Abstract
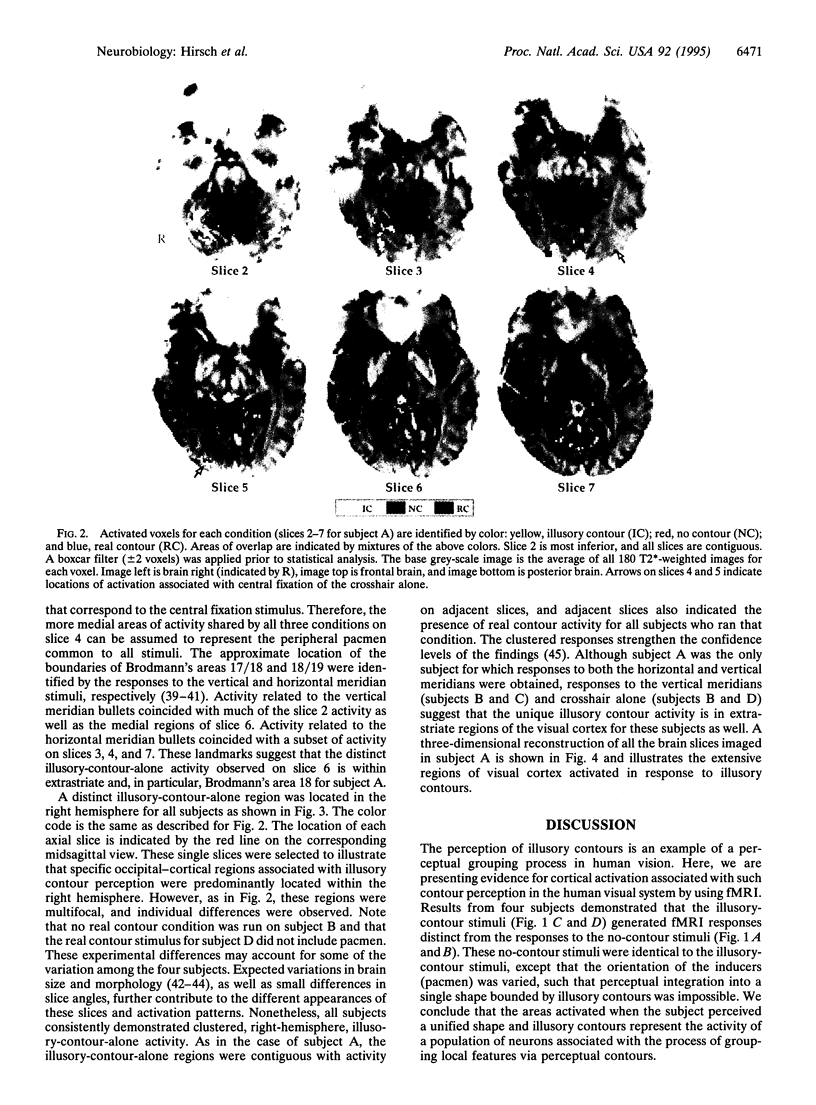
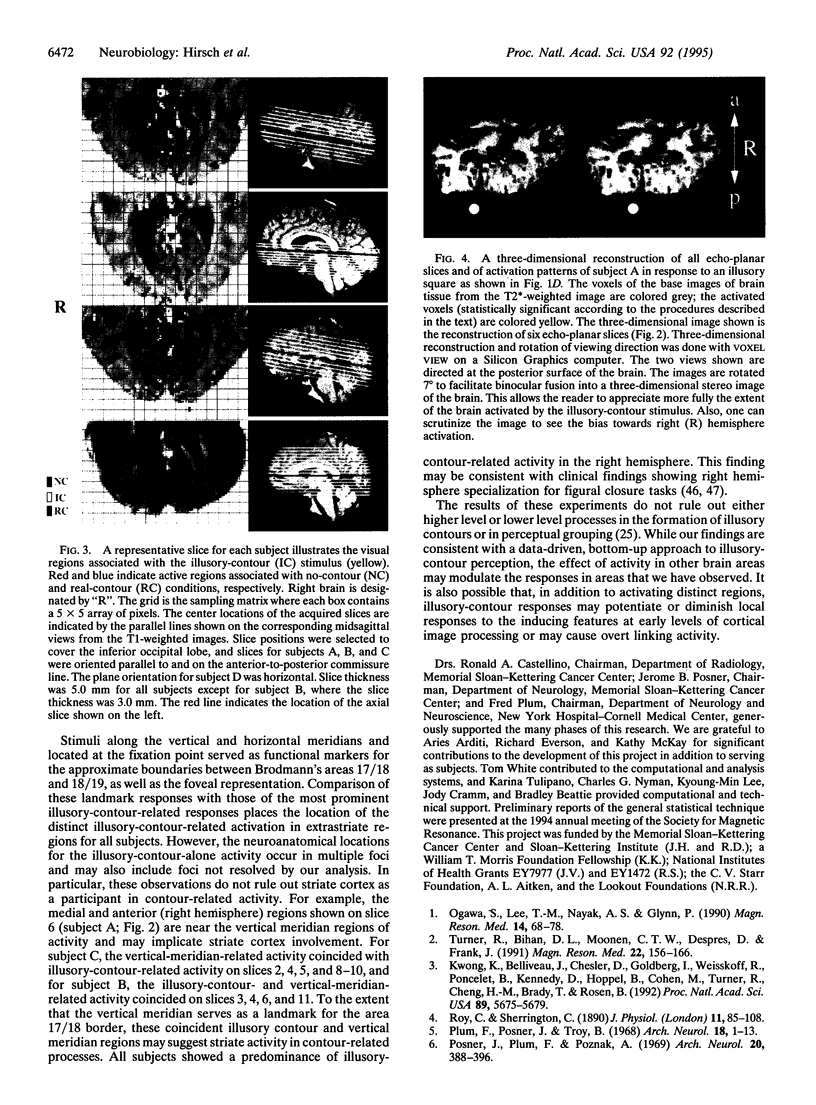
The neural basis for perceptual grouping operations in the human visual system, including the processes which generate illusory contours, is fundamental to understanding human vision. We have employed functional magnetic resonance imaging to investigate these processes noninvasively. Images were acquired on a GE Signa 1.5T scanner equipped for echo planar imaging with an in-plane resolution of 1.5 x 1.5 mm and slice thicknesses of 3.0 or 5.0 mm. Visual stimuli included nonaligned inducers (pacmen) that created no perceptual contours, similar inducers at the corners of a Kanizsa square that created illusory contours, and a real square formed by continuous contours. Multiple contiguous axial slices were acquired during baseline, visual stimulation, and poststimulation periods. Activated regions were identified by a multistage statistical analysis of the activation for each volume element sampled and were compared across conditions. Specific brain regions were activated in extrastriate cortex when the illusory contours were perceived but not during conditions when the illusory contours were absent. These unique regions were found primarily in the right hemisphere for all four subjects and demonstrate that specific brain regions are activated during the kind of perceptual grouping operations involved in illusory contour perception.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandettini P. A., Wong E. C., Hinks R. S., Tikofsky R. S., Hyde J. S. Time course EPI of human brain function during task activation. Magn Reson Med. 1992 Jun;25(2):390–397. doi: 10.1002/mrm.1910250220. [DOI] [PubMed] [Google Scholar]
- Belliveau J. W., Kennedy D. N., Jr, McKinstry R. C., Buchbinder B. R., Weisskoff R. M., Cohen M. S., Vevea J. M., Brady T. J., Rosen B. R. Functional mapping of the human visual cortex by magnetic resonance imaging. Science. 1991 Nov 1;254(5032):716–719. doi: 10.1126/science.1948051. [DOI] [PubMed] [Google Scholar]
- Binder J. R., Rao S. M., Hammeke T. A., Yetkin F. Z., Jesmanowicz A., Bandettini P. A., Wong E. C., Estkowski L. D., Goldstein M. D., Haughton V. M. Functional magnetic resonance imaging of human auditory cortex. Ann Neurol. 1994 Jun;35(6):662–672. doi: 10.1002/ana.410350606. [DOI] [PubMed] [Google Scholar]
