Abstract
A 64-year-old man was admitted to the Sun Yat-Sen University Cancer Center with chief complaints of recurrent abdominal pain and diarrhea for about 3 years and with a history of surgical repair for intestinal perforation owing to stress ulcer. Positron emission tomography (PET)/computed tomography (CT) demonstrated a primary tumor on the pancreatic tail with multifocal liver metastases. Pathological and immunohistochemistry staining revealed the lesion to be a pancreatic neuroendocrine tumor (pNET). According to the latest World Health Organization (WHO, 2013) classification, the tumor was classified as stage IV functional G1 pNET. After referral to the multidisciplinary treatment board (MDT), the patient was started on periodic dose of omeprazole, somatostatin analogues and Interferon α (IFNα) and had scanning follow-ups. Based upon the imaging results, CT-guided radioactive iodine-125 (125I) seeds implantation therapy, radiofrequency ablation therapy (RFA) or microwave ablation technique were chosen for the treatment of the primary tumor. Transarterial chemoembolization (TACE), RFA and microwave ablation techniques were decided upon for liver metastases. The patient showed beneficial response to the treatment with clinically manageable low-grade side effects and attained partial remission (RECIST criteria) with a good quality of life.
Keywords: Pancreatic neuroendocrine tumor (pNET), functional, liver metastases, multidisciplinary team, prognosis
Introduction
Pancreatic neuroendocrine tumors (pNETs) account for a small percentage of all pancreatic malignancies, and due to their insidious course, most present with metastatic disease (1). Anti-proliferative treatments that are available to reduce the tumor burden and to delay tumor progression encompass tumor debulking, molecular targeted therapy, chemoembolization, receptor-targeted radiotherapy, ablative methods and cytotoxic drugs (2-5).
Case report
A 64-year-old man was admitted with complaints of recurrent moderate abdominal pain around the umbilicus, watery diarrhea (approximately 10×/day), heartburn and acid regurgitation for 3 years that had been aggravated since the previous month. His past history included intestinal repair after a jejunal perforation 6 months before and a 10-year history of duodenal ulcers and diabetes. Physical examination revealed no major abnormalities after a full systematic review. Initial laboratory tests yielded significant results for elevated serum gastrin (1,250 pg/mL) and normal results for the tumor markers carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9) and alpha-fetoprotein (AFP). Gastroduodenoscopy followed by biopsy detected multiple sites of chronic gastric cardiac ulcerative inflammation, with multiple other sites detected in the duodenum and upper jejunum. Endoscopic ultrasonography (EUS) revealed a pancreatic tail mass with a size of 28.5 mm × 38.7 mm, without any abnormally enlarged lymph nodes. Further assessment by positron emission tomography (PET)/computed tomography (CT) with enhancement identified a 57 mm × 37 mm malignant mass in the pancreatic tail, with multiple metastatic nodules in liver segments 4, 5, 6 and 7 (sized from 12 mm × 12 mm to 54 mm × 37 mm) (Figure 1). Subsequent immunohistochemical staining after percutaneous transhepatic biopsy showed CgA (+), Syn (+), NSE (+), Gas (+), CD56 (+), CK20 (+), CK19 (+), CK7 (+), CEA (–), CDX2 (–) and Ki67 (1%) (Figure 2). Based on the above-mentioned results, the patient was diagnosed with a functional, well-differentiated stage IV G1 pNET by a consultant pathologist.
Figure 1.
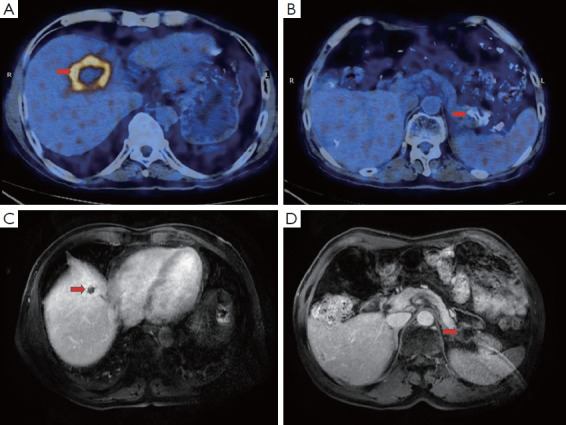
Imaging evaluation of the primary and metastatic lesions before and after treatment. (A,B) PET/CT scanning revealed a 57 mm × 37 mm soft mass in the pancreatic tail and multiple nodules in the liver (sized from 12 mm × 12 mm to 54 mm × 37 mm); (C,D) MRI performed in February 2013 demonstrated a favorable prognosis for the patient, indicating a decreased size for the primary tumor at the pancreatic tail (20 mm × 20 mm) for and the hepatic lesions (sized 12 mm × 12 mm to 30 mm × 20 mm).
