Abstract
Background
Video-assisted thoracic surgery (VATS) has been shown to be a safe alternative to conventional thoracotomy for patients with non-small cell lung cancer (NSCLC). However, popularization of this relatively novel technique has been slow, partly due to concerns about its long-term outcomes. The present study aimed to evaluate the long-term survival outcomes of patients with NSCLC after VATS, and to determine the significant prognostic factors on overall survival.
Methods
Consecutive patients diagnosed with NSCLC referred to one institution for VATS were identified from a central database. Patients were treated by either complete-VATS or assisted-VATS, as described in previous studies. A number of baseline patient characteristics, clinicopathologic data and treatment-related factors were analyzed as potential prognostic factors on overall survival.
Results
Between January 2000 and December 2007, 1,139 patients with NSCLC who underwent VATS and fulfilled a set of predetermined inclusion criteria were included for analysis. The median age of the entire group was 60 years, with 791 male patients (69%). The median 5-year overall survival for Stage I, II, III and IV disease according to the recently updated TNM classification system were 72.2%, 47.5%, 29.8% and 28.6%, respectively. Female gender, TNM stage, pT status, and type of resection were found to be significant prognostic factors on multivariate analysis.
Conclusions
VATS offers a viable alternative to conventional open thoracotomy for selected patients with clinically resectable NSCLC.
Keywords: Non-small cell lung cancer (NSCLC), video-assisted thoracoscopic surgery (VATS), overall survival
Introduction
The use of video-assisted thoracic surgery (VATS) lobectomy for the treatment of lung cancer was first reported in 1992 (1-4). Compared with conventional thoracotomy, VATS is associated with smaller wounds, less postoperative pain, less damage to the chest muscle and respiratory function, lower postoperative levels of inflammatory factors in circulation related to injury, and higher immunity (5-10). Current research shows that VATS lobectomy is relatively safe, with satisfying long-term effects (11-17). However, VATS lobectomy has not been widely used so far, accounting for only 5% of all lobectomy operations in the US, and only 2% to 3% in the United Kingdom (11). Surgeons who are conservative about this surgical approach base their opinion on its limited ability to perform systemic lymphadenectomy, incapability of achieving complete cure and their lack of interest in the new technology.
A recent meta-analysis study has shown that VATS lobectomy is more appropriate than conventional thoracotomy in the treatment of screened early non-small cell lung cancer (NSCLC) patients (18). However, there are only a small number of reports on the application of this technique for the treatment of stage II and III lung cancer patients (14,19-21). The long-term survival for these patients undergoing VATS lobectomy is still unknown (15). Hence, the primary purpose of our study was to assess the long-term survival of VATS lobectomy for clinically resectable NSCLC patients, as well as the prognostic factors associated with the overall survival.
Patients and methods
Enrollment
All patients with NSCLC confirmed by pathology following VATS lobectomy in our department were enrolled. The basic characteristics, treatment details, clinical and pathological data, and outcomes of the patients were used to establish an electronic database. The staging of lung cancer was made according to the seventh edition of the TNM staging criteria newly published by the International Association of Lung Cancer (22,23).
Preoperative preparation
Preoperative preparation included a detailed medical history and physical examination, chest X-ray and chest and abdominal CT scans. Upon ultrasound examination of the liver, patients with clinical symptoms received an additional whole body bone scan and MRI. All patients were subject to real-time staging with VATS before surgery. Only those with good physical condition could receive the procedure. The resection techniques included: lobectomy, pneumonectomy, wedge resection, segmentectomy and sleeve pneumonectomy. Under normal circumstances, radical lobectomy was used when pneumonectomy could not satisfy the need of cure, while segmentectomy was used for peripheral masses in patients who could not tolerate pulmonary lobectomy.
