Abstract
Rituximab therapy may achieve remission of proteinuria in children or adolescents with refractory focal segmental glomerulosclerosis (FSGS), but its effectiveness in adults is uncertain. We describe the case of a 22-year-old Caucasian woman with refractory FSGS that achieved complete and sustained remission of nephrotic syndrome (NS) with one single 375 mg/m2 rituximab infusion. At 4 months after complete circulating B-cell depletion, proteinuria declined from 4.5 to 0.27 g/24 h and serum albumin normalised. Rituximab was well tolerated and allowed complete withdrawal of previous immunosuppression with steroids and azathioprine. The patient was in sustained remission up to month 32, when she experienced a relapse. A second infusion of rituximab (375 mg/m2) achieved prompt proteinuria reduction with no additional immunosuppressants. At 48 months after the initial treatment, this patient is in complete remission without any immunosuppression. This case suggests that rituximab, even a single dose, may safely promote NS remission in adults with refractory FSGS.
Background
Idiopathic focal segmental glomerulosclerosis (FSGS) is a major cause of nephrotic syndrome (NS) in adults and the most common primary glomerular disease underlying end-stage renal disease in the USA.1 Glucocorticoids are first-line treatment and can achieve remission in 50–70% of patients, but a large fraction of them are burdened by frequent relapses.2–4 In steroid-resistant and relapsing cases several approaches have been used to control disease activity including plasmapheresis and add-on therapy with cyclophosphamide, cyclosporine, mycophenolate mofetil and other immunosuppressants.2–5 Chronic immunosuppression, however, seldom achieves persistent remission and is invariably associated with steroid toxicity and serious adverse effects such as gonadotoxicity and sterility, opportunistic infections, malignancies, bone marrow depression and renal toxicity.4
Since the first case described in 2006, rituximab, a chimeric monoclonal antibody targeting the CD20 antigen of B cells, has been reported to safely reduce proteinuria and induce remission in patients with steroid-dependent and multirelapsing FSGS,6–8 recurrent FSGS after transplant9 10 and in children with primary FSGS unresponsive to other immunosuppressive agents.10–12 Conversely, data on the efficacy of rituximab in adult patients with refractory FSGS are still limited. There is evidence of a reduction in proteinuria, but in no case, however, complete remission of proteinuria was obtained.13–15 Of note, in most of these cases multiple rituximab infusions were administered.13–15 This may be clinically relevant since repeated courses of rituximab on top of other immunosuppressive drugs have been reported to increase the risk of opportunistic infections and of hypersensitivity reactions.16 Treatment costs are also remarkable.16 17
In a matched-cohort study in 36 patients with idiopathic membranous nephropathy, we demonstrated that a single rituximab administration was enough to completely deplete B cells and is as effective as the standard four-dose regimen in achieving disease remission, but was virtually devoid of adverse events and less expensive.17 Conceivably, a similar approach might improve the tolerability of rituximab therapy in patients with refractory FSGS who are at increased risk because of previous prolonged exposure to other immunosuppressive medications.
We report the outcome of a woman with FSGS and long-lasting NS despite repeated courses of steroid and other immunosuppressants, who was treated with two single doses of rituximab 3 years apart.
Case presentation
In March 2009, a 22-year-old Caucasian woman was admitted to the Nephrology Unit of the Azienda Ospedaliera Ospedali Riuniti di Bergamo, Italy, due to refractory NS secondary to idiopathic FSGS. Previous medical history included the onset of Raynaud reactivity in 2003 that spontaneously recovered. In March 2006, at the age of 17, the patient developed NS (proteinuria: 6.1 g/24 h; serum albumin 26.8 g/L), with normal renal function (serum creatinine by Jaffe’ method: 68.9 mmol/L; estimated glomerular filtration rate (GFR) by Schwartz formula: 115.6 mL/min/1.73 m2 18) and blood pressure. The patient was also referring diffuse arthralgias at small and large joints. Antinuclear antibodies were faintly positive (1:160), rheumatoid factor and antistreptolysin titre were negative. Complement studies showed normal C3 and C4 levels. Hepatitis B and C virus markers were negative. Renal ultrasound was unremarkable.
