMI in kg/m2), smoking (nonsmoker, current smoker), alcohol consumption (National Institute on Alcohol Abuse and Alcoholism definitions: nondrinkers, moderate drinkers [women ≤7 drinks/week; men ≤14 drinks/week], heavy drinkers [women >7 drinks/week; men >14 drinks/week]), and significant depressive symptoms (score ≥8 on the revised 10-item Center for Epidemiologic Studies Depression Scale24).
Statistical analysis.
Cox proportional hazards models were used to assess the associations between baseline serum 25(OH)D and the risk of incident all-cause dementia and AD. Participants were considered at risk for dementia from baseline (1992–1993) and were censored at death or the end of follow-up in June 1999. All-cause dementia included AD cases, and analyses for AD were censored for non-AD dementia. The proportionality of hazards assumption was assessed using the Schoenfeld residuals technique.25 We analyzed serum 25(OH)D using clinically relevant cutpoints: <25 nmol/L (severely deficient), ≥25 nmol/L to <50 nmol/L (deficient), and ≥50 nmol/L (sufficient).26 Linear trends across categories were tested by entering 25(OH)D groups into models as a continuous rather than a categorical variable. In basic adjusted models, we controlled for age and season of blood collection. In fully adjusted models, we controlled for education, sex, BMI, smoking, alcohol consumption, and depressive symptoms. To investigate any threshold, we used multivariate adjusted penalized smoothing spline plots. Eight outlying participants with 25(OH)D concentrations between 170 and 283 nmol/L were excluded due to imprecision at the extreme end of the distribution (none developed dementia during follow-up).
In secondary analyses, serum 25(OH)D concentrations were analyzed as a continuous rather than a categorical variable. 25(OH)D concentrations were standardized to have a mean of 0 and an SD of 1 to aid interpretation. Because 25(OH)D concentrations were positively skewed, they were normalized using a log transformation. We repeated the main analyses to include adjustments for health conditions that have been identified as potential mediators for the association between serum 25(OH)D concentrations and dementia risk12,13: diabetes (American Diabetes Association guidelines: using oral hypoglycemic agents or insulin, or plasma fasting glucose ≥7.0 nmol/L) and/or hypertension (3 categories; no hypertension: systolic <140 mm Hg and diastolic <90 mm Hg; treated hypertension: hypertensive medication; untreated hypertension: systolic ≥140 mm Hg or diastolic ≥90 mm Hg with no hypertensive medication). We also adjusted for ethnicity (white/black) and examined potential interactions with ethnicity in separate models, although it is recognized that this may represent overadjustment.27 In a further analysis, we adjusted for socioeconomic status indicators: annual income (<$12,000, $12,000–24,999, $25,000–49,999, ≥$50,000, missing) and usual lifetime occupation (professional/technical/managerial/administrative, sales/clerical service, craftsman/machine operator/laborer/farming/forestry, housewife, other/missing). Multivariate adjusted logistic regression models were used to investigate the cross-sectional association between serum 25(OH)D and prevalent all-cause dementia (n = 69) and AD (n = 34).
In sensitivity analyses, we excluded participants who developed all-cause dementia (n = 12) and AD (n = 6) within 1 year of baseline to remove the possibility that any association observed was determined by these “early converters.” p Values were 2-sided throughout, and the type I error rate for statistical significance was set at 0.05. Analyses were performed using Stata SE version 12 (StataCorp, College Station, TX) with the exception of the spline plots, which were fitted in R version 2.15.1 (www.r-project.org).