Operation
The procedure of VATS lobectomy has been reported before (24), which is similar to the method adopted by Shigemura and others (15). VATS is a purely endoscopic technique completed under 100% monitoring, without any retracted intercostal incision. VATS lobectomy requires a small incision in the chest with a hard or soft distraction. During the operation, the visual field is provided by a monitor or this chest incision. Bronchial sleeve resection and complex vascular separation are completed under direct vision, while systematic lymph node dissection, decomposition of adhesions and separation of pulmonary ligaments and fissure was completed via the monitor view.
The choice of complete VATS (c-VATS) or VATS assisted small incision surgical was dependent on preoperative real-time staging. The VATS assisted small incision surgery was indicated for patients whose: tumor diameter was greater than 8 cm; complications were present intraoperatively or preioperatively, such as bleeding, which prevented the use of complete VATS; tumor was too close to the bronchi, requiring the use of sleeve pneumonectomy and bronchial anastomosis; lymph node metastasis had invaded through the external membrane and adhered to the surrounding tissue or blood vessels, or calcified lymph node tuberculosis and other inflammatory lymph nodes had caused adhesions.
Postoperative care and follow up
After surgery, patients at stage IB were advised to receive 4 cycles of third-generation platinum-based adjuvant chemotherapy. If there were no contraindications to chemotherapy, patients at stage II to IV would receive 4 to 6 cycles of third-generation platinum-based adjuvant chemotherapy. For N2 patients who had completed a thorough mediastinal lymph node dissection, not conventional adjuvant radiotherapy was conducted. In the case of enlarged lymph nodes during the follow-up period, adjuvant radiotherapy would be used for these patients.
Postoperative follow-up data were obtained from postoperative visits, imaging data review and cancer patient registration. The hospitalized and perioperative morbidity and mortality were registered for each patient. Routine chest CT scans were carried out after surgery at an interval of 3 months for the first year, 6 months for the second, and once a year afterwards.
Statistical analysis
Survival rates were calculated using the Kaplan-Meier curves, and compared using the log-rank test. Independent prognostic factors were taken in multivariate analysis using the Cox proportional hazards regression model. The survival time was calculated from surgery as a starting point, until death caused by cancer as an end point. Statistical parameters included age, gender, histological type, lymphatic invasion, tumor size, lymph node metastasis, staging as per the 7th edition of TNM, smoking status, VATS lung resection type, and adjuvant chemotherapy. A P value of <0.05 was considered statistically significant. Statistical analysis was conducted in SPSS 11.5 (SPSS, Chicago, IL, USA).
Results
Patients’ characteristics
There were 1,139 patients with NSCLC who were treated with video-assisted thoracoscopic lung resection from January 2000 to December 2007 (Table 1), and in line with our screening criteria from the database. The average follow-up time was 40.5 months after the surgery. Of the 463 patients at stage I, 374 cases (80.8%) were treated with complete VATS lobectomy; 176 out of the 301 patients at stage II (58.5%), 193 out of the 348 patients at stage III (55.5%) and 15 out of the 27 patients at stage IV (55.6%) received video-assisted thoracoscopic small-incision surgery. A total of 57 patients (5.1%) converted to other approaches due to complications, including 52 cases from c-VATS to a-VATS (assisted VATS), one from c-VATS to open chest surgery, and four from a-VATS to open thoracotomy. The reasons for conversion included adhesions around the pulmonary artery in 23 cases, cutter malfunction in four cases, severe pleural adhesions in 21 cases, lung collapse failure in nine cases. These cases were included in the analysis.
Table 1. Summary of baseline patient characteristics, operative data and treatment-related factors of 1,139 patients who underwent video-assisted thoracic surgery for non-small cell lung cancer.
