Abstract
Trochulus oreinos oreinos and T. oreinos scheerpeltzi are two land snail taxa endemic to the Northeastern Austrian Alps, which have been regarded as subspecies of the highly variable, widespread land snail T. hispidus. We analysed these three taxa morphologically and genetically to evaluate whether a delimitation between them is possible and, if so, to resolve their phylogenetic relationships. Shell morphological results revealed high similarity between the two T. oreinos taxa, and that they are clearly separated from T. hispidus. Additionally, the T. oreinos subspecies concur with respect to their habitat preferences, as they are both restricted to rocky high alpine areas, whereas the local form of T. hispidus is distributed over a wider altitudinal range in moist areas and scrubby perennial herb vegetation near water bodies. While the morphological and ecological results allow clear differentiation between T. hispidus and T. oreinos only, analyses of the mitochondrial cytochrome c oxidase subunit I and 16S rRNA genes revealed high sequence divergences between all three taxa, which indicates that they represent old lineages. The two T. oreinos taxa appear as distantly related sister groups, well separated from T. hispidus. Whether T. o. oreinos and T. o. scheerpeltzi should be considered as species cannot be decided at the current state of knowledge.
INTRODUCTION
A large number of land snail species have been described from the Alpine region, including several endemics. Many species have been divided into different infra- or subspecific entities (races, forms etc.), mainly by minor shell morphological features. This differentiation has often been explained by glacial/postglacial events of isolation, displacement, dispersal and (re-)colonization (Adensamer, 1937; Klemm, 1974; Gittenberger, 1991). Moreover, the environment may have triggered special genetic adaptations and/or changes caused by phenotypic plasticity, e.g. smaller shells at higher elevations. Often, subspecific entities have been described from defined altitudinal zones or within the boundaries of particular mountain massifs. Different interpretations and opinions concerning the origin of described morphological variants often resulted in controversial taxonomic conclusions and systematic assignments.
The genus Trochulus Chemnitz, 1786 is one such example. The genus comprises small, pulmonate land snails with flattened or globular, particularly hairy shells, mainly distributed in Central and Western Europe. The CLECOM list (Falkner, Bank & von Proschwitz, 2000) gives up to 18 different species of Trochulus s. s. for central and northern Europe. Two species – Trochulus waldemari (Wagner, 1915) and T. suberectus (Clessin, 1878) – were not included by Falkner et al. (2000), because their distribution ranges are not covered by the CLECOM list. However, the number of recognized species varies among different authors. For example, one of the listed taxa, T. alpicola (Eder, 1921), has not been regarded as an independent species by some authors. Additionally, some authors (e.g. Davis, 2004; Cameron et al., 2006) have expressed objections to some taxonomic decisions in the CLECOM list. Subsequently, Proćków (2009) enumerated 22 species in Trochulus by lumping T. plebeius and T. hispidus at species level and the (sub)genera Petasina and Plicuteria at generic level, based on morphological data.
This study is focused on the poorly known northeastern Alpine endemic T. oreinos (Wagner, 1915), which was originally regarded as regional subspecies of T. hispidus (Linnaeus, 1758), but was later considered as a separate species not closely related to T. hispidus (Falkner, 1982, 1995). According to the current taxonomic view, T. oreinos comprises two subspecies, T. oreinos oreinos (=Fruticicola hispida oreinos) and T. oreinos scheerpeltzi (Mikula, 1957) (=T. hispidus scheerpelzi), which are both endemic to the northeastern Alps in Austria (Fig. 1A, B). Falkner (1982, 1995) based his decision to treat T. o. scheerpeltzi as a subspecies of T. oreinos on ‘differing hair morphology’ of the two T. oreinos taxa. However, he did not provide any details. This is remarkable because the original description of Wagner (1915) characterized T. o. oreinos as hairless, and Mikula (1957) did not mention any hairs in T. o. scheerpeltzi. Because no clear descriptions and pictures are available, knowledge of T. o. oreinos and T. o. scheerpeltzi has been restricted to a few specialists who have inspected specimens collected by the describers and deposited in scientific collections.
Figure 1.
A. Trochulus o. oreinos, specimen from sample site 34. B. Trochulus o. scheerpeltzi, specimen from sample site 45. C. Trochulus hispidus, specimen from sample site 2. Scale bar = 2.0 mm.
Trochulus o. oreinos (Fig. 1A) is found in Lower Austria and Styria at high altitudes (1,600–2,280 m). Its distribution extends from Schneeberg mountain to Totes Gebirge (Klemm, 1974). Reischütz & Reischütz (2009) mentioned rocky grass biotopes and duff as habitats. In the original description Wagner (1915) characterized it by a shiny, finely granular, hairless shell with coarse, irregular ridges. The shell was described as smaller than that of T. hispidus, but ‘more stable’. As additional traits Wagner mentioned a strong lip inside the aperture, visible as a yellow structure from outside, with a tooth-like structure at the basal margin. The type locality is at Hochschwab mountain in Styria at elevations above 2,000 m. So far, no picture of T. o. oreinos has been published.
Trochulus o. scheerpeltzi (Fig. 1B) is found in the mountain ranges of Höllengebirge to Totes Gebirge and in parts of Haller Mauern (Klemm, 1974). Like T. o. oreinos this subspecies is found at high altitudes (1,600–2,300 m) and in a similar habitat – rocky grass biotopes and crevices with duff (Reischütz & Reischütz, 2009). In the original description Mikula (1957) mentioned a groove beneath a clearly visible keel as a trait distinguishing T. o. scheerpeltzi from T. o. oreinos and T. hispidus. The type locality of T. o. scheerpeltzi is ‘Hauptkar’ at the Hohe Nock Mountain in Upper Austria at elevations of 1,600–1,800 m. The only published pictures are those in the original description (Mikula, 1957).
Trochulus hispidus (Fig. 1C) has a wide distribution in Europe, occurring over a broad range of altitudes (up to 2,300 m) and habitats. The distribution covers large parts of Europe from Ireland and France to Kazan and St Petersburg in European Russia. In the north it reaches the Arctic Circle. It does not occur in southernmost parts of Europe (Ložek, 1956). According to Giusti & Manganelli (1987) records from Sardinia are very likely due to confusion with Ichnusotricha berninii. As T. hispidus is a polymorphic species, its systematics have long been the focus of controversy. Forcart (1965) suggested a division of Trichia hispida (nowadays Trochulus hispidus) into two distinct species, Trichia hispida and Trichia concinna. Subsequently, he assigned the two subspecies oreinos and scheerpeltzi to T. concinna. However, Gittenberger, Backhuys & Ripken (1970), followed by various authors including Klemm (1974), Falkner (1982) and Naggs (1985), rejected this theory because large clinal transition zones exist between hispida and concinna and the geographic distribution ranges are not clearly delimited. Shileyko (1978) raised doubts about the justification of the species status of several taxa of the ‘T. hispida group’ (including T. plebeia, T. sericea, T. septentrionalis and T. concinna). Recent papers that have employed molecular biological methods (Pfenninger et al., 2005; Dépraz, Hausser & Pfenninger, 2009) have provided an even more confusing picture, showing several distinct mitochondrial lineages in T. hispidus, and perhaps the occurrence of cryptic species. Proćków (2009) synonymized T. plebeius and T. concinnus with T. hispidus based on an extended morphological analysis.
The questions we wanted to clarify were: (1) Can the three taxa be differentiated morphologically? (2) Are the two T. oreinos taxa and the northeastern Alpine form of T. hispidus genetically differentiated? (3) Do the morphological and genetic data corroborate the species status of T. oreinos? (4) Do the three forms occupy different habitats, elevations and geographic ranges? Using samples covering the entire distribution range of T. o. oreinos and T. o. scheerpeltzi as well as the local forms of T. hispidus we performed morphological and genetic analyses to answer these questions.
The systematics of some species of Trochulus are problematic. This is especially true of T. hispidus with a number of divergent lineages (Pfenninger et al., 2005; Dépraz et al., 2009), among which there may be some cryptic species. However, since no comprehensive phylogenetic study of Trochulus has yet been carried out, we provisionally adopted the classification of the Austrian taxa provided by Reischütz & Reischütz (2007).
