Abstract
Resveratrol (trans-3,5,4′-trihydroxystilbene), a compound found largely in the skins of red grapes and wines, possesses anti-cancer and anti-angiogenic properties and protects the cardiovascular system. However, the molecular mechanisms by which resveratrol inhibits angiogenesis are currently subjects of intense investigation. The purpose of this study was to examine whether FOXO transcription factors mediate anti-angiogenic effects of resveratrol, and whether vascular endothelial growth factor (VEGF) neutralizing antibody can enhance these effects of resveratrol. Inhibition of PI3 kinase (PI3K)/AKT and MEK/ERK pathways synergistically inhibited migration and capillary tube formation of Human Umbilical Vein Endothelial Cells (HUVECs) and further enhanced the anti-angiogenic effects of resveratrol. Inhibitors of AKT and MEK kinase synergistically inhibited cytoplasmic FOXO3a phosphorylation, which was accompanied by its nuclear translocation in HUVECs. Interestingly, inhibition of PI3K/AKT and MEK/ERK pathways synergistically induced FOXO transcriptional activity and inhibited cell migration and capillary tube formation. Antiangiogenic effects of resveratrol were enhanced by inhibitors of AKT and MEK. Phosphorylation-deficient mutants of FOXOs induced FOXO transcriptional activity, inhibited HUVEC cell migration, and capillary tube formation, and also enhanced antiangiogenic effects of resveratrol. Finally, VEGF neutralizing antibody enhanced the anti-proliferative and anti-angiogenic effects of resveratrol. In conclusion, regulation of FOXO transcription factors by resveratrol may play an important role in angiogenesis which is critical for cancer, diabetic retinopathy, rheumatoid arthritis, psoriasis, and cardiovascular disorders.
Keywords: Angiogenesis, FOXO, Resveratrol, Vascular endothelial growth factors (VEGF)
Introduction
Resveratrol is a phytoalexin produced by many plants, and the skin of red grapes is particularly rich in resveratrol which accounts for the “French Paradox.” Besides its protection of the cardiovascular system, it can affect the processes underlying all three stages of carcinogenesis, involving tumor initiation, promotion, and progression [1]. The anti-carcinogenic effects of resveratrol appear to be closely associated with its capacity to interact with multiple molecular targets involved in cancer development, while minimizing toxicity in normal tissues as tested. Resveratrol has been shown to enhance the therapeutic potential of chemotherapeutic drugs or cytotoxic factors for the highly efficient treatment of drug refractory tumor cells [1–3]. Although resveratrol has been shown to inhibit angiogenesis and metastasis, the involvement of FOXO transcription factor in anti-angiogenic effects of resveratrol has never been examined.
Members of the FOXO family, FOXO1 (FKHR), FOXO3 (FKHRL1), and FOXO4 (AFX), are mammalian homologs of DAF-16, which influences life span and energy metabolism in Caenorhabditis elegans. Mammalian FOXO proteins also play important roles in cell cycle arrest, apoptosis, angiogenesis, stress resistance, and energy metabolism [4–7]. The PI3 kinase (PI3K) pathway phosphorylates FOXO proteins through activation of downstream kinase AKT [8–10]. These phosphorylations result in impairment of DNA binding ability and increased binding affinity for the 14-3-3 protein [9, 10]. Newly formed 14-3-3-FOXO complexes are then exported from the nucleus [11], thereby inhibiting FOXO-dependent transcription. Inhibition of the PI3K/AKT pathway leads to dephosphorylation and nuclear translocation of active FKHRL1, FKHR, and AFX, which induce transcription of genes related to cells' cycle arrest and apoptosis [12]. Conversely, loss of PTEN activity causes an increase in AKT activity leading to inhibition of FOXO protein activity through phosphorylation and cytoplasmic sequestration [13]. Recent evidence has demonstrated that the FOXO subfamily induces apoptosis at least in part by regulating transcription of genes such as Bim [14], BCL-6 [15], and Fas Ligand [9, 10], causes cell cycle arrest via controlling the expression of p27/KIP1 [11, 16–18], Gadd45 [19], Cyclin D [20], or inhibits angiogenesis by regulating angiopoietin-2 (Ang-2) and Tie-2 [21]. In some situations, FOXO proteins play a redundant role, whereas in others, their roles in development and physiology are diverse, and genetic loss of the distinct FOXO isoforms results in different phenotypes. For example, mice homozygous for a FOXO1−/− allele, but not FOXO3a−/− or FOXO4−/− mice, die during embryogenesis from defects in vascular development [22, 23]. Although these studies suggest an essential role of FOXO1 in the formation and maturation of the nascent vasculature, relatively little is known about the function and significance of the distinct FOXO family members for the angiogenic activity of endothelial cells and postnatal vessel formation. In mature endothelial cells, angiopoietin 1 (Ang1) modulates endothelial function [24] by inhibiting FOXO1 activity. Furthermore, in addition to phosphorylation, other post-translation regulatory mechanisms such as acetylation can regulate FOXO-dependent gene transcription.
In addition to PI3K/AKT pathway, MAP kinases have been shown to regulate the phosphorylation of FOXO transcription factor. MAP kinases regulate many cellular activities including angiogenesis [25]. They are activated by the dual phosphorylations of neighboring threonine and tyrosine residues in response to various extracellular stimuli [26, 27]. Since FOXO1 contains 15 consensus phosphorylation sites for the mitogen-activated protein kinase (MAPK) family; its transcriptional activity could directly be regulated by MAPKs. In vitro kinase assay showed that FOXO1 was phosphorylated by ERK and p38 but not by JNK [28]. In NIH3T3 cells, epidermal growth factor or anisomycin increased phosphorylation of exogenous FOXO1, which was significantly inhibited by pre-treatment with an MEK1 inhibitor, PD98059, or a p38 inhibitor, SB203580. Phosphopeptide mapping using mutation of phosphorylation sites for MAPK revealed that the nine serine residues in FOXO1 are specifically phosphorylated by ERK and that five of the nine residues are phosphorylated by p38 in vivo. These studies clearly demonstrate phosphorylation-dependent regulation of FOXO by MAPKs.
The discovery of vascular endothelial growth factors (VEGFs) and their receptors has considerably improved the understanding of the development and function of endothelial cells [29, 30]. Each member of the VEGF family appears to have a specific function: VEGF-A induces angiogenesis (i.e., growth of new blood vessels from preexisting ones), placental growth factor mediates both angiogenesis and arteriogenesis (i.e., the formation of collateral arteries from preexisting arterioles), VEGF-C and VEGF-D act mainly as lymphangiogenic factors [30, 31]. The study of the biology of these endothelial growth factors has allowed major progress in the comprehension of the genesis of the vascular system and its abnormalities observed in various pathologic conditions (atherosclerosis and coronary artery disease). Anti-angiogenic therapies that target VEGF and the VEGF receptor (VEGFR) are effective adjuncts to the treatment of solid tumors. The FDA has recently approved the use of two anti-VEGF antibodies, bevacizumab (Avastin), and ranibizumab (Lucentis) which have demonstrated therapeutic utility in blocking VEGF-induced angiogenesis [32–34]. Treatment with bevacizumab is commonly combined with cytotoxic chemotherapy and results in dramatic responses seen on radiographs, prolongation of progression-free survival [31, 35, 36]. Similar results have been shown with small-molecule inhibitors of VEGFR, such as cediranib [36–38]. Dual inhibition of Raf and VEGFR2, NVP-AAL881, has been shown to inhibit on tumor growth, vascularization, and metastasis [39, 40]. Similarly, the combination of agents that inhibit VEGF signaling (e.g., antibody and small molecule) and chemopreventive agent resveratrol may offer several health benefits.