A renal biopsy performed to exclude a renal involvement in the context of a systemic lupus erythematosus showed, of the 23 retrieved glomeruli, 3 glomeruli with segmental sclerosis and 3 with synechiae. None of them had global sclerosis. Glomerular mesangial matrix showed slight focal and segmental increase, with epithelial hyperplasia. Immunofluorescence analysis showed faint glomerular fixation for C3 and IgM, with a focal and segmental distribution pattern. The pattern was consistent with the not otherwise specified variant of FSGS. Treatment with prednisone was promptly initiated on top of ACE inhibitor ramipril. Owing to the young-adult age of the patient, prednisone was started at the adult dosage (1 mg/kg/day) corresponding to 50 mg/day and continued for 5 months, but 24 h proteinuria continued to range between 4 and 8 g. Shortly after prednisone withdrawal, proteinuria increased up to 19 g/24 h, which prompted the decision to reintroduce steroid therapy at a higher dose (1.5 mg/kg corresponding to 75 mg/day) that led to a reduction of proteinuria up to previous levels (5 g/24 h) over a period of 5 months. Prednisone dose was thereafter tapered up to 0.5 mg/kg. After exhaustive information about the pros and contra of cyclosporine, the patient refused this therapy, mainly because of its substantial side effects, in particular nephrotoxicity.19 20 Therefore, prednisone was associated with biweekly photopheresis and leukapheresis, in light of published evidence suggesting that these treatments, by removing activated T cells and by exerting overall immunomodulatory activity have beneficial effect in immune-mediated glomerular diseases.21–23 However, no change in proteinuria occurred. Three months later (July 2007), cyclophosphamide therapy (100 mg/day) was added on steroids up to October 2008 without appreciable changes in proteinuria. A transient proteinuria reduction (from 3.8 g/24 h to 1.6 g/24 h and then rose again up to 4 g/24 h) was observed shortly after three plasmapheresis sessions performed in March 2008. The clinical course was complicated by a Papillomavirus infection that recovered after cyclophosphamide temporary withdrawal. In January 2009, azathioprine therapy was initiated at the dose of 150 mg/day, reduced to 100 mg/day thereafter due to the occurrence of anaemia. In the meanwhile the patient was maintained on 12.5 mg of prednisone per day.
At the admittance in the Nephrology Unit of the Azienda Ospedaliera Ospedali Riuniti di Bergamo, the patient had a persistent NS despite daily therapy with prednisone (12.5 mg), azathioprine (100 mg), ramipril (10 mg), losartan (50 mg), furosemide (50 mg) and simvastatin (20 mg).
Investigations
Genetic analyses performed to assess whether the persistence of NS despite different immunosuppressive therapies could be explained by a primary podocyte defect did not disclose any of the abnormalities in WT1, ACTN4, INF2 and NPHS2 genes so far reported to be associated with FSGS.
Treatment
In light of published cases reporting a potential beneficial effect of B-cell depletion in children with refractory FSGS, we discussed this therapeutic option with the patient who provided her written informed consent to the procedure. Thus, we administered rituximab to the patient in June 2010. At that time, proteinuria was 4.5 g/24 h, total serum proteins 4.6 g/dL, serum albumin 2.6 g/dL and iohexol-measured GFR 80.2 mL/min/1.73 m2. According to the B-cell driven protocol we currently use to treat membranous nephropathy,17 we infused a single 375 mg/m2 rituximab dose after premedication with 10 mg chlorphenamine and 500 mg hydrocortisone.
Outcome and follow-up
Circulating CD19+ B cells were fully depleted since the day after rituximab administration (figure 1A). The infusion was well tolerated and proteinuria promptly declined up to complete remission (0.27 g/24 h at month 4 after treatment; figure 1B), with an increase in serum albumin (figure 1C) and GFR and a reduction in albumin fractional clearance (figure 2). Oedema fully recovered and diuretic therapy was no longer required.
Figure 1.
