Abstract
Purpose
The aim was to investigate desire for children, difficulties achieving a pregnancy, and infertility distress among survivors 3 to 7 years after cancer treatment in reproductive age.
Methods
Cancer survivors were identified in national population-based cancer registries. Eligible subjects presented with selected cancer diagnoses between 2003 and 2007 between the ages of 18 to 45. A postal questionnaire including study-specific questions, the Short-Form 36 Health Survey and the Fertility Problem Inventory, was sent to 810 survivors, and 484 participated (60 % response).
Results
Most survivors who had a pretreatment desire for children still wanted children 3–7 years after treatment, and this group was characterized by young age and being childless at diagnosis. In addition, a substantial group of survivors (n = 55, 17 %) that did not have a pretreatment desire for children had changed their mind about wanting children after treatment. About a third of the survivors with a desire to have children had experienced difficulties achieving a pregnancy after the cancer treatment, and an unfulfilled desire to have children was associated with worse mental health. Survivors presently facing difficulties achieving a pregnancy reported moderate levels of infertility distress and expressed low interest in using gamete donation.
Conclusions
Health professionals in cancer care need to be aware that patients’ plans for future children may change, particularly if they are young and childless. All patients of reproductive age should be provided with adequate information about the impact of cancer treatment on future fertility and fertility preservation.
Keywords: Cancer, Care, Quality of life, Attitudes, Fertility distress, Infertility
Background
The desire for biological children is generally strong, and infertility is a known cause of psychological distress with a negative impact on quality of life (QoL) [1]. Progress in cancer therapy has increased survival rates, and the number of men and women living with a history of cancer is steadily rising [2]. With this development, a growing group of cancer survivors will reach an age where they want to start a family. However, certain cancer treatments may have an adverse impact on reproductive health for both males and females and result in impaired sperm production and permanent loss of oocytes and premature menopause, respectively [3]. The overall probability of parenthood in cancer survivors may be reduced by up to 50 %, as found when comparing with sibling controls [4].
A number of methods can be performed to preserve young cancer patients’ future ability to have children. For men, sperm banking is a simple and effective method that has been used for several decades [5]. For women, fertility preservation (FP) is more demanding and time consuming, as egg retrieval requires invasive methods and often hormone stimulation [5]. When there is no time for such methods, ovarian tissue may be retrieved in order to preserve cortical tissue and primordial follicles [6]. As the feasibility of FP is higher for men than for women, FP is practiced by a larger proportion of male cancer patients compared to females (60 vs. 2–4 %) [7, 8], and a low percentage of female cancer patients are being referred to fertility clinics [9].
Specific guidelines and decision aids have been developed to improve young cancer patients’ access to information on FP options, with particular emphasis on the need for providing timely information to female patients, as some FP methods may require a period of up to 2–3 weeks to accomplish [10–13]. Physicians are encouraged to initiate a discussion on cancer treatment impact on reproductive ability prior to treatment start and to provide information about FP to all patients, regardless of whether they already have children or not [12].
It has been shown that patients who had a desire to have children at the time of cancer treatment are more likely to receive fertility-related information [7] and to be referred to a fertility clinic for FP [14]. However, since qualitative studies with young adult cancer survivors indicate that patients may change their mind regarding future children [15, 16], there is a risk that some patients who decline FP because they do not desire children at the time of their treatment may regret their decision later.
Cancer survivors unable to conceive after treatment have reported lower physical well-being and QoL compared to survivors who have had children after cancer treatment [17, 18]. In addition, cancer survivors who have become infertile report a strain in intimate relationships, being rejected by partners, and difficulties beginning new relationships [19]. Even when there is no confirmed infertility, many survivors express fertility-related distress, including worry regarding premature menopause and fear of future infertility [20, 21]. Cancer survivors who experience infertility can use their cryopreserved sperm, oocytes, or embryos to conceive. They can also choose to use gamete donation or to adopt, although adoption agencies may consider a cancer experience as a contraindication to adopting [22]. However, there is limited knowledge about cancer survivors’ perceptions of, and preferences for, alternative parenthood options [23].
