Abstract
Background
visual and cognitive impairments are common in later life. Yet there are very few cognitive screening tests for the visually impaired.
Objective
to screen for cognitive impairment in the visually impaired.
Methods
case–control study including 150 elderly participants with visual impairment (n = 74) and a control group without visual impairment (n = 76) using vision-independent cognitive tests and cognitive screening tests (MMSE and clock drawing tests (CDT)) which are in part vision dependent.
Results
the scoring of the two groups did not differ in the vision-independent cognitive tests. Visually impaired patients performed poorer than controls in the vision-dependent items of the MMSE (T = 7.3; df: 148; P < 0.001) and in CDT (T = 3.1; df: 145; P = 0.003). No group difference was found when vision-independent items were added to MMSE and CDT. The test score gain by the use of vision-independent items correlated with the severity of visual impairment (P < 0.002).
Conclusion
visually impaired patients benefit from cognitive tests, which do not rely on vision. The more visually impaired the greater the benefit.
Keywords: visual impairment, cognitive impairment, cognitive testing
Introduction
Dementia and visual impairment are among the most common medical conditions in later life [1, 2] and medical services dealing with older adults are likely to encounter patients with dual impairments. Cognitive testing in visually impaired older adults is an under-researched area and many tests rely on vision. Little research has been conducted to develop adjusted cognitive measures for the visually impaired and most studies omitted visual items, rather than offering vision-independent alternatives [3–6]. This study used cognitive tests that do not require vision and the two most commonly used screening tests for cognitive impairment, namely the Mini-Mental-State-Examination (MMSE) [7] and the clock drawing test (CDT) [8, 9] which are partly vision dependent. We hypothesised that vision-independent cognitive testing is more accurate for visually impaired patients.
Methods
Participants
The study was approved by the UK NHS Research Ethics Committee and all participants gave written informed consent prior to inclusion. Of 197 potential participants approached, 150 agreed to take part. Inclusion criteria were age 60 and older and exclusion criteria were any active medical, psychiatric or neurological condition that could affect the ability to handle cognitive screening tests (e.g. hemiplegia). Participants were considered to be visually impaired according to WHO criteria [10], i.e. if best near visual acuity was ≤0.32 (equivalent to LogMAR 0.5).
Visually impaired participants were recruited from the memory assessment service (n = 34) at Newcastle General Hospital and the Department of Ophthalmology (n = 40) at the Royal Victoria Hospital in Newcastle upon Tyne, UK. The majority (71 of 74) had acquired visual impairment (e.g. macular degeneration or glaucoma) and three were congenitally blind. The 76 control participants, i.e. those without visual impairment, were recruited from the local community via advertisement (n = 40) and from the memory assessment service (n = 36).
Procedures
Testing took place at participants’ homes. Demographic data were derived using a structured questionnaire. Binocular best visual acuity at presentation was measured using Landolt Broken Rings Charts [11] at near (test distance 40 cm) with participants wearing their reading glasses if required. Activities of daily living were assessed using Bristol activities of daily living (ADL) [12].
Vision-independent cognitive tests
Three vision-independent cognitive tests were used, including the verbal fluency (FAS test) [13], category fluency test [14] and the Rey auditory verbal learning test (RAVLT) [15]. The FAS test consists of three word naming trials (1 min each) and required the naming of words starting with either F, A or S, whereas the category fluency test involves the naming of as many different animals as possible within 1 min. The number of correct words was counted in both tests. They measure executive and language skills. In RAVLT participants were asked to learn, retain (for 30 min) and to recognise 15 words. The maximum score for each step is 15 and the test measures episodic verbal memory.
