Abstract
Background
Psoriasis symptoms have a significant negative impact on health-related quality of life, impairing physical functioning and well-being.
Objective
To evaluate the impact of brodalumab, a human anti-interleukin-17R monoclonal antibody, on psoriasis symptom severity as measured by a novel patient-reported outcome measure, the Psoriasis Symptom Inventory, and dermatology-specific health-related quality of life as measured by the Dermatology Life Quality Index (DLQI).
Methods
This was a secondary analysis of a phase II, randomized, double-blind, placebo-controlled clinical study of patients with moderate-to-severe psoriasis (n = 198) treated with brodalumab or placebo. This analysis assessed Psoriasis Symptom Inventory scores and DLQI scores over time. Analyses were conducted on all patients who were randomized and received one or more injections of the study drug according to intention to treat using last observation carried forward to impute missing data.
Results
At week 12, subjects in the brodalumab groups had significant improvements in mean Psoriasis Symptom Inventory total scores [8·5 (70 mg), 15·8 (140 mg), 16·2 (210 mg) and 12·7 (280 mg)] compared with placebo (4·8). Mean improvements in DLQI were clinically meaningful (≥ 5·7) in the brodalumab groups (6·2, 9·1, 9·6 and 7·1, respectively) and significantly greater than placebo (3·1). Improvements in Psoriasis Symptom Inventory were observed as early as week 2 and in DLQI by week 4. All eight Psoriasis Symptom Inventory item scores improved significantly among the brodalumab groups by week 12.
Conclusions
Results were from a single randomized clinical trial and may not generalize to broader patient populations. However, treatment with brodalumab provided significant improvement in psoriasis symptoms in patients with moderate-to-severe psoriasis.
What's already known about this topic?
Psoriasis has a significant negative impact on health-related quality of life.
What does this study add?
Brodalumab treatment provided statistically significant improvement in psoriasis symptoms and functional outcomes in patients with moderate-to-severe psoriasis.
These results support the further clinical development of brodalumab to treat patients with moderate-to-severe psoriasis.
Plaque psoriasis is a life-long chronic condition that even when treated can remain bothersome for most patients. The severity of physical symptoms of psoriasis, including itch, pain and discomfort, is correlated with disease-related quality of life and self-reported disease severity.1,2 The primary goal of psoriasis treatment is to control disease symptoms, minimizing their negative effects on the patient's health-related quality of life (HRQoL) associated with impaired physical functioning and well-being.3–5 The psychosocial and physical impairments related to psoriasis affect HRQoL to an extent similar to the effects of other chronic diseases such as depression and hypertension,3,4 with 71% of respondents in a 2008 U.S. National Psoriasis Foundation survey reporting that psoriasis was a significant problem in everyday life; 63% reported significant feelings of being self-conscious, 58% reported feelings of embarrassment and more than a third reported avoiding social activities and limiting dating or intimate interactions.6 Importantly, successful treatment of psoriasis has been shown to improve HRQoL.7,8
Patient-reported outcomes (PROs) are important to understand the patient's perspective of disease and determine how treatments alter the psychosocial and physical impairments related to the disease by providing information not captured by clinician-assessed measures.9–11 However, generic PRO instruments commonly used in dermatology studies, such as the 36-Item Short Form Health Survey, do not assess symptoms specific to psoriasis. While the Dermatology Life Quality Index (DLQI) is a dermatology-specific HRQoL measure frequently used in clinical trials of psoriasis, it includes only a single item on psoriasis-related symptoms and does not adequately provide content coverage for psoriasis-specific symptom measurement. Other PROs used to assess symptoms in psoriasis clinical trials include single items on specific symptoms (e.g. itching, pain) with little documentation of measurement properties.11 The use of a PRO that adequately captures symptoms associated with psoriasis is important in assessing treatment efficacy, as the severity of psoriasis symptoms are the most relevant and important attribute when patients assessed symptoms associated with their psoriasis.12–14 The Psoriasis Symptom Inventory was developed as a psoriasis-specific patient-reported measure of symptom severity12,13 and has demonstrated good reliability and validity in patients with psoriasis.15,16
In this study, we used the Psoriasis Symptom Inventory to assess psoriasis symptoms following treatment with brodalumab (AMG 827), a human monoclonal antibody against interleukin (IL)-17 receptor A (IL-17RA) that blocks the biological activity of the cytokines IL-17A, IL-17F, IL-17A/F, IL-17C and IL-25. In a phase II trial, brodalumab was shown to improve clinical outcomes in patients with moderate-to-severe plaque psoriasis.17 At week 12, there was a > 85% mean improvement in Psoriasis Area and Severity Index (PASI) scores in the 140- and 210-mg brodalumab groups compared with a 16% improvement in the placebo group, and 75% improvement in PASI (PASI 75) was achieved by > 75% of the 140 and 210 mg brodalumab-treated subjects.17 As a secondary analysis of this clinical trial data, we examined severity of psoriasis symptoms (Psoriasis Symptom Inventory) and dermatology-specific quality of life (DLQI) before and after brodalumab treatment.