Previous findings by our group [7] indicate marked sex differences in fertility-related information and use of fertility preservation among young adult cancer patients. Furthermore, we found that patients with a desire to have children were more likely to receive fertility-related information. Based on these findings, the aim of the present study was to investigate reproductive desire among survivors 3 to 7 years after cancer treatment in reproductive age. The following research questions were addressed: (1) Is desire for children among survivors related to their reproductive desire at diagnosis and sociodemographic characteristics? (2) Is fulfillment of a desire for children related to indicators of mental health among survivors? (3) To what extent do survivors with difficulties achieving a pregnancy report infertility distress, and an intention to use assisted reproduction and adoption?
Methods
Sample and procedure
The sample was identified from the Swedish Cancer Registry and selected Quality Cancer Registries, which are population-based registers administered by the Regional Cancer Center (RCC) Uppsala Örebro, and vital status was verified in the National Population Register. The sample consisted of female and male cancer survivors who met the following inclusion criteria: age 18–45 at time of a diagnosis of lymphoma (Hodgkin and non-Hodgkin), acute leukemia (acute lymphatic leukemia and acute myeloid leukemia), testicular cancer, ovarian cancer, or breast cancer treated with chemotherapy. The sample included patients diagnosed from 2003 to 2007 in the Uppsala Örebro health-care region (around two million inhabitants). These diagnoses were selected as they involved treatments requiring gonadotoxic chemotherapy and/or treatment directed at the reproductive organs. A total of 494 women and 316 men met the inclusion criteria and were sent a postal questionnaire in 2010. A maximum of two reminders were sent to nonresponders. Returning a completed questionnaire was considered as giving informed consent. The study was approved by the Regional Ethical Review Board in Stockholm, Sweden (no. 2010/195-31/4).
Measures
Data were obtained using a survey including validated instruments measuring mental health and infertility-related stress as well as study-specific items developed in collaboration with experts in oncology, reproduction, psychology, and cancer care. The face validity and feasibility of the study-specific items were confirmed in a pilot study including a subsample of 66 cancer survivors from the present study.
Mental health
Mental health was measured by the Mental Component Summary (MCS) scale of the Swedish version of the Short-Form 36 Health Survey (SF-36), reported to have high validity and reliability [24, 25]. Based on the eight scales included in the SF-36, two summary scales can be constructed for physical (physical component summary, PCS) and mental health (mental component summary, MCS) [26]. The PCS is primarily a measure of physical function, role function, bodily pain, and general health, whereas the MCS mainly encompasses vitality, social function, and mental health [27].
Infertility-related stress
The Fertility Problem Inventory (FPI) [28] was used to assess infertility-related stress within two dimensions: representations about the importance of parenthood (FPI-dimension RIP) and impact on life domains (FPI-dimension ILD) [29]. The instrument comprises 46 items organized in five subscales and has been shown to have high validity and reliability among patients undergoing infertility treatment [30]. The two subscales need for parenthood and rejection of a childfree lifestyle form the main dimension FPI-dimension RIP. The three subscales sexual concern, social concern, and relationship concern form the main dimension FPI-dimension ILD. The FPI also has a total summary score, global stress, based on the scoring in all five subscales. The responses are given on a 6-grade Likert scale (1 = strongly agree, 6 = strongly disagree); as the FPI was originally developed for patients undergoing infertility treatment, a seventh response alternative was added for the present study, “Not applicable”. All participants in the present study were asked to answer the questions in the FPI-dimension RIP, and the survivors who reported presently facing difficulties achieving a pregnancy were asked to additionally answer the questions in the FPI-dimension ILD. The FPI was translated into Swedish for this study using independent forward and backward translations by professional translators with Swedish and English, respectively, as their first language.
Behavioral intentions in case of infertility
Behavioral intentions in case of infertility were assessed by three items adapted from earlier research [31]. Participants were requested to indicate the likelihood of undergoing in vitro fertilization (IVF), using donor oocytes or sperm, and adopting in case of infertility. The VAS scales had the endpoints “Entirely unlikely” (0 mm) and “Highly likely” (100 mm).
Sociodemographic and reproductive variables
Sociodemographic and clinical variables included age, sex, diagnosis, level of education, and marital status. Reproductive variables included children at the time of diagnosis, children born after cancer treatment, pretreatment desire for (additional) children, and desire for children at time of study, as well as posttreatment and present difficulties achieving a pregnancy. In addition, the frequency of using FP was assessed by study-specific questions, as has previously been reported for the present sample [7].