MATERIAL AND METHODS
Study area and sampling
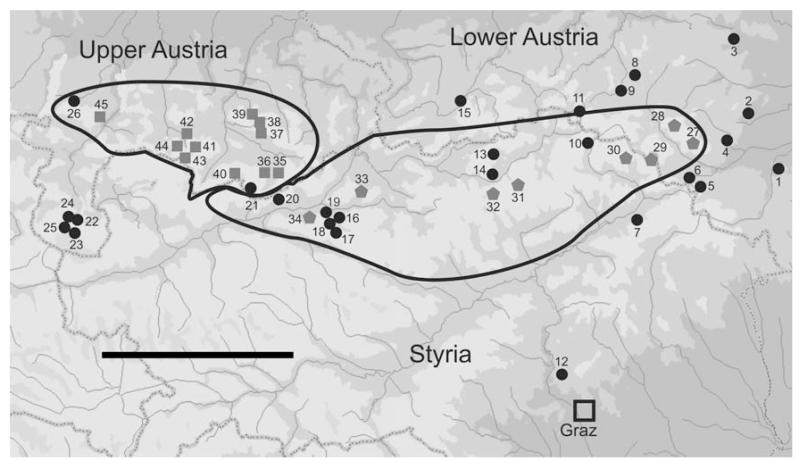
The study area was located in the Northeastern Austrian Alps, including parts of the provinces Upper Austria, Lower Austria and Styria. This covers the distribution ranges of the two T. oreinos taxa (according to Klemm, 1974) and adjacent areas where T. hispidus occurs (Fig. 2). Most sample sites are situated on the limestone bedrock of the Northern Calcareous Alps, one on Palaeozoic limestone of the Grazer Bergland and four on metamorphic rocks of the Central Alps. Most of the study area is characterized by a cool humid Central European climate with heavy precipitation; only the easternmost and southeastern parts are influenced by the warmer and dryer Illyrian and Pannonian climates (Kilian, Müllner & Starlinger, 1994). Both T. oreinos subspecies occur down to an altitude of 1,300–1,450 m, which is also the lower limit of the subalpine zone. Like other marginal Alpine areas, the study area has been the focus of research projects seeking potential glacial refugia (e.g. Schönswetter et al., 2005), because large parts of it remained ice-free during the last glaciations (Van Husen, 1997).
Figure 2.
Sample localities: Trochulus o. oreinos, grey pentagons; T. o. scheerpeltzi, grey squares; T. hispidus, black circles. Numbers correspond to localities in Table 1. Distribution ranges of T. o. oreinos and T. o. scheerpeltzi (according to Klemm, 1974) are delineated with black lines. The scale bar represents 50 km.
Sampling and habitat analysis were carried out at 45 sampling sites (Fig. 2, Table 1). Topotypes of T. o. oreinos (11 specimens from sample site 32) and T. o. scheerpeltzi (11 specimens from sample site 38) were included. Exact positions and elevations of collection sites were determined using GPS. The sampling period extended from May to July in 2007, 2008 and 2009. Adjacent water bodies, vegetation, habitat structure and dominant plant species were recorded. Trochulus hispidus was identified by morphological traits described in the literature (e.g. Ložek, 1956; Gittenberger et al., 1970; Kerney, Cameron & Jungbluth, 1983). Trochulus o. oreinos and T. o. scheerpeltzi were identified using the original descriptions (Wagner, 1915; Mikula, 1957) as well as by comparison with reference specimens (paratypes and syntypes) in the collections of the Natural History Museum, Vienna (NHMW). In total, 327 specimens (225 living animals and 102 empty shells) of the three Trochulus taxa were included (Table 1).
Table 1.
Sampling localities of Trochulus taxa.
| Species | Number of locality | Name of locality | E | T | L | M | S | J | G | A |
|---|---|---|---|---|---|---|---|---|---|---|
| T. hispidus | 1 | Pittental-Schlattenbach | 397 | 3 | 3 | 2 | 0 | 1 | 3 | 2 |
| 2 | Würflach-Johannesbachklamm | 445 | 5 | 3 | 4 | 2 | 1 | 3 | 2 | |
| 3 | Berndorf-Grabenweg | 412 | 6 | 6 | 6 | 0 | 0 | 3 | 3 | |
| 4 | Sierningtal-Stixenstein | 470 | 9 | 4 | 9 | 5 | 0 | 3 | 3 | |
| 5 | Semmering-Maria Schutz | 871 | 3 | 3 | 2 | 0 | 1 | 3 | 2 | |
| 6 | Breitenstein-Adlitzgraben | 650 | 3 | 2 | 3 | 1 | 0 | 2 | 2 | |
| 7 | Fischbacher Alpen-Hauereck | 1,187 | 2 | 2 | 1 | 0 | 1 | 2 | 1 | |
| 8 | Halbachtal-Rossbachklamm | 649 | 5 | 5 | 5 | 0 | 0 | 3 | 3 | |
| 9 | Tiefental-Ochbauer | 739 | 10 | 10 | 10 | 0 | 0 | 3 | 3 | |
| 10 | Frein-Freinbach | 869 | 10 | 10 | 10 | 0 | 0 | 3 | 3 | |
| 11 | Göller-Gscheid | 914 | 10 | 10 | 10 | 0 | 0 | 3 | 3 | |
| 12 | Grazer Bergland-Semriach | 503 | 11 | 10 | 11 | 1 | 0 | 10 | 10 | |
| 13 | Dürradmer-Kräuterin | 1,100 | 3 | 3 | 3 | 0 | 0 | 3 | 3 | |
| 14 | Salzatal-Weichselboden | 660 | 3 | 3 | 2 | 0 | 1 | 3 | 3 | |
| 15 | Dürrenstein-Lechnergraben | 604 | 3 | 3 | 2 | 0 | 1 | 3 | 2 | |
| 16 | Johnsbachtal-Wasserfallmauer | 978 | 5 | 3 | 5 | 2 | 0 | 3 | 3 | |
| 17 | Johnsbachtal-Kölblwirt | 868 | 3 | 3 | 3 | 0 | 0 | 3 | 3 | |
| 18 | Johnsbachtal-Kneippstation | 865 | 3 | 3 | 2 | 0 | 1 | 3 | 2 | |
| 19 | Johnsbachtal-Langriesmündung | 652 | 3 | 3 | 2 | 0 | 1 | 3 | 2 | |
| 20 | Großer Phyrgas-Arlingsattel | 1,425 | 10 | 10 | 10 | 0 | 0 | 2 | 3 | |
| 21 | Warscheneck-Wurzeralmbahn | 810 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | |
| 22 | Hallstatt-Salzberg | 942 | 3 | 3 | 3 | 0 | 0 | 3 | 3 | |
| 23 | Hallstatt-Klausalm | 796 | 4 | 3 | 3 | 1 | 1 | 3 | 2 | |
| 24 | Hallstatt-Sportplatz | 524 | 3 | 3 | 2 | 0 | 1 | 3 | 2 | |
| 25 | Hallstatt-Waldbachstrub | 806 | 4 | 4 | 4 | 0 | 0 | 4 | 4 | |
| 26 | Hochlecken-Taferlklause | 778 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | |
| T. o. oreinos | 27 | Schneeberg-Waxriegel | 1,873 | 41 | 3 | 41 | 38 | 0 | 3 | 3 |
| 28 | Schneeberg-Fadenwände | 1,562 | 7 | 2 | 6 | 5 | 1 | 2 | 1 | |
| 29 | Rax-Bismarksteig | 1,787 | 36 | 6 | 31 | 30 | 5 | 6 | 1 | |
| 30 | Schneealpe-Schauerkogel | 1,664 | 11 | 9 | 11 | 2 | 0 | 3 | 3 | |
| 31 | Hochschwab-Severinkogel | 2,010 | 1* | 1 | 0 | 0 | 0 | 1 | 0 | |
| 32 | Hochschwab-Schiestlhaus | 2,179 | 11 | 11 | 10 | 0 | 1 | 3 | 2 | |
| 33 | Tamischbachturm | 1,940 | 10 | 1 | 10 | 9 | 0 | 3 | 3 | |
| 34 | Admonter Kalbling | 2,026 | 9 | 6 | 9 | 3 | 0 | 6 | 6 | |
| T. o. scheerpeltzi | 35 | Großer Phyrgas-Haller Mauern | 1,900 | 11 | 11 | 10 | 0 | 1 | 3 | 2 |
| 36 | Großer Phyrgas-Westgrat | 2,000 | 3* | 3 | 2 | 0 | 0 | 3 | 3 | |
| 37 | Hohe Nock-Feichtausee | 1,399 | 10 | 10 | 10 | 0 | 0 | 2 | 2 | |
| 38 | Hohe Nock-Hauptkar | 1,704 | 11 | 11 | 11 | 0 | 0 | 3 | 3 | |
| 39 | Hohe Nock-Haltersitz | 1,583 | 10 | 10 | 10 | 0 | 0 | 3 | 2 | |
| 40 | Warscheneck-Toter Mann | 2,028 | 2 | 1 | 2 | 1 | 0 | 1 | 1 | |
| 41 | Großer Priel-Welser Hütte | 1,747 | 9 | 9 | 8 | 0 | 1 | 3 | 2 | |
| 42 | Großer Priel-Hinterer Ackergraben | 1,564 | 2 | 2 | 2 | 0 | 0 | 2 | 2 | |
| 43 | Großer Priel-Schlund | 2,284 | 2 | 2 | 2 | 0 | 0 | 2 | 2 | |
| 44 | Großer Priel-Fleischbanksattel | 2,157 | 12 | 12 | 10 | 0 | 2 | 3 | 1 | |
| 45 | Höllengebirge-Bledigupf | 1,677 | 3 | 1 | 3 | 2 | 0 | 1 | 1 | |
| 327 | 225 | 304 | 102 | 21 | 132 | 111 |
Abbreviations: E, elevation (metres above sea level); T, total number of investigated specimens; L, total number of living specimens (adult and juvenile); M, total number of morphologically investigated specimens (adult; living and empty shells); S, total number of adult empty shells; J, total number of living juvenile specimens; G, total number of genetically investigated specimens (selected living adult and all juvenile specimens); A, total number of specimens investigated genetically and morphologically.