Materials and methods
Study design
This was an analysis of a phase II, randomized, double-blind, placebo-controlled clinical study of patients with moderate-to-severe psoriasis. Subjects with PASI score ≥ 12 and percentage of psoriasis-affected body surface area (BSA) ≥ 10% were eligible for the phase II study. Patients were randomly assigned to receive placebo or brodalumab, at a dose of 70 mg, 140 mg or 210 mg, administered subcutaneously every 2 weeks, or at a dose of 280 mg every 4 weeks. The primary objective of the phase II clinical trial was to establish a dose–response efficacy profile of brodalumab compared with placebo as measured by the percentage improvement in PASI from baseline to week 12. Secondary and exploratory endpoints included PASI 50/75/90/100, affected BSA, score on the static physician's global assessment (sPGA), DLQI score, and Psoriasis Symptom Inventory score. The study protocol was approved by the institutional review board or ethics committee at each participating site. Details of the study and the primary and key secondary endpoints have been published previously.17
Patient-reported outcomes
The Psoriasis Symptom Inventory, an eight-item measure to assess the severity of psoriasis symptoms12,13 was completed by subjects at baseline and weeks 2, 4, 8 and 12. The eight symptoms include itch, redness, scaling, burning, cracking, stinging, flaking and pain. ‘Scaling’ refers to the skin forming plates or scales that represent compacted desquamated layers of stratum corneum, which may then peel off; ‘flaking’ refers simply to loss of dry skin cells. Subjects rated the severity of each symptom on a 5-point Likert-type rating scale ranging from 0 (not at all) to 4 (very severe). Individual item scores were summed for a total score, which ranged from 0 to 32. The Psoriasis Symptom Inventory was administered as two recall versions (24-h and 7-day recall) in this study. The 24-h version was administered to compare performance of the two versions and was administered only at study visits and not as a daily diary; thus it does not give full coverage over the time period of the study. The two Psoriasis Symptom Inventory versions have previously been shown to provide equivalent results.15 This analysis focused on the 7-day version of the Psoriasis Symptom Inventory. Analyses of the Psoriasis Symptom Inventory included mean total and item scores over time, mean improvement in Psoriasis Symptom Inventory total score over time, and proportion of subjects with individual item scores (0–4) for each symptom. Complete improvement of psoriasis-related symptoms was defined as achieving the best possible Psoriasis Symptom Inventory total score of ‘0’.
The DLQI is a 10-item measure assessing a patient's self-assessment of their quality of life and problems associated with dermatological disease encompassing aspects such as symptoms and feelings, daily activities, leisure, work and school, personal relationships and treatment.18 DLQI total scores range from 0 to 30, with lower scores indicating better dermatology-specific quality of life. The DLQI was completed by subjects at baseline and weeks 4, 8 and 12. DLQI total scores were categorized as total score of 0 and total score of 0 or 1 both representative of no effect at all on HRQoL.
Statistical analysis
Analyses were conducted on all patients who were randomized and received one or more injections of the study drug according to the intention-to-treat principle. All analyses were conducted using SAS® version 9·2 (SAS Institute, Cary, NC, U.S.A.). Baseline demographic and disease characteristics were summarized descriptively with means and SD for continuous variables and percentages for categorical variables. Psoriasis Symptom Inventory and DLQI efficacy endpoints were assessed by an analysis of variance (anova) with a generalized linear model adjusting for baseline body mass index group (≤ 35, > 35). Threshold analyses used proportion of subjects meeting threshold values of Psoriasis Symptom Inventory scores of 0 and DLQI scores of 0 or 1. A P-value < 0·05 was required for significance using two-sided hypothesis tests. All P-values are nominal and not adjusted for multiplicity. Last observation carried forward (LOCF) was used to impute missing data.
Results
A total of 198 subjects were enrolled: 160 to brodalumab and 38 to placebo (Table 1). The Psoriasis Symptom Inventory was completed at both baseline and week 12 by 186 (94%) subjects, with a slightly lower proportion of subjects in the placebo group completing the Psoriasis Symptom Inventory at both time points than in the brodalumab groups. Baseline demographics and clinical characteristics were similar across treatment groups; 64% of subjects were male, mean age was 42 years and mean PASI score was 19·1 (Table 1). Details on baseline demographics and disease characteristics have previously been reported.17 The mean Psoriasis Symptom Inventory total score for the full study population was 18·0 and was similar across groups (Table 2). Mean baseline Psoriasis Symptom Inventory item scores, DLQI total scores, and DLQI domain scores were consistent across groups (Table 2).
Table 1.
Baseline demographics and characteristics
| Brodalumab | |||||
|---|---|---|---|---|---|
| Q2W | Q4W | ||||
| Placebo (n = 38) | 70 mg (n = 39) | 140 mg (n = 39) | 210 mg (n = 40) | 280 mg (n = 42) | |
| Sex (male), n (%) | 22 (58) | 22 (56) | 28 (72) | 25 (62) | 30 (71) |
| Race, n (%) | |||||
| White | 32 (84) | 36 (92) | 37 (95) | 34 (85) | 36 (86) |
| Black | 3 (8) | 0 (0) | 1 (3) | 1 (2) | 1 (2) |
| Asian | 1 (3) | 1 (3) | 0 (0) | 2 (5) | 3 (7) |
| Other | 2 (5) | 2 (5) | 1 (3) | 3 (8) | 2 (5) |
| Age (years) | 41·8 (14·4) | 42·1 (11·1) | 44·0 (11·7) | 42·1 (12·2) | 42·3 (12·2) |
| Weight (kg) | 86·9 (20·6) | 88·8 (22·0) | 92·4 (23·7) | 90·4 (20·4) | 91·5 (22·9) |
| PASI | 18·9 (5·9) | 18·8 (5·7) | 19·4 (8·0) | 20·6 (7·8) | 17·9 (5·5) |
| Affected BSA (%) | 23·5 (12·8) | 24·1 (12·8) | 24·9 (16·9) | 25·0 (15·5) | 21·3 (11·0) |
Data represent mean (SD) unless otherwise indicated. BSA, body surface area affected; PASI, Psoriasis Area and Severity Index; Q2W, every 2 weeks; Q4W, every 4 weeks.
Table 2.