Figure 2.
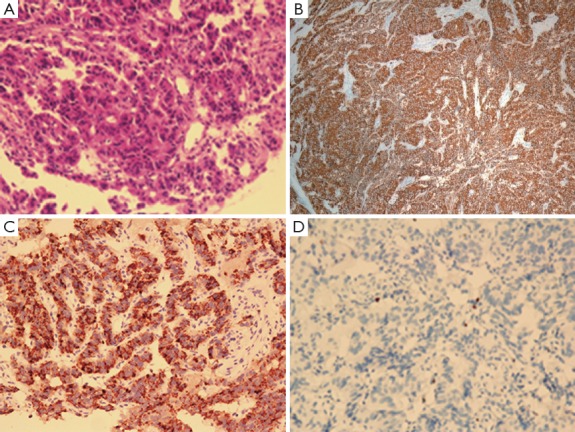
Immunohistochemical staining results (100×magnification). (A) H&E staining; (B) Syn (+); (C) CgA (+); (D) Ki67 (1%).
Due to the distant metastasis, the patient was referred to a multidisciplinary team (MDT) for better therapeutic evaluation. He was consequently prescribed the following medications: omeprazole 40 mg b.i.d., somatostatin analogs (SSAs) (octreotide LAR) 20 mg i.m. q.28d and interferon α (IFNα) 3 MIU i.h. q.o.d. (overall duration). For the metastatic lesions, he first underwent transarterial chemoembolization (TACE) (with doxorubicin 10 mg, mitomycin 10 mg, carboplatin 300 mg, 5-FU 500 mg and iodized oil 22 mL). The patient responded well to the treatment, except that he suffered from minor side effects, such as grade 1 nausea, vomiting and grade 2 leukocytosis.
The patient was closely followed from December 2008 to March 2013 (Figure 3), with re-examination and treatment assessment of the lesions’ progression. In December 2008, CT-guided perisplenic radioactive iodine-125 (125I) seed implantation therapy was performed on the pancreatic tail mass. The main side effects observed were grade 1 vomiting and leukocytosis. A CT scan in January 2009 found that the pancreatic and hepatic lesions had shrunken in size to 35 mm × 28 mm and to between 12 mm × 12 mm and 40 mm × 35 mm, respectively, compared with the sizes detected by PET/CT in October 2008. TACE was then performed in February 2009 for the liver metastases, and the manageable side effects that were observed were grade 1 nausea, vomiting and leukocytosis. CT review in March 2009 showed progressive shrinkage of the hepatic lesions (from 10 mm × 10 mm to 38 mm × 35 mm), whereas no visible changes were detected in the primary tumor’s size (35 mm × 28 mm). Radiofrequency ablation (RFA) therapy was initiated eight days later, and the only side effect noted was grade 1 leukocytosis. Subsequent CT reassessment in April 2009 showed stabilization of the pancreatic tail tumor, sized 35 mm × 28 mm, along with a gradual reduction in the diameters of the intrahepatic lesions to 10 mm × 10 mm and 38 mm × 31 mm. By June 2009, RFA therapy was additionally performed to treat the primary pancreatic tail tumor, and the minor side effect that was observed was grade 2 leukocytosis. Follow-up CT re-evaluation showed liquefactive necrosis, along with a degree of scarring of the primary lesion and stabilization of the intrahepatic lesions.
Figure 3.