One adult living specimen of each of these samples was broken.
Shell morphology
Four shell traits were measured in intact adult specimens (shell diameter, umbilicus diameter, shell height and height of last whorl) with a graduated eyepiece in a stereomicroscope. The definition of adulthood from shell apertural traits was problematic, as presumably adult specimens of T. hispidus often lack an outer lip (Geyer, 1915; Frömming, 1954; Cameron, 1982). Therefore, individuals were defined as ‘adult’ when their shells had a minimum diameter of 5.4 mm, as this was the size of the smallest individual seen with a fully developed lip. This might appear arbitrary, because the standard literature and some collections contain only ‘typical’ specimens with outer lip. However this analysis was intended to include all naturally occurring variants and therefore we had to define a size limit. Altogether 304 specimens (complete specimens and empty shells) from all 45 sample sites were measured and standard variables (mean, variance and standard deviation) were calculated. Hairs (which are present also in juveniles) were inspected in all 327 individuals. Another two adult specimens could be investigated only genetically, because their shells were broken. A total of 111 specimens were investigated using all methods (Table 1). The measurement error is too often neglected in measurements of small (<10 mm) globular shells. The main source of error is the lack of precise measurement points on the shell. Furthermore, the definition of the main axes is not very precise and the projection of the shell in two dimensions is problematic. Measurement error was determined by repeated measurements (10 times) of shell diameter, umbilicus diameter and shell height in 140 empty shells of Trochulus and examination of the distribution of residuals (total 4,200).
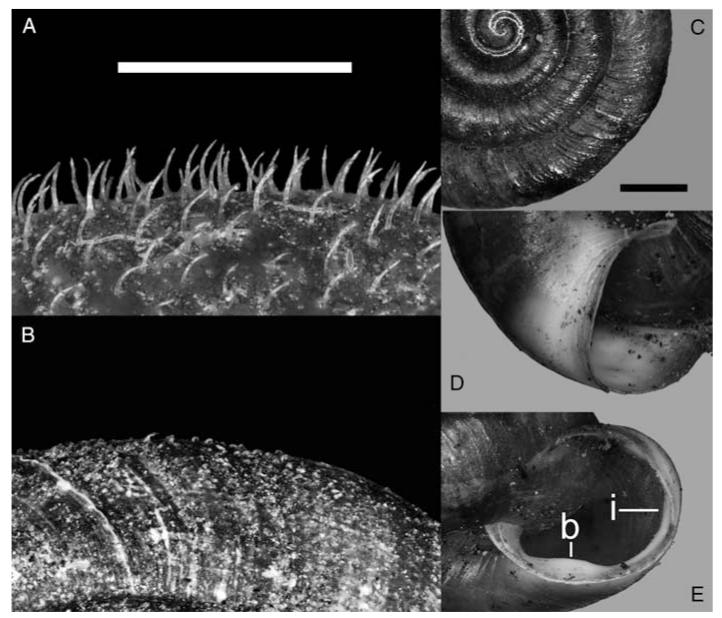
Four additional shell traits were recorded as presence/absence, because they were mentioned as typical for T. o. oreinos in the original description (Wagner, 1915). Three of these were apertural traits: basal tooth, internal rib and paler area around the aperture (Fig. 3). The occurrence of coarse, irregular ridges was also recorded as presence/absence. Ridges were classified as ‘coarse’ if broad ridges (>0.5 mm) were immediately followed by smaller ones (Fig. 3). In T. o. scheerpeltzi a groove beneath the keel was described by Mikula (1957) and was recorded by us in three categories: well developed, partly developed and absent. If the groove was clearly visible with ×16 magnification and covered at least 50% of the circumference, it was characterized as well developed. If it covered less than this and was only weakly visible, it was characterized as partly developed. Measurements of shells were log-transformed. These data together with scores obtained with a correspondence analysis of presence/absence data were used in a canonical discriminant analysis (Tabachnick & Fidell, 1996). Morphometric analyses were performed with programs written by one of us (H.W.). Since we were interested in identifying group differences and the variables responsible for these differences, we used discriminant analysis rather than PCA or ordination techniques that deal with overall variation, which might be dominated by variables that do not contribute to, or even mask, the variation among groups.
Figure 3.
Shell traits. A. Hairs of Trochulus hispidus (specimen from sample site 17). B. Hairs of T. o. scheerpeltzi (specimen from sample site 37). C–E. Trochulus o. oreinos specimen from sample site 29. C. Coarse riffles. D. Pale area around aperture. E. Internal rib (i) and basal tooth (b). Scale bars = 1.0 mm.
To quantify hair length and structure, digital microscopic images were taken of five hairs of 15 specimens (five of each form). Hair lengths were measured by using TPSdig Version 2.14 (Rohlf, 2001). To proof the repeatability of measurements, all hairs were measured twice.
As measuring of hairs takes a lot of time and the different hair morphologies can be easily recognized (Fig. 3), the values from all specimens were assigned to three categories: long hairs (>0.2 mm), short hairs (<0.1 mm) and no hairs.
Genetic analysis
From 132 specimens (adults and juveniles) a partial region of the mitochondrial cytochrome c oxidase subunit I (COI) gene was sequenced. In addition, from representatives of each clade a partial region of the mitochondrial 16S rRNA (16S) gene was sequenced (altogether 38). As outgroup taxa Monacha cantiana and Plicuteria lubomirskii (one specimen each) were analysed for both fragments.