RESULTS
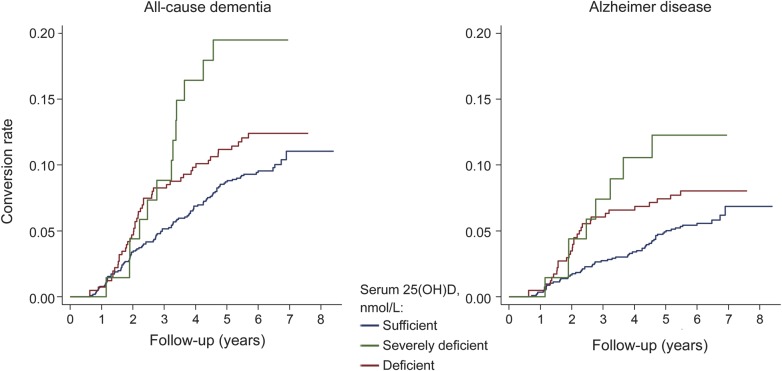
Table 1 displays baseline characteristics for the study population included in the main prospective analyses. Participants were followed up for a mean of 5.6 years (SD 1.6, median 6.1, range 0.1–8.4). During 9,317.5 person-years of follow-up, 171 participants developed all-cause dementia and 102 developed AD. The risk of developing both all-cause dementia and AD was significantly higher in participants who were either 25(OH)D deficient or severely deficient (table 2). In minimally adjusted models, those who were deficient had about a 51% increased risk of all-cause dementia, whereas the increased risk for those who were severely deficient was about 122%. The strength of the association observed for incident AD was similar to that observed for all-cause dementia. Additional adjustment for potential confounders did not alter the pattern of results, and there was a linear trend across groups in all analyses, suggesting