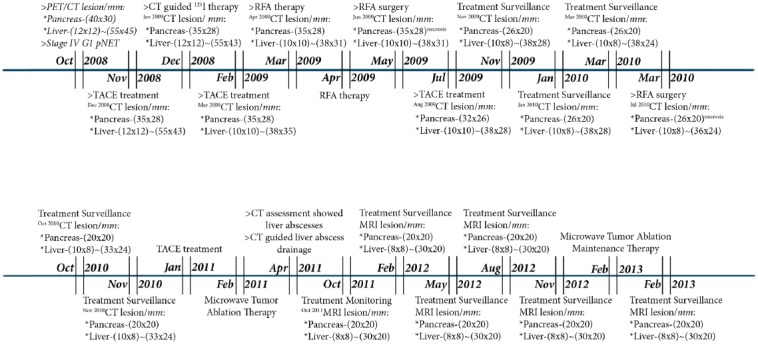
The treatment process and outcome of the patient from October 2008 to February 2013.
The following month, the patient was scheduled for TACE and experienced the same minor side effects as described above. A CT scan in August 2009 showed a slight decrease in the size of the pancreatic tail lesion, to 32 mm × 26 mm, and a slight amelioration of the hepatic lesions, sized from 10 mm × 10 mm to 38 mm × 30 mm. Another scan in November revealed a further decrease in the size of the pancreatic tail lesion, to 26 mm × 20 mm. By January 2010, CT results demonstrated stabilization of the lesions, showing a good response to treatment. A follow-up CT scan in March showed slight amplification of the density of hepatic segments 6 and 7, after which the patient underwent RFA therapy. The minor side effect noted was grade 1 leukocytosis.
A subsequent CT scan in July 2010 showed liquefactive necrosis in part of the pancreatic tail lesion, sized 26 mm × 20 mm, and a decreased density of the hepatic tumor masses, to 10 mm × 10 mm and 35 mm × 30 mm. CT review in October and November 2010 showed gradual amelioration of the primary pancreatic tumor’s density and size (26 mm × 20 mm), and the metastatic hepatic lesions ranged in size from 10 mm × 10 mm to 33 mm × 28 mm. The patient underwent TACE (mitomycin 10 mg, carboplatin 300 mg and iodized oil 6 mL) and tumor microwave ablation (MWA) therapy for the liver metastases in January and February 2011, respectively. The side effects observed were grade 1 nausea, vomiting and grade 1 leukocytosis.
In April 2011, the patient was hospitalized for hyperthermia (38.6 °C) and acute right upper abdominal pain. Blood chemistry showed white blood count (WBC) 13.2 k/uL and neutrophilicgranulocyte (NE) 86%, CT results revealed multiple liver abscesses in segments 4, 6 and 7. The patient was thus referred for CT-guided abscess drainage. Full abdominal magnetic resonance imaging (MRI) evaluation in October 2011 showed a degree of increased activity in the hepatic lesions, which were approximately 12 mm × 12 mm to 30 × 20 mm in size. In an attempt to control the active lesions, the patient was administered MWA therapy, and MRI imaging follow-up was performed every 3 months from February to November 2012. The imaging reports satisfactorily described stabilization, both in size and in density, of the pancreatic tail tumor and the hepatic metastases. The patient was administered additional MWA therapy as maintenance therapy in February 2013, and no side effects were noted. The last MRI, performed in February 2013, demonstrated further stabilization of the lesions, indicating a good response to therapy and a favorable prognosis (Figure 1).
Discussion
pNETs account for a small percentage of all pancreatic malignancies, with an incidence of <1 per 100,000 persons per year in population-based studies from Europe and Asia, but due to their insidious course, most pNETs present with metastatic disease (6,7). These tumors are functional (in 40-55% cases) or nonfunctional (in 45-60% cases), based on the presence or absence, respectively, of a particular clinical syndrome associated with hormone hypersecretion (6). Gastrinomas, being the most common functional, malignant pNET, account for up to 30% of these tumors. Gastrinomas secrete gastrin and are responsible for Zollinger-Ellison syndrome (ZES), which includes ulceration of the gastrointestinal tract, resulting in abdominal pain (79-100%), diarrhea (30-75%) and esophageal symptoms (31-56%) (8). These phenomena can lead to serious complications, such as ulcerative perforation, as in the case of our patient, who had a jejunal perforation due to gastric hyperacidity and underwent reconstructive surgery 6 months prior to his subsequent hospitalization.