- Blamire A. M., Ogawa S., Ugurbil K., Rothman D., McCarthy G., Ellermann J. M., Hyder F., Rattner Z., Shulman R. G. Dynamic mapping of the human visual cortex by high-speed magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):11069–11073. doi: 10.1073/pnas.89.22.11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S., Miklossy J. Occipital cortex in man: organization of callosal connections, related myelo- and cytoarchitecture, and putative boundaries of functional visual areas. J Comp Neurol. 1990 Aug 8;298(2):188–214. doi: 10.1002/cne.902980205. [DOI] [PubMed] [Google Scholar]
- Cohen M. S., Bookheimer S. Y. Localization of brain function using magnetic resonance imaging. Trends Neurosci. 1994 Jul;17(7):268–277. doi: 10.1016/0166-2236(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Constable R. T., McCarthy G., Allison T., Anderson A. W., Gore J. C. Functional brain imaging at 1.5 T using conventional gradient echo MR imaging techniques. Magn Reson Imaging. 1993;11(4):451–459. doi: 10.1016/0730-725x(93)90463-n. [DOI] [PubMed] [Google Scholar]
- Engel S. A., Rumelhart D. E., Wandell B. A., Lee A. T., Glover G. H., Chichilnisky E. J., Shadlen M. N. fMRI of human visual cortex. Nature. 1994 Jun 16;369(6481):525–525. doi: 10.1038/369525a0. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Raichle M. E., Mintun M. A., Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988 Jul 22;241(4864):462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Grosof D. H., Shapley R. M., Hawken M. J. Macaque V1 neurons can signal 'illusory' contours. Nature. 1993 Oct 7;365(6446):550–552. doi: 10.1038/365550a0. [DOI] [PubMed] [Google Scholar]
- Hinke R. M., Hu X., Stillman A. E., Kim S. G., Merkle H., Salmi R., Ugurbil K. Functional magnetic resonance imaging of Broca's area during internal speech. Neuroreport. 1993 Jun;4(6):675–678. doi: 10.1097/00001756-199306000-00018. [DOI] [PubMed] [Google Scholar]
- Horton J. C., Hoyt W. F. Quadrantic visual field defects. A hallmark of lesions in extrastriate (V2/V3) cortex. Brain. 1991 Aug;114(Pt 4):1703–1718. doi: 10.1093/brain/114.4.1703. [DOI] [PubMed] [Google Scholar]
- Horton J. C., Hoyt W. F. The representation of the visual field in human striate cortex. A revision of the classic Holmes map. Arch Ophthalmol. 1991 Jun;109(6):816–824. doi: 10.1001/archopht.1991.01080060080030. [DOI] [PubMed] [Google Scholar]
- Kim S. G., Ashe J., Hendrich K., Ellermann J. M., Merkle H., Uğurbil K., Georgopoulos A. P. Functional magnetic resonance imaging of motor cortex: hemispheric asymmetry and handedness. Science. 1993 Jul 30;261(5121):615–617. doi: 10.1126/science.8342027. [DOI] [PubMed] [Google Scholar]