a monotonic association. Kaplan-Meier plots for unadjusted rates of incident all-cause dementia and AD show clear differences in risk by 25(OH)D concentrations after 2–3 years of follow-up (figure 1). Multivariate adjusted smoothing spline plots suggest that the risk of all-cause dementia and AD markedly increases at 25(OH)D concentrations below 50 nmol/L (figure 2).
Table 1.
Baseline characteristics of 1,658 CHS participants by serum 25(OH)D concentration
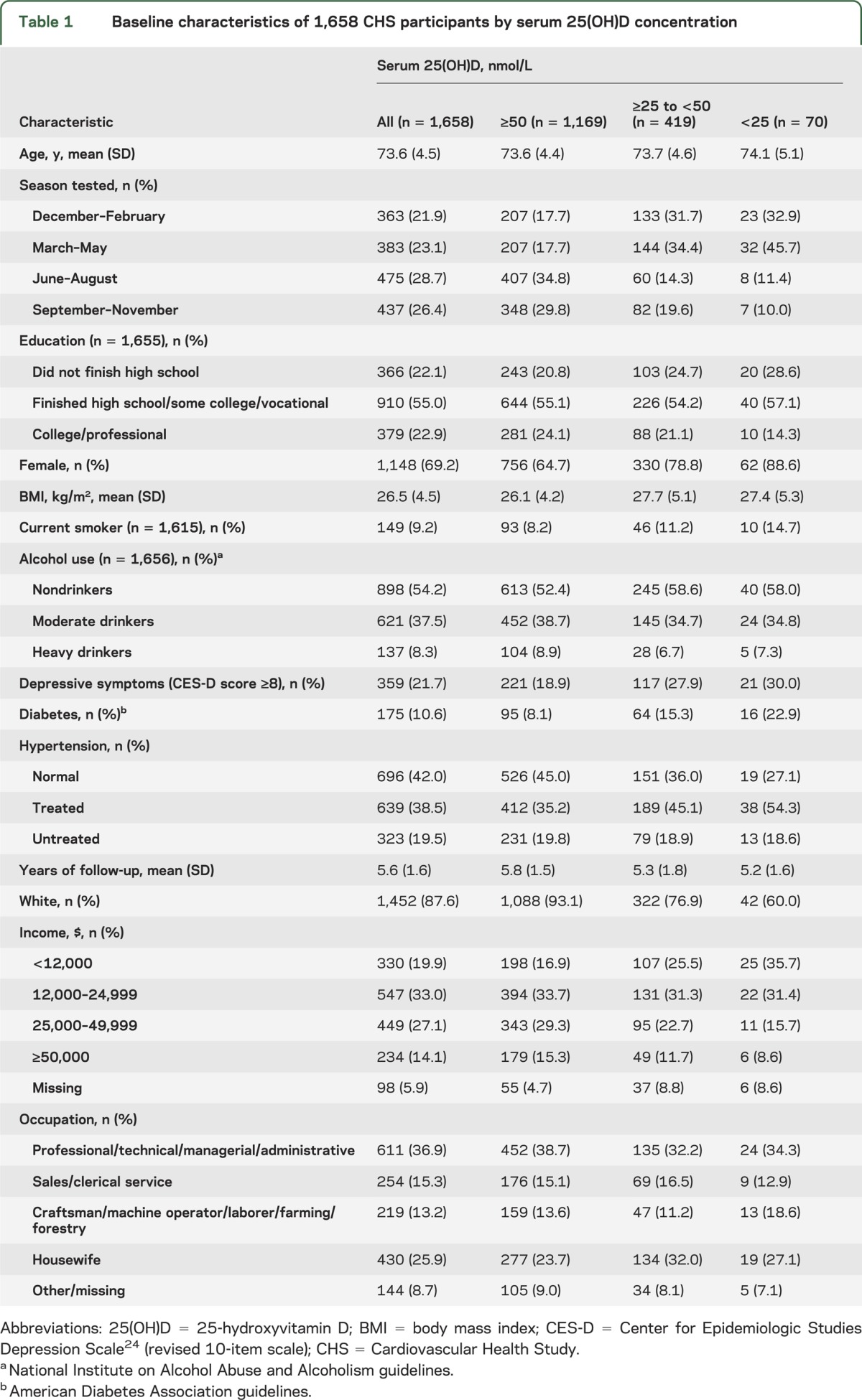
Table 2.
Cox proportional hazards regression models of incident all-cause dementia and Alzheimer disease by serum 25(OH)D concentration
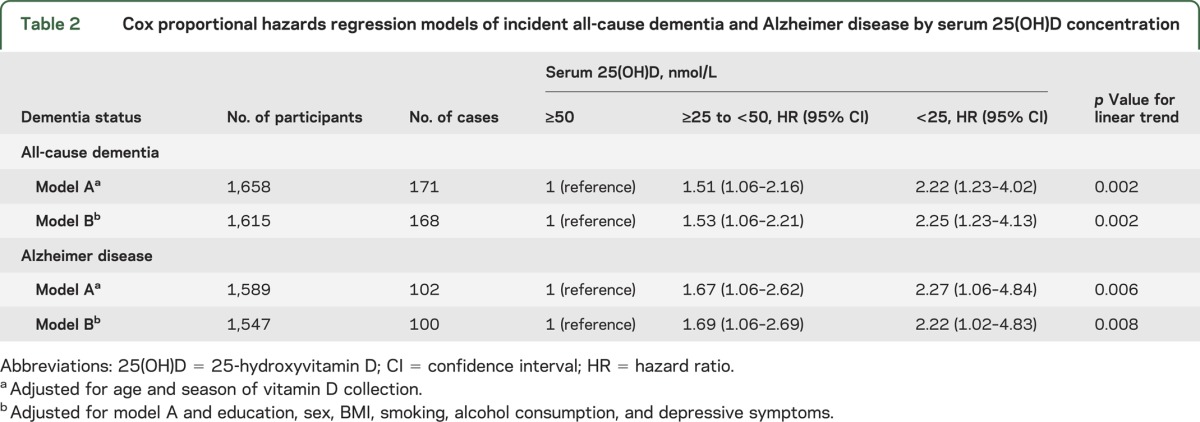
Figure 1. Kaplan-Meier curves for unadjusted rates of all-cause dementia and Alzheimer disease by serum 25-hydroxyvitamin D (25(OH)D) concentrations.
Figure 2. Multivariate adjusted smoothing spline plots showing the hazard ratios for dementia and Alzheimer disease by serum 25(OH)D concentrations.