The purpose of this study was to examine whether the inhibition of PI3K/AKT and MEK/ERK pathways enhanced the antiangiogenic effects of resveratrol through activation of FOXO transcription factors. We have demonstrated that inhibition of PI3K/AKT and MEK/ERK pathways acted synergistically to induce FOXO transcription activity, and inhibit capillary tube formation and migration. Finally, anti-VEGF antibody enhanced the anti-proliferative and anti-angiogenic effects of resveratrol, suggesting the clinical utility of combining humanized VEGF antibody with resveratrol for the treatment of angiogenesis-related diseases. Furthermore, anti-angiogenic effects of resveratrol were regulated through activation of FOXO transcription factors.
Materials and methods
Reagents
Resveratrol was purchased from LKT Laboratories, Inc. (St. Paul, MN, USA). MEK inhibitor (PD98059) and AKT inhibitor-IV were purchased from EMD Biosciences (San Diego, CA, USA). Dual Luciferase Reporter Assay kit was purchased from Promega Corporation (Madison, WI, USA). Anti-human VEGF neutralizing antibodies (catalog number MAB293 mentioned as Ab1, and catalog number AF-291-NA mentioned as Ab2) were purchased from R&D Systems (Minneapolis, MN, USA).
Cell culture and cell proliferation assay
Human umbilical vein endothelial cells (HUVECs) were purchased from Clonetics (Walkersville, MD, USA) and maintained in endothelial cell growth factor medium-2 (EGM2 MV SingleQuots, Clonetics) supplemented with 5% FBS. Stock solutions of the resveratrol were prepared in DMSO and diluted with complete medium, and an equal volume of DMSO (final concentration, 0.05%) was added to the controls. For cell proliferation assay, HUVECs were seeded in 24-well plates and treated with either anti-VEGF Ab1 or anti-VEGF Ab2 in the presence or absence of resveratrol for 48 h. Viable cells were counted under a microscope after staining cells with trypan blue dye.
Capillary tube formation assay
Cell migration assay was performed as we described earlier [41]. In brief, matrigel (100 μl) was added to wells of a 96-well culture plate and allowed to polymerize for 1 h at 37°C. To examine the effects of resveratrol on in vitro angiogenesis, subconfluent HUVECs were resuspended in complete medium and added to matrigel containing wells (1 × 104 cells/well), and exposed to resveratrol or DMSO (control). The plates were incubated at 37°C in a humidified atmosphere of 95% air and 5% CO2. Capillary tube formation was assessed after 24 h by counting the total number of capillary-like tubular structures from three randomly chosen fields using an inverted microscope.
In vitro cell migration assay
Cell migration assay was performed as we described earlier [41]. In brief, migration of HUVEC cells was assessed using Transwell Boyden chamber (Corning, Acton, MA, USA) containing a polycarbonated filter with a pore size of 8-μM. HUVECs (4 × 104 cells in 0.2 ml) cells in complete medium was mixed with desired concentration of resveratrol or DMSO (control), and the cell suspension was added to the upper chamber. The lower chamber contained 0.6 ml of complete medium with the same concentration of resveratrol or DMSO. Migration through the membrane was determined after 24 h of incubation at 37°C. Cells remaining on the topside of the transwell membrane were removed using a cotton swab. The membrane was washed with ice-cold phosphate-buffered saline (PBS). Cells that had migrated to the underside were fixed with 90% methanol and stained with Giemsa. Cell migration was quantified by counting the number of cells per field in five random fields.
Luciferase assay
HUVEC cells were transfected with empty vector, FOXO1-TM, FOXO3a-TM, or FOXO4-TM along with reporter plasmids, p6xDBE-luc, and pRL-TK [23]. The FOXO expression vectors (wild-type and phosphorylation-deficient mutants) and FOXO-luciferase constructs have been described elsewhere [23, 42]. After 24 h, transfection medium was replaced with culture medium and cells were treated with resveratrol (10–20 μM). After incubation of 24 h, the relative luciferase activity, i.e., firefly enzyme activity divided by that of the Renilla enzyme, was determined using Dual Luciferase Reporter Assay System (Promega) according to the manufacturer's protocol.
Western blot analysis
Cells were washed with PBS, collected using a cell scraper, and lysed in RIPA buffer containing protease inhibitor mixture (Sigma-Aldrich, St. Louis, MO, USA). After sonication, the lysate was centrifuged for 30 min at 13000 rpm, and the supernatant was collected. Protein concentration was determined, and equal amounts of protein were subjected to SDS–PAGE (12% polyacrylamide). After electrophoresis, proteins were transferred to polyvinylidene difluoride membrane, blocked 1 h at room temperature with 5% nonfat dry milk, and incubated overnight with primary antibody (dilution 1:1000) in blocking solution. After washing thrice with 0.1% TBS-Tween, the blots were incubated with HRP-conjugated secondary antibody (dilution 1:2000). Proteins were detected using ECL substrate (Thermo Fisher, Pittsburgh, PA, USA).
Immunofluorescence analysis
HUVECs were grown on fibronectin-coated coverslips (Beckton Dickinson, Bedford, MA, USA), washed in PBS, and fixed for 15 min in 4% paraformaldehyde. Cells were permeabilized in 0.1% Triton X-100, washed and blocked in 10% normal goat serum. Cells were incubated with anti-FOXO3a antibody (1:200) for 18 h at 4°C. Cells were then washed and incubated with fluorescently labeled secondary antibodies (1:200) along with DAPI (1 μg/ml) for 1 h at room temperature. Cells were washed and coverslips were mounted using Vectashield (Vector Laboratories, Burlington, CA, USA). Isotype-specific negative controls were included with each staining. Stained cells were mounted and visualized under a fluorescence Olympus microscope (Olympus America Inc., Center Valley, PA, USA). Pictures were captured using a Photometrics Cool-snap CF color camera (Olympus) and SPOT software (Diagnostic Instruments, Inc., Sterling Heights, MI, USA).
Statistical analysis
The mean and SD were calculated for each experimental group. Differences between groups were analyzed by one- or two-way ANOVA using PRISM statistical analysis software (GrafPad Software, Inc., San Diego, CA, USA). Significant differences among groups were calculated at P < 0.05.
Results
Inhibitory effects of resveratrol on HUVEC cell migration and capillary tube formation are enhanced by inhibitors of AKT and MEK1/2
The PI3K/AKT and MEK/ERK pathways have been shown to enhance angiogenesis which plays a critical role in tumor development [13, 43]. Therefore, agents that inhibit angiogenesis can be developed for the treatment of human diseases. Cellular events such as endothelial cell migration and capillary tube formation are important events for angiogenesis. In order to inhibit PI3K/AKT and MEK/ERK pathways, we have used AKT inhibitor IV and PD98059, respectively. AKT inhibitor IV is a cell-permeable benzimidazole compound that inhibits AKT phosphorylation/activation by targeting the ATP binding site of a kinase upstream of AKT, but downstream of PI3K [44]. It has been shown to block AKT-mediated FOXO1 nuclear export and cell proliferation [44]. Unlike phosphatidylinositol analog-based AKT inhibitors, this inhibitor does not affect PI3K [44]. We first examined whether resveratrol inhibits HUVEC cell migration using a modified Boyden Chamber assay (Fig. 1a, b). A large fraction of HUVEC cells migrated to the bottom face of the membrane in control group. Inhibitors of AKT (AKT inhibitor IV) and MEK1/2 (PD98059) alone resulted in inhibition HUVEC cell migration. Similarly, resveratrol inhibited HUVEC cell migration. Interestingly, the combination of AKT inhibitor IV and PD98059 inhibited cell migration in an additive manner. Furthermore, the inhibitory effects of resveratrol on cell migration were further enhanced in the presence of inhibitors of AKT and/or MEK1/2.
Fig. 1.