| Characteristic | No. of patients (%) |
|---|---|
| Gender | |
| Male | 791 (69.4) |
| Female | 348 (30.6) |
| Age, years | |
| Median | 60 |
| Range | 21-85 |
| ≤65 | 740 (65.0) |
| > 65 | 399 (35.0) |
| Smoking status | |
| Non-smoker | 284 (24.9) |
| Smoker | 855 (75.1) |
| FEV1.0 (L) | 2.3±0.6a |
| FEV1.0 (% predicted) | 73.5±8.7a |
| TNM stage | |
| I | 463 (40.6) |
| IA | 252 (22.1) |
| IB | 211 (18.5) |
| II | 301 (26.4) |
| IIA | 179 (15.7) |
| IIB | 122 (10.7) |
| III | 348 (30.6) |
| IIIA | 330 (30.0) |
| IIIB | 18 (1.6) |
| IV | 27 (2.4) |
| pT status | |
| ≤2 | 190 (16.7) |
| >2 to ≤3 | 264 (23.2) |
| >3 to ≤5 | 378 (33.2) |
| >5 to ≤7 | 193 (16.9) |
| >7 | 114 (10.0) |
| pN status | |
| N0 | 607 (53.3) |
| N1/N2 | 532 (46.7) |
| Lymphovascular invasion | |
| Lymphovascular invasion | 556 (48.8) |
| Without lymphovascular invasion | 583 (51.2) |
| Type of resection | |
| Right upper lobectomy | 265 (23.3) |
| Right middle lobectomy | 62 (5.4) |
| Right lower lobectomy | 145 (12.7) |
| Right middle and lower bilobectomy | 52 (4.6) |
| Right upper and middle bilobectomy | 22 (1.9) |
| Left upper lobectomy | 201 (17.6) |
| Left lower lobectomy | 143 (12.6) |
| Pneumonectomy | 61 (5.4) |
| Left | 36 (3.2) |
| Right | 25 (2.2) |
| Segmentectomy | 67 (5.9) |
| Sleeve lobectomy | 121 (10.6) |
| Final pathology | |
| Adenocarcinoma | 546 (47.9) |
| Squamous cell | 437 (38.4) |
| Bronchioalveolar carcinoma | 96 (8.4) |
| Adenocarcinoma—squamous cell carcinoma | 39 (3.4) |
| Large cell | 21 (1.8) |
| Type of VATS approach | |
| c-VATS | 663 (58.2) |
| a-VATS | 476 (41.8) |
| Operative time (minutes) | 168.0±35.0a |
| Blood loss (mL) | 165.6±48.4a |
| Chest tube duration (days) | 3.2±1.8a |
| Postoperative hospital stay (days) | 6.5±2.5a |
| Dissected lymph nodes | 16.5±5.5a |
| Dissected nodal stations | 7.5±1.5a |
| Dissected mediastinal node stations | 6.2±1.1a |
| Dissected intrapulmonary node stations | 2.1±0.5a |
| Adjuvant chemotherapy | |
| Vinorelbine + platinum | 352 (30.9) |
| Gemcitabine + platinum | 287 (25.2) |
| Docetaxel + platinum | 247 (21.7) |
| None | 253 (22.2) |
a, Values are expressed as mean ± standard deviation. FEV1.0, Forced expiratory volume in 1 second; VATS, video-assisted thoracic surgery; c-VATS, complete VATS; a-VATS, assisted VATS.
Morbidity and mortality
There were no intraoperative deaths. Five (0.4%) patients died during the perioperative period due to the following causes: respiratory failure in two cases, pulmonary embolism in two cases, and myocardial infarction in one case. Three patients received a second surgery 8 to 10 days after the video-assisted thoracoscopic lung surgery due to a daily drainage of more than 1,000 mL in one case and more than 500 mL in two. All of them recovered well after the second surgery, and were discharged one week later. In the perioperative period, 1,030 cases (90.4%) patients had no complications. The other 109 cases (9.6%) had one or more complications (Table 2).