Baseline Psoriasis Symptom Inventory and Dermatology Life Quality Index (DLQI) scores
| Brodalumab | |||||
|---|---|---|---|---|---|
| Q2W | Q4W | ||||
| Placebo (n = 38) | 70 mg (n = 39) | 140 mg (n = 39) | 210 mg (n = 40) | 280 mg (n = 42) | |
| Psoriasis Symptom Inventory score, 7-day | |||||
| Total | 19·8 (6·4) | 17·7 (7·4) | 18·2 (6·6) | 18·9 (7·1) | 17·6 (7·0) |
| Itch | 2·8 (0·9) | 2·5 (1·0) | 2·5 (0·9) | 2·7 (0·8) | 2·4 (0·9) |
| Redness | 2·7 (0·8) | 2·6 (0·9) | 2·6 (0·9) | 2·8 (0·9) | 2·5 (0·9) |
| Stinging | 2·2 (1·2) | 1·9 (1·4) | 1·9 (1·1) | 1·8 (1·2) | 1·8 (1·1) |
| Cracking | 2·2 (1·0) | 2·1 (1·1) | 2·2 (1·1) | 2·2 (1·1) | 2·1 (1·2) |
| Scaling | 2·7 (0·9) | 2·6 (0·9) | 2·4 (1·0) | 2·8 (0·8) | 2·5 (1·0) |
| Burning | 2·1 (1·1) | 1·8 (1·3) | 2·1 (1·1) | 1·9 (1·2) | 1·8 (1·1) |
| Flaking | 2·8 (0·9) | 2·6 (1·0) | 2·5 (0·8) | 2·8 (0·8) | 2·6 (0·9) |
| Pain | 2·1 (1·1) | 1·8 (1·3) | 2·1 (1·2) | 2·1 (1·2) | 1·8 (1·2) |
| DLQI score | |||||
| Total | 13·3 (7·0) | 12·4 (7·2) | 11·1 (6·7) | 11·4 (6·4) | 10·8 (6·6) |
| Symptoms and feelings | 4·3 (1·5) | 4·2 (1·4) | 4·0 (1·5) | 3·9 (1·5) | 3·6 (1·6) |
| Daily activities | 3·1 (1·7) | 2·8 (1·8) | 2·4 (1·6) | 2·1 (1·6) | 2·2 (1·8) |
| Leisure | 2·3 (2·3) | 2·2 (2·0) | 1·8 (1·8) | 1·6 (1·8) | 1·9 (1·9) |
| Work and school | 0·9 (1·0) | 0·8 (1·1) | 0·6 (0·8) | 0·6 (0·8) | 0·7 (0·9) |
| Personal relationships | 1·7 (1·7) | 1·4 (1·8) | 1·7 (1·9) | 2·0 (1·8) | 1·4 (1·6) |
| Treatment | 1·0 (1·2) | 0·9 (1·1) | 0·7 (1·0) | 1·2 (1·1) | 1·0 (1·0) |
Data represent mean (SD). Q2W, every 2 weeks; Q4W, every 4 weeks.
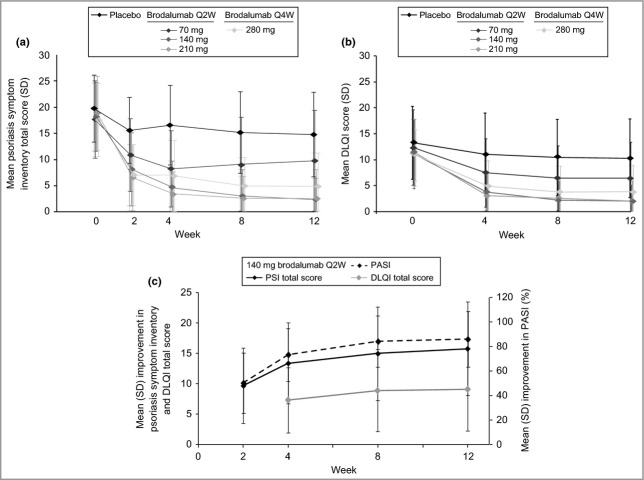
At week 12, subjects in the brodalumab groups had significant improvements in mean Psoriasis Symptom Inventory total score compared with the placebo group (Table 3). Significant differences in mean Psoriasis Symptom Inventory total score and item scores were observed as early as week 2 and were maintained through week 12 for the brodalumab groups (Figs 1a and 2). There were no observable improvements in the 210-mg group over the 140-mg group, but the improvements in the 280-mg group were nominally higher than those of the other brodalumab treatment groups (Table 3; Figs 1 and 2). Similar results were obtained with the 24-h version of the Psoriasis Symptom Inventory (data not shown).
Table 3.