Of all of the cases available on NETs in the Surveillance, Epidemiology, and End Results (SEER) database, 27% included distant metastases, which predominantly occurred in the liver (30-85%) (1). The median survival time for distant metastatic disease was 33 months in patients with G1-G2 pNETs, compared with only 5 months in patients with poorly differentiated carcinomas/neuroendocrine carcinoma (NEC) G3, and survival at 5 years was 35% and less than 5%, respectively (1). Because our patient had a type III metastatic hepatic lesion (diffuse multifocal lesions are found in 60-70% of cases), he was referred to an MDT. Several options were then considered to diminish his metastatic burden and improve his quality of life. Periodic doses of omeprazole, SSAs and IFNα were prescribed for symptomatic relief, and based on follow-up scanning results, CT-guided radioactive 125I seed implantation therapy, RFA therapy, TACE or MWA was chosen for treatment of the tumor lesions.
To date, there have been no randomized clinical trials comparing the efficacy of locoregional therapies with palliative surgery or medical treatment. The choice of a locoregional procedure for liver-targeted therapy depends on local expertise and the extent (the number and size of the lesions) and location of liver involvement. TACE is a common palliative technique that provides embolic blockade of nutrients and oxygen from the tumor-feeding arteries and offers direct chemotherapy to lesions, increasing the concentration of agents more than 20 times, in contrast to systemic chemotherapy. Response rates after embolization vary between 50% and 96% from study to study, and the median duration of the response extends up to 18 months (9,10).
RFA, which uses a probe inserted into a tumor, leads to tumor cell destruction by causing intracellular ion vibration. A clinical study performed at the Cleveland Clinic on 89 patients found that 90% of the patients experienced immediate relief of their symptoms after the procedure, with a mean progression free survival (PFS) of 1.3 years (11). Here, RFA was used as an “adjuvant” due to its limitations in eradicating multiple lesions greater than 3 cm in size. Just as other therapeutic procedures are accompanied by certain complications, the liver abscess experienced by our patient is one of the common side effects of this technique. MWA is another procedure that is similar to RFA, except that a nonionizing form of radiation causes extremely rapid oscillation of the water within tissues to generate heat, which in turn leads to coagulative necrosis. The intratumoral temperatures induced by MWA are consistently higher than those that can be achieved with RFA, and multiple lesions can be ablated during the same procedure.
Previous studies have shown that SSAs, IFNs and their combinations have comparable anti-proliferative effects when used during disease progression (12). Moreover, IFNα can yield disease stabilization in 40-50% of patients, so a dose of 3 MIU i.h. q.o.d. was indicated for our patient. As demonstrated by multiple studies, both SSAs and IFNs have been shown to be very effective for the symptomatic control of functional pNETs. In the United States, it has been further proven that higher doses of octreotide LAR (30 mg every 4 weeks) significantly lengthens the time of tumor progression compared with placebo (P=0.000072). The PROMID study (13) showed that octreotide LAR results in 66.7% disease stabilization in patients with metastatic neuroendocrine tumors, with 2.4% of recipients showing partial remission. However, due to differences in sex, race and body surface area, the effect of higher doses of octreotide LAR in Asians remains undetermined.
Regarding the chemotherapeutic regimen, the most commonly used cytotoxic agents are doxorubicin and streptozotocin (14). To date, streptozotocin has not been marketed in China, so even our experienced oncologist is unfamiliar with its efficacy and side effects. Doxorubicin, mitomycin, carboplatin and 5-FU regimens have thus been very commonly used effective approaches.
In 2011, a prospective, randomized phase 3 clinical trial (RADIANT-3) (15) showed a promising antitumor benefit for everolimus in patients with advanced progressive pNETs, with relatively minor drug-related adverse events. At a dose of 10 mg once daily, the estimated risk of progression or death compared with placebo was reduced to 65%. Promisingly, everolimus was also found to achieve partial tumor responses in at least 5% of the patients enrolled. Meanwhile, another randomized controlled study in 2011, performed by Raymond et al. (16), confirmed that continuous daily administration of sunitinib at a dose of 37.5 mg once daily improved PFS plus overall survival compared with placebo among patients with advanced pNETs. The objective response rate was found to be 9.3%, with two patients showing a complete response and six showing partial responses. To date, it is unclear whether sunitinib and everolimus, if combined, would have synergistic effects and limited toxicities.