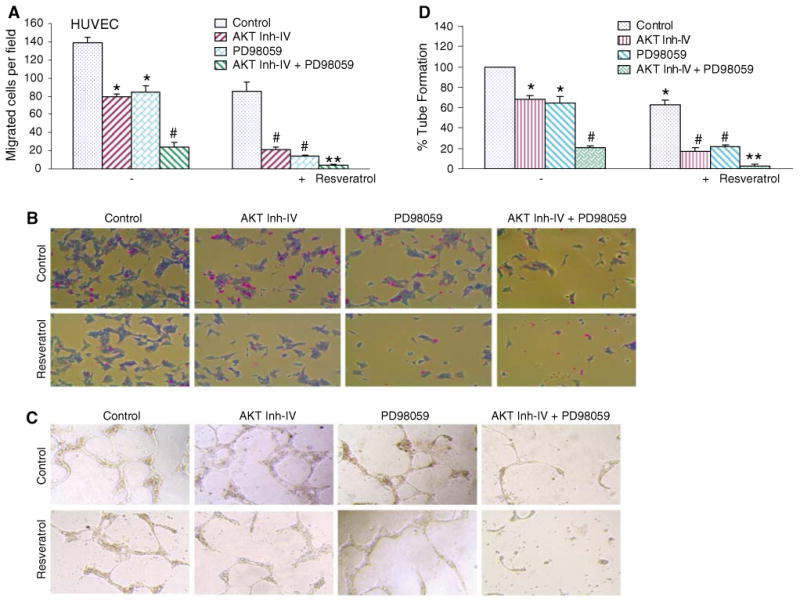
Inhibition of cell migration and capillary tube formation by inhibitors PI3K/AKT and MEK/ERK pathways are enhanced resveratrol. a Migration of HUVEC cells was assessed using Transwell Boyden chamber containing a polycarbonated filter. HUVECs (4 × 104 cells) were pretreated with AKT inhibitor IV (1 μM) and/or MEK1/2 inhibitor PD98059 (10 μM) for 2 h, followed by treatment with resveratrol (20 μM) or DMSO (control). Migration through the membrane was determined after 24 h of incubation at 37°C. Cells that had migrated to the lower chamber were fixed with 90% methanol, stained with giemsa, quantified by counting the number of cells under a microscope. Data represent mean ± SD. * and # significantly different from control, P < 0.05. b HUVEC cells were treated as described in (a). Cells that had migrated to the lower chamber were fixed with 90% methanol, and photographed with a digital camera attached to a microscope. c HUVECs (10 × 104) were seeded in 24-well plates containing matrigel, and pretreated with AKT inhibitor IV (1 μM) and/or MEK1/2 inhibitor PD98059 (10 μM) for 2 h, followed by treatment with resveratol (20 μM) or DMSO (control) for 24 h. Capillary tube structures were photographed with a digital camera attached to a microscope. d HUVECs cells were seeded and treated as described in (c). Capillary tubes were counted under a microscope. Data represent mean ± SD. * and # significantly different from control, P < 0.05
We next examined the interactive effects of PI3K/AKT and MEK/ERK pathways on capillary tube formation by HUVEC on growth factor-reduced matrigel, which is a well-accepted technique to measure in vitro angiogenesis [45]. AKT inhibitor IV, PD98059, and resveratrol alone inhibited capillary tube formation (Fig. 1c, d). The treatment of cells with AKT inhibitor IV and PD98059 resulted in synergistic inhibition of capillary tube formation. Interestingly, the inhibitory effects of resveratrol on capillary tube formation were further enhanced in the presence of AKT inhibitor IV and/or PD98059. These data suggest that the inhibition of PI3K/AKT and MEK/ERK pathways acts synergistically to inhibit migration and capillary tube formation by HUVEC cells, and regulation of these two pathways can significantly control angiogenesis.
The inhibitory effects of resveratrol on HUVEC cell migration and capillary tube formation can be enhanced by phosphorylation deficient mutants of FOXO (FOXO1-TM and FOXO3A-TM)
We and others have demonstrated that phosphorylation-deficient mutants of FOXO inhibit in vitro angiogenesis [23, 45–47]. All the three AKT phosphorylation sites in this FOXO proteins were mutated to alanines, allowing it to escape the phosphorylation-induced cytoplasmic sequestration by AKT and localize exclusively in the nucleus. We therefore examined the involvement of FOXO transcription factors in resveratrol-induced migration and capillary tube formation by HUVEC cells (Fig. 2). Since dephosphorylated FOXO transcription factors translocate to nucleus and induce gene transcription, we used phosphorylation-deficient mutants of FOXO (constitutively active) to activate its transcriptional activity. Overexpression of phosphorylation-deficient mutants of FOXO (FOXO1-TM, FOXO3a-TM, or FOXO4-TM) inhibited migration and capillary tube formation by HUVEC cells. The inhibitory effects of resveratol on HUVEC cell migration and capillary tube formation were further enhanced by over-expressing FOXO1-TM, FOXO3a-TM, or FOXO4-TM. These data suggest that resveratrol may inhibit angiogenesis through activation of FOXO transcription factors.
Fig. 2.
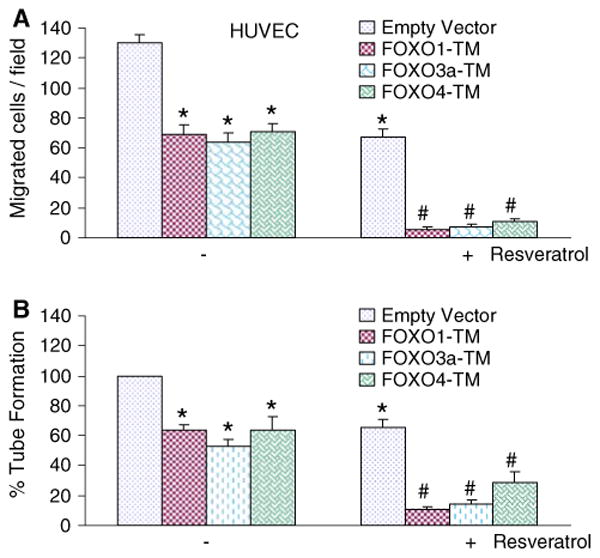
Phosphorylation-deficient mutants of FOXO transcription factor enhance the inhibitory effects of resveratrol on HUVEC migration and capillary tube formation. a HUVEC (4 × 104) cells were transiently transfected with empty vector, FOXO1-TM, FOXO3A-TM, or FOXO4-TM along with pCMV-LacZ vector (as transfection control) and treated with or without resveratrol (20 μM). Migration through the membrane was determined after 24 h of incubation at 37°C. Cells that had migrated to the lower chamber were fixed with 90% ethanol, stained with Geimsa, quantified by counting the number of cells under a microscope. Data represent mean ± SD. * and # significantly different from control, P < 0.05. b HUVEC (4 × 104) cells were transiently transfected with empty vector, FOXO1-TM, FOXO3A-TM, or FOXO4-TM along with pCMV-LacZ vector (as transfection control) and treated with or without resveratrol (20 μM) for 24 h. Capillary tubes were counted under a microscope. Data represent mean ± SD. * and # significantly different from control, P < 0.05
Resveratrol-induced FOXO activity in HUVEC cells can be enhanced by AKT inhibitor IV and PD98059, or phosphorylation-deficient mutants of FOXO
We next examined whether inhibition of PI3K/AKT and MEK/ERK pathways act synergistically to induce FOXO transcriptional activity in a luciferase reporter gene assay which measures FOXO function, and how inhibition of these two pathways regulate resveratrol-induced FOXO activity (Fig. 3a). AKT inhibitor IV, PD98059, and resveratrol alone induced FOXO transcriptional activity. The combination of AKT inhibitor IV and PD98059 had a synergistic effect on FOXO activity. Furthermore, resveratrol-induced FOXO transcriptional activity was further enhanced in the presence of either AKT inhibitor IV or PD98059. These data suggest that inhibition of PI3K/AKT and MEK/ERK pathways act synergistically to induce FOXO transcriptional activity, and inhibition of these two pathways further enhanced resveratrol-induced FOXO activity.