Improvement from Baseline in Psoriasis Symptom Inventory and Dermatology Life Quality Index (DLQI) total scores
| Brodalumab | |||||
|---|---|---|---|---|---|
| Q2W | Q4W | ||||
| Placebo (n = 38) | 70 mg (n = 39) | 140 mg (n = 39) | 210 mg (n = 40) | 280 mg (n = 42) | |
| Psoriasis Symptom Inventory total score | |||||
| Week 2 | |||||
| n | 30 | 37 | 34 | 37 | 41 |
| Mean (SD) | 4·2 (5·2) | 7·3 (7·1) | 9·7 (6·2) | 12·3 (7·4) | 10·5 (7·1) |
| P-value | 0·06 | 0·001 | < 0·0001 | 0·0001 | |
| Week 4 | |||||
| n | 33 | 37 | 37 | 39 | 41 |
| Mean (SD) | 3·2 (6·8) | 9·9 (8·3) | 13·4 (6·7) | 15·5 (6·5) | 10·7 (7·0) |
| P-value | < 0·0001 | < 0·0001 | < 0·0001 | < 0·0001 | |
| Week 8 | |||||
| n | 33 | 37 | 37 | 39 | 41 |
| Mean (SD) | 4·5 (6·2) | 9·3 (9·4) | 15·0 (7·7) | 16·3 (6·4) | 12·6 (7·0) |
| P-value | 0·0072 | < 0·0001 | < 0·0001 | < 0·0001 | |
| Week 12 | |||||
| n | 33 | 37 | 37 | 39 | 41 |
| Mean (SD) | 4·8 (6·5) | 8·5 (9·2) | 15·8 (7·7) | 16·2 (7·2) | 12·7 (8·1) |
| P-value | 0·042 | < 0·0001 | < 0·0001 | < 0·0001 | |
| DLQI total score | |||||
| Week 4 | |||||
| n | 36 | 37 | 39 | 39 | 40 |
| Mean (SD) | 2·4 (5·1) | 5·2 (6·2) | 7·3 (5·4) | 8·2 (5·9) | 6·3 (5·2) |
| P-value | 0·033 | 0·0002 | < 0·0001 | 0·003 | |
| Week 8 | |||||
| n | 37 | 37 | 39 | 40 | 41 |
| Mean (SD) | 3·0 (5·3) | 6·1 (6·9) | 8·9 (6·8) | 9·4 (5·8) | 7·1 (5·8) |
| P-value | 0·030 | < 0·0001 | < 0·0001 | 0·003 | |
| Week 12 | |||||
| n | 37 | 37 | 39 | 40 | 41 |
| Mean (SD) | 3·1 (6·6) | 6·2 (7·0) | 9·1 (6·8) | 9·6 (6·1) | 7·1 (6·8) |
| P-value | 0·047 | 0·0001 | < 0·0001 | 0·007 | |
P-value is based on a generalized linear model adjusting for body mass index group (≤ 35, > 35) and is for comparison between each brodalumab group and placebo without multiplicity adjustment; last observation carried forward was used to impute missing data. Q2W, every 2 weeks; Q4W, every 4 weeks.
Fig 1.
Mean Psoriasis Symptom Inventory (7-day) and Dermatology Life Quality Index (DLQI) total scores over time. (a) Mean (SD) Psoriasis Symptom Inventory total score by treatment group: brodalumab 70 mg every 2 weeks (Q2W) arm (P < 0·005), 140- and 210-mg Q2W arms (P < 0·0001) and 280 mg every 4 weeks (Q4W) arms (P < 0·0001) at week 2 to week 12. (b) Mean (SD) DLQI total score by treatment group. (c) Mean (SD) improvement in Psoriasis Symptom Inventory total score, DLQI total score and Psoriasis Area and Severity Index (PASI) score for the 140-mg brodalumab group.
Fig 2.
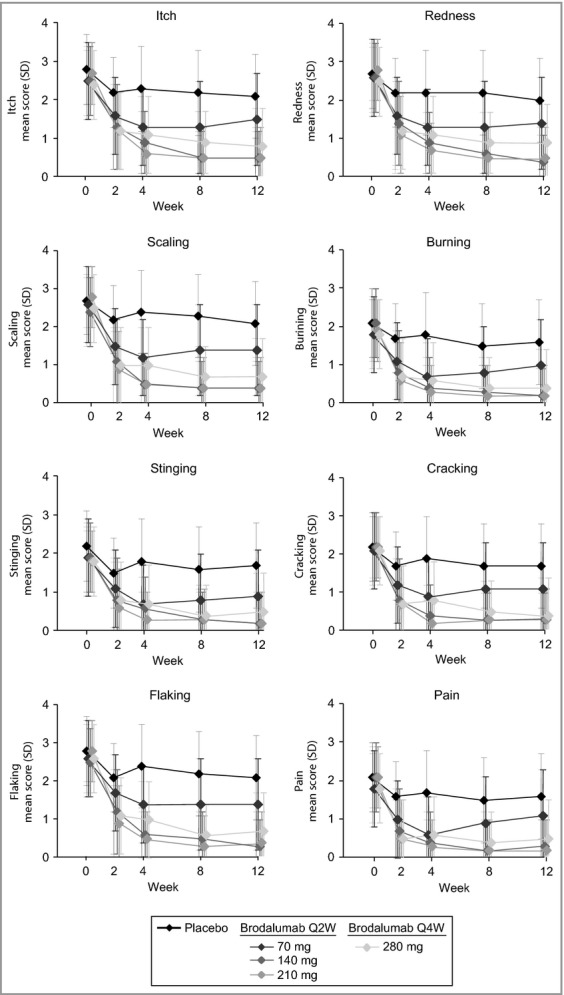
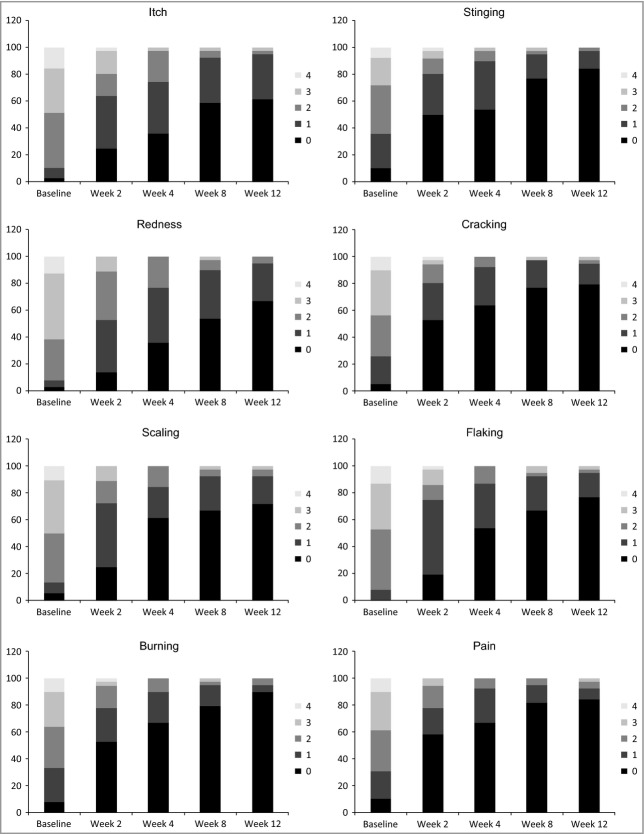
Mean (SD) Psoriasis Symptom Inventory (7-day) item scores over time. Itch (P ≤ 0·002), burning (P ≤ 0·002), redness (P ≤ 0·0009), scaling (P ≤ 0·0001), flaking (P < 0·0004), cracking (P < 0·0004), stinging (P < 0·02) and pain (P ≤ 0·0008) at week 2 to week 12. Q2W, every 2 weeks; Q4W, every 4 weeks.
