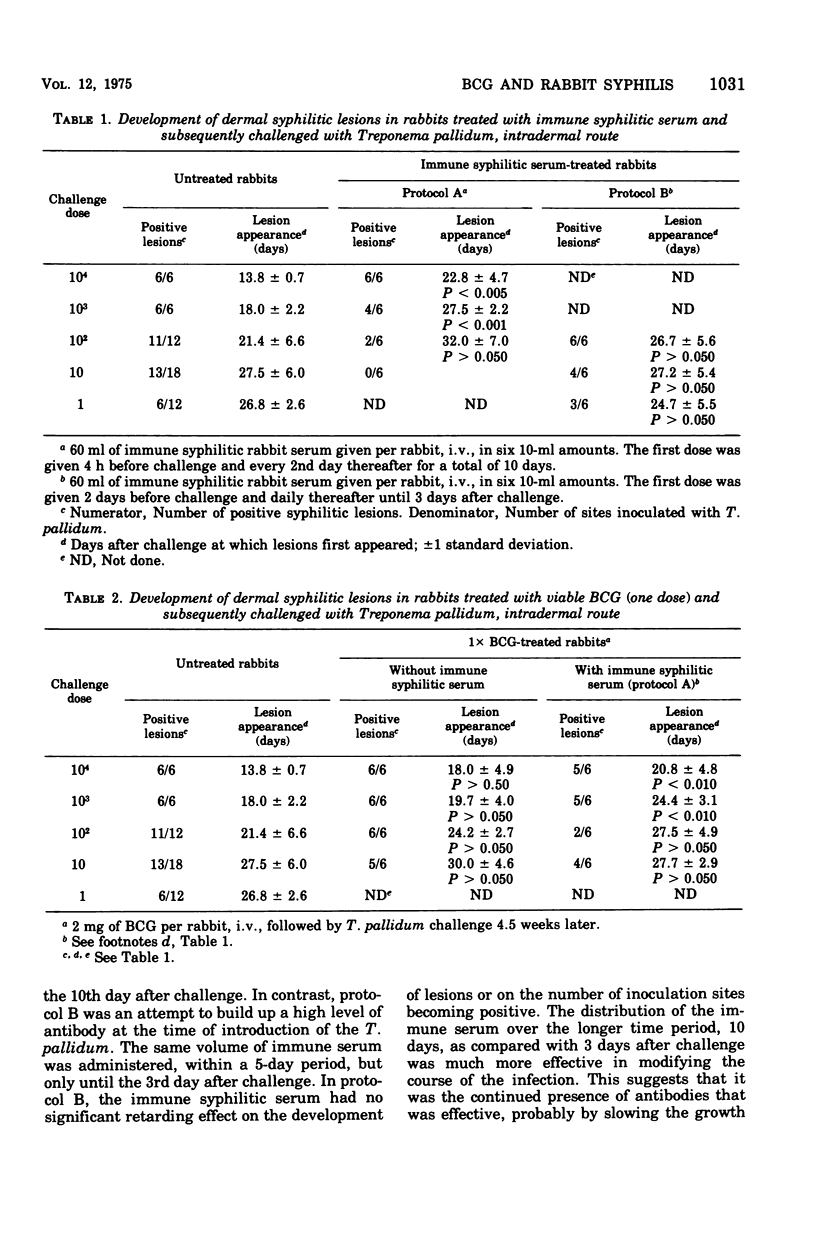
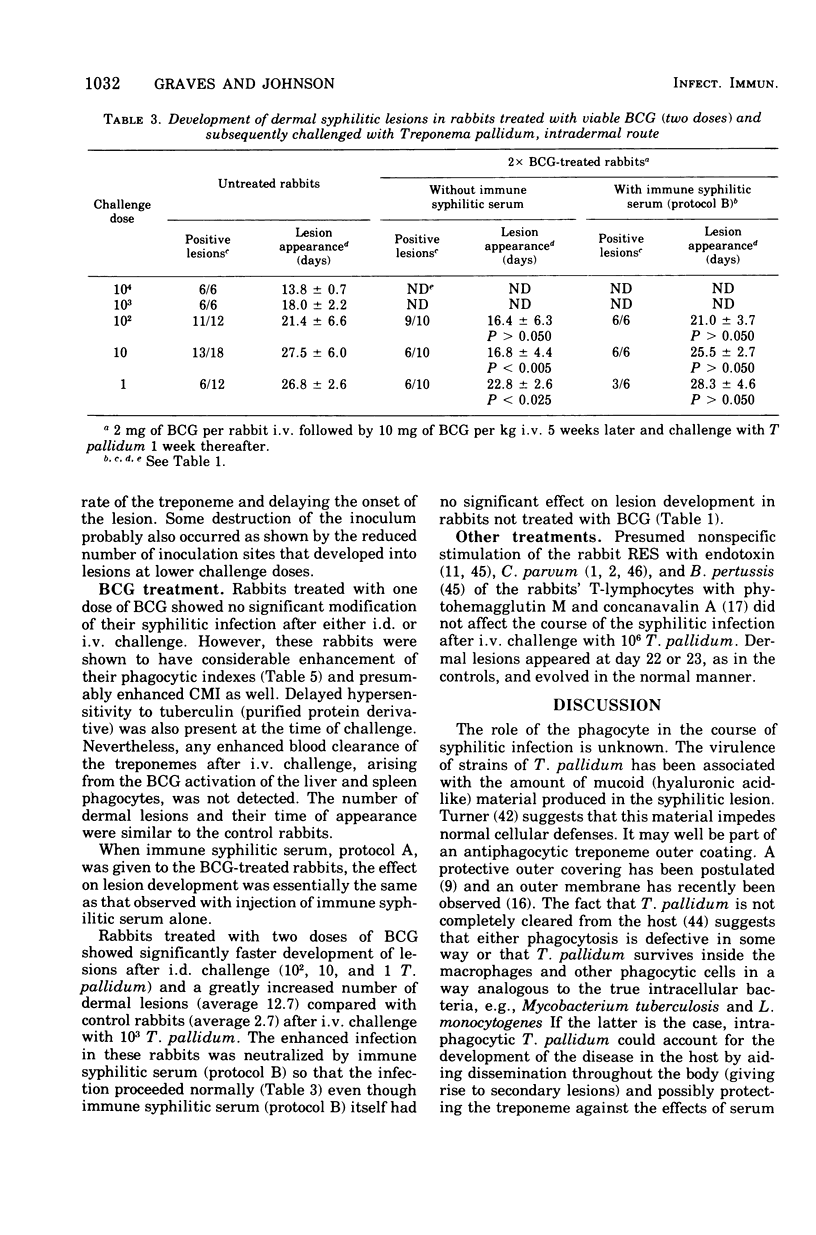
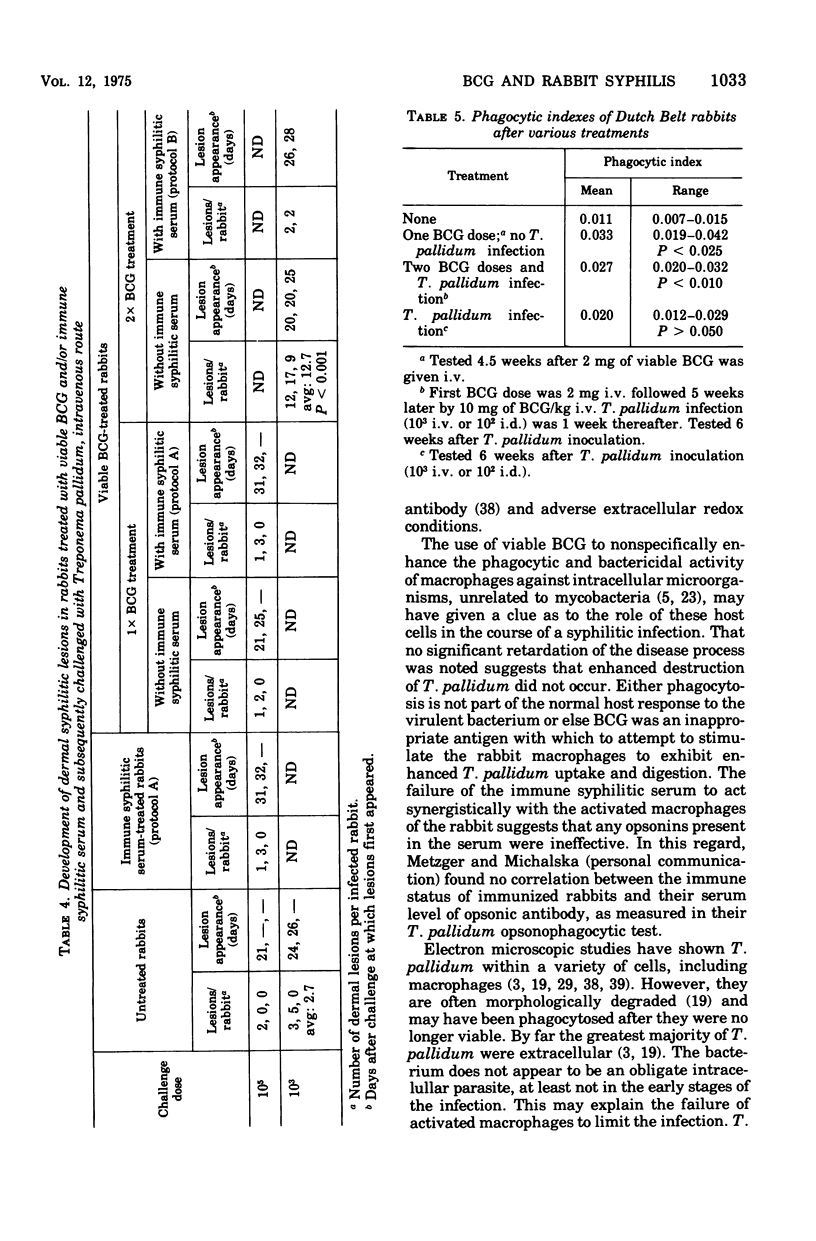
Abstract
Stimulation of the rabbit reticuloendothelial system with viable Mycobacterium bovis (strain BCG), and other agents, had no effect on the development of syphilitic lesions after intradermal or intravenous inoculation with graded doses of Treponema pallidum (virulent Nichol's strain; mean infective doses less than 10). The simultaneous administration of immune syphilitic rabbit serum retarded the development of lesions, but this appeared to be due solely to the immune serum, suggesting no synergism between the activated reticuloendothelial system and the anti-T. pallidum antibodies. The administration of two doses of BCG enhanced syphilitic lesion development in the rabbit.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlam C., Broughton E. S., Scott M. T. Enhanced resistance of mice to infection with bacteria following pre-treatment with Corynebacterium parvum. Nat New Biol. 1972 Feb 16;235(59):219–220. doi: 10.1038/newbio235219a0. [DOI] [PubMed] [Google Scholar]
- Adlam C., Scott M. T. Lympho-reticular stimulatory properties of Corynebacterium parvum and related bacteria. J Med Microbiol. 1973 Aug;6(3):261–274. doi: 10.1099/00222615-6-3-261. [DOI] [PubMed] [Google Scholar]
- Azar H. A., Pham T. D., Kurban A. K. An electron microscopic study of a syphilitic chancre. Engulfment of Treponema pallidum by plasma cells. Arch Pathol. 1970 Aug;90(2):143–150. [PubMed] [Google Scholar]
- Blanden R. V., Lefford M. J., Mackaness G. B. The host response to Calmette-Guérin bacillus infection in mice. J Exp Med. 1969 May 1;129(5):1079–1107. doi: 10.1084/jem.129.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges J. S., Johnson W. D., Jr Inhibition of multiplication of Toxoplasma gondii by human monocytes exposed to T-lymphocyte products. J Exp Med. 1975 Feb 1;141(2):483–496. doi: 10.1084/jem.141.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTIANSEN S. Protective layer covering pathogenic treponemata. Lancet. 1963 Feb 23;1(7278):423–425. doi: 10.1016/s0140-6736(63)92309-2. [DOI] [PubMed] [Google Scholar]