Statistical methods
Differences in the distributions of categorical variables were assessed by using chi-square tests and differences in continuous variables by t test or ANOVA (post hoc Games-Howell as assumption of normal distribution was violated). For the analyses of present difficulties achieving a pregnancy, only women aged ≤40 were included in the analyses. Statistical analyses were performed using SPSS (IBM SPSS Statistics 20, IBM, New York, USA). A level of p ≤ 0.05 was used to indicate statistical significance.
Results
Sample characteristics
Out of the 810 eligible cancer survivors 3–7 years post-diagnosis, seven had no known address. Of 803 survivors who were contacted, 484 completed the survey (156 men and 328 women), yielding a 60 % response rate. A comparison between responders and nonresponders showed that a higher percentage of women participated compared to men (67 vs. 50 %, χ2 = 22.977, df = 1, p < 0.001), but no differences were found with regard to age at diagnosis, age at the time of study, or time since diagnosis. Differences regarding sociodemographic and reproductive characteristics between male and female participants are presented in Table 1.
Table 1.
Sociodemographic and clinical characteristics of participants
| Characteristics | Men (n = 156) | Women (n = 328) | p |
|---|---|---|---|
| Age at time of study, mean (SD) | 37.8 (7.2) | 42.8 (6.9) | <0.00a |
| Time since diagnosis, mean (SD) | 4.9 (1.4) | 5.0 (1.4) | NS |
| Diagnosis, no. (%) | |||
| Breast cancer | – | 245 (74.7) | – |
| Ovarian cancer | – | 17 (5.2) | – |
| Lymphoma | 35 (22.4) | 50 (15.2) | NS |
| Leukemia | 10 (6.4) | 16 (4.9) | NS |
| Testicular cancer | 111 (71.2) | – | – |
| Marital status at time of study, no. (%) | NS | ||
| Living alone | 31 (19.9) | 64 (19.5) | |
| Married/cohabiting | 125 (80.1) | 264 (80.5) | |
| Educational level at time of studyb, no. (%) | NS | ||
| No university education | 105 (67.3) | 197 (60.4) | |
| University education | 51 (32.7) | 129 (39.6) | |
| Children at time of study, no. (%) | 97 (62.2) | 272 (82.9) | <0.001c |
| Children born after diagnosis, no. (%) | 36 (23.0) | 24 (7.3) | <0.001c |
| Desire for children at time of study, no. (%) | 52 (33.3) | 43 (13.1) | <0.001c |
| Posttreatment difficulties achieving a pregnancy, no. (%) | 32 (20.5) | 36 (11.0) | 0.007c |
| Current difficulties achieving a pregnancy, no. (%) | 23 (14.7) | 30 (9.1) | NS |
aBetween sex, two-tailed t test
bTwo women did not answer the question
cBetween sex, two-tailed χ2 test
Desire for children before cancer treatment and 3–7 years post-diagnosis
Individuals with a desire for children before treatment
Among participants who reported a pretreatment desire for children (71 men and 81 women), there were significant differences in age and parenthood status between those whose desire for children remained persistent 3–7 years post-diagnosis and those who reported a change in desire (Table 2). Being older and already having children at the time of diagnosis characterized those survivors who changed their mind and no longer had a desire to have children when 3 to 7 years had passed. In addition, 16 individuals in this group had children between the cancer diagnosis and the time of the study and had subsequently fulfilled their desire to have children.
Table 2.