Table 2. Summary of perioperative complications after video-assisted thoracic surgery for non-small cell lung cancer in 1,139 patients.
| Complicationsa | No. of patients (%) |
|---|---|
| None | 1,030 (90.4) |
| Air leak (lasting ≥7 days) | 45 (4.0) |
| Atrial fibrillation | 28 (2.5) |
| Serous drainage (requiring drainage ≥7 days) | 17 (1.5) |
| Pneumonia | 12 (1.1) |
| Subcutaneous air (requiring reinsertion of chest tube or subcutaneous catheter) | 10 (0.9) |
| Myocardial infarction | 7 (0.6) |
| Empyema | 2 (0.2) |
| Atelectasis | 3 (0.3) |
| Anastomotic fistula | 2 (0.2) |
a, Some patients had more than one complication.
Overall survival
The 5-year survival rates of patients at stages IA, IB, IIA, IIB, IIIA, IIIB and IV were 77.9% (95% CI, 71.4-84.4%), 65.8% (95% CI, 58.0-73.6%), 54.9% (95% CI, 45.5-64.3%), 37.0% (95% CI, 26.4-47.6%), 30.3% (95% CI, 23.6-37.0%), 22.2% (95% CI, 11.6-44.9%), and 28.6% (95% CI, 6.6-50.6%), respectively (Figure 1).
Figure 1.
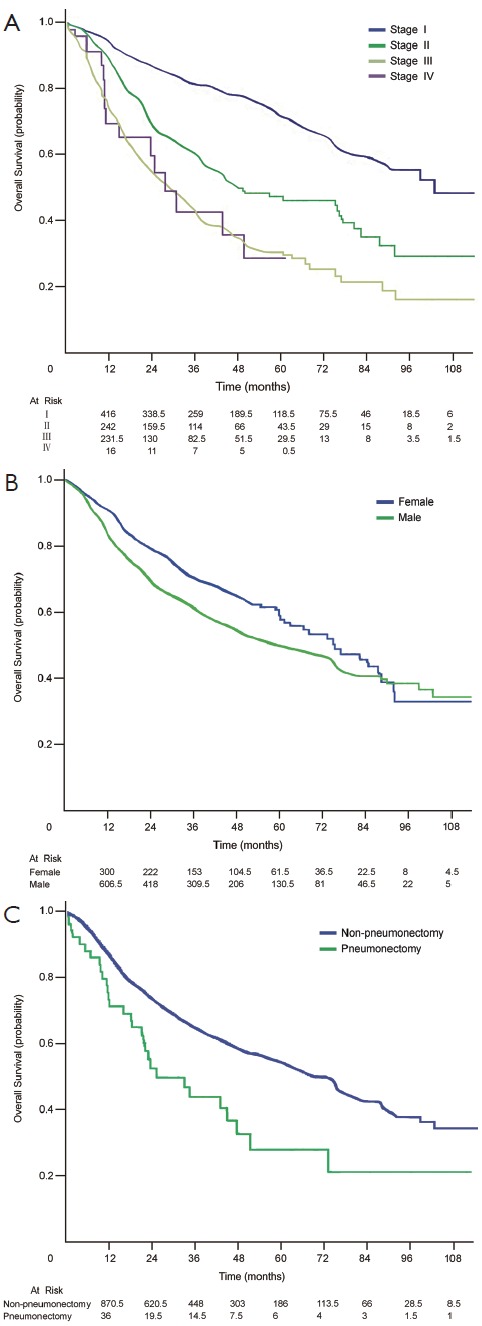
Kaplan-Meier survival curves for patients with non-small cell lung cancer after video-assisted thoracic surgery, stratified according to (A) TNM stage (P<0.001); (B) gender (P=0.009) and (C) type of resection (P=0.01).
Prognostic factors
Log-rank test analysis showed that sex (P=0.009), primary tumor status (P<0.001), lymph node status (P<0.001), TNM staging (P<0.001), lymphatic invasion (P<0.001), and the type of lung resection (P=0.01) were significant risk factors for the overall survival. However, age (P=0.37), smoking status (P=0.31), type of video-assisted thoracoscopic surgery (P=0.39), histological type (P=0.14) and adjuvant chemotherapy (P=0.91) were not related to long-term survival. Multivariate analysis showed that women, primary tumor status, TNM stage, and type of lung resection were significant independent risk factors (Table 3).