- Chieregato G., Faldarini G. La stimolazione pluriantigenica in vitro dei linfociti di soggetti luetici nei vari stadi della malattia. Minerva Dermatol. 1968 Jun;43(6):264–269. [PubMed] [Google Scholar]
- Claflin J. L., Larson C. L. Infection-immunity in tularemia: specificity of cellular immunity. Infect Immun. 1972 Mar;5(3):311–318. doi: 10.1128/iai.5.3.311-318.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festenstein H., Abrahams C., Bokkenheuser V. Runting syndrome in neonatal rabbits infected with Treponema pallidum. Clin Exp Immunol. 1967 May;2(3):311–320. [PMC free article] [PubMed] [Google Scholar]
- HANNA E. E., WATSON D. W. ALTERATION OF RES PHAGOCYTIC FUNCTION BY PYROGEN CONTAMINATED COLLOIDAL CARBON. Proc Soc Exp Biol Med. 1965 Apr;118:865–869. doi: 10.3181/00379727-118-29992. [DOI] [PubMed] [Google Scholar]
- Janot C., Grandidier M., Pupil P., Thomas J. L., Beurey J., de Lavergne E. Le test de transformation lymphoblastique au cours de la syphilis. Presse Med. 1971 Oct 16;79(43):1901–1904. [PubMed] [Google Scholar]
- Johnson R. C., Ritzi D. M., Livermore B. P. Outer envelope of virulent Treponema pallidum. Infect Immun. 1973 Aug;8(2):291–295. doi: 10.1128/iai.8.2.291-295.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T., Youmans G. P. Nonspecific inhibition of growth of intracellular Listeria monocytogenes by lymphocyte culture products. Infect Immun. 1974 Feb;9(2):472–474. doi: 10.1128/iai.9.2.472-474.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird S. M., Thorburn A. L. Assessment of the "luotest" in late syphillis. Br J Vener Dis. 1966 Jun;42(2):119–121. doi: 10.1136/sti.42.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderdale V., Goldman J. N. Serial ultrathin sectioning demonstrating the intracellularity of T. Pallidum. An electron microscopic study. Br J Vener Dis. 1972 Apr;48(2):87–96. doi: 10.1136/sti.48.