Cognitive screening tests requiring vision
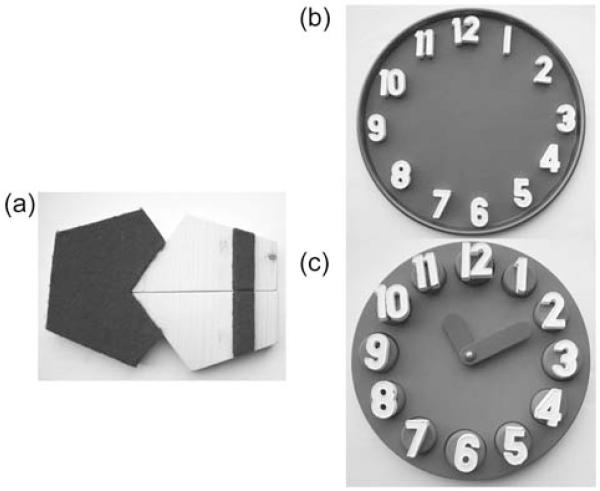
The MMSE [7] (© Psychological Assessment Resources, Inc.) tests five distinct cognitive domains: orientation, working memory, attention, language, praxis and memory. It includes vision-independent and vision-dependent test items. The three items relying on vision are the reading, writing, overlapping pentagon copying tasks. In the naming task visually impaired participants can touch the presented object (watch and pencil) before naming it and this task is therefore not considered vision dependent. Three vision-independent items (VI-items) were added before or after the MMSE (alternative order between successive participants): participants were (i) instructed verbally to ‘close your eyes’; (ii) to speak out a sentence and (iii) to assemble a pentagon, using three-shaped and textured wooden pieces (see Figure 1a). Each correct answer scored 1 point (max. 3 points).
Figure 1.
(a) The three pieces pentagon assembling task used shaped and textured wooden pieces and the instruction was to assemble the pentagons according to a template. (b) In the clock assembly task a metallic tray and 12 magnetic numbers were provided to the participant. The instruction was to arrange the numbers as they would appear on the clock face. (c) In the clock setting task participants were asked to set the time to 10 min past 11 o’clock.
The CDT is a vision-dependent task and evaluates comprehension, memory, visuo-spatial abilities, abstract thinking and executive function [16]. An alternative vision-independent task (i.e. the clock test for the visually impaired (CTVI)) was developed and had two parts: A clock assembly task (see Figure 1b) and a clock reading task (see Figure 1c). Both relied on haptic perception. The clock assembly task involved arranging 12 magnetic numbers on a circular metallic tray, with the verbal instruction ‘Please arrange these numbers as they would appear on a clock face’. On a separate 3D clock, participants had to set the time to 10 min past 11 o’clock. The Manos 10-item rating [8] which allows the scoring for accuracy of time setting was used for CDT and CTVI. All participants did both tests. The order was alternated between successive participants.
Statistical analysis
All data were examined for normality using the Kolmogorov–Smirnov test. The demographics, clinical characteristics and cognitive test measures were compared between patients and controls using independent sample parametric (T-tests) or non-parametric tests (Mann–Whitney U tests) depending on the data distribution. Pearson Chi-square was used for two group comparison of categorical data. Pearson or Spearman correlations were used to establish the relationship between the severity of visual impairment and the potential gain in scoring when comparing VI-items and the vision-dependent items of MMSE or CTVI and CDT, respectively. Cronbach’s alpha was used to test internal consistency and reliability of VI-items and vision-dependent items of MMSE and of CTVI and CDT. A P-value ≤ 0.05 was considered as a significant result.
Results
Demographic and cognitive function of the control and visually impaired groups are summarised in Table 1.
Table 1.