Table 3. Univariate and multivariate analysis of prognostic factors for overall survival in 1,139 patients with non-small cell lung cancer after video-assisted thoracic surgery.
| Characteristic | Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|---|
| 5-yr survival, % (95% CI) | P value | HR (95% CI) | P value | ||
| Gender | 0.009 | 0.01 | |||
| Male | 50.0 (45.7-54.3) | Reference group | |||
| Female | 58.6 (51.9-65.3) | 0.746 (0.596-0.934) | |||
| Age, years | 0.37 | – | – | ||
| ≤65 | 53.2 (48.7-57.7) | ||||
| >65 | 51.8 (45.7-57.9) | ||||
| Smoking status | 0.31 | – | – | ||
| Non-smoker | 55.9 (49.2-62.6) | ||||
| Smoker | 51.5 (47.2-55.8) | ||||
| Type of VATS approach | 0.39 | – | – | ||
| c-VATS | 55.7 (51.2-60.2) | ||||
| a-VATS | 48.4 (42.3-54.5) | ||||
| Final pathology | 0.14 | – | – | ||
| Non-squamous cell | 50.7 (46.4-55.0) | ||||
| Squamous cell | 58.1 (51.4-64.8) | ||||
| TNM stage | <0.001 | 0.01 | |||
| I | 72.2 (67.1-77.3) | Reference group | |||
| II | 47.5 (40.4-54.6) | 1.516 (1.020-2.252) | |||
| III | 29.8 (23.3-36.3) | 2.207 (1.342-3.629) | |||
| IV | 28.6 (6.6-50.6) | 1.722 (0.636-4.665) | |||
| pT status | <0.001 | <0.001 | |||
| ≤2 | 68.2 (59.6-76.8) | Reference group | |||
| >2 to ≤3 | 63.6 (56.0-71.2) | 0.863 (0.601-1.239) | |||
| >3 to ≤5 | 50.8 (44.3-57.3) | 1.314 (0.947-1.812) | |||
| >5 to ≤7 | 30.7 (21.5-39.9) | 1.830 (1.246-2.687) | |||
| >7 | 37.6 (25.8-49.4) | 1.817 (1.180-2.798) | |||
| pN status | <0.001 | 0.55 | |||
| N0 | 67.1 (62.4-71.8) | Reference group | |||
| N1/N2 | 35.5 (30.0-41.0) | 0.807 (0.400-1.628) | |||
| Lymphovascular invasion | <0.001 | 0.11 | |||
| No | 68.3 (63.6-73.0) | Reference group | |||
| Yes | 35.0 (29.7-40.3) | 1.862 (0.875-3.964) | |||
| Type of resection | 0.01 | 0.04 | |||
| Non-pneumonectomy | 53.9 (50.2-57.6) | Reference group | |||
| Pneumonectomy | 28.0 (12.1-43.9) | 1.509 (1.026-2.219) | |||
| Adjuvant chemotherapy | 0.91 | – | – | ||
| Vinorelbine + platinum | 52.3 (48.6-56.0) | ||||
| Gemcitabine + platinum | 58.1 (51.4-64.8) | ||||
| Docetaxel + platinum | 50.7 (46.4-55.0) | ||||
| None | 59.6 (39.4-79.8) | ||||
CI, confidence interval; HR, hazard ratio; VATS, video-assisted thoracic surgery; c-VATS, complete-VATS; a-VATS, assisted-VATS.
Discussion
Currently, there are very few reports on the long-term survival of patients with NSCLC following video-assisted thoracic surgery with a sample of more than 100 cases (12-14,16,25-28). This study is one of the studies with the largest number of subjects that include both long-term and short-term outcomes. Our overall 5-year survival rate is similar to those reported in other studies (14,27,28). At present, only one study reported the survival rate for stage III patients (28.6%), which is close to our result of 29.8% (11). However, due to differences in the inclusion criteria, removal techniques and choice of TNM staging systems, caution should be made when comparing the survival results from two surgical units. The long-term survival results of this study are similar to those of conventional thoracotomy research and the study of 1,532 patients recently by Japanese investigators (29).