Fig. 3.
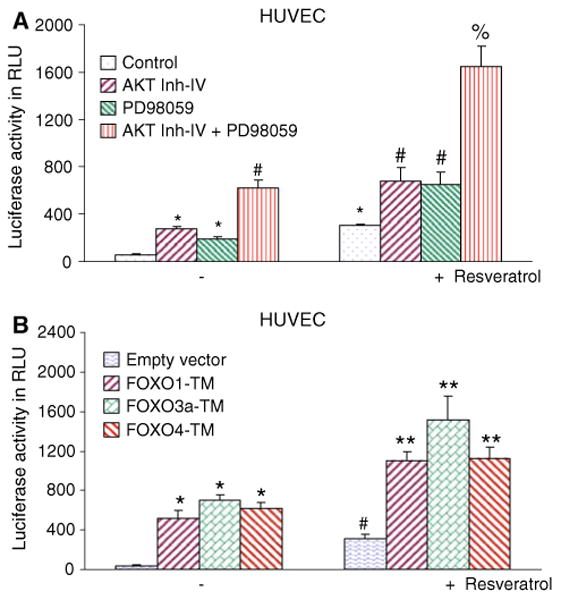
Inhibitors of PI3K/AKT and MEK/ERK pathways or phosphorylation deficient mutants of FOXO-enhanced resveratrol-induced FOXO transcriptional activity in HUVEC cells. a Inhibition of PI3K/AKT and MEK/ERK pathways synergistically enhanced resveratrol-induced FOXO activity in HUVEC cells. HUVEC cells were transiently transfected with 6× DBE-luciferase and pRL-TK plasmids for 24 h [42]. After transfection, HUVEC cells were pretreated with AKT inhibitor IV (1 μM) and/or MEK1/2 inhibitor PD98059 (10 μM) for 2 h, followed by treatment with or without resveratrol (20 μM) for 24 h. Cells were harvested for firefly/Renilla luciferase assays using the Dual-Luciferase Reporter Assay System (Promega). Luciferase counts were normalized using Renilla luciferase transfection control. Data represent the mean ± SD. *, #, and % significantly different from respective controls, P < 0.05. b Phosphorylation deficient mutants of FOXO enhanced resveratrol-induced FOXO transcriptional activity in HUVEC cells. HUVEC cells were transiently transfected with empty vector or constructs encoding FOXO1-TM, FOXO3a-TM, or FOXO4-TM together with 6× DBE-luciferase and pRL-TK plasmids for 24 h [42]. After transfection, cells were washed, treated with resveratrol (20 μM) for 24 h, and harvested for firefly/Renilla luciferase assays using the Dual-Luciferase Reporter Assay System (Promega). Luciferase counts were normalized using Renilla luciferase transfection control. Data represent the mean ± S.D. *, #, and ** significantly different from respective controls, P < 0.05
We next examined whether resveratrol induces transcriptional activation of FOXO in the presence or absence FOXO1-TM, FOXO3a-TM, or FOXO4-TM (phosphorylation-deficient triple mutant) (Fig. 3). HUVEC cells were transfected with p6xDBE-luciferase reporter construct in the presence or absence of plasmids expressing FOXO1-TM, FOXO3a-TM, or FOXO4-TM. After transfection, cells were treated with resveratrol for 24 h, and luciferase activity was measured. Transfection of cells with plasmids expressing FOXO1-TM, FOXO3a-TM, or FOXO4-TM-induced FOXO transcriptional activity compared with the empty vector (control). Resveratrol-induced FOXO transcriptional activity was further enhanced in the presence of phosphorylation-deficient mutants of FOXO1, FOXO3a, and FOXO4. These data indicate that inhibition of PI3K/AKT and MEK/ERK pathways act synergistically to activate FOXO transcription factors, and activation of FOXO transcription factors further enhances the anti-angiogenic effects of resveratrol. These results indicate that resveratrol enhances the function of FOXO proteins independent of effects on phosphorylation by AKT, since those sites have been mutated. This may be because deacetylation of FOXO proteins enhances DNA binding activity, as suggested by Daitoku et al. [48]. The enhanced DNA binding activity also serves to limit the availability of FOXO proteins for phosphorylation by AKT [48]. So, rather than enhancing dephosphorylation of phospho-FOXO proteins, resveratrol may be simply inhibiting the rephosphorylation of FOXO proteins after they have been dephosphorylated. Thus, resveratrol treatment inhibits the accumulation of phospho-FOXO, either by inhibiting the phosphorylation of FOXO proteins or by promoting the dephosphorylation of phospho-FOXO.
Dephosphorylation of FOXO3a by AKT inhibitor IV and/or PD98059 is enhanced by resveratrol in HUVEC cells
Since AKT inhibitor IV, PD98059, and/or resveratrol inhibited in vitro angiogenesis by inducing FOXO transcriptional activity which is regulated by its dephosphorylation and subsequent nuclear translocation, we measured the phosphorylation and nuclear translocation of FOXO3a by Western blot analysis and immunofluorescence, respectively. Treatment of HUVEC cells with resveratrol, AKT Inh IV, or PD98059 inhibited FOXO3a phosphorylation (Fig. 4a, b). The combination of resveratrol with AKT Inh IV and PD98059 inhibited FOXO3a phosphorylation than single agent alone. The inhibition of PI3K/AKT and MEK/ERK pathways together, or in the presence of resveratrol, completely inhibited FOXO3a phosphorylation. These data suggest that dephosphorylation of FOXO3a is an important event for antiangiogenic effects of resveratrol or PI3K/AKT and MEK/ERK pathways.
Fig. 4.
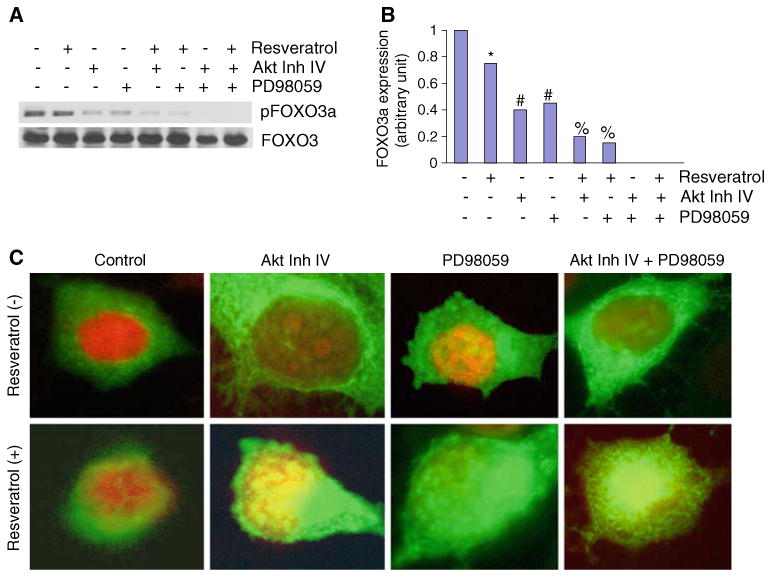
Inhibition of FOXO3a phosphorylation by inhibitors of AKT, MEK/ERK, and resveratrol. a HUVEC cells were pretreated with AKT inhibitor IV (1 μM) and/or MEK1/2 inhibitor PD98059 (10 μM) for 2 h, followed by treatment with or without resveratrol (20 μM) for 24 h. Cells were harvested and cytoplasmic fractions were prepared. Crude proteins were subjected to Western blot analysis to measure the expression of phospho-FOXO3a and total FOXO3a. b Expression density of pFOXO3a. The expression density of phospho-FOXO3a protein was quantified from Western blot reported in (a), and plotted on Y-axis. The Control band was normalized to 1. *, #, and % significantly different from respective control, P < 0.05. c Nuclear translocation of FOXO3a. HUVECs were seeded in chambered slides and treated with or without resveratrol (20 μM) for 18 h. Cells were fixed, permeabilized, and stained with anti-FOXO3a antibody at 4°C for 18 h. After washing, cells were stained with DAPI (nuclear staining) and secondary antibody conjugated with FITC, and visualized under a fluorescence microscope. Green color FOXO3a, red color nucleus (for clarity the color of nucleus was changed from blue to red), yellow colocalization of FOXO3a to nucleus (Color figure online)
We next measured the translocation of FOXO3a to nucleus in response to treatment with resveratrol, AKT Inh IV, PD98059, or their combinations (Fig. 4c). Treatment of HUVEC cells with resveratrol, AKT Inh IV, or PD98059 resulted in translocation of FOXO3a to the nucleus. The combination of resveratrol with AKT Inh IV or PD98059 caused greater translocation of FOXO3a to the nucleus than single agent alone. The combination of AKT Inh IV and PD98059 together, or in the presence of resveratrol, also resulted in nuclear translocation of FOXO3a. These data suggest that dephosphorylation and nuclear translocation of FOXO3a are critical for antiangiogenic effects of resveratrol or PI3K/AKT and MEK/ERK pathways.