The rapidity of the response varied by item with a majority of subjects achieving a score of 0 by week 2 for the burning, stinging, cracking and pain items (Fig. 3). Improvements for the other four symptoms occurred later, but among the brodalumab treatment groups all items were significantly different at week 12 compared with placebo (Fig. 3).
Fig 3.
Proportion of subjects achieving Psoriasis Symptom Inventory (7-day) score of 0 over time for the 140-mg brodalumab group. 5-point Likert-type scale: 0, no symptoms; 4, severe symptoms.
At week 12, DLQI total scores and domain scores were significantly lower in the brodalumab groups than in the placebo group17 (Figs 1b and 4). Lower DLQI total scores were observed as early as week 4, the earliest time point measured (Fig. 1b), and improvements in the 140-, 210- and 280-mg brodalumab groups were clinically meaningful (change of ≥ 5·7)19 as early as week 4 (Table 3). DLQI scores were significantly improved across all domains at week 12 in all brodalumab groups (Fig. 4).
Fig 4.
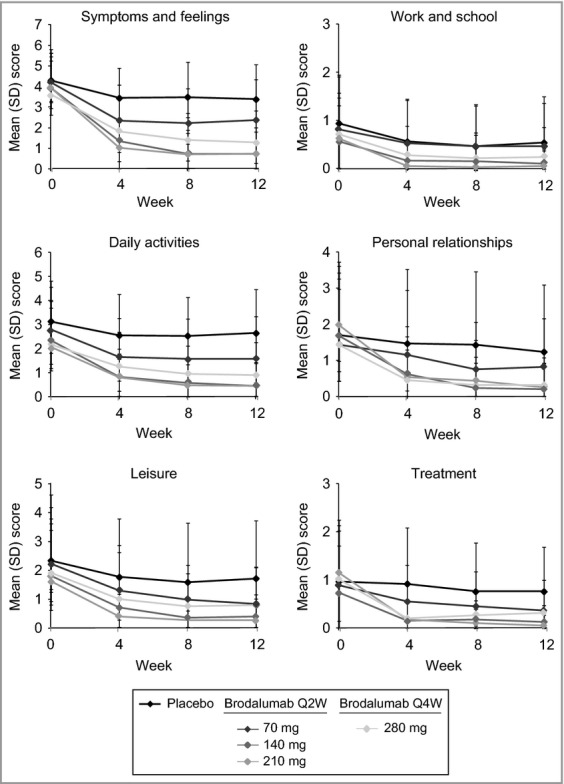
Dermatology Life Quality Index (DLQI) domain scores over time. Q2W, every 2 weeks; Q4W, every 4 weeks.
The proportion of subjects with complete improvement of psoriasis-related symptoms (Psoriasis Symptom Inventory total score of ‘0’) at week 12 was significantly greater in the brodalumab groups than in the placebo group (Fig. 5; Table 4). At week 12, 41% and 55% of subjects in the 140- and 210-mg groups, respectively, had a Psoriasis Symptom Inventory total score of ‘0’ compared with 0% of subjects in the placebo group (P < 0·0001). A significant proportion of subjects had a Psoriasis Symptom Inventory total score of ‘0’ as early as week 2 in the 210-mg group and by week 4 in the 140-mg group (Table 4). The proportions of subjects with complete or almost complete improvement of dermatological-related impairment of quality of life (DLQI total score of ‘0’ and ‘0 or 1’) at week 12 were significantly greater in the brodalumab groups than in the placebo group (Fig. 5; Table 4). At week 12, 46% and 58% of subjects in the 140- and 210-mg groups, respectively, had a DLQI total score of ‘0’ compared with 0% of subjects in the placebo group (P < 0·0001). The proportions of subjects with no impairment to health-related quality of life (DLQI total score 0 or DLQI total score 0 or 1) were significantly greater at all time points for the brodalumab groups compared with placebo, except for the 70-mg group at week 4 (Table 4).
Fig 5.