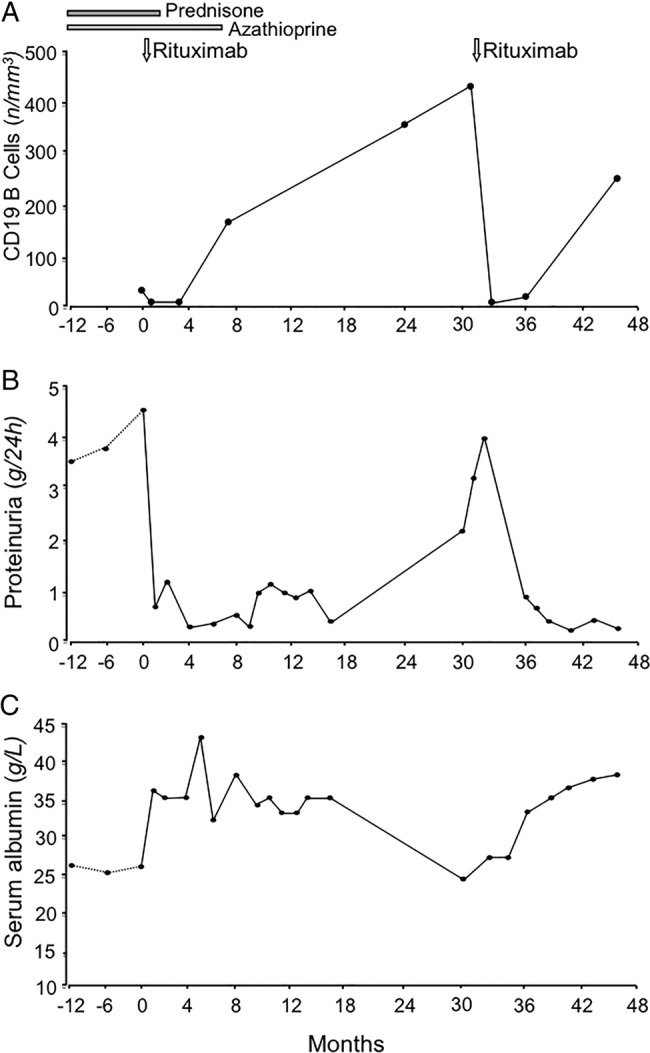
Changes in CD19+ circulating B cells (A), 24 h proteinuria (B) and serum albumin (C) levels before and up to 48 months after the first rituximab administration.
Figure 2.
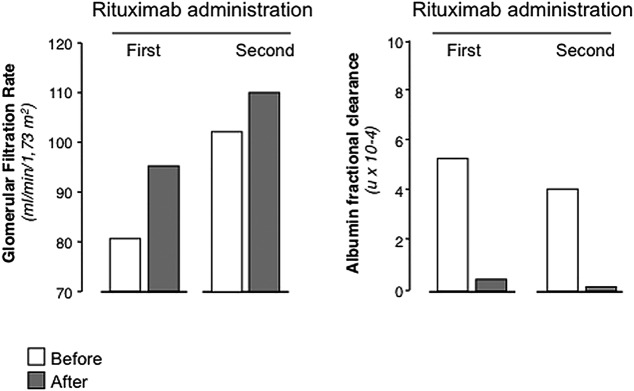
Change in glomerular filtration rate (left panel) and albumin fractional clearance (right panel) before (white bars) and at 12 months after (grey bars) each rituximab administration.
Steroid and azathioprine therapy was progressively tapered up to withdrawal in 1 and 6 months, respectively. Circulating CD19+ B cells recovered to normal levels in 10 months after rituximab infusion. The patient was in sustained remission and in good general condition up to month 32 after rituximab infusion, when NS relapsed. Infusion of a second 375 mg/m2 rituximab dose induced a prompt proteinuria remission (figure 1). Iohexol-measured GFR approximated the normal range (80–120 mL/min/1.73 m2) after the first rituximab administration and further improved after the second one, albumin fractional clearance normalised after both rituximab administrations (figure 2). At 48 months after the first infusion, the patient is well and off-treatment with no sign of the NS (table 1).
Table 1.
Biological parameters before (month 0) and at 48 months after first rituximab treatment
| Months after rituximab treatment |
||
|---|---|---|
| 0 | 48 | |
| Serum creatinine (μmol/L) | 71.6 | 48.6 |
| Serum proteins (g/L) | 46 | 60 |
| Total cholesterol (mmol/L) | 5.01 | 3.8 |
| Triglicerides (mmol/L) | 1.4 | 0.38 |
| B lymphocytes (cells/mm3) | 35 | 0.2 |
The patient filled out the Short Form 36 (SF-36) Health Survey (V.1) before and after receiving the second infusion of rituximab. The SF-36 is a measure of health status consisting of eight scaled scores, which are the weighted sums of the questions in their section. Each scale is directly transformed into a 0–100 scale on the assumption that each question carries equal weight. The lower the score is, the more disable the patient is. A score between 40 and 60 is considered normal. Before rituximab, our patient had remarkably low scores that increased above the normality threshold after rituximab therapy (figure 3).