Neutralization of VEGF by anti-VEGF antibody enhances anti-proliferative and anti-angiogenic effects of resveratrol
Angiogenesis, the growth of new blood vessels, is required for a variety of normal proliferative processes and neo-plastic growth and metastasis [49]. VEGF stimulates vascular endothelial cell proliferation and it is a potent inducer of physiological and pathological angiogenesis [50]. It is a soluble protein secreted by a wide variety of cell types, and the inhibition of VEGF by antibody has been shown to inhibit angiogenesis [32–34]. We therefore examined the involvement of VEGF on antiangiogenic effects of resveratrol by using VEGF antibody. In order to neutralize the secreted VEGF, we have used two anti-human VEGF antibodies (Anti-VEGF Ab1, catalog number MAB293; and anti-VEGF Ab2, catalog number AF-293-NA). Both of these antibodies possess specificity to human VEGF165 and human VEGF121, but with varying VEGF neutralizing bioactivity (0.04–0.08 μg/ml Ab1 versus 0.01–0.03 μg/ml ab2 will neutralize 50% of the bioactivity due to 10 ng/ml of rhVEGF cell proliferation assay). Anti-VEGF Ab1, anti-VEGF Ab2, and resveratrol alone inhibited HUVEC cell proliferation (Fig. 5a). The combination of resveratrol with either anti-VEGF Ab1 or anti-VEGF Ab2 further inhibited cell proliferation. These data suggest that VEGF acts as a growth factor and neutralization of VEGF enhances anti-proliferative effects of resveratrol.
Fig. 5.
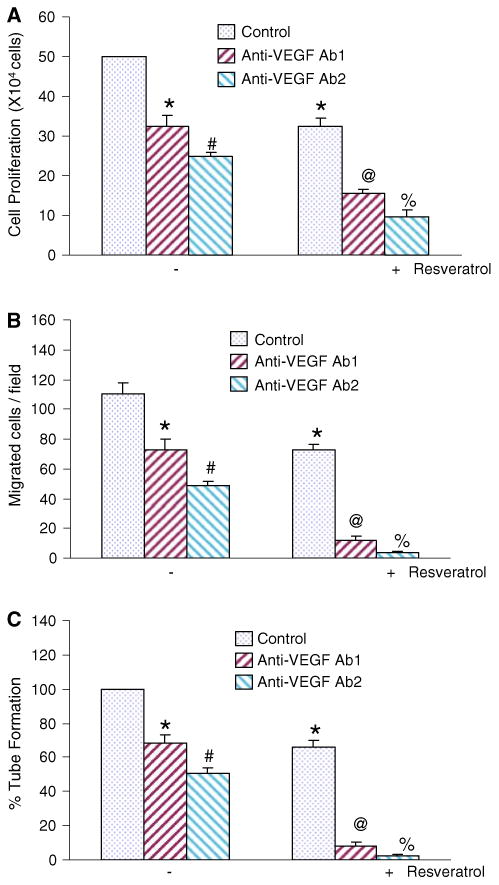
Neutralization of VEGF enhances the anti-proliferative and anti-angiogenic effects of resveratrol. HUVEC cells were co-treated with either anti-VEGF Ab1 (2 μg/ml) or anti-VEGF Ab2 (5 μg/ml) in the presence or absence of resveratrol (20 μM) for 48 h. At the end of incubation period, cell proliferation (a), cell migration (b), and percent tube formation were determined as described in Experimental Procedures. Data represent the mean ± SD. *, #, @, or % significantly different from respective controls, P < 0.05
Since VEGF binds to VEGF-receptors and can induce endothelial cell mitosis, invasion, and eventually capillary tube formation, we sought to examine the effects of neutralizing VEGF on HUVEC cell migration and capillary tube formation (Fig. 5b, c). Anti-VEGF Ab1, anti-VEGF Ab2, and resveratrol alone inhibited HUVEC migration and capillary tube formation. The combination of resveratrol with either anti-VEGF Ab1 or anti-VEGF Ab2 further inhibited HUVEC cell migration and capillary tube formation. These data suggest that anti-angiogenic effects of resveratrol are exerted through production of VEGF.
Discussion
Resveratrol (3,5,4′-trihydroxystilbene) is a naturally occurring phytoalexin produced by a wide variety of plants in response to stress, injury, and fungal infection. Resveratrol has been shown to suppress diverse cellular events associated with each step of carcinogenesis, i.e., tumor initiation, promotion, and progression [3]. To the best of our knowledge, this is the first study to demonstrate that inhibition of PI3K/AKT and MEK/ERK pathways acted synergistically to induce activation of FOXO transcription factors and enhanced the anti-angiogenic effects of resveratrol in HUVEC cells. Inhibitors of AKT and MEK synergistically inhibited cytoplasmic phosphorylation of FOXO3a, and enhanced its nuclear translocation in HUVECs. Specifically, cell migration and capillary tube formation were inhibited by inhibitors of AKT and MEK, and phosphorylation-deficient mutants of FOXO1, FOXO3a, and FOXO4. Similar to this study, we have recently demonstrated that (i) another chemopreventive agent EGCG inhibited markers of angiogenesis, invasion, and metastasis in vitro and in nude mice [45], (ii) inhibition of PI3K/AKT and MEK/ERK pathways converge to regulate angiogenesis through activation of FOXO transcription factors [43], and (iii) the FOXO transcription factors mediate anti-angiogenic effects of EGCG [43].
Angiogenesis, the sprouting of new capillaries from the preexistent blood vessels, is of central importance in many biological processes, including embryonic vascular development and differentiation, wound healing, and organ regeneration [51, 52]. In addition, angiogenesis plays a major role in pathological conditions such as diabetic retinopathy, rheumatoid arthritis, psoriasis, cardiovascular diseases, tumor growth, and metastasis [53, 54]. During angiogenesis, endothelial cells migrate, proliferate, organize into tube-like structures, and play an active role in tissue remodeling. In this study, resveratrol inhibited cell migration and capillary tube formation, and these beneficial effects of resveratrol were further enhanced in the presence of MEK/ERK kinase inhibitor, pointing a positive role of ERK in angiogenesis and metastasis. Furthermore, inhibition of PI3K/AKT and MEK/ERK pathways together resulted in synergistic inhibition of cell migration and capillary tube formation through activation of FOXO transcription factors.