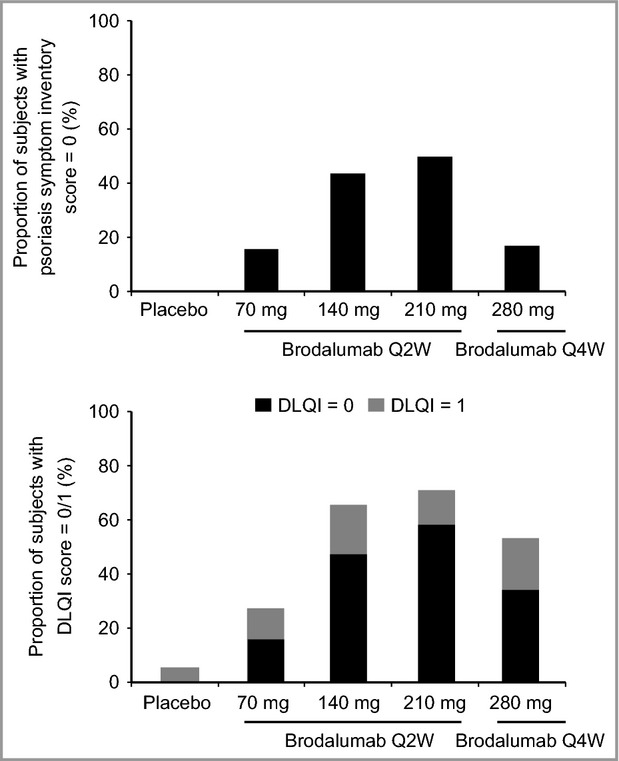
Proportion of subjects with a complete or almost complete response at week 12. (a) Proportion of subjects with a Psoriasis Symptom Inventory total score of 0 at week 12. (b) Proportion of subjects with a Dermatology Life Quality Index (DLQI) score of 0 or 1. Q2W, every 2 weeks; Q4W, every 4 weeks.
Table 4.
Psoriasis Symptom Inventory and Dermatology Life Quality Index (DLQI) threshold scores over time
| Brodalumab | |||||
|---|---|---|---|---|---|
| Q2W | Q4W | ||||
| Placebo (n = 38) | 70 mg (n = 39) | 140 mg (n = 39) | 210 mg (n = 40) | 280 mg (n = 42) | |
| Proportion of subjects with Psoriasis Symptom Inventory total score = 0 | |||||
| Baseline | |||||
| n/N (%) | 0/33 (0) | 0/37 (0) | 0/37 (0) | 0/39 (0) | 0/42 (0) |
| P-value | N/A | N/A | N/A | N/A | |
| Week 2 | |||||
| n/N (%) | 0/34 (0) | 4/38 (10) | 2/36 (6) | 4/38 (10) | 6/41 (15) |
| P-value | 0·047 | 0·154 | 0·043 | 0·020 | |
| Week 4 | |||||
| n/N (%) | 0/37 (0) | 2/38 (5) | 6/39 (15) | 14/40 (35) | 9/41 (22) |
| P-value | 0·161 | 0·012 | < 0·0001 | 0·002 | |
| Week 8 | |||||
| n/N (%) | 0/37 (0) | 6/38 (16) | 17/39 (44) | 20/40 (50) | 7/41 (17) |
| P-value | 0·012 | < 0·0001 | < 0·0001 | 0·007 | |
| Week 12 | |||||
| n/N (%) | 0/37 (0) | 7/38 (18) | 16/39 (41) | 22/40 (55) | 13/41 (32) |
| P-value | 0·006 | < 0·0001 | < 0·0001 | 0·0001 | |
| Proportion of subjects with DLQI total score = 0 | |||||
| Baseline | |||||
| n/N (%) | 0/37 (0) | 0/37 (0) | 0/39 (0) | 0/40 (0) | 1/42 (2) |
| P-value | N/A | N/A | N/A | 0·341 | |
| Week 4 | |||||
| n/N (%) | 0/36 (0) | 3/38 (8) | 8/39 (20) | 14/39 (36) | 8/40 (20) |
| P-value | 0·088 | 0·004 | < 0·0001 | 0·004 | |
| Week 8 | |||||
| n/N (%) | 0/37 (0) | 5/38 (13) | 17/39 (44) | 21/40 (52) | 10/41 (24) |
| P-value | 0·023 | < 0·0001 | < 0·0001 | 0·0009 | |
| Week 12 | |||||
| n/N (%) | 0/37 (0) | 6/38 (16) | 18/39 (46) | 23/40 (58) | 14/41 (34) |
| P-value | 0·012 | < 0·0001 | < 0·0001 | < 0·0001 | |
| Proportion of subjects with DLQI total score = 0/1 | |||||
| Baseline | |||||
| n/N (%) | 0/37 (0) | 0/37 (0) | 2/39 (5) | 0/40 (0·0) | 2/42 (5) |
| P-value | N/A | 0·161 | N/A | 0·175 | |
| Week 4 | |||||
| n/N (%) | 2/36 (6) | 8/38 (21) | 14/39 (36) | 22/39 (72) | 12/40 (30) |
| P-value | 0·054 | 0·002 | < 0·0001 | 0·006 | |
| Week 8 | |||||
| n/N (%) | 2/37 (5) | 9/38 (24) | 24/39 (62) | 29/40 (72) | 18/41 (44) |
| P-value | 0·026 | < 0·0001 | < 0·0001 | < 0·0001 | |
| Week 12 | |||||
| n/N (%) | 2/37 (5) | 10/38 (26) | 25/39 (64) | 29/40 (72) | 23/41 (56) |
| P-value | 0·015 | < 0·0001 | < 0·0001 | < 0·0001 | |
P-value is based on Cochran–Mantel–Haenszel test stratified by body mass index group (≤ 35, > 35) and is for comparison between each brodalumab group and placebo without multiplicity adjustment; last observation carried forward was used to impute missing data. N/A, not applicable; Q2W, every 2 weeks; Q4W, every 4 weeks.