Figure 3.
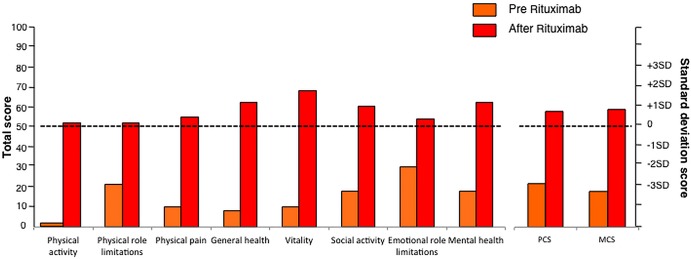
Short Form 36 (SF-36) form-derived individual standardised indexes (left) and synthetic indexes (Physical Component Summary (PCS) and Mental Component Summary (MCS)) (right) before (orange) and after (red) rituximab treatment. The horizontal dotted lines indicate expected mean values among healthy subjects matched for age and gender (for PCS and MCS indexes) and from the general Italian population (for the other indexes), respectively. SF-36 form was taken from ref. 37.
Discussion
We reported the case of an adult patient with refractory FSGS who achieved complete and sustained NS remission after rituximab administration. Treatment was well tolerated and allowed interruption of concomitant immunosuppression. A second dose of rituximab administered 3 years apart to treat a recurrence of the disease again achieved prompt and sustained remission with no need of any additional immunosuppression. Importantly, the patient had a remarkable improvement in her quality of life after rituximab therapy. Before referral to our nephrology unit, virtually all therapeutic interventions commonly used to promote proteinuria remission had been attempted sequentially according to established guidelines. Steroids were appropriately dosed and other treatments including plasmapheresis, photopheresis, leukapheresis, cyclophosphamide and azathioprine were attempted at the recommended regimens, but without any remarkable effects. Despite the available evidence on the efficacy of calcineurin inhibitors,24–26 the patient refused to receive this treatment, due to the unproven superiority of cyclosporine over other treatments such as antiproliferative agents,27 the high risk of recurrences after treatment interruption and, more importantly, the risk of nephrotoxicity.19 20
Thus, finding that, shortly after rituximab infusion, proteinuria persistently declined and did not relapse even after withdrawal of any concomitant immunosuppression, is consistent with the possibility of a cause and effect relationship between rituximab administration and disease recovery. This causal relationship was definitely proven by the similar treatment effect observed after the second dose of rituximab administered to treat the recurrence.
This is also the evidence that remission can be achieved in adults with refractory FSGS by a single 375 mg/m2 rituximab administration. These findings extend to recent evidence in adults that a single administration of rituximab resulted in rapid clearance of circulating B cells and achieved persistent remission of NS in children with refractory FSGS.12 28
These data have major clinical implications, since provide the evidence that rituximab therapy allows safely withholding treatment with steroid and immunosuppressive drugs, avoiding their major toxicity. Moreover, achieving disease remission with a single rituximab administration, in addition to remarkably reduce acute treatment risks, may be instrumental to limit long-term complications.17 In particular, decreasing the risk of sensitisation, may allow patient re-treatment in case of disease recurrence.17
The mechanisms underlying the response to rituximab when previous treatments had failed are still unclear. Until recently, FSGS has been considered as an immune disorder sustained by a still elusive permeability factor, possibly originating from T lymphocytes, responsible for the induction of proteinuria.10 29 However, evidence that FSGS may occasionally occur in patients with B-cell-related autoimmune disorders argue for a B-cell involvement in the pathogenesis of the disease.30 Moreover, further data suggest that the permeability factor could be, or could bind to, an immunoglobulin.31 Thus, if B cells play a role in FSGS pathogenesis, failure of previous unspecific immunosuppression might be explained by incomplete and/or only transient inhibition of autoreactive B cells, whereas full and prolonged depletion of these pathogenic clones could explain the sustained response to rituximab. Whether rituximab may also act through mechanisms including interference with antigen presentation, expansion of regulatory T cells and modulation of natural killer cell and macrophage activity is an additional possibility that merits further investigation.32 Similar mechanisms have been suggested to explain the efficacy of rituximab therapy in idiopathic membranous nephropathy patients with NS refractory to treatment with unselective immunosuppressants.33