Members of the FOXO family of transcription factors have been postulated to be tumor suppressors because of their established roles in cell-cycle arrest, apoptosis, DNA-damage repair, and scavenging of reactive oxygen species. Recently, several animal model studies have shown that the FOXO proteins are indeed tumor suppressors. Furthermore, FOXO proteins have recently been implicated in the negative regulation of signaling by the hypoxia-inducible factor 1 during vascular development, raising the possibility that the FOXO proteins suppress not only tumor formation, but also tumor angiogenesis and, possibly, metastasis. FOXO1 and FOXO3a are the most abundant FOXO isoforms in mature endothelial cells and that overexpression of constitutively active FOXO1 or FOXO3a significantly inhibits endothelial cell migration and tube formation in vitro [47]. Silencing of either FOXO1 or FOXO3a gene expression led to a profound increase in the migratory and sprout-forming capacity of endothelial cells. The FOXO1-deficient mice died around embryonic day 11 because of defects in the bronchial arches and impaired vascular development of embryos and yolk sacs [23]. Gene expression profiling showed that FOXO1 and FOXO3a specifically regulate a nonredundant but overlapping set of angiogenesis- and vascular remodeling-related genes. Whereas Ang-2 was exclusively regulated by FOXO1, eNOS, which is essential for postnatal neovascularization, was regulated by FOXO1 and FOXO3a. Consistent with these findings, constitutively active FOXO1 and FOXO3a repressed eNOS protein expression. In vivo, FOXO3a deficiency increased eNOS expression and enhanced postnatal vessel formation and maturation. Nuclear expression of FOXO4 resulting in the suppression of various response to hypoxia, including decreased VEGF, glucose transporter-1, and erythropoietin expression [55]. In our study, all the three forms of FOXO proteins appear to play significant role in inhibiting angiogenesis.
FOXO1 contains 15 consensus phosphorylation sites for the MAPK family. The phosphorylation of FOXO1 was demonstrated by ERK and p38 MAPK but not by JNK [28]. Epidermal growth factor or anisomycin increased phosphorylation of exogenous FOXO1, which was significantly inhibited by pretreatment with an MEK 1 inhibitor, PD98059, or a p38 inhibitor, SB203580 [28]. Phosphopeptide mapping using mutation of phosphorylation sites for MAPK revealed that the nine serine residues in FOXO1 are specifically phosphorylated by ERK and that five of the nine residues are phosphorylated by p38 in vivo [28]. These data suggest that FOXO1 is specifically phosphorylated by ERK and p38, and that this phosphorylation regulates the function of FOXO1. In this study, MEK inhibitor and phosphorylation-deficient mutants of FOXO activated FOXO transcription factor, resulting in inhibition of capillary tube formation and HUVEC cell migration. Resveratrol further enhanced the inhibitory effects of MEK inhibitor on capillary tube formation and cell migration by HUVECs. These data suggest that anti-angiogenic effects of resveratrol can be mediated through inhibition of MEK/ERK pathway and activation FOXO transcription factor.
Activation of PI3K/AKT pathway leads to phosphorylation and nuclear exclusion of the transcription factor FOXO [56]. Inhibition of AKT phosphorylation causes dephosphorylation of FOXO1 proteins followed by their nuclear translocation and induction of gene transcription such as angiogenesis-related molecule Ang-2 [24], a competitive antagonist of Ang-1, and eNOS [57]. Ang-1 has potential therapeutic applications in inducing angiogenesis, enhancing endothelial cell survival, and preventing vascular leakage. Ang-1 inhibits FOXO1 and thereby the expression of its antagonist Ang-2 [46]. In contrast, this regulation implies a positive feedback loop in which an increase in Ang-2 expression, blocking Ang-1 effects, leads indirectly to activation of FOXO1, resulting in a further increase in the expression of Ang-2 and other FOXO1 target genes. In this study, AKT inhibitor IV blocks capillary tube formation and HUVEC cell migration through transcription activation of FOXO, which may regulate the expression of angiogenesis-related genes.
Anti-angiogenic therapies that target VEGF and the VEGFR are effective adjuncts to the treatment of solid tumors. VEGF is a key molecule that orchestrates the formation and function of vascular networks [50]. Impaired regulation of angiogenesis is implicated in a number of pathologic states [58]. For instance, neoplasias exhibit uncontrolled angiogenesis, whereas ischemia and states of vascular insufficiency involve reduced VEGF activity [59]. As the role of VEGF has been elucidated in these disease processes, its therapeutic role has been developed [50]. The Food and Drug Administration has approved several anti-VEGF agents (e.g., bevacizumab, Avastin) for treating colorectal, breast, lung, and kidney cancer or age-related macular degeneration [31]. Treatment with bevacizumab is commonly combined with cytotoxic chemotherapy and results in dramatic responses seen on radiographs, prolongation of progression-free survival, and less need for corticosteroids. VEGF-inducing agents have also been used experimentally to induce angiogenesis in patients with critical limb ischemia. Similar results have been shown with small-molecule inhibitors of VEGFR, such as cediranib. In this study, VEGF neutralizing antibody enhanced the anti-proliferative and anti-angiogenic effects of resveratrol. Because resveratrol and VEGF antibody act on two different pathways critical to tumor growth and dissemination, administering these drugs concomitantly may confer additional clinical benefits to cancer patients with advanced disease, by virtue of their complementary (or additive) antitumor activity. As more knowledge is gathered about the biology of VEGF and its receptors, there is greater promise for therapeutic modulation of VEGF expression.
In conclusion, we have demonstrated that inhibition of PI3K/AKT and Ras/MEK/ERK pathways interact synergistically to activate FOXO transcription factors which, in turn, inhibit angiogenesis. Furthermore, inhibition of both of these pathways further enhances the anti-angiogenic effects of resveratrol. The activation of FOXO transcription factors through inhibition of Ras/MEK/ERK and PI3K/AKT pathways may have physiological significance in management of diabetic retinopathy, rheumatoid arthritis, psoriasis, cardiovascular diseases, and cancer. The combination of VEGF neutralizing antibody with resveratrol may have additional health benefits than single agent alone. Since activation of FOXO transcription factors inhibits angiogenesis, the development of small molecules that block phosphorylation of FOXO which result in its activation could be developed as anti-angiogenic drugs.
Acknowledgments
We thank our lab members for critical reading of the manuscript. We also thank Dr. Noboru Motoyama (National Institute for Longevity Sciences, Obu, Aichi, Japan) and Dr. Tatsuo Furuyama (Sonoda Women's University, Amagasaki, Hyogo, Japan) for providing FOXO expression plasmids and FOXO-luciferase construct (pGL3-6X DBE), respectively. The study was initiated at the University of Texas Health Science Center at Tyler. The project was supported by NIH R01CA114469 (R.K.S.) and the Department of Veterans Affairs Merit Review Program (T.G.U.).
Contributor Information
Rakesh K. Srivastava, Department of Pharmacology, Toxicology and Therapeutics, The University of Kansas Medical Center, Kansas City, KS 66160-7410, USA; Department of Medicine, The University of Kansas Medical Center, Kansas City, KS 66160-7410, USA
Terry G. Unterman, Department of Medicine, College of Medicine and Jesse Brown VA Medical Center, University of Illinois at Chicago, Chicago, IL 60612, USA; Department of Physiology and Biophysics, College of Medicine and Jesse Brown VA Medical Center, University of Illinois at Chicago, Chicago, IL 60612, USA
Sharmila Shankar, Email: sshankar@kumc.edu, Department of Pathology & Laboratory Medicine, The University of Kansas Medical Center, 3901 Rainbow Blvd., Mail Stop 3045, Kansas City, KS 66160-7410, USA.