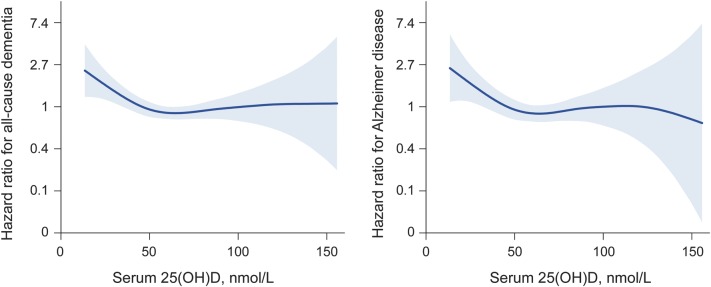
Models adjusted for age, season of vitamin D collection, education, sex, body mass index, smoking, alcohol consumption, and depressive symptoms. Hazard ratios centered on median serum 25-hydroxyvitamin D (25(OH)D) concentrations.
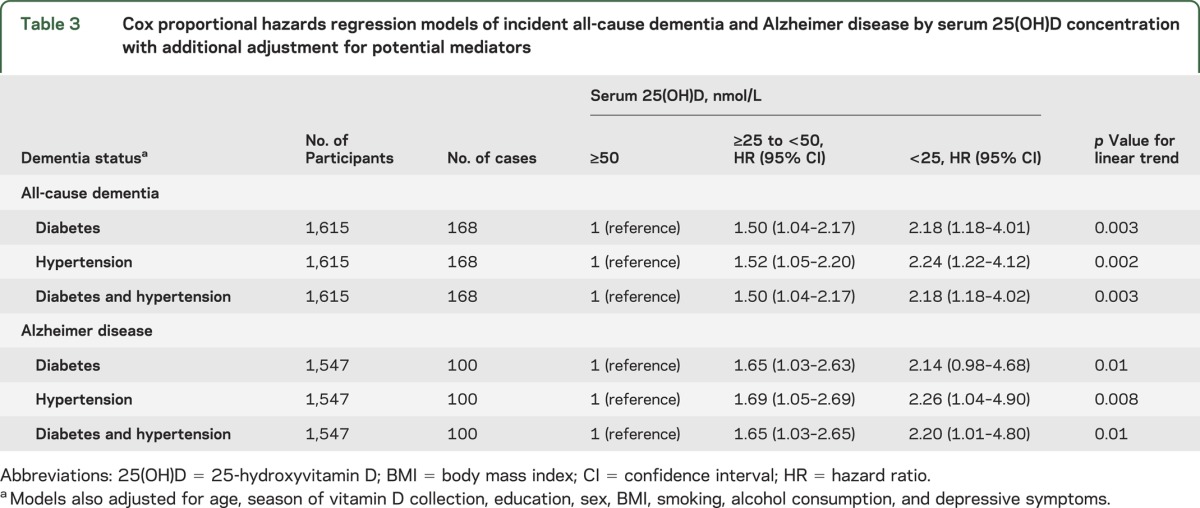
Secondary analyses incorporating continuous 25(OH)D concentrations gave a similar pattern of results. The multivariate adjusted risks for incident all-cause dementia and incident AD reduced by 18% (hazard ratio [HR] = 0.82, 95% confidence interval [CI]: 0.70–0.97, p = 0.02) and 20% (HR = 0.80, 95% CI: 0.65–0.99, p = 0.04), respectively, for each 1 SD increase in log-transformed 25(OH)D. Additional adjustment for diabetes or hypertension did not change the pattern of results for either incident all-cause dementia or AD, suggesting that these conditions are unlikely to mediate the observed associations (table 3). Adjustment for ethnicity attenuated the main results slightly but did not change the overall pattern of results, and there were no significant interactions (table e-1 on the Neurology® Web site at Neurology.org). Additional adjustment for income and occupation did not change the associations either (table e-2). The odds of prevalent all-cause dementia and AD at baseline in participants who were severely 25(OH)D deficient were 3–6 times higher than those with sufficient 25(OH)D, with a linear trend across groups (table e-3). After excluding participants who developed all-cause dementia and AD within 1 year of baseline, the multivariate adjusted HRs in participants who were severely 25(OH)D deficient and deficient compared to participants with sufficient 25(OH)D concentrations were 2.42 (95% CI: 1.33–4.39) and 1.54 (95% CI: 1.06–2.28) for incident all-cause dementia (p for linear trend = 0.001) and 2.36 (95% CI: 1.08–5.16) and 1.69 (95% CI: 1.04–2.73) for AD (p for linear trend = 0.007). This suggests that the association is not driven by “early converters.”