- Koizuka I., Yano H., Nagahara M., Mochizuki R., Seo R., Shimada K., Kubo T., Nogawa T. Functional imaging of the human olfactory cortex by magnetic resonance imaging. ORL J Otorhinolaryngol Relat Spec. 1994 Sep-Oct;56(5):273–275. doi: 10.1159/000276672. [DOI] [PubMed] [Google Scholar]
- Kwong K. K., Belliveau J. W., Chesler D. A., Goldberg I. E., Weisskoff R. M., Poncelet B. P., Kennedy D. N., Hoppel B. E., Cohen M. S., Turner R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamme V. A., van Dijk B. W., Spekreijse H. Contour from motion processing occurs in primary visual cortex. Nature. 1993 Jun 10;363(6429):541–543. doi: 10.1038/363541a0. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Lee T. M., Nayak A. S., Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990 Apr;14(1):68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Menon R. S., Tank D. W., Kim S. G., Merkle H., Ellermann J. M., Ugurbil K. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J. 1993 Mar;64(3):803–812. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S., Tank D. W., Menon R., Ellermann J. M., Kim S. G., Merkle H., Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R. C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971 Mar;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Plum F., Posner J. B., Troy B. Cerebral metabolic and circulatory responses to induced convulsions in animals. Arch Neurol. 1968 Jan;18(1):1–13. doi: 10.1001/archneur.1968.00470310015001. [DOI] [PubMed] [Google Scholar]
- Posner J. B., Plum F., Van Poznak A. Cerebral metabolism during electrically induced seizures in man. Arch Neurol. 1969 Apr;20(4):388–395. doi: 10.1001/archneur.1969.00480100064010. [DOI] [PubMed] [Google Scholar]
- Purpura K. P., Victor J. D., Katz E. Striate cortex extracts higher-order spatial correlations from visual textures. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8482–8486. doi: 10.1073/pnas.91.18.8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher J., Caviness V. S., Jr, Steinmetz H., Galaburda A. M. Topographical variation of the human primary cortices: implications for neuroimaging, brain mapping, and neurobiology. Cereb Cortex. 1993 Jul-Aug;3(4):313–329. doi: 10.1093/cercor/3.4.313. [DOI] [PubMed] [Google Scholar]