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levene G. M., Turk J. L., Wright D. J., Grimble A. G. Reduced lymphocyte transformation due to a plasma factor in patients with active syphilis. Lancet. 1969 Aug 2;2(7614):246–247. doi: 10.1016/s0140-6736(69)90010-5. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. Resistance to intracellular infection. J Infect Dis. 1971 Apr;123(4):439–445. doi: 10.1093/infdis/123.4.439. [DOI] [PubMed] [Google Scholar]
- Medici M. A. The immunoprotective niche--a new pathogenic mechanism for syphilis, the systemic mycoses and other infectious diseases. J Theor Biol. 1972 Sep;36(3):617–625. doi: 10.1016/0022-5193(72)90012-4. [DOI] [PubMed] [Google Scholar]
- Metzger M., Michalska E., Podwińska J., Smogór W. Immunogenic properties of the protein component of Treponema pallidum. Br J Vener Dis. 1969 Dec;45(4):299–304. doi: 10.1136/sti.45.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzadra G., Sapuppo A., Lazzaro C., Buzzoni A. The "in vitro" lymphocyte blast transformation and syphilis. Arch Belg Dermatol Syphiligr. 1969 Oct-Dec;25(4):385–388. [PubMed] [Google Scholar]
- Miller J. N. Immunity in experimental syphilis. VI. Successful vaccination of rabbits with Treponema pallidum, Nichols strain, attenuated by -irradiation. J Immunol. 1973 May;110(5):1206–1215. [PubMed] [Google Scholar]
- Musher D. M., Schell R. F., Knox J. M. In vitro lymphocyte response to Treponema refringens im human syphilis. Infect Immun. 1974 Apr;9(4):654–657. doi: 10.1128/iai.9.4.654-657.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovcinnikov N. M., Delektorskij V. V. Electron microscopy of phagocytosis in syphilis and yaws. Br J Vener Dis. 1972 Aug;48(4):227–248. doi: 10.1136/sti.48.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perine P. L., Weiser R. S., Klebanoff S. J. Immunity to syphilis. I. Passive transfer in rabbits with hyperimmune serum. Infect Immun. 