A piece of foot tissue was extracted with QIAgen Blood and Tissue Kit. Primers were based on those used by Gittenberger, Piel & Groenenberg (2004) for COI and by Pfenninger, Posada & Magnin, (2003) for 16S. Primers were optimized on the basis of alignments of published sequences of several snail species. Primer sequences for COI: COIfolmerfwd 5′-GGTCAACAATCATAAAGATATTGG-3′ (LCO1490 modified from Folmer et al., 1994) and COIschneckrev 5′-TATACTTCTGGATGACCAAAAAATCA-3′ (H2198-Alb modified from Gittenberger et al., 2004). Primer sequences for 16S: 16Sfw 5′-CGCAGTACTCTGACTGTGC-3′ (Pfenninger et al., 2003) and 16S_sch_rev 5′-CG CCGGTCTGAACTCAGATC-3′ (16Srev modified from Pfenninger et al., 2003). Resulting fragment sizes were 705 bp (COI) and about 395 bp (16S), respectively. PCR was performed on a Master gradient thermocycler (Eppendorf) in 50 μl with 1 U Taq DNA polymerase (Roche), 1 μM of each primer and 0.2 mM of each dNTP (Boehringer Mannheim). Each PCR comprised 35 reaction cycles with the following annealing temperatures: 50°C (COI) and 55°C (16S). Control reactions for both DNA extractions and PCR amplifications were carried out. PCR products were purified using the QIAquick PCR Purification kit (QIAGEN) and analysed with direct sequencing (both directions). Sequencing using the amplification primers was performed by AGOWA (Berlin, Germany).
For the COI sequences the alignment was straightforward as there were no insertions or deletions. Alignment of 16S sequences was performed with Tcoffee (Notredame, Higgins & Heringa, 2000) and adjusted manually. Neighbour-joining (NJ; Saitou and Nei, 1987) dendrograms were calculated with ClustalX v. 2.0.12 (Larkin et al., 2007) using p-distances. Bootstrap analyses were performed with 1,000 replicates. For calculation of models of sequence evolution the sequences were collapsed to haplotypes using Collapse1.2 (Posada, 2004). The resulting dataset was used applying the Akaike information criterion corrected for small sample size (AICc) as implemented in the jModelTest v. 0.1.1. (Posada, 2008); the selected models were HKY + I + G for the COI dataset, GTR + G for the 16S dataset and GTR + I + G for the combined (COI + 16S) dataset. Bayesian analyses (BI) were performed using MrBayes v. 3.1.2 (Huelsenbeck & Ronquist, 2001). Runs were started with random trees and performed for 2 million generations each with four Markov chains, and a sampling frequency of every 100th generation. Those trees generated prior to stationarity were discarded as burn-in and were not included in the calculation of the consensus trees.
Numbers of haplotypes and haplotype diversity and average p-distances (gaps excluded) were calculated with ARLEQUIN v. 3.11 (Excoffier, Laval & Schneider, 2005). The sequences determined in this study are deposited in GenBank under the accession numbers HQ204370–HQ204503 (COI) and HQ204504–HQ204543 (16S).
RESULTS
Shell morphology
Trochulus hispidus shows higher variation in all shell measurements than the T. oreinos forms, which are at least 0.5 mm smaller (Table 2). Of the four measurements, shell width and umbilicus width differed between the two T. oreinos taxa. Although the T. oreinos subspecies are smaller and less variable than T. hispidus, ranges overlap. Small specimens of T. hispidus (e.g. those from sample site 17 at Sierningtal-Stixenstein) overlap the range of T. oreinos. Therefore, shell measurements alone are not a suitable discriminating character for the three taxa. Although the systematic measurement error was relatively high (with 1% error probability, from ±0.14 to ±0.18 mm), it did not compromise these results (see Table 2). Measures showed small differences between T. o. oreinos and T. o. scheerpeltzi, but large differences between both taxa and T. hispidus.
Table 2.
Summary of shell measurements (mm) of Trochulus taxa.
| Range | ME | Mean | SD | SE | ||
|---|---|---|---|---|---|---|
| T. o. oreinos, 8 sample sites, n = 118 | WS | 5.4–7.5 | 0.15 | 6.37 | 0.43 | 0.04 |
| WU | 0.8–1.6 | 0.14 | 1.24 | 0.15 | 0.01 | |
| SH | 2.8–4.2 | 0.18 | 3.42 | 0.28 | 0.03 | |
| HW | 2.0–2.8 | 0.17 | 2.40 | 0.19 | 0.02 | |
| T. o. scheerpeltzi, 11 sample sites, n = 70 | WS | 5.6–7.5 | 0.15 | 6.50 | 0.38 | 0.05 |
| WU | 0.9–1.5 | 0.14 | 1.16 | 0.13 | 0.02 | |
| SH | 2.8–4.0 | 0.18 | 3.43 | 0.25 | 0.03 | |
| HW | 2.0–2.9 | 0.17 | 2.39 | 0.18 | 0.02 | |
| T. hispidus, 26 sample sites, n = 116 | WS | 5.5–8.4 | 0.15 | 7.18 | 0.65 | 0.06 |
| WU | 1.0–2.3 | 0.14 | 1.59 | 0.22 | 0.02 | |
| SH | 2.9–4.7 | 0.18 | 3.79 | 0.36 | 0.03 | |
| HW | 2.1–3.5 | 0.17 | 2.80 | 0.26 | 0.02 |
Abbreviations: WS, width of shell; WU, width of umbilicus; SH, shell height; HW, height of last whorl; ME, measurement error; SD, standard deviation; SE, standard error of the mean.
At first sight T. hispidus has remarkably longer hairs than both T. oreinos subspecies. Hair length of T. hispidus ranges from 0.21 to 0.31 mm (mean 0.27 mm), of T. o. oreinos from 0.03 to 0.09 mm (mean 0.06 mm) and of T. o. scheerpeltzi from 0.04 to 0.08 mm (mean 0.06 mm). Additionally, hairs of the T. oreinos subspecies are often curled or strongly bent, while those of T. hispidus are only slightly bent. The problem with this trait is that elder specimens and empty shells often lack hairs. For example, of the 118 specimens investigated in T. o. oreinos, 86 were hairless, but of these individuals 78 were empty shells. Among T. o. scheerpeltzi individuals 59 out of 70 were hairy, most of them collected alive. Among the 116 specimens of T. hispidus 106 showed the characteristic long hairs.
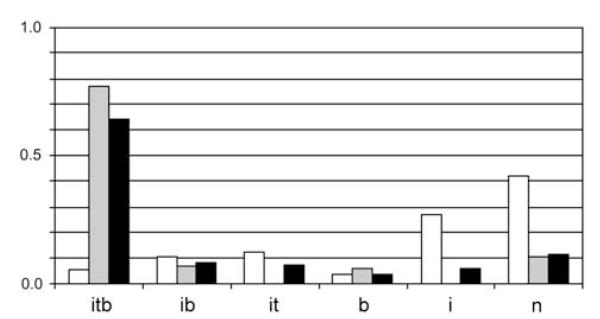
The T. oreinos taxa show strong development of the lip and aperture (internal rib, basal tooth, pale around aperture), while in T. hispidus these traits occur only occasionally and rarely in combination (Fig. 4). Most specimens of T. hispidus show only an internal rib or even none of these traits. Both T. oreinos forms consistently show strong irregular riffles, while only 8 of 116 specimens of T. hispidus have this character. The groove beneath the keel proved not to be a constant character of T. o. scheerpeltzi as two specimens were found in which this trait was virtually absent. On the other hand, nine specimens of T. o. oreinos were found in which a faint groove was present, five of them from site 34 (Tamischbachturm). At this same site three T. o. oreinos with a well-developed groove were found.
Figure 4.
Combination of apertural traits of Trochulus o. oreinos, T. o. scheerpeltzi and T. hispidus in proportion values. White bars, T. hispidus; grey bars, T. o. oreinos; black bars, T. o. scheerpeltzi. itb: internal rib, basal tooth, pale area around aperture; ib: internal rib, pale area around aperture; b: pale area around aperture; i: internal rib; n: none of these traits.