Desire for children 3–7 years post-diagnosis among survivors in relation to pretreatment desire for children and demographic factors
| Characteristics | Pretreatment desire for children (n = 152) | p | No pretreatment desire for children (n = 329) | p | ||||
|---|---|---|---|---|---|---|---|---|
| Posttreatment definite desire (n = 77) | Posttreatment possible desire (n = 41) | Posttreatment no desire (n = 34) | Posttreatment definite desire (n = 18) | Posttreatment possible desire (n = 37) | Posttreatment no desire (n = 274) | |||
| Age at study (mean, SD) | 33.4 (6.7) | 37.2 (4.8) | 39.7 (4.5) | <0.001a | 30.7 (6.3) | 36.9 (7.5) | 45.4 (4.4) | <0.001b |
| Sex, no. (%) | NS | <0.001c | ||||||
| Women | 35 (45.5) | 24 (58.5) | 22 (64.7) | 8 (44.4) | 18 (48.6) | 220 (80.3) | ||
| Men | 42 (54.5) | 17 (41.5) | 12 (35.3) | 10 (55.6) | 19 (51.4) | 54 (19.7) | ||
| Children, no. (%) | ||||||||
| At diagnosis | 16 (20.8) | 18 (43.9) | 26 (76.5) | <0.001d | 1 (5.6) | 24 (64.9) | 251 (91.6) | <0.001d |
| Born after diagnosis | 15 (19.5) | 19 (43.3) | 16 (47.1) | 0.002e | 0 (-) | 1 (2.7) | 8 (2.9) | NS |
aANOVA, post hoc Games-Howell, significant difference between “definite desire” and “possible desire,” and between “definite desire” and “no desire”
bANOVA, post hoc Games-Howell, significant difference between all groups
cChi-square test, significant difference between definite desire and no desire, and between possible desire and no desire
dChi-square test, significant difference between all groups
eChi-square test, significant difference between definite desire and no desire, and between definite desire and possible desire
About half of those with a pretreatment desire for children reported a definite desire for children 3–7 years later; this group was characterized by younger age and lower frequency of children than the remaining groups.
Individuals with no desire for children before treatment
Among participants who reported having no pretreatment desire for children (83 men and 246 women), there were significant differences between those who kept this standpoint 3–7 years post-diagnosis and those who reported a change in desire (Table 2). Being older, female, and already having children at the time of diagnosis characterized those survivors who continued to feel no desire for (additional) children. One of six survivors reported that their desire had changed from no pretreatment desire for children to a definite or possible desire to have children 3–7 years later. These groups were characterized by younger age and lower percentage of children at diagnosis. The reported use of FP was relatively frequent among the men who had changed their standpoint to a definite (90 %) or possible desire for children (53 %), while none of the women in these groups had performed any FP.
Mental health in relation to fulfillment of a pretreatment desire for children
Among survivors with a pretreatment desire to have children, those who had had children after the cancer treatment rated their mental health (SF-36 MCS) as better (n = 49, M = 47.76) compared to those who had not had any children (n = 98, M = 43.03) [t(145) = 2.28, p = 0.024].
Difficulties achieving a pregnancy, infertility distress, and behavioral intentions
At the time of the study, 83 survivors (51 men aged 24–43 and 32 women aged 22–39) reported a desire to have children. Of these, about half had not tried to conceive (n = 46), and 13 % (n = 11) had not experienced any difficulties achieving a pregnancy. One in three survivors (n = 26) reported having experienced difficulties conceiving after cancer treatment, and 19 of these reported that they presently experienced such difficulties.
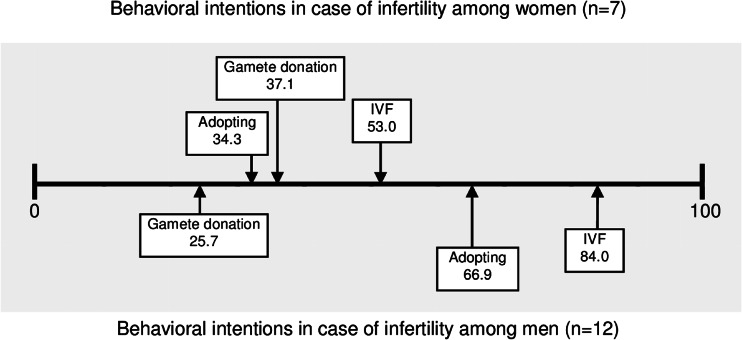
The 19 survivors who presently faced difficulties achieving a pregnancy (12 men and 7 women) represented 22.3 % of the survivors with a desire to have children at time of study. The mean age was 33 years (men, 24–42; women, 23–38), all but one woman was married or cohabiting, and 42 % had previous children (4 men and 4 women). Of the 12 men, 9 had cryopreserved sperm, and none of the women had undergone any FP. The level of infertility distress in the subgroup is presented in Table 3. The survivors’ behavioral intentions in case of infertility are presented in Fig. 1
Table 3.