In this study, we have found prognostic factors significantly associated with the overall survival, including sex, pathological T stage and type of resection, which are also independent risk factors in the multivariate analysis. We have further confirmed the finding that female patients tend to have better long-term survival outcomes than pair-matched male patients after surgery (30-34). There have been a number of explanations for this, but we believe that it may be caused by multiple factors. Current studies have attached importance to the relationship between exogenous or endogenous estrogen (35), genetic and emotional factors and NSCLC in female patients (36). In the newly released seventh edition of TNM staging criteria, T1 and T2 are further subdivided into T1a (≤2 cm), T1b (>2 cm, ≤3 cm), T2a (>3 cm, ≤5 cm) and T2b (>5 cm, ≤7 cm). Our research shows that pathological T status is a significant prognostic factors in multifactorial analysis, which further confirms that the subdivision of T staging in the seventh edition of the TNM staging is reasonable (37). In addition, the type of lung resection is also a significant prognostic factor, as patients undergoing total lung resection have poorer outcomes compared with those receiving localized resection (38,39). The study by Alexiou and colleagues shows that, in 485 stage I patients with NSCLC treated with surgery, the 111 patients who received total resection had significantly worse outcomes than the other 374 patients who received localized resection (40).
Fourteen studies reported that the conversion rate from VATS to other surgical operations ranged from 0% to 15.7% (18). It is difficult to compare the results from different centers because of the differences in patient selection and the presence of the learning curves of the operation. Recently, Jones and others conducted a retrospective case-control study of the outcomes in patients whose VATS was converted to thoracotomy compared with those undergoing conventional thoracotomy. The study indicated that there were no significant differences in the short-term and long-term survival rates between the two groups (31). At present, the largest reported VATS lobectomy study was done by McKenna, in which he described 1,072 operations for 1,100 cases, with a conversion rate of 2.5% (14). In the present cohort of 1,139 patients, 57 (5.0%) patients converted to open thoracotomy at the time of surgery. Our experience is that during surgery, the complete VATS could be converted to VATS assisted surgery, or even a small-incision procedure without damage to the chest muscles, or eventually open thoracotomy, rather than direct conversion to thoracotomy.
In conclusion, this study shows that in experienced facilities, complete VATS and VATS assisted surgery can serve as an alternative to conventional thoracotomy for clinically resectable NSCLC. In the near future, VATS surgery will be widely used due to its minimal perioperative invasiveness and satisfactory long-term survival outcomes. Multivariate analysis shows that the female sex, earlier TNM stage, smaller tumor diameters and smaller lung tumor resection area are prognostic factors in favor of the long-term survival. Of course, a prospective multicenter randomized controlled study will provide conclusive results for the comparison between VATS and other surgical techniques.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Roviaro G, Rebuffat C, Varoli F, et al. Videoendoscopic pulmonary lobectomy for cancer. Surg Laparosc Endosc 1992;2:244-7 [PubMed] [Google Scholar]