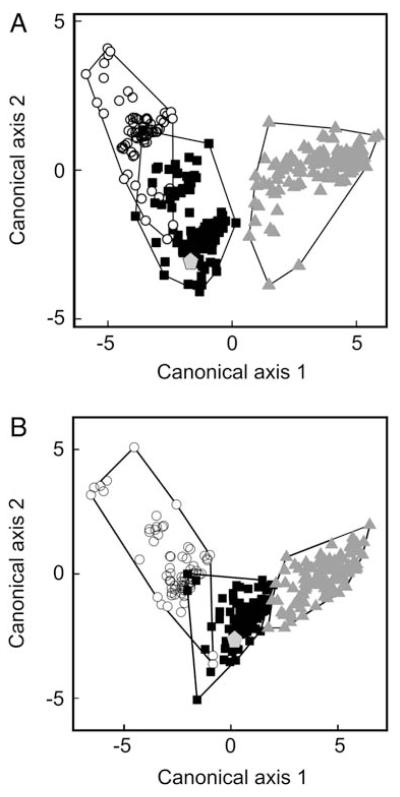
The occurrence of coarse riffles could not be included in the discriminant analysis, as it is a constant trait of both T. oreinos forms. The variables which most strongly influenced the results of the analysis were the qualitative characters (hair length, groove, internal rib, basal tooth, pale area), especially the apertural traits. The dominance of these factors on the first axis caused a visible ‘horseshoe’ effect (Fig. 5A). The results show a clear differentiation between T. o. scheerpeltzi and T. hispidus. Trochulus o. oreinos and T. hispidus are visibly differentiated but still close. The two T. oreinos taxa show an overlap in the discriminant analyses (polygons in Fig. 5A) mainly caused by the occurrence of a groove beneath the keel of some specimens of T. o. oreinos. The first axis explains 84% and the second 16% of the total variation. The first axis separates T. hispidus from the other populations, and correlates negatively with the scores associated with the first reciprocal ordering component (mainly defined by the groove beneath the keel and hair length), and positively with all linear measurements, indicating a general size difference. Trochulus o. scheerpeltzi scores higher on the second axis, which is mainly characterized by a negative correlation with the scores derived from the second axis of the correspondence analysis (mainly defined by strong development of the groove beneath the keel) (Table 3). Hair length could not be analysed in several shells which had obviously lost the hairs (empty shells and some live-collected). However, as this is an essential discriminant trait, we included it in our analysis. An additional discriminant analysis excluding hair length is shown in Figure 5B. It reveals the same groups, although T. o. oreinos and T. hispidus are less clearly separated from each other.
Figure 5.
Discriminant analysis of Trochulus o. scheerpeltzi, T. o. oreinos and T. hispidus. The outlier was treated separately as we considered it a malformation (see text). A. Discriminant analysis including hair traits. Wilks’ Lambda = 4.6328E–02, F14,590 = 153.7, P < 0.00001. B. Discriminant analysis excluding hair traits. Wilks’ Lambda = 4.2035E–02, F14,588 = 120.1, P < 0.00001. Grey triangles, T. hispidus; white circles, T. o. scheerpeltzi; black squares, T. o. oreinos; grey pentagon, T. hispidus (outlier).
Table 3.
Correspondence analysis of Trochulus taxa: linear discriminant coefficients of linear discriminant.
| Variable | Linear discriminant coefficients |
|
|---|---|---|
| 1 | 2 | |
| WS | −0.35081 | −0.26637 |
| WU | 0.57778 | 0.11054 |
| SH | 0.28020 | 0.69537 |
| HW | 0.47491 | 0.34116 |
| ROC1 | −0.44336 | 0.05416 |
| ROC2 | 0.10844 | −0.46297 |
| ROC3 | −0.17531 | 0.31563 |
Abbreviations: WS, width of shell; WU, width of umbilicus; SH, shell height; HW, height of last whorl; ROC1–3, reciprocal order components.
There is one outlier of T. hispidus, a rather small, hairless, empty shell (site 4) which has an internal rib and a pale area around the aperture. This outlier was not included in the computation of the discriminant function, but was subsequently scored to show its position (Fig. 5).
Habitat selection and elevation
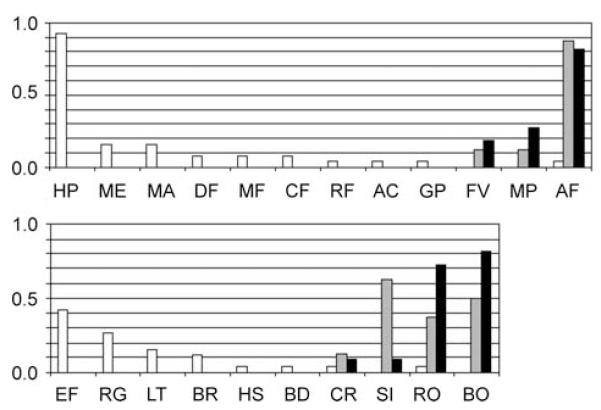
Trochulus o. oreinos was found at elevations from 1,562 to 2,179 m, T. o. scheerpeltzi from 1,399 to 2,157 m and T. hispidus from 397 to 1,425 m (Table 1). Both T. oreinos taxa were found to be restricted to rocky habitats, mostly among sparse alpine grass or in vegetation-free areas and mountain pine shrubbery, while T. hispidus preferred moist habitats, in particular tall perennial herbs, often near water bodies (Fig. 6). Trochulus hispidus inhabits a wider range of vegetation types and landscape structures than both T. oreinos forms.
Figure 6.
Habitat (top) and landscape structure (below) preferences of the three Trochulus taxa in proportion values. White bars, T. hispidus; grey bars, T. o. oreinos; black bars, T. o. scheerpeltzi. Abbreviations: HP, high perennial herbs; ME, meadow; MA, marsh; DF, deciduous forest; MF, mixed forest; CF, coniferous forest; RF, riparian forest; AC, alder carr; GP, garden/park; FV, free of vegetation; MP, mountain pine shrubbery; AG, alpine grassland; EF, edge of forest; RG, riverbank grove; LT, losse trees and shrubs; BR, boundary ridge; HS, hedgerows and shrubs; BD, bank/dam; CR, canyon/rock face; SI, single stones; RO, rocks; BO, boulders.
Molecular analysis
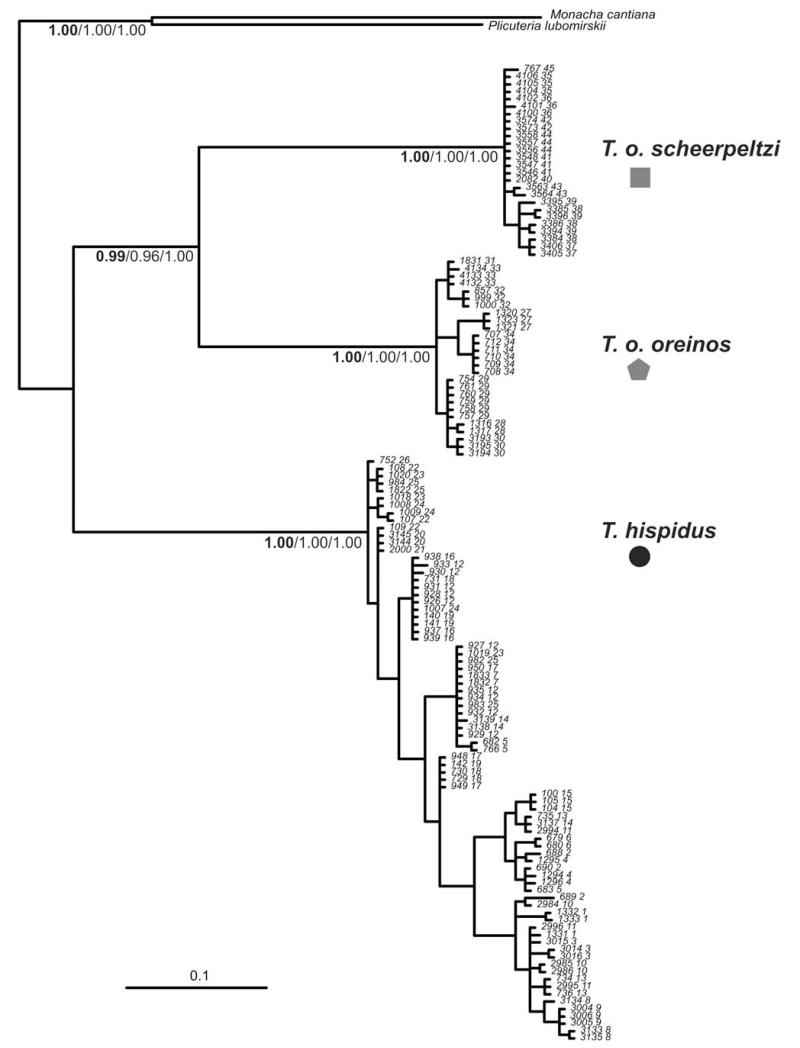
Among the 132 individuals analysed for COI, 51 different haplotypes were detected. In the 38 individuals from which 16S sequence was obtained, 19 different haplotypes were observed. In the BI tree based on the COI dataset (Fig. 7) three clearly differentiated groups are found: (1) T. hispidus, (2) T. oreinos scheerpeltzi and (3) T. oreinos oreinos. The same topology was obtained from both 16S and combined datasets, and with a different tree-building algorithm (NJ; data not shown). All three taxa are highly supported (maximum posterior probability) in analyses of all three datasets. The two T. oreinos taxa are well-supported sister groups in all trees. The genetic distances among the three clades are high. Average p-distances in the COI sequences are 13.3% between the two T. oreinos taxa. Between T. hispidus and T. o. oreinos, and T. o. scheerpeltzi, the values are 14.4% and 16.0%, respectively. The average p-distance for the 16S sequences is 13.7% between the two T. oreinos taxa. Between T. hispidus and T. o. oreinos and T. o. scheerpeltzi the values are 18.0% and 17.4%, respectively. Both T. oreinos subspecies have a somewhat lower haplotype diversity (T. o. scheerpeltzi: 0.88 and T. o. oreinos: 0.70) compared to T. hispidus (0.95), but the number of sample sites is different in all three (T. hispidus 26, T. o. oreinos 8, T. o. scheerpeltzi, 11). In T. oreinos specimens from one locality had either the same or very similar haplotypes, while in T. hispidus quite distinct haplotypes coexist even within one locality (e.g. sample site 25).
