Abstract
Objective
To determine the clinicopathological characteristics, and evaluate the appropriate extent of lymph node dissection in distal gastric cancer patients with comparable T category.
Methods
A retrospective study was conducted on 570 distal gastric cancer patients, who underwent gastric resection with D2 nodal dissection, which was performed by the same surgical team from January 1997 to January 2011. We compared the differences in lymph node metastasis rates and metastatic lymph node ratios between different T categories. Additionally, we investigated the impact of lymph node metastasis in the 7th station on survival rate of distal gastric cancer patients with the same TNM staging.
Results
Among the 570 patients, the overall lymph node metastasis rate of advanced distal gastric cancer was 78.1%, and the metastatic lymph node ratio was 27%. The lymph node metastasis rate in the 7th station was similar to that of perigastric lymph nodes. There was no statistical significance in patients with the same TNM stage (stage II and III), irrespective of the metastatic status in the 7th station.
Conclusions
Our results suggest that to a certain extent, it is reasonable to include lymph nodes in the 7th station in the D1 lymph node dissection.
Keywords: Stomach neoplasms, lymph node excision, lymphatic metastasis
Introduction
There is an ongoing debate on the extent of lymph node dissection required in all stages of gastric cancer, especially in advanced gastric cancer (1). According to the Japanese Gastric Cancer Treatment Protocols, lymph nodes in the 7th station were considered as compartment II node in the 13th edition, but were transferred to compartment I in the 14th edition. Additionally, lymph node staging was based on the number of metastatic lymph nodes, which is identical to the UICC guidelines version of 2010 (2,3).
Currently, T category is the only variable that can be precisely evaluated before surgery, as compared to other variables (4). There are specific features and patterns of clinical pathology and lymph node metastasis among distal gastric cancer patients with different T categories.
In this retrospective study, we analyzed the pattern and features of distal gastric cancer with different T categories based on our clinical data. The aim was to provide clinical evidence for a reasonable protocol for the extent of lymph node dissection during surgery, especially for the lymph nodes in the 7th station.
Material and methods
Subjects
Between January 1997 and January 2011, 1,635 patients with gastric cancer were treated with gastric resection and D2 nodal dissection in our medical center. The medical history, clinical symptoms and physical examination were evaluated after the patients were admitted to the hospital. Blood tests, chest radiography, CT scan, tumor marker studies and endoscopy were routinely performed before the surgery.
According to Rohde classification (5), distal gastric cancer is defined as a tumor located in the lower-third of the stomach. A total of 786 patients had distal gastric cancer, which was confirmed by pathological diagnosis. Of these, 216 patients were excluded because of a diagnosis of multiple carcinomas or non-adenocarcinomas, or for undergoing non-radical surgery, or for receiving palliative therapy. Therefore, in this study, we retrospectively enrolled 570 patients with distal gastric cancer, which included 379 males and 191 females. As shown in Table 1, the clinicopathological characteristics of patients with different T categories were collated based on age, tumor size, tumor differentiation, WHO pathology type (6), and distant metastasis.
Table 1. General conditions of the 570 patients.
| Categories | T1 | T2 | T3 | T4 |
|---|---|---|---|---|
| Age (years)0 | 55.28±13.3 | 58.06±11.92 | 57.33±12.66 | 55.85±13.23 |
| Operation time (min) | 250.87±76.33*4 | 260.65±64.50 | 279.22±68.01 | 280.40±86.93*1 |
| Tumor diameter (cm) | 2.76±2.09*3,4 | 3.16±1.53*3,4 | 4.94±2.61*1,2 | 5.84±3.23*1,2 |
| Gender (male/female)0 | 39/61 | 22/41 | 77/194 | 53/83 |
0, differences were not statistically significant; *, the difference was statistically significant. 1,2,3,4, the difference between the groups has statistical significance by t-test.
Methods
Surgery method
All patients underwent extended D1, standard D2 and extended D2 lymphadenectomy based on the Japanese Gastric Cancer Treatment Protocols (13th edition) (7). Of these, 29 patients with early-stage distal gastric cancer underwent extended D1 surgery, 456 patients underwent standard D2 surgery, and 92 patients underwent D2+ surgery.
D2 surgery requires a routine lymph node dissection of perigastric lymph nodes and lymph nodes from stations 7, 8, 9, 11 to 12 along with gastrectomy. Extended D2 surgery requires additional lymph node dissection of station 16 or other extra-perigastric lymph nodes based on the preoperative and intraoperative findings. For most patients with distal gastric cancer, Billroth II gastrojejunostomy was the reconstruction method employed after gastrectomy.