Intriguingly, the present case had a persistent remission, despite full B-cell recovery. Similar outcomes were observed in patients with idiopathic membranous nephropathy achieving remission of the NS that persisted also after re-emergence of circulating B cells.17 A plausible interpretation of the above findings is that, independent from the total number of circulating B cells, only re-emergence of autoreactive clones producing nephritogenic antibodies is associated with disease relapse. Rituximab is able to promote persistent disease remission in about 50% of steroid-dependent cases.7 On demand further rituximab administrations—the same approach that we employed for patients with membranous nephropathy17 and that we also followed in the present case—appears to be an effective strategy to treat disease recurrence. This approach is possibly safer than chronic, add-on therapy with antiproliferative agents to retard/prevent recovery of autoreactive B cells and prolong remission after rituximab therapy.34
Independent of the above, the close association between B-cell depletion and disease remission consistently observed in our present case and in any previous series of patients given rituximab for idiopathic NS or membranous nephropathy,6–17 28 can be taken to suggest that B-cell-mediated immune mechanisms appear to mediate the response to treatment, whereas the role of non-immunological direct effects of rituximab on podocyte35 36 although possible, remains unproven.
In conclusion, the present case may support the use of single-dose rituximab therapy in adult patients with refractory FSGS. Mechanistic studies evaluating the effect of rituximab on possible mediators of disease activity may be instrumental to a more in-depth knowledge of FSGS pathogenesis and further improvements in patient care.
Patient's perspective.
Below we report an interview recently conducted with the patient to understand her perspective of the disease and treatment.
Q: How did your life change after you were diagnosed with the kidney disease?
A: It completely changed my life. I was an ordinary teenager who used to be very physically active: I often ran, skated, etc. Then I started having nausea and an unbearable heaviness in the body due to the weight gain, which led me to restrain from outdoor activities. I also often experienced sleepiness and fatigue. All these had negative effects on my life, and even worse, I did not know what was going on with my body initially.
Q: How did the immunosuppression therapy affect you?
A: I was terrified by the idea of receiving steroids and other immunosuppressants. When I started steroid therapy, I had nausea, vomiting, tachycardia, vertigo, fatigue and burning sensation on my arm while taking my blood. During apheresis sessions I frequently had hypotensive episodes and when the needle touched my vein I was terrified. At one time, this made me even pull the needle out myself. The sessions were always associated with some disturbance, either during the session or shortly after. I frequently had nausea and felt cold. Therapy was worse than the disease itself.
Q: What happened when you stopped the apheresis therapy?
A: Cyclophosphamide made me weak and my nausea never went away. I was also feeling either chill or heat.
Q: How would you describe in one word the period when you were on immunosuppressive therapy?
A: Inhumane.
Q: How did the disease and immunosuppression affect your physical aspect?
A: It changed my body, I started having stretch marks, scars, leg swellings, and enlarged veins.
Q: Did the disease change your social relationships?
A: It reduced the time I would spend with my friends, the opportunities to go outside for sports, and time for myself.
Q: How did the disease affect your life overall?
A: I had to change my lifestyle and stop my education. I was able to finish high school, but I could not go to college, due to the frequent admissions to the hospital and the drug toxicities. So I started to work. I actually found a job that I really liked, but I had to quit the job, too, since I felt sick again. I was full of anger, frustration, and disappointment.
Q: What did you think when you first started Rituximab?
A: I could not believe it. Initially, I was scared to be disappointed again because I had doubts about the efficacy of the drug. Then, I noticed I felt much better soon after the infusion. I have still been tired for the following 2 months, but I have not felt hot, or tense muscle on my back. I regained my strength and I could concentrate better. Then, proteinuria disappeared.
Q: What did you feel when you had a relapse?
A: I had the same symptoms of when they first discovered the disease, but without the adverse effects of immunosuppression.
Q: What did you think when you realised you could repeat rituximab?
A: I was less scared and had more faith. I was not taking any immunosuppressive drug, so I was feeling better.
Q: How do you feel now?
A: I no longer feel dependent on someone else for doing things for me. I've got back my life.
Learning points.