Figure 7.
Bayesian tree of the COI sequences of Trochulus species. Posterior probabilities of the main groups are indicated at the nodes (COI/16S/COI + 16S). Posterior probabilities of the depicted tree (COI) are in bold. Labelling of the individuals include individual number and locality (before the space; as in Table 1). Monacha cantiana and Plicuteria lubomirskii were used as outgroups.
DISCUSSION
The results obtained by the two different approaches (morphological and genetic) agree only in some aspects. The clear differentiation between Trochulus hispidus and the two T. oreinos taxa is supported by hair and shell morphology, genetic analysis and ecological preferences. In contrast, a clear differentiation between T. o. oreinos and T. o. scheerpeltzi is only found in the genetic analyses, but not in morphology or ecological preferences.
Differentiation between T. hispidus and T. oreinos
Trochulus o. oreinos and T. o. scheerpeltzi are very similar to each other shell morphology and ecology, while T. hispidus differs from these taxa in both respects and also appears to be more variable. Hair morphology discriminates the T. oreinos subspecies from T. hispidus, and this character is also useful for juvenile specimens. The basic pattern for hair length and distance between hairs in Trochulus is supposed to be a stable trait as it is determined by glands in the mantle (Kaiser, 1966). Other shell morphological traits also show clear differences between T. oreinos subspecies and T. hispidus. A constant trait of both T. oreinos subspecies are the coarse riffles. Furthermore, only 20% of both T. oreinos subspecies, but nearly 70% of T. hispidus, did not show fully developed apertural traits. Seasonality cannot explain this observation, as the snails were collected at similar times of the year. A lack of fully developed apertural traits in adult specimens of T. hispidus has been reported several times in the literature. Weinland (1883) mentioned that it is difficult to find specimens of T. hispidus with a fully developed lip. A similar finding was reported by Geyer (1915), who noticed that in Upper Austrian populations of T. hispidus many adult specimens did not show a lip.
Shell measurements alone are not enough for a reliable differentiation. Furthermore, these traits can also be influenced by the environment (Davis, 2004). Our results show that T. hispidus is more variable than both T. oreinos subspecies. Other authors have also described substantial shell variability in T. hispidus, which can result in difficulties in species recognition (Ložek, 1956; Forcart, 1965; Naggs, 1985; Von Proschwitz, 1993; Proćków 2009). However, in contrast to previous studies, we found no specimens with pronounced globular shells.
With respect to habitat preferences a clear difference was revealed between T. hispidus and T. oreinos. Both T. oreinos subspecies are restricted to rocky areas with alpine vegetation at high elevations, whereas T. hispidus extends over a larger range, predominantly in lower areas, preferring moist habitats with a well-developed herb layer, often close to water bodies. The small overlap in the altitudinal distribution of T. hispidus and T. oreinos subspecies might be due to the geomorphological conditions of the northern Calcareous Alps. Springs and creeks usually discharge at the base of the mountain massifs, while plateaus and slopes at high elevation remain dry (Lieb, 1991). Where damp habitats extend up to higher elevation, T. hispidus can be found above 1,000 m, as at the sampling sites ‘Fischbacher Alpen-Hauereck’ (1,180 m) and ‘Haller Mauern-Arlingsattel’ (1,425 m). The wide distribution of T. hispidus even in formerly glaciated areas of Austria might be due to the fact that rivers and creeks act as linear corridors along which the snails can easily disperse (actively or passively via rafting). The occurrence of T. o. scheerpeltzi at 1,399 m at the site ‘Hohe Nock-Feichtausee’ is the lowest one ever recorded for this taxon, as the known range is 1,600–2,300 m (Klemm, 1974). Nevertheless, this site fits the habitat preferences of T. o. scheerpeltzi because it is an azonal deep habitat of high alpine vegetation resulting from the cool microclimate of a sunless shady slope with northern exposure.
Our genetic data are in accordance with the morphological and ecological differentiation between T. hispidus and T. oreinos. We cannot conclude that T. oreinos and T. hispidus are sister species, owing to our limited sampling of other taxa, and because the phylogenetic status and monophyly of T. hispidus are unclear (see above). This underlines the need for a complete phylogeny of the genus. The high genetic distances (mean distance for COI 15.2%) (only slightly higher than those between the two T. oreinos subspecies: mean distance for COI 13.3%) suggest that the differentiation between the lineages leading to these two species might have taken place before the Pleistocene glaciations. Also, the difference in the morphological variability in T. oreinos and T. hipidus is reflected in the haplotype variation, T. hispidus showing higher haplotype diversity than the other two taxa. The haplotype diversity found within the T. oreinos taxa is rather low (0.88 and 0.70). Direct comparison of the haplotype diversity is problematic, since the number of sampled localities is higher in T. hispidus than in the two T. oreinos taxa, but it is still meaningful since we sampled the whole distribution range of the two T. oreinos taxa. Hence, it cannot be expected that the diversity will increase much by including more localities and samples. Only a part of the distribution range was sampled T. hispidus, but well-differentiated haplotypes can be found within a single locality. The low haplotype diversity within the two T. oreinos taxa is remarkable, since the populations live on isolated mountain peaks. This suggests past extinction and recolonization events, perhaps during glacial periods. Other studies have reported highly divergent lineages within T. hispidus (Pfenninger et al., 2005; Dépraz et al., 2009). However, in our analysis all T. hispidus from the foothills of the northeastern Austrian Alps belong to a single haplogroup, distantly related to the haplogroups of T. hispidus published so far (Pfenninger et al., 2005, Dépraz et al., 2009). Thus, our analysis reveals yet another lineage that morphologically resembles T. hispidus.