Chemotherapy method
Neoadjuvant chemotherapy was given to the patients before surgery based on clinical staging indications. Postoperative adjuvant chemotherapy was given to patients based on pathological TNM staging. Before 2002, patients were mainly treated with 5-fluorouracil (5-FU)/calcium folinate (CF) regimen, but this regimen was replaced with oxaliplatin/5-FU after 2002. The indications of adjuvant chemotherapy were based on the NCCN guidelines.
Data processing
The resected specimens and lymph nodes of all patients were meticulously handled and classified by experienced surgeons for pathological examination. After receiving the pathology reports, the locations and the numbers of retrieved lymph nodes were comprehensively documented in the surgery records.
Lymph nodes were classified in compliance with the D level criteria of the Japanese Gastric Cancer Treatment Protocols (13th edition) (7). When reporting the data for constructing a formal database, the pathological TNM staging should fulfill the criterion from the 7th edition of TNM staging (3).
Based on our detailed records, we compared the differences in clinicopathological characteristics among patients with different T categories. We also compared the differences in lymph node metastasis ratio (LNR) and metastatic lymph node ratio (MLR) for distal gastric cancer patients with different T categories. Additionally, we analyzed the risk factors for lymph node metastasis in distal gastric cancer.
Follow-up visit
The patients were followed up through letters, re-diagnosis and telephone calls. The visit interval was 6 months. The terminal points of follow-up visit was December 20th, 2012.
Statistical analysis
All data were analyzed with the Statistical Package for the Social Sciences (SPSS 17.0) software. Student’s t-test was used to compare the differences in quantitative data, chi-square test was used to analyze the categorical data, and survival was evaluated by the Kaplan-Meier method and compared by log-rank test.
The stepwise entry and removal method was adopted to construct a logistic regression model for univariate and multivariate analyses. Probabilities for stepwise entry and removal were set at 0.05 and 0.10, respectively.
Results
Comparison of clinicopathological characteristics
The clinicopathological characteristics of distal gastric cancer patients with different T categories are shown in Table 1.
The radical resection rate was 72.5%. There were no significant differences in age and sex among patients with different T categories. However, there were statistically significant differences in tumor size, lymph node metastasis rate, Borrmann classification and tumor differentiation.
Relationship between lymph node metastasis and clinicopathological characteristics in distal gastric cancer
Logistic regression was adopted for univariate and multivariate analyses (Table 2).
Table 2. Multivariate logistic regression analysis on the factors influencing lymph node metastases.
| Items | Single factor |
Multi-factor |
|||||
|---|---|---|---|---|---|---|---|
| P value | OR | 95% CI of OR | P value | OR | 95% CI of OR | ||
| Gender | 0.316 | 1.205 | 0.837-1.734 | ||||
| Age | 0.539 | 0.949 | 0.802-0.1.123 | ||||
| Distant metastasis | 0 | 2.063 | 1.567-6.791 | 0.066 | 1.398 | 0.712-2.171 | |
| Tumor diameter | 0 | 3.192 | 2.059-3.948 | 0.028 | 1.804 | 1.381-2.623 | |
| Borrmman | 0 | 2.21 | 1.694-2.883 | 0.053 | 1.598 | 0.912-2.105 | |
| Differentiation | 0 | 2.158 | 1.606-2.901 | 0.061 | 1.473 | 0.838-2.090 | |
| Infiltration | 0 | 2.923 | 2.367-3.608 | 0.011 | 2.31 | 1.841-2.897 | |
Age (1: <40, 2: 41-50, 3: 51-60, 4: 51-60, 5: 61-70), gender (0: female, 1: male), operation time (min), tumor diameter (cm), Borrmman type (1: I, 2: II, 3: III, 4: IV), depth of invasion (1: T1, 2: T2, 3: T3, 4: T4), lymph node metastasis (1: N1, 2: N2, 3: N3).
Univariate analysis of the relationship between lymph node metastasis and clinicopathological characteristics in distal gastric cancer showed that tumor size, distant metastasis, differentiation, and depth of invasion were risk factors for lymph node metastasis. Multivariate analysis indicated that depth of invasion and tumor size were independent prognostic factors for patients with lymph node involvement.