Table 3.
Cox proportional hazards regression models of incident all-cause dementia and Alzheimer disease by serum 25(OH)D concentration with additional adjustment for potential mediators
DISCUSSION
We have conducted what is to our knowledge the first large, prospective, population-based study to examine vitamin D concentrations in relation to a comprehensive adjudicated assessment of dementia and AD. We observed a strong monotonic association between 25(OH)D concentrations and the risk of both incident all-cause dementia and AD. This association was robust to adjustment for a range of potential confounders and the exclusion of dementia cases that occurred within a year of baseline.
The 2 previous studies that have investigated vitamin D and incident dementia have produced conflicting results. The first found that severe vitamin D deficiency was associated with non-AD dementia but not AD risk.16 The second found that severe vitamin D deficiency was associated with AD but not vascular dementia risk.17 However, the first study incorporated a small sample of high-functioning women (n = 40), and the lack of association with AD may reflect limited statistical power. The second study relied on registry data for dementia diagnoses, which may have resulted in considerable misclassification. Our results establish that low 25(OH)D concentrations are linked to an increased risk of incident all-cause dementia and AD, and they are consistent with studies suggesting a link with cognitive impairment1,3,12,26,28 and cognitive decline.13–15 Few studies have examined potential mediators of this association, although there was no evidence in the present study or the InCHIANTI13 study for mediation by diabetes or hypertension.
A threshold below which the risk of dementia increases markedly has previously been hypothesized to lie in the 25–50 nmol/L range.12 The optimal level of vitamin D for general health remains controversial, with the Institute of Medicine recommending 50 nmol/L and the Endocrine Society recommending 75 nmol/L.29,30 A post hoc analysis of the Women's Health Initiative randomized controlled trial discovered that a relatively low dose of vitamin D (400 IU) in combination with calcium (1,000 mg) did not protect against dementia over a mean follow-up period of 7.8 years in women who had relatively high serum vitamin D levels at baseline (mean of 49 nmol/L in a small subsample).31 Our results clarify that the threshold above which older adults are unlikely to benefit from supplementation with regard to dementia risk is likely to lie in the region of 50 nmol/L when 25(OH)D concentrations are measured using LC-MS. This therefore adds to the ongoing debate regarding optimal vitamin D levels for different health outcomes.
A number of potential mechanisms linking low vitamin D levels with the risk of dementia have been identified.32 Vitamin D receptors are expressed throughout the brain, including areas involved in memory such as the hippocampus and dentate gyrus.6 Similarly, the enzyme that synthesizes the active form of vitamin D, 1α-hydroxylase, is produced in several cerebral regions. The active form of vitamin D, 1,25dihydroxy-vitamin D3 (1,25-D3), regulates neurotrophin expression, such as nerve growth factor, neurotrophin 3, and glial-derived neurotrophic factor,11 and the survival, development, and function of neural cells.33 In vitro, vitamin D stimulates macrophages, which increases the clearance of amyloid plaques, a hallmark of AD.7,8 Vitamin D also reduces amyloid-induced cytotoxicity and apoptosis in primary cortical neurons.9 A recent study found that amyloid-β induction of induced nitric oxide synthase, part of the inflammatory process of AD, is dependent on the disruption of the vitamin D-vitamin D receptor pathway.34 Vitamin D supplementation ameliorates age-related decline in learning and memory in aged rats.35 In addition, vitamin D deficiency has been linked to cerebrovascular pathology. Meta-analyses establish that 25(OH)D deficiency is associated with an increased risk of incident stroke,36 particularly ischemic stroke.10 A cross-sectional study of 318 elderly adults found that 25(OH)D deficiency was associated with increased white matter hyperintensity volume and a greater number of large vessel infarcts.37 In summary, low vitamin D concentrations may increase the risk of dementia and AD through both neurodegenerative and vascular mechanisms.