- 2.Landreneau RJ, Hazelrigg SR, Ferson PF, et al. Thoracoscopic resection of 85 pulmonary lesions. Ann Thorac Surg 1992;54:415-9; discussion 419-20 [DOI] [PubMed] [Google Scholar]
- 3.Stanley DG. Thoracoscopic lobectomy. J Tenn Med Assoc 1992;85:463-4 [PubMed] [Google Scholar]
- 4.Lewis RJ, Sisler GE, Caccavale RJ. Imaged thoracic lobectomy: should it be done? Ann Thorac Surg 1992;54:80-3 [DOI] [PubMed] [Google Scholar]
- 5.Dylewski MR, Lazzaro RS. Robotics-The answer to the Achilles’ heel of VATS pulmonary resection. Chin J Cancer Res 2012;24:259-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao C, Manganas C, Ang SC, et al. A meta-analysis of unmatched and matched patients comparing video-assisted thoracoscopic lobectomy and conventional open lobectomy. Ann Cardiothorac Surg 2012;1:16-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lacin T, Swanson S.Current costs of video-assisted thoracic surgery (VATS) lobectomy. J Thorac Dis 2013;5:S190-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimizu K, Okita R, Nakata M.Clinical significance of the tumor microenvironment in non-small cell lung cancer. Ann Transl Med 2013;1:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang Y, Wakelee HA. Adjuvant chemotherapy of completely resected early stage non-small cell lung cancer (NSCLC). Transl Lung Cancer Res 2013;2:403-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bar J, Urban D, Borshtein R, et al. EGFR mutation in lung cancer: tumor heterogeneity and the impact of chemotherapy. Chin Clin Oncol 2013;2:2. [DOI] [PubMed] [Google Scholar]
- 11.Walker WS, Codispoti M, Soon SY, et al. Long-term outcomes following VATS lobectomy for non-small cell bronchogenic carcinoma. Eur J Cardiothorac Surg 2003;23:397-402 [DOI] [PubMed] [Google Scholar]
- 12.Simone CB, II, Jones JA. Palliative care for patients with locally advanced and metastatic non-small cell lung cancer. Ann Palliat Med 2013;2:178-88 [DOI] [PubMed] [Google Scholar]
- 13.Roviaro G, Varoli F, Vergani C, et al. Long-term survival after videothoracoscopic lobectomy for stage I lung cancer. Chest 2004;126:725-32 [DOI] [PubMed] [Google Scholar]
- 14.McKenna RJ, Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6 [DOI] [PubMed] [Google Scholar]
- 15.Shigemura N, Hsin MK, Yim AP. Segmental rib resection for difficult cases of video-assisted thoracic surgery. J Thorac Cardiovasc Surg 2006;132:701-2 [DOI] [PubMed] [Google Scholar]
- 16.Congregado M, Merchan RJ, Gallardo G, et al. Video-assisted thoracic surgery (VATS) lobectomy: 13 years’ experience. Surg Endosc 2008;22:1852-7 [DOI] [PubMed] [Google Scholar]
- 17.Watanabe A, Mishina T, Ohori S, et al. Is video-assisted thoracoscopic surgery a feasible approach for clinical N0 and postoperatively pathological N2 non-small cell lung cancer? Eur J Cardiothorac Surg 2008;33:812-8 [DOI] [PubMed] [Google Scholar]
- 18.Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62 [DOI] [PubMed] [Google Scholar]
- 19.Lewis RJ, Caccavale RJ, Sisler GE, et al. One hundred video-assisted thoracic surgical simultaneously stapled lobectomies without rib spreading. Ann Thorac Surg 1997;63:1415-21; discussion 1421-2 [DOI] [PubMed] [Google Scholar]
- 20.Mahtabifard A, Fuller CB, McKenna RJ., Jr Video-assisted thoracic surgery sleeve lobectomy: a case series. Ann Thorac Surg 2008;85:S729-32 [DOI] [PubMed] [Google Scholar]
- 21.Watanabe A, Ohori S, Nakashima S, et al. Feasibility of video-assisted thoracoscopic surgery segmentectomy for selected peripheral lung carcinomas. Eur J Cardiothorac Surg 2009;35:775-80; discussion 780 [DOI] [PubMed] [Google Scholar]