In adult refractory, focal segmental glomerulosclerosis rituximab can safely induce sustained proteinuria remission and allows complete withdrawal of concomitant immunosuppression.
A repeat treatment for disease recurrence can be equally effective and well tolerated.
Compared to the standard four-dose regimen, administration of one single (375 mg/m2) dose is equally effective but may decrease the risk of sensitisation and late complications, and is remarkably less expensive.
Evidence that remission persists after reconstitution of circulating B cells, suggests that the long-term benefit of rituximab may be mediated by specific and prolonged depletion of autoreactive B-cell clones.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kitiyakara C, Eggers P, Kopp JB. Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis 2004;44:815. [PubMed] [Google Scholar]
- 2.D'Agati VD, Kaskel FJ, Falk RJ. Focal segmental glomerulosclerosis. N Engl J Med 2011;365:2398–411 [DOI] [PubMed] [Google Scholar]
- 3.Hogan J, Mohan P, Appel GB. Diagnostic tests and treatment options in glomerular disease: 2014 update. Am J Kidney Dis 2014;63:656–66 [DOI] [PubMed] [Google Scholar]
- 4.Van Husen M, Kemper MJ. New therapies in steroid-sensitive and steroid-resistant idiopathic nephrotic syndrome. Pediatr Nephrol 2011;26:881–92 [DOI] [PubMed] [Google Scholar]
- 5.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO clinical practice guidelines for glomerulonephritis. Kidney Int Suppl 2012;2:139–274 [Google Scholar]
- 6.Ravani P, Ponticelli A, Siciliano C, et al. Rituximab is a safe and effective long-term treatment for children with steroid and calcineurin inhibitor-dependent idiopathic nephrotic syndrome. Kidney Int 2013;84:1025–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruggenenti P, Ruggiero B, Cravedi P, et al. Rituximab in steroid-dependent or frequently-relapsing idiopathic nephrotic syndrome. J Am Soc Nephrol 2014;25:850–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinha A, Bagga A. Rituximab therapy in nephrotic syndrome: implications for patients’ management. Nat Rev Nephrol 2013;9:154–69 [DOI] [PubMed] [Google Scholar]
- 9.Pescovitz MD, Book BK, Sidner RA. Resolution of recurrent focal segmental glomerulosclerosis proteinuria after rituximab treatment. N Engl J Med 2006;354:1961–3 [DOI] [PubMed] [Google Scholar]
- 10.Marasà M, Kopp JB. Monoclonal antibodies for podocytopathies: rationale and clinical responses. Nat Rev Nephrol 2009;5:337–48 [DOI] [PubMed] [Google Scholar]
- 11.Gulati A, Sinha A, Jordan SC, et al. Efficacy and safety of treatment with rituximab for difficult steroid-resistant and -dependent nephrotic syndrome: multicentric report. Clin J Am Soc Nephrol 2010;5:2207–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito S, Kamei K, Ogura M, et al. Survey of rituximab treatment for childhood-onset refractory nephrotic syndrome. Pediatr Nephrol 2013;28:257–64 [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Fresnedo G, Segarra A, González E, et al. Rituximab treatment of adult patients with steroid-resistant focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 2009;4:1317–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters HP, van de Kar NC, Wetzels JF. Rituximab in minimal change nephropathy and focal segmental glomerulosclerosis: report of four cases and review of the literature. Neth J Med 2008;66:408–15 [PubMed] [Google Scholar]
- 15.Kisner T, Burst V, Teschner S, et al. Rituximab treatment for adults with refractory nephrotic syndrome: a single-center experience and review of the literature. Nephron Clin Pract 2012;120:c79–85 [DOI] [PubMed] [Google Scholar]
- 16.Ruggenenti P, Cravedi P, Remuzzi G. Rituximab for membranous nephropathy and immune disease: less might be enough. Nat Clin Pract Nephrol 2009;5:76–7 [DOI] [PubMed] [Google Scholar]