Patterns of lymph node metastasis in distal gastric cancer with different T categories
Evaluation of lymph node metastasis rate
General data
Among the 570 patients, 358 patients had lymph node metastasis with a metastasis rate of 62.8%. A mean node yield of 26.24±15.11 was obtained from each patient, and a mean of 4.19±5.8 positive nodes were detected. The rate of lymph node metastasis for T1 stage was 18%, and that of advanced distal gastric cancer was 78.1%. Due to variations in the T categories, there were significant differences in lymph node metastasis rates among different stations. In patients with advanced gastric cancer, the highest rate of lymph nodes metastasis was detected in No. 6, and the others were sorted in descending order as follows: No. 3, No. 5, No. 4, No. 7, No. 8, No. 1, No. 12, No. 9 and No. 11 (Table 3).
Table 3. Lymph node metastasis rate with comparable T category.
| Stage number | T1 [%] (n=100) | T2 (%) (n=63) | T3 (%) (n=271) | T4 (%) (n=136) | Chi-square | P |
|---|---|---|---|---|---|---|
| 1 | 3 [3] | 3 (4.8) | 39 (14.4) | 21 (15.4) | 14.123 | 0.003 |
| 2 | 0 [0] | 0 (0) | 0 (0) | 5 (3.7) | 0.035 | 0.851 |
| 3 | 5 [5] | 11 (17.5) | 113 (41.7) | 59 (43.4) | 58.258 | 0 |
| 4 | 4 [4] | 7 (11.1) | 72 (26.6) | 42 (30.9) | 32.856 | 0 |
| 5 | 5 [5] | 5 (7.9) | 82 (30.3) | 47 (34.6) | 42.327 | 0 |
| 6 | 6 [6] | 16 (25.4) | 138 (50.9) | 80 (58.8) | 84.928 | 0 |
| 7 | 4 [4] | 5 (7.9) | 71 (26.2) | 31 (22.8) | 29.389 | 0 |
| 8 | 1 [1] | 4 (6.3) | 58 (21.4) | 34 (25.0) | 33.072 | 0 |
| 9 | 0 [0] | 1 (1.6) | 14 (5.2) | 11 (8.1) | 3.646 | 0.162 |
| 10 | 0 [0] | 0 (0) | 1 (0.4) | 4 (2.9) | 4.937 | 0.026 |
| 11 | 1 [1] | 0 (0) | 11 (4.1) | 9 (6.6) | 4.59 | 0.101 |
| 12 | 1 [1] | 1 (1.6) | 29 (10.7) | 18 (13.2) | 16.535 | 0.001 |
| 13 | 0 [0] | 0 (0) | 7 (2.6) | 5 (3.7) | 0.378 | 0.538 |
| 14 | 0 [0] | 0 (0) | 8 (3.0) | 4 (2.9) | 0 | 0.995 |
| 15 | 0 [0] | 0 (0) | 2 (0.7) | 6 (4.4) | 6.343 | 0.012 |
| 16 | 0 [0] | 0 (0) | 7 (2.6) | 5 (3.7) | 0.378 | 0.538 |
| Total | 18/100 [18] | 25/63 (39.7) | 206/271 (76.0) | 109/136 (80.1) | 138.11 | 0 |
n, number of patients; data format, patients had lymph node metastasis (lymph node metastasis rate); lymph node metastasis rate, patients had lymph node metastasis/n, 100%; total, patients had lymph node metastasis/all patients.
Comparison of metastatic lymph node rate
As shown in Table 4, the total number of extracted lymph nodes was 14,876, and the number of metastatic lymph nodes was 2,363, with a metastasis rate of 15.8%. There was significant correlation between the metastatic lymph node ratio and depth of invasion. Metastatic lymph node ratio gradually increased with advancing T stage. The metastatic lymph node rate was 2.2% in T2 patients, 26.7% in patients with advanced disease, and was especially high in T4 patients (37.7%). There was significant difference in metastatic lymph node ratio according to T categories (Table 5).