References
- 1.Shankar S, Singh G, Srivastava RK. Chemoprevention by resveratrol: molecular mechanisms and therapeutic potential. Front Biosci. 2007;12:4839–4854. doi: 10.2741/2432. [DOI] [PubMed] [Google Scholar]
- 2.Sagar SM, Yance D, Wong RK. Natural health products that inhibit angiogenesis: a potential source for investigational new agents to treat cancer-Part 1. Curr Oncol. 2006;13:14–26. doi: 10.3747/co.v13i1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shankar S, Siddiqui I, Srivastava RK. Molecular mechanisms of resveratrol (3, 4, 5-trihydroxy-trans-stilbene) and its interaction with TNF-related apoptosis inducing ligand (TRAIL) in androgen-insensitive prostate cancer cells. Mol Cell Biochem. 2007;304:273–285. doi: 10.1007/s11010-007-9510-x. [DOI] [PubMed] [Google Scholar]
- 4.Galili N, Davis RJ, Fredericks WJ, Mukhopadhyay S, Rauscher FJ, III, Emanuel BS, Rovera G, Barr FG. Fusion of a fork head domain gene to PAX3 in the solid tumour alveolar rhabdomyosarcoma. Nat Genet. 1993;5:230–235. doi: 10.1038/ng1193-230. [DOI] [PubMed] [Google Scholar]
- 5.Anderson MJ, Viars CS, Czekay S, Cavenee WK, Arden KC. Cloning and characterization of three human forkhead genes that comprise an FKHR-like gene subfamily. Genomics. 1998;47:187–189. doi: 10.1006/geno.1997.5122. [DOI] [PubMed] [Google Scholar]
- 6.Hillion J, Le Coniat M, Jonveaux P, Berger R, Bernard OA. AF6q21, a novel partner of the MLL gene in t(6;11)(q21;q23), defines a forkhead transcriptional factor subfamily. Blood. 1997;90:3714–3719. [PubMed] [Google Scholar]
- 7.Borkhardt A, Repp R, Haas OA, Leis T, Harbott J, Kreuder J, Hammermann J, Henn T, Lampert F. Cloning and characterization of AFX, the gene that fuses to MLL in acute leukemias with a t(X;11)(q13;q23) Oncogene. 1997;14:195–202. doi: 10.1038/sj.onc.1200814. [DOI] [PubMed] [Google Scholar]
- 8.Van Der Heide LP, Hoekman MF, Smidt MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J. 2004;380:297–309. doi: 10.1042/BJ20040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 10.Guo S, Rena G, Cichy S, He X, Cohen P, Unterman T. Phosphorylation of serine 256 by protein kinase B disrupts transactivation by FKHR and mediates effects of insulin on insulin-like growth factor-binding protein-1 promoter activity through a conserved insulin response sequence. J Biol Chem. 1999;274:17184–17192. doi: 10.1074/jbc.274.24.17184. [DOI] [PubMed] [Google Scholar]
- 11.Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–787. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, Sellers WR. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol Cell Biol. 2000;20:8969–8982. doi: 10.1128/mcb.20.23.8969-8982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10:1201–1204. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- 15.Tang TT, Dowbenko D, Jackson A, Toney L, Lewin DA, Dent AL, Lasky LA. The forkhead transcription factor AFX activates apoptosis by induction of the BCL-6 transcriptional repressor. J Biol Chem. 2002;277:14255–14265. doi: 10.1074/jbc.M110901200. [DOI] [PubMed] [Google Scholar]
- 16.Dijkers PF, Medema RH, Pals C, Banerji L, Thomas NS, Lam EW, Burgering BM, Raaijmakers JA, Lammers JW, Koenderman L, Coffer PJ. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27(KIP1) Mol Cell Biol. 2000;20:9138–9148. doi: 10.1128/mcb.20.24.9138-9148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cappellini A, Tabellini G, Zweyer M, Bortul R, Tazzari PL, Billi AM, Fala F, Cocco L, Martelli AM. The phosphoinositide 3-kinase/Akt pathway regulates cell cycle progression of HL60 human leukemia cells through cytoplasmic relocalization of the cyclin-dependent kinase inhibitor p27(Kip1) and control of cyclin D1 expression. Leukemia. 2003;17:2157–2167. doi: 10.1038/sj.leu.2403111. [DOI] [PubMed] [Google Scholar]
- 18.Burgering BM, Kops GJ. Cell cycle and death control: long live Forkheads. Trends Biochem Sci. 2002;27:352–360. doi: 10.1016/s0968-0004(02)02113-8. [DOI] [PubMed] [Google Scholar]
- 19.Tran H, Brunet A, Grenier JM, Datta SR, Fornace AJ, Jr, DiStefano PS, Chiang LW, Greenberg ME. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science. 2002;296:530–534. doi: 10.1126/science.1068712. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt M, Fernandez de Mattos S, van der Horst A, Klompmaker R, Kops GJ, Lam EW, Burgering BM, Medema RH. Cell cycle inhibition by FoxO forkhead transcription factors involves downregulation of cyclin D. Mol Cell Biol. 2002;22:7842–7852. doi: 10.1128/MCB.22.22.7842-7852.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 22.Hosaka T, Biggs WH, III, Tieu D, Boyer AD, Varki NM, Cavenee WK, Arden KC. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci USA. 2004;101:2975–2980. doi: 10.1073/pnas.0400093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furuyama T, Kitayama K, Shimoda Y, Ogawa M, Sone K, Yoshida-Araki K, Hisatsune H, Nishikawa S, Nakayama K, Ikeda K, Motoyama N, Mori N. Abnormal angiogenesis in Foxo1 (Fkhr)-deficient mice. J Biol Chem. 2004;279:34741–34749. doi: 10.1074/jbc.M314214200. [DOI] [PubMed] [Google Scholar]
- 24.Daly C, Wong V, Burova E, Wei Y, Zabski S, Griffiths J, Lai KM, Lin HC, Ioffe E, Yancopoulos GD, Rudge JS. Angiopoietin-1 modulates endothelial cell function and gene expression via the transcription factor FKHR (FOXO1) Genes Dev. 2004;18:1060–1071. doi: 10.1101/gad.1189704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang B, Cao DJ, Sainz I, Colman RW, Guo YL. Different roles of ERK and p38 MAP kinases during tube formation from endothelial cells cultured in 3-dimensional collagen matrices. J Cell Physiol. 2004;200:360–369. doi: 10.1002/jcp.20025. [DOI] [PubMed] [Google Scholar]
- 26.Woessmann W, Meng YH, Mivechi NF. An essential role for mitogen-activated protein kinases, ERKs, in preventing heat-induced cell death. J Cell Biochem. 1999;74:648–662. doi: 10.1002/(sici)1097-4644(19990915)74:4<648::aid-jcb14>3.3.co;2-y. [DOI] [PubMed] [Google Scholar]
- 27.Kyriakis JM, Avruch J. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem. 1996;271:24313–24316. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- 28.Asada S, Daitoku H, Matsuzaki H, Saito T, Sudo T, Mukai H, Iwashita S, Kako K, Kishi T, Kasuya Y, Fukamizu A. Mitogen-activated protein kinases, Erk and p38, phosphorylate and regulate Foxo1. Cell Signal. 2007;19:519–527. doi: 10.1016/j.cellsig.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Dai J, Rabie AB. VEGF: an essential mediator of both angiogenesis and endochondral ossification. J Dent Res. 2007;86:937–950. doi: 10.1177/154405910708601006. [DOI] [PubMed] [Google Scholar]