Our study has a number of strengths. The study sample was relatively diverse as it was population-based and included white and African-American men and women. A recent systematic review raised the possibility that the consistent observational associations between vitamin D levels and a wide range of health conditions may simply reflect reverse causation.38 However, in this study reverse causation is made less likely by the fact that participants were ambulatory and relatively healthy at baseline (their outdoor activity was not likely to be limited by impaired function linked to the onset of dementia). The long follow-up and exclusion of prevalent dementia and incident dementia occurring within a year of baseline also make reverse causation less likely. All-cause dementia and AD in the CHS were diagnosed by a committee of neurologists and psychiatrists using a comprehensive range of data, including neuroimaging, according to international criteria (NINCDS-ADRDA).23 Our study also has several limitations. While the CHS is multiethnic, it did not incorporate people of Hispanic or other ethnicities. Due to the exclusion of participants with cardiovascular disease and stroke at baseline, there were few cases of incident vascular dementia (n = 15). It was therefore not possible to investigate the relationship between vitamin D concentrations and incident vascular dementia due to a lack of statistical power, and further research is necessary to investigate generalizability to older adults with vascular dysfunction. In a cohort with a greater burden of vascular and metabolic dysfunction it would also be interesting to investigate these factors as time-varying covariates. The representativeness of our final sample may have been reduced due to the inability to include participants with insufficient serum volume for 25(OH)D measurement (n = 945) as well as those lost to follow-up (n = 596). It is possible that the delay between obtaining the blood samples in 1992–1993 and measuring 25(OH)D concentrations in 2008 could have introduced measurement error; however, this is unlikely to have introduced systematic bias. Despite the wide range of information (including repeat neuroimaging) available to the committee diagnosing all-cause dementia and AD, a degree of misclassification is still likely. In particular, many cases of AD may actually reflect a mixture of pathologies, so caution should be exercised when considering potential mechanisms. As with all observational studies, unmeasured confounding is possible, and our findings do not in themselves demonstrate a causal relationship.
We found a strong association between baseline vitamin D concentrations and the risk of incident all-cause dementia and AD over a mean of 5.6 years of follow-up in ambulatory older adults free from vascular conditions at baseline. Further studies are necessary to replicate our findings and extend them to more diverse populations. It would be useful to conduct prospective studies to investigate the association between vitamin D concentrations and incident vascular dementia and neuroimaging abnormalities. Our findings support the hypothesis that vitamin D may be neuroprotective and that “sufficiency” in the context of dementia risk may be in the region of 50 nmol/L. This information is likely to prove useful in improving the design and reducing the cost of randomized controlled trials investigating whether vitamin D supplements can be used to delay or prevent the onset of dementia and AD in older adults.
Supplementary Material
GLOSSARY
- 25(OH)D
25-hydroxyvitamin D
- AD
Alzheimer disease
- BMI
body mass index
- CHS
Cardiovascular Health Study
- CI
confidence interval
- HR
hazard ratio
- LC-MS
liquid chromatography-tandem mass spectrometry
- NINCDS-ADRDA
National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Mr. Littlejohns: drafting and revising the manuscript for content, study concept and design, analysis and interpretation of data, and statistical analysis. Dr. Henley: revising the manuscript for content, study concept and design, analysis and interpretation of data, and statistical analysis. Dr. Lang: revising the manuscript for content, interpretation of data. Dr. Annweiler: revising the manuscript for content, interpretation of data. Dr. Beauchet: revising the manuscript for content, interpretation of data. Dr. Chaves: revising the manuscript for content, interpretation of data, obtaining funding. Dr. Fried: revising the manuscript for content, interpretation of data, acquisition of data. Dr. Kestenbaum: revising the manuscript for content, interpretation of data, acquisition of data, obtaining funding. Dr. Kuller: revising the manuscript for content, interpretation of data, acquisition of data. Dr. Langa: revising the manuscript for content, interpretation of data, acquisition of data, obtaining funding. Dr. Lopez: revising the manuscript for content, interpretation of data, acquisition of data. Dr. Kos: revising the manuscript for content, study concept and design, analysis and interpretation of data. Dr. Soni: revising the manuscript for content, study concept and design, analysis and interpretation of data, statistical analysis. Dr. Llewellyn: drafting and revising the manuscript for content, study concept and design, analysis and interpretation of data, acquisition of data, statistical analysis, study supervision and coordination, obtaining funding.