Infertility distress among female and male survivors with present difficulties achieving a pregnancy
| Variables | Women (n = 7) | Men (n = 12) | Possible range |
|---|---|---|---|
| Fertility Problem Inventory (FPI)a, mean (SD) | |||
| Global stress | 178.2 (43.2) | 125.6 (24.3) | 46–276 |
| FPI-dimension RIP | 76.7 (18.4) | 64.7 (9.3) | 18–108 |
| Rejection of childfree lifestyle | 32.8 (7.9) | 27.9 (3.9) | 8–48 |
| Need for parenthood | 43.8 (10.6) | 38.1 (8.2) | 10–60 |
| FPI-dimension ILD | 95.6 (28.9) | 60.9 (18.4) | 28–168 |
| Sexual concern | 25.1 (10.7) | 18.7 (6.2) | 8–48 |
| Social concern | 33.6 (8.3) | 21.6 (8.2) | 10–60 |
| Relationship concern | 31.5 (13.9) | 22.1 (9.2) | 10–60 |
RIP representations about the importance of parenthood, ILD impact on life domains
aHigh values indicated high level of infertility-related distress
Fig. 1.
Famale and male behavioral intentions in case of infertility.0 = Entirely Unlikely and 100 = Higly likely
Discussion
The present results show that, among cancer survivors who reported a pretreatment desire for children, one third had succeeded in having children, and a majority wanted (additional) children 3–7 years post-diagnosis. Interestingly, one out of six of the survivors who reported no pretreatment desire for children had subsequently changed their mind about wanting children. Not fulfilling a desire for children was associated with worse mental health compared to fulfilling the desire. Survivors with difficulties achieving a pregnancy reported moderate levels of infertility distress and low interest in using gamete donation treatment.
Our results indicate that the desire to have children among cancer survivors is related to pretreatment reproductive desire, age, and parenthood status. The desire for children persisted among those who were younger and often childless, which is in line with earlier studies showing that survivors’ desire to have children remains persistent through the cancer treatment [18, 23, 32]. In contrast, older survivors, who to a larger extent had fulfilled their reproductive desire, no longer reported wanting children 3 to 7 years after diagnosis. Our study did not investigate the reasons for no longer wanting children, but earlier research has reported that additional explanations for why survivors change their mind to not want children could include fear of cancer recurrence and of transmitting the cancer risk to offspring [18, 23, 32].
One out of six of the participants who reported having no pretreatment desire to have children had changed their mind about wanting children several years after diagnosis. Those who reported a definite desire for children at the time of study were characterized by their young age and by having no children. Since pretreatment desire to have children has been reported to have a significant influence on the probability of receiving fertility-related treatment information [7], young adult cancer patients with no firm desire for children at the start of treatment may be at risk of not receiving adequate information about the impact of treatment on future reproductive ability and information about FP options. While it is essential to respect each patient’s standpoint regarding future children, these findings stress the importance to also discuss fertility-related aspects of treatment and FP with those patients who have no expressed desire for children, particularly those of young age and with no previous children. This is also supported in a previous research showing that firm plans regarding future children do not fully predict the desire to pursue fertility preservation [33].
Among survivors with a pretreatment desire for children, those with an unfulfilled desire reported lower mental health scores compared to those who had succeeded in having children after the treatment. This is in line with findings by Canada and Schover [18] indicating that women who had not been able to conceive after cancer treatment had more intrusive thoughts, used more avoidance strategies, and reported a higher level of emotional distress about infertility than women who had had children. In the present study, the poorer outcomes in mental health among those with an unfulfilled desire for children compared with those who had been able to have children may not be exclusively attributable to infertility distress, and other factors may be involved explaining the outcome, for example, worse physical health affecting the mental health.
In the present study, about a third of the survivors with a desire to have children had experienced difficulties conceiving after the cancer treatment. This can be compared with the worldwide prevalence of infertility at approximately 15 % [34]. The level of infertility distress among female and male survivors with present difficulties achieving a pregnancy was in line with values reported for 525 couples referred for infertility treatment [35], supporting recent findings of no differences in mental health between infertile women with and without cancer experience [36]. According to our results, both men and women reported a fairly low likelihood of using gamete donation in case of infertility, but women were slightly more positive towards using donated eggs than men were towards using donated sperm. An earlier research has been inconclusive concerning attitudes towards using gamete donation among men and women in the general population [37].