Demographic data and results
| Control group (n = 76) | Visually impaired group (n = 74) | Statistics | |
|---|---|---|---|
| Gender (m:f) | 38:38 | 27:47 | Chi-square 2.7; df: 1; P = 0.095; ns* |
| Age (years) | 77.5 (6.0) | 79.7 (7.5) | T = −2.0; df 148; P = 0.048 |
| Education (years) | 10.8 (3.6) | 10.1 (3.2) | T = 1.3 ; df 148; P = 0.20; ns |
| Bristol ADL (max. 60) | 6.0 (8.7) | 11.2 (9.2) | U 3967; P < 0.001** |
| Visual acuity (decimals) | 0.50 (0.13) | 0.14 (0.11) | T = 18.2; df 147; P < 0.001 |
| Vision-independent cognitive tests | |||
| Verbal fluency (mean per min) | 10.6 (6.3) | 10.4 (6.4) | T = 0.2; df 143; P = 0.85; ns |
| Category fluency (mean per min) | 14.9 (7.4) | 14.3 (7.6) | T = 0.46; df 144; P = 0.64; ns |
| RAVLT-immediate recall (max. 15) | 7.0 (4.3) | 7.2 (4.0) | T = −0.23; df 99; P = 0.82; ns |
| RAVLT-delayed recall (max. 15) | 6.6 (4.6) | 7.2 (4.7) | T = −0.66; df 94; P = 0.51; ns |
| RAVLT-recognition (max. 15) | 12.7 (3.1) | 12.2 (4.6) | T = 0.613; df 87; P = 0.54; ns |
| MMSE vision-independent items (max. 27) | 22.6 (5.2) | 22.2 (5.9) | T = 0.5; df 148;P = 0.63; ns |
| Vision-dependent test items | |||
| MMSE vision-dependent items (max. 3) | 2.6 (0.5) | 1.7 (1.0) | T = 7.3; df 148;P < 0.001 |
| Clock drawing test (CDT) (max. 10) | 7.7 (3.1) | 5.9 (3.7) | T = 3.1; df 145; P = 0.003 |
| Adjusted vision-independent test items | |||
| VI-items (max.3) | 2.8 (0.5) | 2.7 (0.6) | T = 1.0; df 143; P = 0.303; ns |
| CTVI (max. 10) | 8.2 (2.9) | 7.9 (3.4) | T = 0.44; df 131; P = 0.664; ns |
Mean and standard deviation (SD) unless otherwise reported. ns, not significant; RAVLT: Rey auditory verbal learning test; MMSE vision-dependent items include: reading ‘Close your eyes’; writing a sentence and copying overlapping pentagons; vision-independent items (VI-items) added to MMSE include: following the verbal command ‘Close your eyes’; speaking a sentence and pentagon assembling. CTVI, clock test for the visually impaired.
Pearson Chi-square.
Mann–Whitney U Test; otherwise independent sample T-test.
There were no gender and education differences between the groups, but the visually impaired group was slightly older, more impaired in Bristol ADL and had, as expected, poorer visual acuity. No group differences were found when FAS test, category fluency and RAVLT were compared. The moderately to severely cognitively impaired participants in both groups were not able to do RAVLT leaving 47 visually impaired and 54 in the control participants for the group comparison on this test.
The scoring of the MMSE (vision-independent items) ranged from 9 to 27 in the control group and from 8 to 27 in the visually impaired group. The visually impaired group performed significantly worse on MMSE items requiring vision and on CDT than controls. No group differences were found when VI-items or the CTVI were compared. Within the control group internal consistencies of vision-dependent MMSE items and VI-items (Cronbach’s alpha 0.79) and of CDT and CTVI (Cronbach’s alpha 0.83) were high. Visually impaired patients were found to benefit most from VI-items and CTVI. There was a significant correlation between visual acuity and number of test points gained by the use of VI-items (rho = −0.719; P < 0.0001) as well as with the CTVI (rho = −0.370; P = 0.002).
Discussion
Visually impaired patients benefited most from vision-independent cognitive testing that were based on haptic and auditory perception. No group difference was found in vision-independent tests, suggesting that the differences found in vision-dependent test items reflected visual rather than cognitive impairments. Only the visually impaired patients benefited from the vision-independent items (VI-items and CTVI) and the more visual impairment the greater the benefit. The order of vision-dependent and vision-independent test items was altered and therefore results cannot be explained by order effects.