- Roy C. S., Sherrington C. S. On the Regulation of the Blood-supply of the Brain. J Physiol. 1890 Jan;11(1-2):85–158.17. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schad L. R., Trost U., Knopp M. V., Müller E., Lorenz W. J. Motor cortex stimulation measured by magnetic resonance imaging on a standard 1.5 T clinical scanner. Magn Reson Imaging. 1993;11(4):461–464. doi: 10.1016/0730-725x(93)90464-o. [DOI] [PubMed] [Google Scholar]
- Schneider W., Noll D. C., Cohen J. D. Functional topographic mapping of the cortical ribbon in human vision with conventional MRI scanners. Nature. 1993 Sep 9;365(6442):150–153. doi: 10.1038/365150a0. [DOI] [PubMed] [Google Scholar]
- Shulman R. G., Blamire A. M., Rothman D. L., McCarthy G. Nuclear magnetic resonance imaging and spectroscopy of human brain function. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3127–3133. doi: 10.1073/pnas.90.8.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz H., Seitz R. J. Functional anatomy of language processing: neuroimaging and the problem of individual variability. Neuropsychologia. 1991;29(12):1149–1161. doi: 10.1016/0028-3932(91)90030-c. [DOI] [PubMed] [Google Scholar]
- Turner R., Jezzard P., Wen H., Kwong K. K., Le Bihan D., Zeffiro T., Balaban R. S. Functional mapping of the human visual cortex at 4 and 1.5 tesla using deoxygenation contrast EPI. Magn Reson Med. 1993 Feb;29(2):277–279. doi: 10.1002/mrm.1910290221. [DOI] [PubMed] [Google Scholar]
- Turner R., Le Bihan D., Moonen C. T., Despres D., Frank J. Echo-planar time course MRI of cat brain oxygenation changes. Magn Reson Med. 1991 Nov;22(1):159–166. doi: 10.1002/mrm.1910220117. [DOI] [PubMed] [Google Scholar]
- Wasserstein J., Zappulla R., Rosen J., Gerstman L., Rock D. In search of closure: subjective contour illusions, Gestalt completion tests, and implications. Brain Cogn. 1987 Jan;6(1):1–14. doi: 10.1016/0278-2626(87)90042-x. [DOI] [PubMed] [Google Scholar]
- Woods R. P., Mazziotta J. C., Cherry S. R. MRI-PET registration with automated algorithm. J Comput Assist Tomogr. 1993 Jul-Aug;17(4):536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- von der Heydt R., Peterhans E., Baumgartner G. Illusory contours and cortical neuron responses. Science. 1984 Jun 15;224(4654):1260–1262. doi: 10.1126/science.6539501. [DOI] [PubMed] [Google Scholar]
- von der Heydt R., Peterhans E. Mechanisms of contour perception in monkey visual cortex. I. Lines of pattern discontinuity. J Neurosci. 1989 May;9(5):1731–1748. doi: 10.1523/JNEUROSCI.09-05-01731.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]