The present study is based on a large population-based sample including female and male cancer survivors, and the results are judged to be representative for men and women diagnosed in reproductive age receiving treatment with a possible negative impact on fertility. While the relatively low response rate, particularly among males, is a threat to external validity, we could not detect any response bias regarding age at diagnosis, age at time of study, or time since diagnosis, and the educational level of study participants was in parity to the general Swedish population [38]. While women are commonly found to participate to a higher extent in survey studies, the difference in response rate could also be related to a general difference in women’s and men’s valuation of the studied issues [28, 39]. Whether those who declined participation had severe health problems to a larger extent than those who participated is unfortunately not known. However, it could also be so that those who were better off and had moved on in life after being treated for cancer chose to not to participate. The instrument measuring behavioral intentions (VAS) has been used earlier, with satisfactory outcome [31, 40]. Visual analog scales have been shown to be valuable tools for assessing different aspects of mood and well-being [41] and demonstrate high reliability and validity [41, 42]. Some caution is advised when drawing conclusions from self-reported retrospective data, such as the pretreatment desire for children in the present study. Also, the results from the subgroup of survivors with current difficulties achieving a pregnancy should be considered as tentative as no statistical analyses could be performed due to low statistical power.
Conclusions
The results of our study indicate that a substantial group of patients without a desire for children at the time of cancer treatment may change their mind about wanting children several years after the treatment. Cancer health-care professionals need, therefore, to be aware that patients’ plans for future children may change, particularly if the patients are young and childless at time of diagnosis. An unfulfilled desire to have children is associated with worse mental health in men and women diagnosed with cancer in reproductive age. Information on potentially negative effects of cancer treatment on reproduction and on options for fertility preservation should be provided to all individuals presenting with cancer in reproductive age.
Acknowledgments
We thank all women and men who participated in this research. We also thank the Regional Cancer Center (RCC) Uppsala Örebro for providing assistance with identifying study participants. This study was supported by grants from the Swedish Cancer Society (2010/877) and by Karolinska Institutet faculty funds (6549/10-225).
Conflict of interest
The authors have indicated no potential conflict of interest.
Previous presentations
There were no previous presentations of the results.
References
- 1.Chachamovich JR, Chachamovich E, Ezer H, Fleck MP, Knauth D, Passos EP. Investigating quality of life and health-related quality of life in infertility: a systematic review. J Psychosom Obstet Gynaecol. 2010;31(2):101–110. doi: 10.3109/0167482X.2010.481337. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Wallberg KA. Principles of cancer treatment: impact on reproduction. Adv Exp Med Biol. 2012;732:1–8. doi: 10.1007/978-94-007-2492-1_1. [DOI] [PubMed] [Google Scholar]
- 4.Madanat LM, Malila N, Dyba T, Hakulinen T, Sankila R, Boice JD, Jr, Lahteenmaki PM. Probability of parenthood after early onset cancer: a population-based study. Int J Cancer. 2008;123(12):2891–2898. doi: 10.1002/ijc.23842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez-Wallberg KA, Oktay K. Fertility preservation medicine: options for young adults and children with cancer. J Pediatr Hematol Oncol. 2010;32(5):390–396. doi: 10.1097/MPH.0b013e3181dce339. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Wallberg KA, Oktay K. Recent advances in oocyte and ovarian tissue cryopreservation and transplantation. Best Pract Res Clin Obstet Gynaecol. 2012;26(3):391–405. doi: 10.1016/j.bpobgyn.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armuand GM, Rodriguez-Wallberg KA, Wettergren L, Ahlgren J, Enblad G, Hoglund M, Lampic C. Sex differences in fertility-related information received by young adult cancer survivors. J Clin Oncol. 2012;30(17):2147–2153. doi: 10.1200/JCO.2011.40.6470. [DOI] [PubMed] [Google Scholar]