Table 4. Lymph node metastasis rate and metastatic lymph node rate.
| Categories | T1 (number of patients) | T2 (number of patients) | T3 (number of patients) | T4 (number of patients) |
|---|---|---|---|---|
| Distant metastasis | 0 | 2 | 32 | 37 |
| Peritoneum | 0 | 1 | 24 | 32 |
| Liver | 0 | 0 | 3 | 9 |
| Other | 0 | 1 | 5 | 3 |
| Differentiation | T1 | T2*4 | T3 | T4*2 |
| High | 24 | 4 | 3 | 3 |
| Moderately | 20 | 21 | 509 | 21 |
| Poorly or none | 56 | 38 | 209 | 112 |
| Lymph node metastasis rate | 18/100*2,3,4 | 25/63*1,4 | 206/271*1 | 109/136*1,2 |
| Metastatic lymph node ratio | 54/2,493 (2.2)*3,4 | 98/1,529 (6.6)*3,4 | 1,874/7,021 (26.7)*1,2 | 1,156/3,069 (37.7)*1,2 |
| B-categories | T2*3,4 | T3*1,4 | T4*1,3 | |
| 1 | 1 | 10 | 6 | |
| 2 | 25 | 53 | 11 | |
| 3 | 35 | 181 | 94 | |
| 4 | 3 | 21 | 15 | |
| Pathological type | ||||
| 1 | 85 | 63 | 223 | 110 |
| 2 | 3 | 0 | 24 | 10 |
| 3 | 12 | 0 | 24 | 16 |
0, differences were not statistically significant; *, the difference was statistically significant. 1,2,3,4, the difference between the groups has statistical significance by Chi-square test.
Table 5. Metastatic lymph node ratio with comparable T category.
| Stage number | T1 | T2 | T3 | T4 | Statistical value | P value |
|---|---|---|---|---|---|---|
| 1 | 6/166 (3.6) | 3/141 (2.1) | 87/568 (15.3) | 60/239 (25.1) | 59.186 | 0 |
| 2 | 0/23 (0) | 0/14 (0) | 0/164 (0) | 13/51 (25.5) | 0.667 | 0.414 |
| 3 | 6/346 (1.7) | 23/247 (9.3) | 344/987 (34.9) | 198/479 (41.3) | 227.713 | 0 |
| 4 | 13/320 (4.1) | 12/154 (7.8) | 189/813 (23.2) | 140/411 (34.1) | 117.499 | 0 |
| 5 | 11/186 (5.9) | 11/108 (10.2) | 182/557 (32.7) | 111/190 (58.4) | 145.838 | 0 |
| 6 | 17/362 (4.7) | 38/224 (17.0) | 476/1,141 (41.7) | 272/559 (48.7) | 246.198 | 0 |
| 7 | 5/328 (1.5) | 14/220 (6.4) | 180/825 (21.8) | 84/293 (28.7) | 115.232 | 0 |
| 8 | 1/259 (0.4) | 10/140 (7.1) | 101/523 (19.3) | 80/211 (37.9) | 128.02 | 0 |
| 9 | 0/84 (0) | 4/47 (8.5) | 22/204 (10.8) | 17/60 (28.3) | 13.297 | 0.001 |
| 10 | 0/22 (0) | 0/7 (0) | 2/51 (3.9) | 6/31 (19.4) | ||
| 11 | 1/52 (1.9) | 0/12 (0) | 24/75 (32.0) | 11/33 (33.3) | 18.729 | 0 |
| 12 | 1/173 (0.6) | 2/110 (1.8) | 78/517 (15.1) | 27/128 (21.1) | 42.221 | 0 |
| 13 | 0/13 (0) | 0/110 (0) | 8/36 (22.2) | 17/58 (29.3) | 0.572 | 0.45 |
| 14 | 0/5 (0) | 0/1 (0) | 9/71 (12.7) | 11/19 (57.9) | 17.732 | 0 |
| 15 | 0/27 (0) | 0/0 (0) | 3/35 (8.6) | 10/38 (26.3) | 3.919 | 0.048 |
| 16 | 0/2 (0) | 26/94 (27.7) | 45/85 (52.9) | 8.358 | 0.004 | |
| Total | 54/2,493 (2.2) | 98/1,529 (6.4) | 1,874/7,021 (26.7) | 1,156/3,069 (37.7) | 1,291.77 | 0 |
Data format, metastatic lymph nodes/extracted lymph nodes (%).
Survival analyses of patients with same pathological stage based on the lymph node status of No. 7
For stage II patients, there were 48 cases with lymph node metastases. Of these, 11 patients were detected with No. 7 lymph node metastasis, while the remaining 37 patients did not have No. 7 involvement. The calculated 5-year overall survival rate of stage II patients with lymph node involvement was 68%, with No. 7 lymph node metastasis was 59%, and without No. 7 lymph node metastasis was 70%, which were not statistically significant (P=0.666, Figure 1).