- 22.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14 [DOI] [PubMed] [Google Scholar]
- 23.Groome PA, Bolejack V, Crowley JJ, et al. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007;2:694-705 [DOI] [PubMed] [Google Scholar]
- 24.He J, Yang Y, Chen M.Lobectomy by video-assisted thoracoscopic surgery. Zhonghua Wai Ke Za Zhi 1996;34:76-8 [PubMed] [Google Scholar]
- 25.Athanassiadi K, Kakaris S, Theakos N, et al. Muscle-sparing versus posterolateral thoracotomy: a prospective study. Eur J Cardiothorac Surg 2007;31:496-9; discussion 499-500 [DOI] [PubMed] [Google Scholar]
- 26.Thomas P, Doddoli C, Yena S, et al. VATS is an adequate oncological operation for stage I non-small cell lung cancer. Eur J Cardiothorac Surg 2002;21:1094-9 [DOI] [PubMed] [Google Scholar]
- 27.Gharagozloo F, Tempesta B, Margolis M, et al. Video-assisted thoracic surgery lobectomy for stage I lung cancer. Ann Thorac Surg 2003;76:1009-14; discussion 1014-5 [DOI] [PubMed] [Google Scholar]
- 28.Iwasaki A, Shirakusa T, Shiraishi T, et al. Results of video-assisted thoracic surgery for stage I/II non-small cell lung cancer. Eur J Cardiothorac Surg 2004;26:158-64 [DOI] [PubMed] [Google Scholar]
- 29.Kameyama K, Takahashi M, Ohata K, et al. Evaluation of the new TNM staging system proposed by the International Association for the Study of Lung Cancer at a single institution. J Thorac Cardiovasc Surg 2009;137:1180-4 [DOI] [PubMed] [Google Scholar]
- 30.He JX. First National Forum on minimally invasive treatment of lung cancer and consensus of 20 controversial issues. Chin J Oncol 2008;30:157-8. [Google Scholar]
- 31.Jones RO, Casali G, Walker WS. Does failed video-assisted lobectomy for lung cancer prejudice immediate and long-term outcomes? Ann Thorac Surg 2008;86:235-9 [DOI] [PubMed] [Google Scholar]
- 32.Minami H, Yoshimura M, Miyamoto Y, et al. Lung cancer in women: sex-associated differences in survival of patients undergoing resection for lung cancer. Chest 2000;118:1603-9 [DOI] [PubMed] [Google Scholar]
- 33.Ferguson MK, Wang J, Hoffman PC, et al. Sex-associated differences in survival of patients undergoing resection for lung cancer. Ann Thorac Surg 2000;69:245-9; discussion 249-50 [DOI] [PubMed] [Google Scholar]
- 34.Alexiou C, Onyeaka CV, Beggs D, et al. Do women live longer following lung resection for carcinoma? Eur J Cardiothorac Surg 2002;21:319-25 [DOI] [PubMed] [Google Scholar]
- 35.Templeton AK, Miyamoto S, Babu A, et al. Cancer stem cells: progress and challenges in lung cancer. Stem Cell Investigation 2014;1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhan P, Qian Q, Wan B, et al. Prognostic value of TTF-1 expression in patients with non-small cell lung cancer: a meta-analysis. Transl Cancer Res 2013;2:25-32 [Google Scholar]
- 37.Heyneman LE, Herndon JE, Goodman PC, et al. Stage distribution in patients with a small (< or = 3 cm) primary nonsmall cell lung carcinoma. Implication for lung carcinoma screening. Cancer 2001;92:3051-5 [DOI] [PubMed] [Google Scholar]
- 38.Gajra A, Newman N, Gamble GP, et al. Impact of tumor size on survival in stage IA non-small cell lung cancer: a case for subdividing stage IA disease. Lung Cancer 2003;42:51-7 [DOI] [PubMed] [Google Scholar]
- 39.Wisnivesky JP, Yankelevitz D, Henschke CI. The effect of tumor size on curability of stage I non-small cell lung cancers. Chest 2004;126:761-5 [DOI] [PubMed] [Google Scholar]
- 40.Alexiou C, Beggs D, Onyeaka P, et al. Pneumonectomy for stage I (T1N0 and T2N0) nonsmall cell lung cancer has potent, adverse impact on survival. Ann Thorac Surg 2003;76:1023-8 [DOI] [PubMed] [Google Scholar]