- 17.Cravedi P, Ruggenenti P, Sghirlanzoni MC, et al. Titrating rituximab to circulating B cells to optimize lymphocytolytic therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 2007;2:932–7 [DOI] [PubMed] [Google Scholar]
- 18.Schwartz GJ, Gauthier B. A simple estimate of glomerular filtration rate in adolescent boys. J Pediatr 1985;106:522–6 [DOI] [PubMed] [Google Scholar]
- 19.Frassinetti Castelo Branco Camurça Fernandes P, Bezerra Da Silva G, Jr, De Sousa Barros FA, et al. Treatment of steroid-resistant nephrotic syndrome with cyclosporine: study of 17 cases and a literature review. J Nephrol 2005;18:711–20 [PubMed] [Google Scholar]
- 20.Hamasaki Y, Yoshikawa N, Nakazato H, et al. Prospective 5-year follow-up of cyclosporine treatment in children with steroid-resistant nephrosis. Pediatr Nephrol 2013;28:765–71 [DOI] [PubMed] [Google Scholar]
- 21.Yokoyama H, Shimizu M, Wada T, et al. The beneficial effects of lymphocytapheresis for treatment of nephrotic syndrome. Ther Apher 2002;6:167–73 [DOI] [PubMed] [Google Scholar]
- 22.Russo GE, Cavallini M, Centi A, et al. Extracorporeal photochemotherapy for the treatment of glomerulopathies with associated nephrotic syndrome. J Nephrol 2010;23:85–9 [PubMed] [Google Scholar]
- 23.Yokoyama H, Wada T, Zhang W, et al. Advances in apheresis therapy for glomerular diseases. Clin Exp Nephrol. 2007;11:122–7 [DOI] [PubMed] [Google Scholar]
- 24.Lieberman KV, Tejani A. A randomized double-blind placebo-controlled trial of cyclosporine in steroid-resistant idiopathic focal segmental glomerulosclerosis in children. J Am Soc Nephrol 1996;7:56–63 [DOI] [PubMed] [Google Scholar]
- 25.Ponticelli C, Rizzoni G, Edefonti A, et al. A randomized trial of cyclosporine in steroid-resistant idiopathic nephrotic syndrome. Kidney Int 1993;43:1377–84 [DOI] [PubMed] [Google Scholar]
- 26.Cattran DC, Appel GB, Hebert LA, et al. A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. North America Nephrotic Syndrome Study Group. Kidney Int 1999;56:2220–6 [DOI] [PubMed] [Google Scholar]
- 27.Gipson DS, Trachtman H, Kaskel FJ, et al. Clinical trial of focal segmental glomerulosclerosis in children and young adults. Kidney Int. 2011;80:868–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakayama M, Kamei K, Nozu K, et al. Rituximab for refractory focal segmental glomerulosclerosis. Pediatr Nephrol 2008;23:481–5 [DOI] [PubMed] [Google Scholar]
- 29.Savin VJ, Sharma R, Sharma M, et al. Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N Engl J Med 1996;334:878–83 [DOI] [PubMed] [Google Scholar]
- 30.Hertig A, Droz D, Lesavre P, et al. SLE and idiopathic nephrotic syndrome: coincidence or not? Am J Kidney Dis 2002;40:1179–84 [DOI] [PubMed] [Google Scholar]
- 31.Dantal J, Godfrin Y, Koll R, et al. Antihuman immunoglobulin affinity immunoadsorbtion strongly decreases proteinuria in patients with relapsing nephroticsyndrome. J Am Soc Nephrol 1998;9:1709–15 [DOI] [PubMed] [Google Scholar]
- 32.Kessel A, Rosner I, Toubi E. Rituximab: beyond simple B cell depletion. Clin Rev Allergy Immunol 2008;34:74–9 [DOI] [PubMed] [Google Scholar]
- 33.Cravedi P, Sghirlanzoni MC, Marasà M, et al. Efficacy and safety of rituximab second-line therapy for membranous nephropathy: a prospective, matched-cohort study. Am J Nephrol 2011;33:461–8 [DOI] [PubMed] [Google Scholar]
- 34.Filler G, Huang SH, Sharma AP. Should we consider MMF therapy after rituximab for nephrotic syndrome? Pediatr Nephrol 2011;26:1759–62 [DOI] [PubMed] [Google Scholar]
- 35.Fornoni A, Sageshima J, Wei C, et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med 2011;3:85ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan AC. Rituximab's new therapeutic target: the podocyte actin cytoskeleton. Sci Transl Med 2011;3:85ps21. [DOI] [PubMed] [Google Scholar]
- 37.Apolone G, Mosconi P, Ware JE., Jr Questionario sullo stato di salute SF-36, A. Milano: Guerrini e Associati, 1997 [Google Scholar]