In this report, we described a case whose treatment strategy was well planned and supervised by an MDT. Compared with the initial sizes of both the primary tumor and the metastatic liver lesions, a decrease in size of >30% was demonstrated in imaging evaluations. Thus, the patient can be characterized as having experienced partial remission based on the RECIST criteria (17). Additionally, complete remission of the presenting symptoms and treatment-related side effects was observed. To date, the patient has a favorable quality of life and above-average overall survivability, despite living with liver metastases, indicating a potentially favorable prognosis.
Acknowledgements
Funding: The work was supported by the National Natural Science Foundation of China (Grant No. 81172080 and 81201773) and the Specialized Research Fund for Doctoral Program of Higher Education of China (Grant No. 20120171120114) and Science and Technology Projects of Guangdong Province (2011B031800181).
Disclosure: The authors declare no conflict of interest.
References
- 1.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72 [DOI] [PubMed] [Google Scholar]
- 2.Pavel M, Baudin E, Couvelard A, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2012;95:157-76 [DOI] [PubMed] [Google Scholar]
- 3.Jensen RT, Cadiot G, Brandi ML, et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: functional pancreatic endocrine tumor syndromes. Neuroendocrinology 2012;95:98-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falconi M, Bartsch DK, Eriksson B, et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms of the digestive system: well-differentiated pancreatic non-functioning tumors. Neuroendocrinology 2012;95:120-34 [DOI] [PubMed] [Google Scholar]
- 5.Kulke MH, Benson AB, 3rd, Bergsland E, et al. Neuroendocrine tumors. J Natl Compr Canc Netw 2012;10:724-64 [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Carbonero R, Capdevila J, Crespo-Herrero G, et al. Incidence, patterns of care and prognostic factors for outcome of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): results from the National Cancer Registry of Spain (RGETNE). Ann Oncol 2010;21:1794-803 [DOI] [PubMed] [Google Scholar]
- 7.Lombard-Bohas C, Mitry E, O'Toole D, Louvet C, et al. Thirteen-month registration of patients with gastroenteropancreatic endocrine tumours in France. Neuroendocrinology 2009;89:217-22 [DOI] [PubMed] [Google Scholar]
- 8.Jensen RT, Niederle B, Mitry E, et al. Gastrinoma (duodenal and pancreatic). Neuroendocrinology 2006;84:173-82 [DOI] [PubMed] [Google Scholar]
- 9.Roche A, Girish BV, de Baère T, et al. Trans-catheter arterial chemoembolization as first-line treatment for hepatic metastases from endocrine tumors. Eur Radiol 2003;13:136-40 [DOI] [PubMed] [Google Scholar]
- 10.Vogl TJ, Naguib NN, Zangos S, et al. Liver metastases of neuroendocrine carcinomas: interventional treatment via transarterial embolization, chemoembolization and thermal ablation. Eur J Radiol 2009;72:517-28 [DOI] [PubMed] [Google Scholar]
- 11.Mazzaglia PJ, Berber E, Milas M, et al. Laparoscopic radiofrequency ablation of neuroendocrine liver metastases: a 10-year experience evaluating predictors of survival. Surgery 2007;142:10-9 [DOI] [PubMed] [Google Scholar]
- 12.Faiss S, Pape UF, Böhmig M, et al. Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors--the International Lanreotide and Interferon Alfa Study Group. J Clin Oncol 2003;21:2689-96 [DOI] [PubMed] [Google Scholar]
- 13.Rinke A, Müller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 2009;27:4656-63 [DOI] [PubMed] [Google Scholar]
- 14.Kouvaraki MA, Ajani JA, Hoff P, et al. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol 2004;22:4762-71 [DOI] [PubMed] [Google Scholar]
- 15.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364:514-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364:501-13 [DOI] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16 [DOI] [PubMed] [Google Scholar]