Vision-independent items were pragmatically selected based on previous research and clinical experience with visually impaired patients. The pentagon task (part of VI-items) and the clock assembling task (part of CTVI) were alternative tasks to drawing, as visual construction embraces drawing and assembling. Furthermore both tests combine visual perception, executive and motor function [17, 18], but drawing relies mainly on visual perception, whereas assembling relies on perception and haptic function. Previous reseach found an association between spatial perception and assembling [19] which further emphases the similarities of the tests.
As reported in previous studies [20, 21] visually impaired participants had more ADL impairments than controls. This illustrates that both perception and cognition are contributing to independent functioning in daily living [22]. Thus if visual impairment remains unrecognised, ADL impairment can falsely be attributed to cognitive impairment. This potentially contributes to over-diagnosis of dementia, to misjudging the severity of dementia [23] or to premature stopping of antidementia drugs [24]. For health professionals dealing with elderly patients, the screening for visual and cognitive impairment is equally important.
There are some limitations to this study. Firstly, the pentagon assembling and the CTVI are bulky. They have been designed for this study and are handmade which limits their accessibility. Secondly, error possibilities in vision-dependent and vision-independent tasks are slightly different. For example, only having a single set of digits in the CTVI may reduce the number of repetition errors in the CTVI compared with the CDT. However, overall the similarities in scoring in vision-dependent and vision-independent test items suggest comparable difficulties. Thus while the VI-items and CTVI used were not a perfect surrogate, we would argue that they are better than standard vision-dependent items for patients with visual impairment. Novel, validated vision-independent cognitive screening tests will need to be developed in the near future to ensure equal access to early, accurate diagnosis of dementia and treatment.
Key points.
Commonly used cognitive screening tests are vision-dependent which limits their use for visually impaired or blind patients.
Visually impaired or blind patients benefit from cognitive tests that do not require vision.
Vision-independent cognitive tests are important to ensure accurate diagnosis and equal access to treatment.
Acknowledgments
Funding This work was supported by Newcastle upon Tyne Hospitals NHS Trust, UK. This work was also supported by the UK NIHR Biomedical Research Centre for Ageing and Age-related disease award to the Newcastle upon Tyne Hospitals NHS Foundation Trust.
Sponsor’s role None.
Footnotes
Conflicts of interest None declared.
References
- 1.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–7. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jefferis JM, Mosimann UP, Clarke MP. Cataract and cognitive impairment: a review of the literature. Br J Ophthalmol. 2011;95:17–23. doi: 10.1136/bjo.2009.165902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busse A, Sonntag A, Bischkopf J, Matschinger H, Angermeyer MC. Adaptation of dementia screening for vision-impaired older persons: administration of the Mini-Mental State Examination (MMSE) J Clin Epidemiol. 2002;55:909–15. doi: 10.1016/s0895-4356(02)00449-3. [DOI] [PubMed] [Google Scholar]
- 4.Clemons TE, Rankin MW, McBee WL. Cognitive impairment in the Age-Related Eye Disease Study: AREDS report no. 16. Arch Ophthalmol. 2006;124:537–43. doi: 10.1001/archopht.124.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reyes-Ortiz CA, Kuo YF, DiNuzzo AR, Ray LA, Raji MA, Markides KS. Near vision impairment predicts cognitive decline: data from the Hispanic Established Populations for Epidemiologic Studies of the Elderly. J Am Geriatr Soc. 2005;53:681–6. doi: 10.1111/j.1532-5415.2005.53219.x. [DOI] [PubMed] [Google Scholar]