- 8.Letourneau JM, Ebbel EE, Katz PP, Katz A, Ai WZ, Chien AJ, Melisko ME, Cedars MI, Rosen MP. Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer. 2012;118(6):1710–1717. doi: 10.1002/cncr.26459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yee S, Buckett W, Campbell S, Yanofsky R, Barr RD. A national study of the provision of oncofertility services to female patients in Canada. J Obstet Gynaecol Can. 2012;34(9):849–858. doi: 10.1016/S1701-2163(16)35384-1. [DOI] [PubMed] [Google Scholar]
- 10.Garvelink MM, Ter Kuile MM, Fischer MJ, Louwe LA, Hilders CG, Kroep JR, Stiggelbout AM. Development of a Decision Aid about fertility preservation for women with breast cancer in the Netherlands. J Psychosom Obstet Gynaecol. 2013;34(4):170–178. doi: 10.3109/0167482X.2013.851663. [DOI] [PubMed] [Google Scholar]
- 11.Peate M, Meiser B, Cheah BC, Saunders C, Butow P, Thewes B, Hart R, Phillips KA, Hickey M, Friedlander M. Making hard choices easier: a prospective, multicentre study to assess the efficacy of a fertility-related decision aid in young women with early-stage breast cancer. Br J Cancer. 2012;106(6):1053–1061. doi: 10.1038/bjc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, Beck LN, Brennan LV, Oktay K. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24(18):2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Wallberg KA, Oktay K. Fertility preservation during cancer treatment: clinical guidelines. Cancer Manag Res. 2014;6:105–117. doi: 10.2147/CMAR.S32380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams E, Hill E, Watson E. Fertility preservation in cancer survivors: a national survey of oncologists’ current knowledge, practice and attitudes. Br J Cancer. 2013;108(8):1602–1615. doi: 10.1038/bjc.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter J, Lewin S, Abu-Rustum N, Sonoda Y. Reproductive issues in the gynecologic cancer patient. Oncology (Williston Park) 2007;21(5):598–606. [PubMed] [Google Scholar]
- 16.Connell S, Patterson C, Newman B. A qualitative analysis of reproductive issues raised by young Australian women with breast cancer. Health Care Women Int. 2006;94(1):94–110. doi: 10.1080/07399330500377580. [DOI] [PubMed] [Google Scholar]
- 17.Wenzel L, Dogan-Ates A, Habbal R, Berkowitz R, Goldstein DP, Bernstein M, Kluhsman BC, Osann K, Newlands E, Seckl MJ, Hancock B, Cella D. Defining and measuring reproductive concerns of female cancer survivors. J Natl Cancer Inst Monogr. 2005;34:94–98. doi: 10.1093/jncimonographs/lgi017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canada AL, Schover LR. The psychosocial impact of interrupted childbearing in long-term female cancer survivors. Psychooncology. 2012;21(2):134–143. doi: 10.1002/pon.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penrose R, Beatty L, Mattiske J, Koczwara B. The psychosocial impact of cancer-related infertility on women: a review and comparison. Clin J Oncol Nurs. 2013;17(2):188–193. doi: 10.1188/13.CJON.188-193. [DOI] [PubMed] [Google Scholar]
- 20.Rosen A, Rodriguez-Wallberg KA, Rosenzweig L. Psychosocial distress in young cancer survivors. Semin Oncol Nurs. 2009;25(4):268–277. doi: 10.1016/j.soncn.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Peate M, Meiser B, Hickey M, Friedlander M. The fertility-related concerns, needs and preferences of younger women with breast cancer: a systematic review. Breast Cancer Res Treat. 2009;116(2):215–223. doi: 10.1007/s10549-009-0401-6. [DOI] [PubMed] [Google Scholar]
- 22.Rosen A. Third-party reproduction and adoption in cancer patients. J Natl Cancer Inst Monogr. 2005;34:91–93. doi: 10.1093/jncimonographs/lgi021. [DOI] [PubMed] [Google Scholar]
- 23.Schover LR, Rybicki LA, Martin BA, Bringelsen KA. Having children after cancer. A pilot survey of survivors’ attitudes and experiences. Cancer. 1999;86(4):697–709. doi: 10.1002/(SICI)1097-0142(19990815)86:4<697::AID-CNCR20>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan M, Karlsson J, Ware JE., Jr The Swedish SF-36 Health Survey–I. Evaluation of data quality, scaling assumptions, reliability and construct validity across general populations in Sweden. Soc Sci Med. 1995;41(10):1349–1358. doi: 10.1016/0277-9536(95)00125-Q. [DOI] [PubMed] [Google Scholar]