Figure 1.
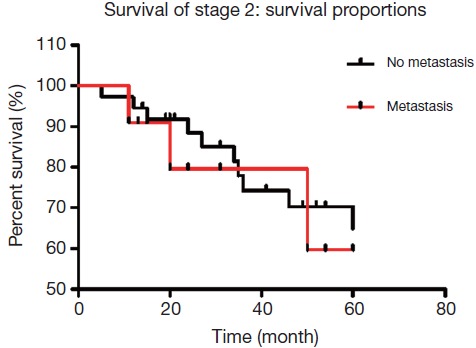
For stage II patients, there were 48 cases with lymph node metastases. Of these, 11 patients were detected with No. 7 lymph node metastasis, while the other 37 patients had no involvement of No. 7 lymph nodes. The calculated 5-year overall survival rate of stage II patients with lymph node involvement was 68%, with No. 7 lymph node metastasis was 59%, and without No. 7 lymph node metastasis was 70%, which were not statistically significant (P=0.666).
A total of 226 patients were diagnosed with stage III distal gastric cancer, and lymph node metastases were found in 165 patients. Among patients with lymph node metastases, 79 patients had No. 7 lymph node metastasis, while 86 cases did not have No. 7 lymph node metastasis. The 5-year overall survival rate of patients with lymph node involvement was 34.4%, with No. 7 lymph node metastasis was 28.4%, and without No. 7 lymph node metastasis was 39.1%, which were not statistically significant according to Kaplan-Meier analysis (P=0.542, Figure 2).
Figure 2.
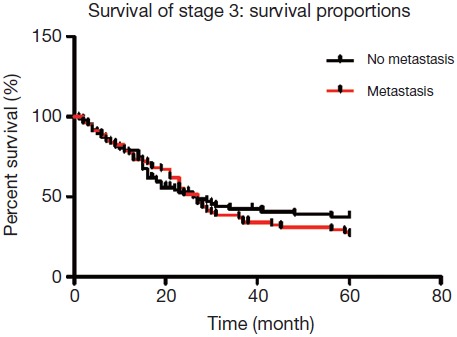
A total of 226 patients were diagnosed with stage III distal gastric cancer, and lymph node metastases were found in 165 patients. Among patients with lymph node metastases, 79 cases had No. 7 lymph node metastasis, while 86 cases did not have No. 7 lymph node metastasis. The 5-year overall survival rate of patients with lymph node involvement was 34.4%, with No. 7 lymph node metastasis was 28.4%, and without No. 7 lymph node metastasis was 39.1%, which were not statistically significant according to Kaplan-Meier analysis (P=0.542).
A total of 77 patients were diagnosed with stage IV distal gastric cancer, and lymph node metastases were found in 40 patients. Of these, 20 patients had No. 7 lymph node involvement, while the remaining 20 cases did not have lymphatic invasion. The 5-year overall survival rate of patients was 7.2%, with No. 7 lymph node metastasis was 5%, and without No. 7 lymph node metastasis was 12%, which were not statistically significant (P=0.852, Figure 3).
Figure 3.
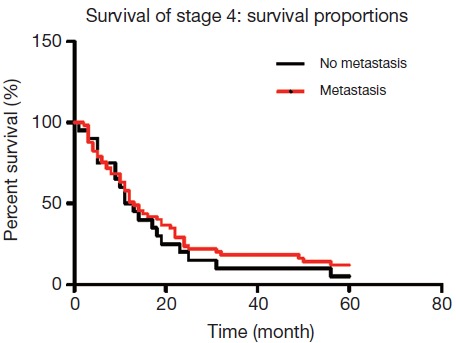
A total of 77 patients were diagnosed with stage IV distal gastric cancer, and lymph node metastases were found in 40 patients. Of these, 20 patients had No. 7 lymph node involvement, while the other 20 cases did not have lymphatic invasion. The 5-year overall survival rate of patients was 7.2%, with No. 7 lymph node metastasis was 5%, and without No. 7 lymph node metastasis was 12%, which were not statistically significant (P=0.852).