- 6.Reischies FM, Geiselmann B. Age-related cognitive decline and vision impairment affecting the detection of dementia syndrome in old age. Br J Psychiatry. 1997;171:449–51. doi: 10.1192/bjp.171.5.449. [DOI] [PubMed] [Google Scholar]
- 7.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 8.Manos PJ, Wu R. The ten point clock test: a quick screen and grading method for cognitive impairment in medical and surgical patients. Int J Psychiatr Med. 1994;24:229–44. doi: 10.2190/5A0F-936P-VG8N-0F5R. [DOI] [PubMed] [Google Scholar]
- 9.Thalmann B, Spiegel R, Stahelin HB, et al. Dementia screening in general practice: optimised scoring for the clock drawing test. Brain Aging. 2002;2:36–43. [Google Scholar]
- 10.WHO, editor. WHO. Classification of Ophthalmic Disorders. Clinical Descriptions and Diagnositc Guidelines. Churchill and Livingstone; Geneva: 2003. [Google Scholar]
- 11.Hohmann A, Haase W. Development of visual line acuity in humans. Ophthalmic Res. 1982;14:107–12. doi: 10.1159/000265180. [DOI] [PubMed] [Google Scholar]
- 12.Bucks RS, Ashworth DL, Wilcock GK, Siegfried K. Assessment of activities of daily living in dementia: development of the Bristol Activities of Daily Living Scale. Age Ageing. 1996;25:113–20. doi: 10.1093/ageing/25.2.113. [DOI] [PubMed] [Google Scholar]
- 13.Benton AL, Hamsher K, editors. Multilingual Aphasia Examination. AJA; Iowa City: 1989. [Google Scholar]
- 14.Benton AL, Sivan AB. Problems and conceptual issues in neuropsychological research in aging and dementia. J Clin Neuropsychol. 1984;6:57–63. doi: 10.1080/01688638408401196. [DOI] [PubMed] [Google Scholar]
- 15.Spreen OS, Stauss E. A Compendium of Neuropsychological Tests. OUP; New York: 1998. [Google Scholar]
- 16.Shulman KI, Shulman KI. Clock-drawing: is it the ideal cognitive screening test? Int J Geriatr Psychiatry. 2000;15:548–61. doi: 10.1002/1099-1166(200006)15:6<548::aid-gps242>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 17.Benton AL. Constructional apraxia and the minor hemisphere. Confinia Neurologica. 1967;29:1–16. [PubMed] [Google Scholar]
- 18.Wechsler D. Wechsler Adult Intelligence Scale (WAIS) Manual. The Psychological Corporation; New York: 1955. [Google Scholar]
- 19.Capruso DX, Hamsher K. Constructional ability in two- versus three-dimensions: relationship to spatial vision and locus of cerebrovascular lesion. Cerebral Cortex. 2011;47:696–705. doi: 10.1016/j.cortex.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Haymes SJ, Johnston AW, Heyes AD. Relationship between vision impairment and ability to perform activities of daily living. Ophthal Physiol Opt. 2002;22:79–91. doi: 10.1046/j.1475-1313.2002.00016.x. [DOI] [PubMed] [Google Scholar]
- 21.Daien VP, Pérès K, Villain M, Colvez A, Delcourt C, Carrière I. Visual impairment, optical correction, and their impact on activity limitations in elderly persons: the POLA Study. Arch Intern Med. 2011;171:1206–7. doi: 10.1001/archinternmed.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitson HE, Cousins SW, Burchett BM, Hybels CF, Pieper CF, Cohen HJ. The combined effect of visual impairment and cognitive impairment on disability in older people. J Am Geriatr Soc. 2007;55:885–91. doi: 10.1111/j.1532-5415.2007.01093.x. [DOI] [PubMed] [Google Scholar]
- 23.Sikkes SA, de Lange-de Klerk ES, Pijnenburg YA, Scheltens P, Uitdehaag BM. A systematic review of Instrumental Activities of Daily Living scales in dementia: room for improvement. J Neurol Neurosurg Psychiatry. 2009;80:7–12. doi: 10.1136/jnnp.2008.155838. [DOI] [PubMed] [Google Scholar]
- 24.Kempen JH, Krichevsky M, Feldman ST. Effect of visual impairment on neuropsychological test performance. J Clin Exp Neuropsychol. 1994;16:223–31. doi: 10.1080/01688639408402633. [DOI] [PubMed] [Google Scholar]