Differentiation between T. o. oreinos and T. o. scheerpeltzi
While the morphological differentiation between T. hispidus and T. oreinos is straightforward, the distinction between T. o. oreinos and T. o. scheerpeltzi is difficult, as there are no apparent differences in hair and shell morphology, size and habitat selection. The only discriminating characters mentioned in the literature are the different geographical ranges and the groove beneath the keel. However, our results indicate that the groove is not a constant trait of T. o. scheerpeltzi as some specimens lack this characteristic, whereas specimens with a groove were found at the western sample sites of T. o. oreinos. The discriminant analysis (Fig. 5) showed overlap of the two taxa. These findings are in accordance with reports of intermediate forms between the two T. oreinos taxa (Mikula, 1957; Falkner, 1970, 1982) and suggest why the two forms have been classified as subspecies. However, from the molecular results there is no indication of hybridization between T. o. oreinos and T. o. scheerpeltzi. The distribution of mitochondrial haplotypes is completely in accordance with the distribution ranges of the two taxa; no haplotypes of T. o. oreinos were found in the range of T. o. scheerpeltzi or vice versa. It could be hypothesized that the groove is just a result of phenotypic plasticity and/or connected with a yet unknown adaptation to climate or habitat conditions. As the distribution ranges of both subspecies belong to the same geological formation, it is unlikely that geological factors could be of importance. In this region there is a transition between two local climatic zones – western vs eastern part of the northern margin of the northeastern Alps (as defined by Kilian et al., 1994). The transition between these two climatic zones covers the eastern part of the distribution area of T. o. scheerpeltzi as well as the western part of the T. o. oreinos distribution (Fig. 2), i.e. regions where some morphologically atypical individuals (T. o. oreinos possessing a groove, T. o. scheerpeltzi lacking a groove) were detected. Subtle climate differences might in some way influence the formation of a partially or fully developed groove. Another explanation for the presence of a groove in the specimens from Tamischbachturm is that they are malformations, although it is hard to explain why this should have happened coincidentally to many individuals.
The genetic data present a different picture. Trochulus o. scheerpeltzi and T. o. oreinos are well separated. The p-distance values for COI and 16S are surprisingly high. It has been suggested that terrestrial gastropods might have a substantially higher substitution rate in their mtDNA than that reported for other animal groups (Thomaz, Guiller & Clarke, 1996; Hayashi & Chiba, 2000; Davison, 2002; Van Riel et al., 2005). However, this is not a plausible explanation for the surprisingly high distances found between the Trochulus taxa, since preliminary analysis of nuclear 5.8S rRNA, ITS2 and 28S rRNA sequences support the hypothesis of an old split (LK, unpublished results). It should be mentioned that many studies report moderate evolutionary rates in molluscs (e.g. Pfenninger & Magnin, 2001; Haase et al., 2003; Ketmaier, Giusti & Caccone, 2006). Hence, our data suggest a long separation of T. o. scheerpeltzi and T. o. oreinos.
The shell morphological traits and ecological preferences do not provide strong arguments to raise the two subspecies T. o. oreinos and T. o. scheerpeltzi to species level. They also do not differ in the gross anatomy of the reproductive organs (Mikula, 1957). More detailed anatomical analyses (e.g. cross-sections of penis and vagina) should be performed to search for differences in these traits. Applying the ecological and phylogenetic species concepts, T. o. oreinos and T. o. scheerpeltzi are not unequivocally separated, as they inhabit the same habitat and no diagnostic morphological traits have been detected so far. The status of T. oreinos as an independent species separated from T. hispidus (Falkner, 1982) is corroborated by morphological (groove, hairs) and ecological (habitat preferences) arguments. The clear genetic separation corresponding to the geographical distributions did not provide any indication for interbreeding between the two taxa. Nevertheless, in the light of the biological species concept further analyses (e.g. ecophysiological, anatomical, breeding experiments) are needed to test the potential for interbreeding before a final decision can be made about species or subspecies status.
ACKNOWLEDGEMENTS
The Austrian Science Foundation supported this work (FWF Proj.-No. 19592-B17). The Friends of the Museum of Natural History Vienna provided financial support for travel expenses. We are very grateful to Wilhelm Pinsker and Luis Cabahía for many valuable discussions and critical comments on the manuscript. We are also obliged to Kurt Jordaens for numerous helpful suggestions. We thank Barbara Däubl and Laura Zopp for excellent technical assistance in the lab as well as during field collections. For help during collection trips we thank Barbara Däubl, Philipp Haselwanter, Wilhelm Pinsker, Anatoly Shileyko, Erich Weigant, Laura Zopp and Sabine Zwierschitz. Special thanks are due to Helmut Baminger, Reiko Slapnik and Agnes Bisenberger for collecting T. oreinos specimens on Admonter Kalbling. Funding to pay the Open Access publication charges for this article was provided by the Austrian Science Foundation.
REFERENCES
- ADENSAMER W. Cylindrus obtusus (Draparnaud 1805), seine relikthafte Verbreitung und geringe Variabilität, sowie zoogeographisch-phylogenetische Betrachtungen über alpine Gastropoden überhaupt. Archiv für Molluskenkunde. 1937;69:66–115. [Google Scholar]
- CAMERON RAD. Life histories, density and biomass in a woodland snail community. Journal of Molluscan studies. 1982;48:159–166. [Google Scholar]
- CAMERON RAD, POKRYSZKO BM, RIEDEL A, WIKTOR A. Checklists, systematics and the Clecom initiative: an alternative view from Europe. Malacologia. 2006;49:225–230. [Google Scholar]
- DAVIS GM. Species check-lists: death or revival of the nouvelle école? Malacologia. 2004;46:227–231. [Google Scholar]
- DAVISON A. Land snails as a model to understand the role of history and selection in the origins of biodiversity. Population Ecology. 2002;44:129–136. [Google Scholar]
- DÉPRAZ A, HAUSSER J, PFENNINGER M. A species delimitation approach in the Trochulus sericeus/hispidus complex reveals two cryptic species within a sharp contact zone. Bio Med Central Evolutionary Biology 2009. 2009;9:171. doi: 10.1186/1471-2148-9-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EXCOFFIER L, LAVAL G, SCHNEIDER S. Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evolutionary Bioinformatics Online. 2005;1:47–50. [PMC free article] [PubMed] [Google Scholar]
- FALKNER G. Molluskenfunde aus Österreich (Auszüge aus dem Katalog der Molluskensammlung) Museum of Natural History; Vienna: 1970. Unpublished manuscript deposited at the library of the 3rd Zoological Department. [Google Scholar]
- FALKNER G. Zur Problematik der Gattung Trichia (Pulmonata, Helicidae) in Mitteleuropa. Mitteilungen der Deutschen Malakologischen Gesellschaft. 1982;3:30–33. [Google Scholar]
- FALKNER G. Beiträge zur Nomenklatur europäischer Binnenmollusken, VII. Nomenklaturnotizen zu europäischen Hygromiidae (Gastropoda: Stylommatophora) Heldia. Münchner malakologische Mitteilungen. 1995;2:97–107. [Google Scholar]
- FALKNER G, BANK RA, von PROSCHWITZ T. Check-list of the non-marine Molluscan Species group taxa of the states of Northern, Atlantic and Central Europe (CLECOM I) Heldia. Münchner malakologische Mitteilungen. 2000;4:1–76. [Google Scholar]
- FOLMER O, BLACK M, HOEH W, LUTZ R, VRIJENHOEK R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biolology and Biotechnology. 1994;3:294–299. [PubMed] [Google Scholar]