STUDY FUNDING
The CHS was supported by contracts HHSN268201200036C, HHSN268200800007C, N01 HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided by AG023629, AG20098, AG15928, and HL084443 from the National Institute on Aging. A full list of principal CHS investigators and institutions can be found at www.chs-nhlbi.org. Additional support was also provided by NIRG-11-200737 from the Alzheimer's Association, the Mary Kinross Charitable Trust, the James Tudor Foundation, the Halpin Trust, the Age Related Diseases and Health Trust, and the Norman Family Charitable Trust (to D.J.L.). This report presents independent research supported by the UK National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) for the South West Peninsula. None of the funding sources had any role in the design of the study; in the analysis and interpretation of the data; or in the preparation of the manuscript. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health in England. The NIH was involved in the original design and conduct of the CHS and in the data collection methods.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Balion C, Griffith LE, Strifler L, et al. Vitamin D, cognition, and dementia: a systematic review and meta-analysis. Neurology 2012;79:1397–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Annweiler C, Llewellyn DJ, Beauchet O. Low serum vitamin D concentrations in Alzheimer's disease: a systematic review and meta-analysis. J Alzheimers Dis 2013;33:659–674 [DOI] [PubMed] [Google Scholar]
- 3.Annweiler C, Montero-Odasso M, Llewellyn DJ, Richard-Devantoy S, Duque G, Beauchet O. Meta-analysis of memory and executive dysfunctions in relation to vitamin D. J Alzheimers Dis 2013;37:147–171 [DOI] [PubMed] [Google Scholar]
- 4.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–281 [DOI] [PubMed] [Google Scholar]
- 5.Thies W, Bleiler L. 2013 Alzheimer's disease facts and figures. Alzheimers Dement 2013;9:208–245 [DOI] [PubMed] [Google Scholar]
- 6.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat 2005;29:21–30 [DOI] [PubMed] [Google Scholar]
- 7.Masoumi A, Goldenson B, Ghirmai S, et al. 1alpha,25-dihydroxyvitamin D3 interacts with curcuminoids to stimulate amyloid-beta clearance by macrophages of Alzheimer's disease patients. J Alzheimers Dis 2009;17:703–717 [DOI] [PubMed] [Google Scholar]
- 8.Mizwicki MT, Menegaz D, Zhang J, et al. Genomic and nongenomic signaling induced by 1α,25(OH)2-vitamin D3 promotes the recovery of amyloid-β phagocytosis by Alzheimer's disease macrophages. J Alzheimers Dis 2012;29:51–62 [DOI] [PubMed] [Google Scholar]
- 9.Dursun E, Gezen-Ak D, Yilmazer S. A novel perspective for Alzheimer's disease: vitamin D receptor suppression by amyloid-β and preventing the amyloid-β induced alterations by vitamin D in cortical neurons. J Alzheimers Dis 2011;23:207–219 [DOI] [PubMed] [Google Scholar]
- 10.Brøndum-Jacobsen P, Nordestgaard BG, Schnohr P, Benn M. 25-hydroxyvitamin D and symptomatic ischemic stroke: an original study and meta-analysis. Ann Neurol 2013;73:38–47 [DOI] [PubMed] [Google Scholar]
- 11.Annweiler C, Montero-Odasso M, Hachinski V, Seshadri S, Bartha R, Beauchet O. Vitamin D concentration and lateral cerebral ventricle volume in older adults. Mol Nutr Food Res 2013;57:267–276 [DOI] [PubMed] [Google Scholar]
- 12.Dickens AP, Lang IA, Langa KM, Kos K, Llewellyn DJ. Vitamin D, cognitive dysfunction and dementia in older adults. CNS Drugs 2011;25:629–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llewellyn DJ, Lang IA, Langa KM, et al. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med 2010;170:1135–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slinin Y, Paudel ML, Taylor BC, et al. 25-Hydroxyvitamin D levels and cognitive performance and decline in elderly men. Neurology 2010;74:33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slinin Y, Paudel M, Taylor BC, et al. Association between serum 25(OH) vitamin D and the risk of cognitive decline in older women. J Gerontol A Biol Sci Med Sci 2012;67:1092–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Annweiler C, Rolland Y, Schott AM, Blain H, Vellas B, Beauchet O. Serum vitamin D deficiency as a predictor of incident non-Alzheimer dementias: a 7-year longitudinal study. Dement Geriatr Cogn Disord 2011;32:273–278 [DOI] [PubMed] [Google Scholar]