Discussion
The Psoriasis Symptom Inventory is a novel PRO instrument for evaluating the severity of psoriasis symptoms12,13 that has demonstrated good reliability and validity in patients with psoriasis.15,16 Previous analyses using pooled data from this study found that correlations between the Psoriasis Symptom Inventory and DLQI total scores ranged from 0·55 to 0·8216 providing strong evidence of convergent validity of the Psoriasis Symptom Inventory. Known groups validity was also supported; mean Psoriasis Symptom Inventory total change scores were significantly different among PASI groups (P < 0·001) with greater change scores found for higher levels of PASI improvement categories (P < 0·01).16 In this study we used the Psoriasis Symptom Inventory to assess symptom severity and the DLQI to assess dermatology-specific quality of life in subjects with psoriasis following treatment with brodalumab. Brodalumab treatment resulted in significant improvements in psoriasis symptoms of patients with moderate-to-severe psoriasis as measured by the Psoriasis Symptom Inventory. These improvements in psoriasis symptoms were rapid, with significant improvements observed as early as week 2. Brodalumab treatment also resulted in significant improvement in dermatology-specific quality of life, as measured by the DLQI, as early as week 4. These PRO improvements were consistent with the clinical effect of brodalumab of improved PASI,17 suggesting that improvements in psoriasis symptoms may lead to improvements in HRQoL.
The Psoriasis Symptom Inventory has been reported to be responsive to changes in psoriasis severity. Psoriasis Symptom Inventory total scores differentiated groups based on PASI and sPGA scores, with mean scores significantly larger in those patients rated by clinicians as having more severe psoriasis.16 In the current study, numerically increasing trend among doses regarding Psoriasis Symptom Inventory response was observed, similar to the dose response observed for the clinical effect of brodalumab as assessed by PASI improvements;17 however, the current study was not adequately powered to differentiate formally among doses, especially the top three doses. The observed changes in Psoriasis Symptom Inventory total scores for the 140- and 210-mg doses were more than twice the baseline SD, indicating large improvements in symptoms.
Significant improvement in DLQI for brodalumab vs. placebo surpassed the disease-specific minimal important difference of 5·7 for this measure19 as early as week 4, demonstrating that brodalumab can rapidly provide clinically meaningful HRQoL improvements in patients with moderate-to-severe psoriasis. Moreover, > 40% of subjects in the 140- and 210-mg brodalumab groups had complete improvement in psoriasis symptom severity (Psoriasis Symptom Inventory = 0) and in impairment to quality of life (DLQI = 0) by week 8, whereas no subjects in the placebo group had complete improvement in Psoriasis Symptom Inventory or DLQI at any time point. These results signify the positive impact of brodalumab treatment on the debilitating effects of psoriasis.
All items in the Psoriasis Symptom Inventory were found to change in the same direction over time and each item demonstrated a significant improvement vs. placebo. Although all the Psoriasis Symptom Inventory items scores improved significantly among the brodalumab groups by week 12, some symptoms improved more rapidly than others. Over 40% of subjects in the 140-mg brodalumab group had complete improvement of the burning, stinging, cracking and pain items by week 2, whereas the improvements in the other symptoms were not reported until week 4 (scaling and flaking) or week 8 (itch and redness). These results suggest that treatment may relieve some psoriasis symptoms faster than other, more persistent, symptoms. This is consistent with anecdotal patient reports of burning, stinging and bleeding accompanying cracking or sloughing of severe plaques, while itch and redness persist until the plaques are resolved (personal communication, Mona Martin). Additional research with larger samples is needed to explore these differences in symptom remission. Little content overlap exists between the Psoriasis Symptom Inventory and DLQI; although the DLQI contains one symptom item, it is a broad questionnaire on overall symptoms in contrast to the specific questions on individual symptoms in the Psoriasis Symptom Inventory.
Overall, this study demonstrated that substantial clinical improvements across treatment groups in patients with psoriasis treated with brodalumab were associated with significant improvements in HRQoL and symptoms as measured by two dermatology-specific measures. Brodalumab improved psoriasis symptoms measured by the Psoriasis Symptom Inventory as well as more distal impacts on HRQoL measured by the DLQI. The combination of symptoms and HRQoL provide a comprehensive assessment of patients' experience with the disease and treatment. Distressing symptoms can often cause impairment to HRQoL. Previous research has demonstrated a high correlation between the Psoriasis Symptom Inventory and DLQI,16 thus it is possible that improvement in symptom severity can be accompanied by improvement in dimensions of HRQoL. More research to evaluate the relationship between psoriasis symptoms and HRQoL may help to understand better the impact of symptom improvement in patients with psoriasis. This would allow for the use of the Psoriasis Symptom Inventory in research settings to better evaluate symptoms from the patient's perspective. Often, physicians' perspectives of controlled disease do not align with the patient's perspective. Therefore the Psoriasis Symptom Inventory, as a PRO of psoriasis symptom severity, can serve as another tool for evaluating the impact of psoriasis symptoms and treatment effectiveness.
There were a number of limitations of this study. The results were from a relatively small study. Therefore, statistical results should be interpreted with caution. Data were from a phase II clinical trial, and study inclusion and exclusion criteria and demographic profile of patients willing to participate in a clinical trial may impact generalizability of the results. The 12-week evaluation captured only short-term improvements and these results need to be confirmed by larger, phase III studies. There was some evidence of differential discontinuation rates between the placebo and brodalumab groups, and the use of LOCF imputation may have introduced some bias into the treatment comparisons favouring the placebo group. However, the large changes in symptoms and HRQoL observed suggest that the bias may be minimal. This study focused on the 7-day version of the Psoriasis Symptom Inventory, because the 24-h version was completed only at designated study visits and not administered as a daily diary. In a separate study, the 24-h version and the 7-day version were found to be comparable with respect to measurement properties.15 The 24-h version administered as a daily diary may be more suitable in a clinical trial setting where rapid change in symptoms is expected. For clinical practice or in an observational research setting, the 7-day version will be more suitable due to the convenience of the administration schedule. Additional research is being undertaken to evaluate the interpretability of Psoriasis Symptom Inventory change scores and thresholds. Similar to the DLQI score of 0, the clinical relevance of the best possible score of the Psoriasis Symptom Inventory (Psoriasis Symptom Inventory = 0) is apparent as it is an indicator of the subject reporting no severity for all eight psoriasis-specific symptoms.