- 25.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36) I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Ware JE, Jr, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33(4 Suppl):AS264–279. [PubMed] [Google Scholar]
- 27.Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res. 2001;10(5):405–413. doi: 10.1023/A:1012588218728. [DOI] [PubMed] [Google Scholar]
- 28.Newton CR, Sherrard W, Glavac I. The Fertility Problem Inventory: measuring perceived infertility-related stress. Fertil Steril. 1999;72(1):54–62. doi: 10.1016/S0015-0282(99)00164-8. [DOI] [PubMed] [Google Scholar]
- 29.Moura-Ramos M, Gameiro S, Canavarro MC, Soares I. Assessing infertility stress: re-examining the factor structure of the Fertility Problem Inventory. Hum Reprod. 2012;27(2):496–505. doi: 10.1093/humrep/der388. [DOI] [PubMed] [Google Scholar]
- 30.Gourounti K, Anagnostopoulos F, Vaslamatzis G. Psychometric properties and factor structure of the Fertility Problem Inventory in a sample of infertile women undergoing fertility treatment. Midwifery. 2011;27(5):660–667. doi: 10.1016/j.midw.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Lampic C, Svanberg AS, Karlstrom P, Tyden T. Fertility awareness, intentions concerning childbearing, and attitudes towards parenthood among female and male academics. Hum Reprod. 2006;21(2):558–564. doi: 10.1093/humrep/dei367. [DOI] [PubMed] [Google Scholar]
- 32.Senkus E, Gomez H, Dirix L, Jerusalem G, Murray E, Van Tienhoven G, Westenberg AH, Bottomley A, Rapion J, Bogaerts J, Di Leo A, Neskovic-Konstantinovic Z (2013) Attitudes of young patients with breast cancer toward fertility loss related to adjuvant systemic therapies. EORTC study 10002 BIG 3-98. Psychooncology. doi:10.1002/pon.3384 [DOI] [PubMed]
- 33.Peate M, Meiser B, Friedlander M, Zorbas H, Rovelli S, Sansom-Daly U, Sangster J, Hadzi-Pavlovic D, Hickey M. It's now or never: fertility-related knowledge, decision-making preferences, and treatment intentions in young women with breast cancer—an Australian fertility decision aid collaborative group study. J Clin Oncol. 2011;29(13):1670–1677. doi: 10.1200/JCO.2010.31.2462. [DOI] [PubMed] [Google Scholar]
- 34.Petraglia F, Serour GI, Chapron C. The changing prevalence of infertility. Int J Gynaecol Obstet: Off Org Int Fed Gynaecol Obstet. 2013 doi: 10.1016/j.ijgo.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Peterson BD, Newton CR, Rosen KH. Examining congruence between partners’ perceived infertility-related stress and its relationship to marital adjustment and depression in infertile couples. Fam Process. 2003;42(1):59–70. doi: 10.1111/j.1545-5300.2003.00059.x. [DOI] [PubMed] [Google Scholar]
- 36.Carter J, Raviv L, Applegarth L, Ford JS, Josephs L, Grill E, Sklar C, Sonoda Y, Baser RE, Barakat RR. A cross-sectional study of the psychosexual impact of cancer-related infertility in women: third-party reproductive assistance. J Cancer Surviv. 2010;4(3):236–246. doi: 10.1007/s11764-010-0121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hudson N, Culley L, Rapport F, Johnson M, Bharadwaj A. “Public” perceptions of gamete donation: a research review. Public Underst Sci. 2009;18(1):61–77. doi: 10.1177/0963662507078396. [DOI] [PubMed] [Google Scholar]
- 38.Statistics Sweden (2009) Utbildningsnivå efter kommun och kön 2011. http://www.scb.se/Pages/ProductTables____9575.aspx. Accessed 21 Dec 2012
- 39.Brase GL, Brase SL. Emotional regulation of fertility decision making: what is the nature and structure of “baby fever”? Emotion. 2012;12(5):1141–1154. doi: 10.1037/a0024954. [DOI] [PubMed] [Google Scholar]
- 40.Tyden T, Svanberg AS, Karlstrom PO, Lihoff L, Lampic C. Female university students’ attitudes to future motherhood and their understanding about fertility. Eur J Contracept Reprod Health Care. 2006;11(3):181–189. doi: 10.1080/13625180600557803. [DOI] [PubMed] [Google Scholar]
- 41.Ahearn EP. The use of visual analog scales in mood disorders: a critical review. J Psychiatr Res. 1997;31(5):569–579. doi: 10.1016/S0022-3956(97)00029-0. [DOI] [PubMed] [Google Scholar]
- 42.Wewers ME, Lowe NK. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health. 1990;13(4):227–236. doi: 10.1002/nur.4770130405. [DOI] [PubMed] [Google Scholar]