- 17.Afzal S, Bojesen SE, Nordestgaard BG. Reduced 25-hydroxyvitamin D and risk of Alzheimer's disease and vascular dementia. Alzheimers Dement 2014;10:296–302 [DOI] [PubMed] [Google Scholar]
- 18.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991;1:263–276 [DOI] [PubMed] [Google Scholar]
- 19.Kestenbaum B, Katz R, de Boer I, et al. Vitamin D, parathyroid hormone, and cardiovascular events among older adults. J Am Coll Cardiol 2011;58:1433–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitzpatrick AL, Kuller LH, Ives DG, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc 2004;52:195–204 [DOI] [PubMed] [Google Scholar]
- 21.De Boer IH, Levin G, Robinson-Cohen C, et al. Serum 25-hydroxyvitamin D concentration and risk for major clinical disease events in a community-based population of older adults: a cohort study. Ann Intern Med 2012;156:627–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phinney KW. Development of a standard reference material for vitamin D in serum. Am J Clin Nutr 2008;88:511S–512S [DOI] [PubMed] [Google Scholar]
- 23.Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N. Evaluation of dementia in the cardiovascular health cognition study. Neuroepidemiology 2003;22:1–12 [DOI] [PubMed] [Google Scholar]
- 24.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401 [Google Scholar]
- 25.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika 1982;69:239–241 [Google Scholar]
- 26.Llewellyn DJ, Lang IA, Langa KM, Melzer D. Vitamin D and cognitive impairment in the elderly U.S. population. J Gerontol A Biol Sci Med Sci 2011;66:59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brehm JM, Schuemann B, Fuhlbrigge AL, et al. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol 2010;126:52–58.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Llewellyn D, Langa K. Serum 25-hydroxyvitamin D concentration and cognitive impairment. J Geriatr Psychiatry 2009;22:188–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press; 2011 [PubMed] [Google Scholar]
- 30.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–1930 [DOI] [PubMed] [Google Scholar]
- 31.Rossom RC, Espeland MA, Manson JE, et al. Calcium and vitamin D supplementation and cognitive impairment in the women's health initiative. J Am Geriatr Soc 2012;60:2197–2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gezen-Ak D, Yilmazer S, Dursun E. Why vitamin D in Alzheimer's disease? the hypothesis. J Alzheimers Dis 2014;40:257–269 [DOI] [PubMed] [Google Scholar]
- 33.Fernandes de Abreu DA, Eyles D, Féron F. Vitamin D, a neuro-immunomodulator: implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology 2009;34(suppl 1):S265–S277 [DOI] [PubMed] [Google Scholar]
- 34.Dursun E, Gezen-Ak D, Yilmazer S. A new mechanism for amyloid-β induction of iNOS: vitamin D-VDR pathway disruption. J Alzheimers Dis 2013;36:459–474 [DOI] [PubMed] [Google Scholar]
- 35.Briones TL, Darwish H. Vitamin D mitigates age-related cognitive decline through the modulation of pro-inflammatory state and decrease in amyloid burden. J Neuroinflammation 2012;9:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Song Y, Manson JE, et al. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes 2012;5:819–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buell JS, Weiner DE, Tucker L, Usda JM. 25-Hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology 2010;74:18–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol 2014;2:76–89 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.