In conclusion, brodalumab treatment provided significant improvement in psoriasis symptoms and functional outcomes in patients with moderate-to-severe psoriasis. These improvements could be distinguished between treatment with brodalumab and placebo as well as between brodalumab dose regimens. These findings demonstrate that observed clinical improvements in disease severity in response to brodalumab17 parallel important improvements in patient-reported symptoms and HRQoL in patients with moderate-to-severe psoriasis.
Acknowledgments
We acknowledge medical writing and editorial assistance provided by Jon Nilsen, PhD (Amgen, Inc.).
References
- 1.Verhoeven EW, Kraaimaat FW, van de Kerkhof PC, et al. Prevalence of physical symptoms of itch, pain and fatigue in patients with skin diseases in general practice. Br J Dermatol. 2007;156:1346–9. doi: 10.1111/j.1365-2133.2007.07916.x. [DOI] [PubMed] [Google Scholar]
- 2.Yosipovitch G, Goon A, Wee J, et al. The prevalence and clinical characteristics of pruritus among patients with extensive psoriasis. Br J Dermatol. 2000;143:969–73. doi: 10.1046/j.1365-2133.2000.03829.x. [DOI] [PubMed] [Google Scholar]
- 3.Bhosle MJ, Kulkarni A, Feldman SR, Balkrishnan R. Quality of life in patients with psoriasis. Health Qual Life Outcomes. 2006;4:35. doi: 10.1186/1477-7525-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sampogna F, Sera F, Abeni D. Measures of clinical severity, quality of life, and psychological distress in patients with psoriasis: a cluster analysis. J Invest Dermatol. 2004;122:602–7. doi: 10.1046/j.0022-202X.2003.09101.x. [DOI] [PubMed] [Google Scholar]
- 5.Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9:136–9. doi: 10.1046/j.1087-0024.2003.09102.x. [DOI] [PubMed] [Google Scholar]
- 6.National Psoriasis Foundation. 2008. Survey panel snapshot, Available from: http://psoriasis.org/document.doc?id=193 (last accessed 28 October 2013)
- 7.Revicki DA, Willian MK, Menter A, et al. Relationship between clinical response to therapy and health-related quality of life outcomes in patients with moderate to severe plaque psoriasis. Dermatology. 2008;216:260–70. doi: 10.1159/000113150. [DOI] [PubMed] [Google Scholar]
- 8.Shikiar R, Heffernan M, Langley RG, et al. Adalimumab treatment is associated with improvement in health-related quality of life in psoriasis: patient-reported outcomes from a phase II randomized controlled trial. J Dermatolog Treat. 2007;18:25–31. doi: 10.1080/09546630601121060. [DOI] [PubMed] [Google Scholar]
- 9.Marquis P, Arnould B, Acquadro C, Roberts WM. Patient-reported outcomes and health-related quality of life in effectiveness studies: pros and cons. Drug Dev Res. 2006;67:193–201. [Google Scholar]
- 10.Otuki MF, Reis RC, Cabrini D. Patient-reported outcomes in psoriasis research and practice. Br J Dermatol. 2011;165:1361–2. doi: 10.1111/j.1365-2133.2011.10469.x. [DOI] [PubMed] [Google Scholar]
- 11.Shikiar R, Bresnahan BW, Stone SP, et al. Validity and reliability of patient reported outcomes used in psoriasis: results from two randomized clinical trials. Health Qual Life Outcomes. 2003;1:53. doi: 10.1186/1477-7525-1-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin ML, McCarrier K, Bushnell DM, et al. Validation of the Psoriasis Symptom Inventory (PSI), a patient reported outcome measure. Presented at the 20th European Academy of Dermatology and Venereology Congress, Lisbon, Portugal, 20–24 October 2011.
- 13.Martin ML, McCarrier K, Chiou C-F, et al. Development of a new patient reported measure for assessing symptoms of psoriiasis. J Am Acad Dermatol. 2012;66(Suppl.1):AB 190. [Google Scholar]
- 14.Martin ML, McCarrier K, Bushnell DM, et al. Validation of the Psoriasis Symptom Inventory (PSI), a patient-reported outcome measure. J Am Acad Dermatol. 2012;66(Suppl. 1):AB 207. doi: 10.3109/09546634.2012.742950. [DOI] [PubMed] [Google Scholar]
- 15.Bushnell DM, Martin ML, McCarrier K, et al. Validation of the Psoriasis Symptom Inventory (PSI), a patient-reported outcome measure to assess psoriasis symptom severity. J Dermatolog Treat. 2013;24:356–60. doi: 10.3109/09546634.2012.742950. [DOI] [PubMed] [Google Scholar]
- 16.Revicki DA, Jin Y, Wilson HD, et al. Reliability and validity of the Psoriasis Symptom Inventory in patients with moderate to severe psoriasis. J Dermatolog Treat. 2013 doi: 10.3109/09546634.2013.769042. Jun 11 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181–9. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- 18.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–16. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 19.Shikiar R, Willian MK, Okun MM, et al. The validity and responsiveness of three quality of life measures in the assessment of psoriasis patients: results of a phase II study. Health Qual Life Outcomes. 2006;4:71. doi: 10.1186/1477-7525-4-71. [DOI] [PMC free article] [PubMed] [Google Scholar]