- 30.Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 2008;11:109–119. doi: 10.1007/s10456-008-9099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khosravi Shahi P, Fernandez Pineda I. Tumoral angiogenesis: review of the literature. Cancer Invest. 2008;26:104–108. doi: 10.1080/07357900701662509. [DOI] [PubMed] [Google Scholar]
- 32.Sirohi B, Smith K. Bevacizumab in the treatment of breast cancer. Expert Rev Anticancer Ther. 2008;8:1559–1568. doi: 10.1586/14737140.8.10.1559. [DOI] [PubMed] [Google Scholar]
- 33.Socinski MA. Bevacizumab as first-line treatment for advanced non-small cell lung cancer. Drugs Today (Barc) 2008;44:293–301. doi: 10.1358/dot.2008.44.4.1212302. [DOI] [PubMed] [Google Scholar]
- 34.Lien S, Lowman HB. Therapeutic anti-VEGF antibodies. Handb Exp Pharmacol. 2008;181:131–150. doi: 10.1007/978-3-540-73259-4_6. [DOI] [PubMed] [Google Scholar]
- 35.Beatty GL, Giantonio BJ. Bevacizumab and oxaliplatin-based chemotherapy in metastatic colorectal cancer. Expert Rev Anticancer Ther. 2008;8:683–688. doi: 10.1586/14737140.8.5.683. [DOI] [PubMed] [Google Scholar]
- 36.Wheatley-Price P, Shepherd FA. Targeting angiogenesis in the treatment of lung cancer. J Thorac Oncol. 2008;3:1173–1184. doi: 10.1097/JTO.0b013e318187220f. [DOI] [PubMed] [Google Scholar]
- 37.Bradley DP, Tessier JJ, Lacey T, Scott M, Jurgensmeier JM, Odedra R, Mills J, Kilburn L, Wedge SR. Examining the acute effects of cediranib (RECENTIN, AZD2171) treatment in tumor models: a dynamic contrast-enhanced MRI study using gadopentate. Magn Reson Imaging. 2008;27:377–384. doi: 10.1016/j.mri.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 38.Heckman CA, Holopainen T, Wirzenius M, Keskitalo S, Jeltsch M, Yla-Herttuala S, Wedge SR, Jurgensmeier JM, Alitalo K. The tyrosine kinase inhibitor cediranib blocks ligand-induced vascular endothelial growth factor receptor-3 activity and lymphangiogenesis. Cancer Res. 2008;68:4754–4762. doi: 10.1158/0008-5472.CAN-07-5809. [DOI] [PubMed] [Google Scholar]
- 39.Lang SA, Brecht I, Moser C, Obed A, Batt D, Schlitt HJ, Geissler EK, Stoeltzing O. Dual inhibition of Raf and VEGFR2 reduces growth and vascularization of hepatocellular carcinoma in an experimental model. Langenbecks Arch Surg. 2008;393:333–341. doi: 10.1007/s00423-008-0292-8. [DOI] [PubMed] [Google Scholar]
- 40.Lang SA, Schachtschneider P, Moser C, Mori A, Hackl C, Gaumann A, Batt D, Schlitt HJ, Geissler EK, Stoeltzing O. Dual targeting of Raf and VEGF receptor 2 reduces growth and metastasis of pancreatic cancer through direct effects on tumor cells, endothelial cells, and pericytes. Mol Cancer Ther. 2008;7:3509–3518. doi: 10.1158/1535-7163.MCT-08-0373. [DOI] [PubMed] [Google Scholar]
- 41.Shankar S, Chen Q, Sarva K, Siddiqui I, Srivastava RK. Curcumin enhances the apoptosis-inducing potential of TRAIL in prostate cancer cells: molecular mechanisms of apoptosis, migration and angiogenesis. J Mol Signal. 2007;2:10–18. doi: 10.1186/1750-2187-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Furukawa-Hibi Y, Kobayashi Y, Chen C, Motoyama N. FOXO transcription factors in cell-cycle regulation and the response to oxidative stress. Antioxid Redox Signal. 2005;7:752–760. doi: 10.1089/ars.2005.7.752. [DOI] [PubMed] [Google Scholar]
- 43.Shankar S, Chen Q, Srivastava RK. Inhibition of PI3K/AKT and MEK/ERK pathways act synergistically to enhance antiangiogenic effects of EGCG through activation of FOXO transcription factor. J Mol Signal. 2008;3:7–16. doi: 10.1186/1750-2187-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kau TR, Schroeder F, Ramaswamy S, Wojciechowski CL, Zhao JJ, Roberts TM, Clardy J, Sellers WR, Silver PA. A chemical genetic screen identifies inhibitors of regulated nuclear export of a Forkhead transcription factor in PTEN-deficient tumor cells. Cancer Cell. 2003;4:463–476. doi: 10.1016/s1535-6108(03)00303-9. [DOI] [PubMed] [Google Scholar]
- 45.Shankar S, Ganapathy S, Hingorani SR, Srivastava RK. EGCG inhibits growth, invasion, angiogenesis and metastasis of pancreatic cancer. Front Biosci. 2008;13:440–452. doi: 10.2741/2691. [DOI] [PubMed] [Google Scholar]
- 46.Chlench S, Mecha Disassa N, Hohberg M, Hoffmann C, Pohlkamp T, Beyer G, Bongrazio M, Da Silva-Azevedo L, Baum O, Pries AR, Zakrzewicz A. Regulation of Foxo-1 and the angiopoietin-2/Tie2 system by shear stress. FEBS Lett. 2007;581:673–680. doi: 10.1016/j.febslet.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 47.Potente M, Urbich C, Sasaki K, Hofmann WK, Heeschen C, Aicher A, Kollipara R, DePinho RA, Zeiher AM, Dimmeler S. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest. 2005;115:2382–2392. doi: 10.1172/JCI23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daitoku H, Hatta M, Matsuzaki H, Aratani S, Ohshima T, Miyagishi M, Nakajima T, Fukamizu A. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc Natl Acad Sci USA. 2004;101:10042–10047. doi: 10.1073/pnas.0400593101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 50.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 51.Folkman J. Fundamental concepts of the angiogenic process. Curr Mol Med. 2003;3:643–651. doi: 10.2174/1566524033479465. [DOI] [PubMed] [Google Scholar]
- 52.Folkman J. Angiogenesis and proteins of the hemostatic system. J Thromb Haemost. 2003;1:1681–1682. doi: 10.1046/j.1538-7836.2003.00344.x. [DOI] [PubMed] [Google Scholar]
- 53.Folkman J. Angiogenesis inhibitors: a new class of drugs. Cancer Biol Ther. 2003;2:S127–S133. [PubMed] [Google Scholar]
- 54.Folkman J. Angiogenesis and apoptosis. Semin Cancer Biol. 2003;13:159–167. doi: 10.1016/s1044-579x(02)00133-5. [DOI] [PubMed] [Google Scholar]
- 55.Tang TT, Lasky LA. The forkhead transcription factor FOXO4 induces the down-regulation of hypoxia-inducible factor 1 alpha by a von Hippel-Lindau protein-independent mechanism. J Biol Chem. 2003;278:30125–30135. doi: 10.1074/jbc.M302042200. [DOI] [PubMed] [Google Scholar]
- 56.Huang H, Tindall DJ. FOXO factors: a matter of life and death. Future Oncol. 2006;2:83–89. doi: 10.2217/14796694.2.1.83. [DOI] [PubMed] [Google Scholar]
- 57.Potente M, Fisslthaler B, Busse R, Fleming I. 11, 12-Epoxyeicosatrienoic acid-induced inhibition of FOXO factors promotes endothelial proliferation by down-regulating p27Kip1. J Biol Chem. 2003;278:29619–29625. doi: 10.1074/jbc.M305385200. [DOI] [PubMed] [Google Scholar]
- 58.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 59.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]