1973 Nov;8(5):787–790. doi: 10.1128/iai.8.5.787-790.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purtilo D. T., Walsh G. P., Storrs E. E., Banks I. S. Impact of cool temperatures on transformation of human and armadilio lymphocytes (Dasypus novemcinctus, Linn.) as related to leprosy. Nature. 1974 Mar 29;248(447):450–452. doi: 10.1038/248450a0. [DOI] [PubMed] [Google Scholar]
- Sapuppo A., Chiarenza A., Lazzaro C. T.P.I.-test e trasformazione blastica dei linfociti in vitro. Minerva Dermatol. 1967 Jan;42(1):12–13. [PubMed] [Google Scholar]
- Schell R. F., Musher D. M. Detection of nonspecific resistance to Listeria monocytogenes in rabbits infected with Treponema pallidum. Infect Immun. 1974 Apr;9(4):658–662. doi: 10.1128/iai.9.4.658-662.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepetjian M., Salussola D., Thivolet J. Attempt to protect rabbits against experimental syphilis by passive immunization. Br J Vener Dis. 1973 Aug;49(4):335–337. doi: 10.1136/sti.49.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes J. A., Miller J. N. Intracellular location of Treponema pallidum (Nichols strain) in the rabbit testis. Infect Immun. 1971 Sep;4(3):307–314. doi: 10.1128/iai.4.3.307-314.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes J. A., Miller J. N., Kalan A. J. Treponema pallidum within cells of a primary chancre from a human female. Br J Vener Dis. 1974 Feb;50(1):40–44. doi: 10.1136/sti.50.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff M. F., McBride W. H., Dunbar N. Tumour growth, phagocytic activity and antibody response in Corynebacterium parvum-treated mice. Clin Exp Immunol. 1974 Jul;17(3):509–518. [PMC free article] [PubMed] [Google Scholar]
- Wright D. J., Grimble A. S. Why is the infectious stage of syphilis prolonged? Br J Vener Dis. 1974 Feb;50(1):45–49. doi: 10.1136/sti.50.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]