Discussion
Comparison of clinicopathological characteristics based on different depths of invasion (T category) in patients with distal gastric cancer indicated that there were statistically significant differences in tumor size and lymph node metastasis rate (Table 1). Saito et al. (8) suggested that tumor size, depth of invasion, lymph node metastasis and lymphatic vessels invasion were independent prognostic factors of gastric cancer. Kawaguchi et al. (9) demonstrated that lymph node metastasis correlated with tumor size. Seto et al. (10) found that the lymph node metastasis rate of low-differentiation tumors was significantly higher as compared to that of high-differentiation tumors. Through univariate and multivariate analyses, we found that T category and tumor size were risk factors that influenced lymph node metastasis. While the preoperative imaging examinations detected enlarged lymph nodes, it was difficult to gauge whether these lymph nodes were infiltrated. This suggested that we should focus on the clinical T staging examination reports and tumor size during preoperative evaluation of tumor staging. These parameters enable surgeons to objectively assess the possibility of lymph node metastasis.
Xu et al. (11) reported that presence of metastasis in compartment II nodes of early gastric cancers were confined to No. 7 and No. 8a lymph nodes, with a metastasis rate of 5.9% in mucosal carcinoma and 22.4% in submucosal carcinoma. Another study asserted that the lymph node metastasis rates of mucosal and submucosal carcinomas were about 3% and 20%, respectively (12). Maruyama et al. (13) showed that the metastases of compartment II lymph nodes were more likely to occur in advanced gastric cancer. According to their findings, the metastasis rates of No. 7 and No. 8 were 23% and 25%, respectively. This suggests that the evaluation of invasion depth plays an important role in estimating lymph node metastases. For TNM staging system, it was difficult to determine the N staging before surgery. However, we can obtain more accurate T staging by CT scan and endoscopic ultrasonography (4). The status of lymph node involvement differs with T staging, which can help in determining the extent of required lymphadenectomy. Our results showed that as the depth of invasion increased, the lymph nodes gradually metastasized, and spread from compartment I to compartment II. The lymph node metastasis rate of early gastric cancer was 18%, and was confined to the regional nodes around the stomach. The metastasis rate was only 2% in compartment II nodes, but rose to 9.5% in T2 tumors, and 81% in T4 tumors. The metastasis rate and ratio of each lymph node station increased in response to the depth of invasion. Therefore, the difference in T category was able to provide clinical evidence for choosing reasonable extent of required lymphadenectomy.
In our group, the metastasis rate of advanced gastric cancer was 73%, which was similar to 70% shown in previous reports (14). de Manzoni et al. (15) analyzed the positive rates of compartment II nodes based on different T categories and found that it was 0% in T2 tumors, 20% in T3 tumors, and 29% in T4 tumors. Also, the positive rates of No. 7 to No. 12 ranged from 20% to 50%, which were lower than those of No. 1 to No. 3. The metastasis rate of No. 6 nodes in our study was the highest, followed by No. 3, No. 1, No. 5, No. 4, No. 7, No. 8, No. 11, No. 12, and other nodes. Lymph node metastases were commonly found to spread from compartment I to compartment II. Moreover, metastasis rate of each station increased with the metastatic ratio, both of which were coincident. In terms of the distant lymph nodes, the metastasis rates of No. 13 and No. 14 were both 0% in tumors under T2 stage, while both increased to 2.9% in tumors above T3 stage. Additionally, the lymph node metastasis ratios in these two stations were 26% and 22%, respectively. Although further research is required to determine the clinical significance of the above-mentioned lymph nodes, it is important to accurately evaluate the lymph node status of each station before and during surgery, if the advanced distal gastric cancer has infiltrated beyond the serosal layer (16). It is necessary to meticulously investigate those lymph nodes with high metastasis rate and ratio. More extensive lymphadenectomy or selective extended D2 dissection is needed to achieve radical resection.
Arai (17) proposed a classification system, which was based on the anatomical distribution of vascular and lymphatic systems. According to this classification system, No. 7 was defined as the compartment I node, No. 16 and No. 112 as the compartment III nodes, and the other nodes as compartment II. According to the Japanese Gastric Cancer Treatment Protocols, No. 7 was considered as compartment II node in the 13th edition, but was transferred to compartment I in the 14th edition. Our data demonstrated that No. 7 was positive in T1 tumors, with a metastasis rate of 4%. Additionally, metastasis rate of the other compartment II nodes was only 3%. For advanced diseases, the metastasis rate of No. 7 was lower than that of No. 3, No. 5 and No. 6, and its metastasis rate and ratio were similar to those of perigastric lymph nodes. This indicated the importance of considering No. 7 as the compartment I node requiring dissection, and the rationality of classifying it into the D1 lymphadenectomy extent. In our study, we also conducted survival analysis of patients with the same TNM stage based on whether the No. 7 nodes were involved. The results showed no statistical significance in survival rates between stage II and III patients, which illustrated the necessity and scientific basis of dissecting No. 7 during D1 lymphadenectomy from a different perspective.