- FORCART L. New researches on Trichia hispida (Linnaeus) and related forms. Proceedings of the First Malacological Congress (London 1962) 1965:79–93. [Google Scholar]
- FRÖMMING E. Biologie der mitteleuropäischen Landgastropoden. Duncker & Humpold; Berlin: 1954. [Google Scholar]
- GEYER D. Über die Molluskenfauna des Salzkammergutes und ihre Beziehung zum Diluvium in Schwaben. Verhandlungen der kaiserlich-königlichen zoologischen Gesellschaft Wien. 1915;64:270–189. [Google Scholar]
- GITTENBERGER E. Altitudinal variation and adaptive zones in Arianta arbustorum: a new look at a widespread species. Journal of Molluscan Studies. 1991;57:99–109. [Google Scholar]
- GITTENBERGER E, BACKHUYS W, RIPKEN Th.E.J. De Landslakken van Nederland. Bibliothek van de Koninklijke Nederlandse Natuurhistorische Vereniging; Amsterdam: 1970. [Google Scholar]
- GITTENBERGER E, PIEL WH, GROENENBERG DSJ. The Pleistocene glaciations and the evolutionary history of the polytypic snail species Arianta arbustorum (Gastropoda, Pulmonata, Helicidae) Molecular Phylogenetics and Evolution. 2004;30:64–73. doi: 10.1016/s1055-7903(03)00182-9. [DOI] [PubMed] [Google Scholar]
- GIUSTI F, MANGANELLI G. On some Hygromiidae (Gastropoda: Helicoidea) living in Sardinia and in Corsica (Studies on the Sardinian and Corsican Malacofauna VI) Bolletino Malacologico. 1987;23:123–206. [Google Scholar]
- HAASE M, MISOF B, WIRTH T, BAMINGER H, BAUR B. Mitochondrial differentiation in a polymorphic land snail: evidence for Pleistocene survival within the boundaries of permafrost. Journal of Evolutionary Biology. 2003;16:415–428. doi: 10.1046/j.1420-9101.2003.00542.x. [DOI] [PubMed] [Google Scholar]
- HAYASHI M, CHIBA S. Intraspecific diversity of mitochondrial DNA in the land snail Euhadra peliomphala (Bradybaenidae) Biological Journal of the Linnean Society. 2000;70:391–401. [Google Scholar]
- HUELSENBECK JP, RONQUIST F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- KAISER P. Bau, Entwicklung und Regeneration des Haarkleides von Trichia hispida (Linnaeus) zugleich ein Beispiel für eine einfache Musterbildung im Tierreich. Archiv für Molluskenkunde. 1966;95:111–122. [Google Scholar]
- KERNEY MP, CAMERON RAD, JUNGBLUTH JH. Die Landschnecken Nord- und Mitteleuropas. Parey, Hamburg: 1983. [Google Scholar]
- KETMAIER V, GIUSTI F, CACCONE A. Molecular phylogeny and historical biogeography of the land snail genus Solatopupa (Pulmonata) in the peri-Tyrrhenian area. Molecular Phylogenetics and Evolution. 2006;39:439–451. doi: 10.1016/j.ympev.2005.12.008. [DOI] [PubMed] [Google Scholar]
- KILIAN W, MÜLLNER F, STARLINGER F. Die forstlichen Wuchsgebiete Österreichs. Eine Naturraumgliederung nach waldökologischen Gesichtspunkten. Forstliche Bundesversuchsanstalt; Wien: 1994. [Google Scholar]
- KLEMM W. Die Verbreitung der rezenten Land-Gehaüse-Schnecken in Österreich. Denkschriften der Österrerreichischen Akademie der Wissenschaften (mathematisch-naturwissenschaftliche Klasse) 1974;117:1–503. [Google Scholar]
- LARKIN MA, BLACKSHIELDS G, BROWN NP, CHENNA R, McGETTIGAN PA, McWILLIAM H, VALENTIN F, WALLACE IM, WILM A, LOPEZ R, THOMPSON JD, GIBSON TJ, HIGGINS DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- LIEB GK. Eine Gebietsgliederung der Steiermark aufgrund naturräumlicher Gegebenheiten. Mitteilungen der Abteilung für Botanik des Landesmuseums Joanneum Graz. 1991;20:1–30. [Google Scholar]
- LOŽEK V. Klíč československých měkkýšů. Vydavateľstvo slovenskej akadémie vied; Bratislava: 1956. [Google Scholar]
- MIKULA E. Trochulus hispidus scheerpeltzi n. Subsp. Archiv für Molluskenkunde. 1957;86:91–92. [Google Scholar]
- NAGGS F. Some preliminary results of a morphometric multivariate analysis of the Trichia (Pulmonata: Helicidae) species group in Britain. Journal of Natural History. 1985;19:1217–1230. [Google Scholar]
- NOTREDAME C, HIGGINS DG, HERINGA J. T-coffee: a novel method for fast and accurate multiple sequence alignment. Journal of Molecular Biology. 2000;302:205–217. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
- PFENNINGER M, HRABÁKOVÁ M, STEINKE D, DÉPRAZ A. Why do snails have hairs? A Bayesian inference of character evolution. Bio Med Central Evolutionary Biology. 2005;5:59. doi: 10.1186/1471-2148-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFENNINGER M, MAGNIN F. Phenotypic evolution and hidden speciation in Candidula unifasciata ssp. (Helicellinae, Gastropoda) inferred by 16S variation and quantitative shell traits. Molecular Ecology. 2001;10:2541–2554. doi: 10.1046/j.0962-1083.2001.01389.x. [DOI] [PubMed] [Google Scholar]
- PFENNINGER M, POSADA D, MAGNIN F. Evidence for survival of Pleistocene climatic changes in Northern refugia by the land snail Trochoidea geyeri (Soós 1926) (Helicellinae, Stylommatophora) Bio Med Central Evolutionary Biology. 2003;3:8. doi: 10.1186/1471-2148-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSADA D. Collapse 1.2. Describing haplotypes from sequence alignment. 2004 http://darwin.uvigo.es/software/collapse.html.
- POSADA D. jModelTest: phylogenetic model averaging. Molecular Biology and Evolution. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- PROĆKÓW M. The Genus Trochulus CHEMNITZ, 1786 (Gastropoda: Pulmonata: Hygromiidae) – a taxonomic Revision. Folia Malacologica. 2009;17:101–176. [Google Scholar]
- REISCHÜTZ A, REISCHÜTZ PL. Rote Liste der Weichtiere (Mollusca) Österreichs. In: Zulka P, editor. Rote Listen gefährdeter Tiere Österreichs. Checklisten, Gefährdungsanalysen, Handlungsbedarf. Teil 2. Böhlauverlag, Wien: 2007. pp. 363–433. [Google Scholar]
- REISCHÜTZ A, REISCHÜTZ PL. Mollusca (Weichtiere) In: Rabitsch W, Essl F, editors. Endemiten – Kostbarkeiten in Österreichs Pflanzen- und Tierwelt. Naturwissenschaftlicher Verein für Kärnten & Umweltbundesamt GmbH, Klagenfurt & Wien; 2009. pp. 318–376. [Google Scholar]
- ROHLF FJ. TPSdig, program version 2.14. Department of ecology and evolution, State University of New York, Stony Brook; New York: 2001. [Google Scholar]
- SAITOU N, NEI M. The neighbor-joining method: a new method for reconstruction of phylogenetic trees. Molecular Biology and Evolution. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- SCHÖNSWETTER P, STEHLIK I, HOLDEREGGER R, TRIBSCH A. Molecular evidence for glacial refugia of mountain plants in the European Alps. Molecular Ecology. 2005;14:3547–3555. doi: 10.1111/j.1365-294X.2005.02683.x. [DOI] [PubMed] [Google Scholar]
- SHILEYKO AA. On the systematics of Trichia s. lat. (Pulmonata: Helicoidea: Hygromiidae) Malacologia. 1978;17:1–56. [Google Scholar]
- TABACHNICK B, FIDELL L. Using multivariate statistics. Harper Collins College Publishers; New York: 1996. [Google Scholar]
- THOMAZ D, GUILLER A, CLARKE B. Extreme divergence of mitochondrial DNA within species of pulmonate land snails. Proceedings of the Royal Society of London, Series B. 1996;263:363–368. doi: 10.1098/rspb.1996.0056. [DOI] [PubMed] [Google Scholar]
- VAN HUSEN D. Die Ostalpen in den Eiszeiten. Geologische Bundesanstalt; Wien: 1997. [Google Scholar]
- VAN RIEL P, JORDAENS K, VAN HOUTTE N, MARTINS AM, VERHAGEN R, BACKELJAU T. Molecular systematics of the endemic Leptaxini (Gastropoda: Pulmonata) on the Azores islands. Molecular Phylogenetics and Evolution. 2005;37:132–43. doi: 10.1016/j.ympev.2005.03.019. [DOI] [PubMed] [Google Scholar]
- VON PROSCHWITZ T. On the spread and development of the anthropochorous element in the land-snail fauna of the province of Dalsland (SW Sweden) Mitteilungen der Deutschen Malakozoologischen Gesellschaft. 1993;50/51:15–32. [Google Scholar]
- WAGNER AJ. Beiträge zur Anatomie und Systematik der Stylomatophoren aus dem Gebiet der Monarchie und der angrenzenden Balkanländer. Denkschriften der Österrerreichischen Akademie der Wissenschaften (mathematisch-naturwissenschaftliche Klasse) 1915;91:429–498. [Google Scholar]
- WEINLAND DF. Zur Molluskenfauna von Würthembergisch Franken. Jahresheft des Vereins für vaterländische Naturkunde in Württemberg. 1883;39:112–127. [Google Scholar]