Conclusions
In conclusion, as the T categories of distal gastric cancer advanced, the lymph node metastasis rate and ratio also increased, with certain significance and patterns of lymph node metastasis. The precise staging before surgery could help surgeons to choose the appropriate surgical procedure, and standardize the lymphadenectomy. The requirement of dissecting No. 7 in the new version of Japanese Gastric Cancer Treatment Protocols was reasonable and scientific.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Bickenbach K, Strong VE. Comparisons of Gastric Cancer Treatments: East vs. West. J Gastric Cancer 2012;12:55-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma, Fourteenth Edition (in Japanese). Tokyo: Kanehara, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Sobin LH, Gospodarowicz MK, Wittekind C. eds. TNM classification of malignant tumours. New York: Wiley, 2010. [Google Scholar]
- 4.Seevaratnam R, Cardoso R, Mcgregor C, et al. How useful is preoperative imaging for tumor, node, metastasis (TNM) staging of gastric cancer? A meta-analysis. Gastric Cancer 2012;15:S3-18 [DOI] [PubMed] [Google Scholar]
- 5.Rohde H, Bauer P, Stützer H, et al. Proximal compared with distal adenocarcinoma of the stomach: differences and consequences. Br J Surg 1991;78:1242-8 [DOI] [PubMed] [Google Scholar]
- 6.Pathology and genetics of tumours of the digestive system. Lyon: IARC Press, 2000. [Google Scholar]
- 7.Japanese Classification of Gastric Carcinoma-2nd English Edition. Gastric Cancer 1998;1:10-24 [DOI] [PubMed] [Google Scholar]
- 8.Saito H, Osaki T, Murakami D, et al. Macroscopic tumor size as a simple prognostic indicator in patients with gastric cancer. Am J Surg 2006;192:296-300 [DOI] [PubMed] [Google Scholar]
- 9.Kawaguchi A, Nagao S, Takebayashi K, et al. Long-term outcome of endoscopic semiconductive diode laser irradiation therapy with injection of indocyanine green for early gastric cancer. J Gastroenterol Hepatol 2008;23:1193-9 [DOI] [PubMed] [Google Scholar]
- 10.Seto Y, Shimoyama S, Kitayama J, et al. Lymph node metastasis and preoperative diagnosis of depth of invasion in early gastric cancer. Gastric Cancer 2001;4:34-8 [DOI] [PubMed] [Google Scholar]
- 11.Xu YY, Huang BJ, Sun Z, et al. Risk factors for lymph node metastasis and evaluation of reasonable surgery for early gastric cancer. World J Gastroenterol 2007;13:5133-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunisaki C, Akiyama H, Nomura M, et al. Significance of longterm follow-up of early gastric cancer. Ann Surg Oncol 2006;13:363-9 [DOI] [PubMed] [Google Scholar]
- 13.Maruyama K, Gunvén P, Okabayashi K, et al. Lymph node metastases of gastric cancer. General pattern in 1931 patients. Ann Surg 1989;210:596-602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crucitti F, Doglietto GB, Bellantone R, et al. Stomach cancer: a study of 117 consecutive resected cases and results of R2-R3 gastrectomy. Int Surg 1991;76:23-6 [PubMed] [Google Scholar]
- 15.de Manzoni G, Morgagni P, Roviello F, et al. Nodal abdominal spread in adenocarcinoma of the cardia. Results of a multicenter prospective study. Gastric Cancer 1998;1:146-51 [DOI] [PubMed] [Google Scholar]
- 16.Yokota T, Ishiyama S, Saito T, et al. Lymph node metastasis as a significant prognostic factor in gastric cancer: a multiple logistic regression analysis. Scand J Gastroenterol 2004;39:380-4 [DOI] [PubMed] [Google Scholar]
- 17.Arai K.Contradictions in Japanese classification of gastric carcinoma (JCGC) and a proposal for revision. Gan To Kagaku Ryoho 2007;34:2325-8 [PubMed] [Google Scholar]


