Summary
Fulminant pneumococcal infection is a fatal pneumococcal infection that tends to occur in immunocompromised hosts, such as patients who are asplenic or on immunosuppressant therapy. We experienced a case of a 73-year-old Japanese man with a medical history of coronary stent implantation and catheter ablation for atrial flutter who presented with dyspnoea at rest. The patient was diagnosed with streptococcal pneumonia based on a urine antigen test and CT. Despite the use of effective antibiotics and systemic therapies, his clinical course was rapidly progressive and he died 18 h after admission. This case of fulminant pneumococcal infection is reported along with the autopsy findings.
Background
Streptococcus pneumoniae is a leading cause of community-acquired pneumonia. The frequency of this organism in patients with community-acquired pneumonia in Japan was reported to be 24.8% (Japan Respiratory Society Guideline, adult community-acquired pneumonia 2007). Pneumococcal infection is usually treatable with appropriate antibiotics; however, some cases of streptococcal pneumonia present with a rapidly progressive clinical course and have a fatal outcome.1 These severe conditions are considered as either fulminant or overwhelming pneumococcal infections.2 The incidence of fulminant pneumococcal infection varies depending on several factors, including age, comorbidities and immunological status. In this report, a case of fulminant pneumococcal infection that was resistant to multidisciplinary treatment is described with the autopsy findings.
Case presentation
A 73-year-old Japanese man was emergently admitted to our hospital with dyspnoea at rest. He had a history of coronary stent implantation 1 year before admission and underwent percutaneous catheter ablation for atrial flutter 10 months before admission. He had smoked 50 cigarettes per day from the age of 25 to 65 years. Dyspnoea at rest occurred 6 h before the emergent admission and gradually worsened.
Physical examination findings on admission were as follows: Glasgow Coma Scale score 15, blood pressure 155/96 mm Hg, pulse rate 113/min, temperature 36.5°C, respiratory rate 32/min and oxygen saturation 82% on oxygen at 10 L/min with a partial rebreathing mask. Jugular venous distention was present. On chest auscultation, there were no extra heart sounds and no murmurs, with rales in the expiratory phase on the left side of the lung field. Purpura was present on the trunk. Lower extremity oedema was absent, but his extremities were cold.
Chest radiography showed an infiltrate in the left lung field and cardiomegaly (figure 1). Thoracic CT revealed widespread consolidation in the left upper lobe and bilateral emphysematous changes (figure 2). The ECG showed atrial fibrillation and no significant ST-T segment changes compared with the previous ECG in our institution. Transthoracic echocardiography showed a mildly impaired left ventricular ejection fraction, hypokinesis of the anterior wall of the left ventricle and dilatation of the left atrium. Thrombi were not seen in the left atrium or the left atrial appendage and the inferior vena cava was collapsed. Blood examinations showed an elevated white cell count (28 800/μL), mild anaemia (haemoglobin concentration 10.5 g/dL) and increased levels of lactate dehydrogenase (234 U/mL) and C reactive protein (26.6 mg/dL). Renal function was decreased (serum creatinine value 2.3 mg/dL and estimated glomerular filtration rate 22.4 mL/min). The N-terminal pro brain-type natriuretic peptide value was 18 917 ng/L. A urinary antigen test for S. pneumoniae (Binax NOW Streptococcus pneumoniae) was positive. The patient had not been diagnosed with a pneumococcal infection within several months before admission.
Figure 1.
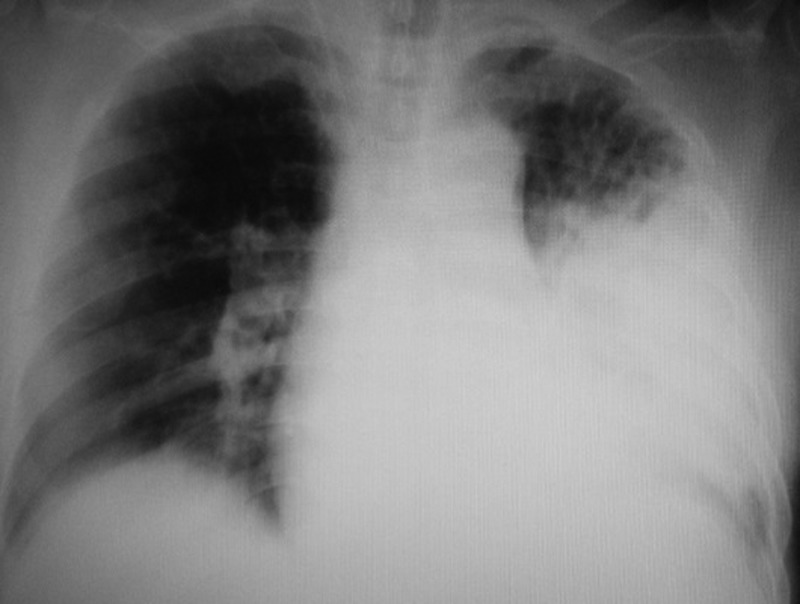
Chest radiograph on admission.
Figure 2.
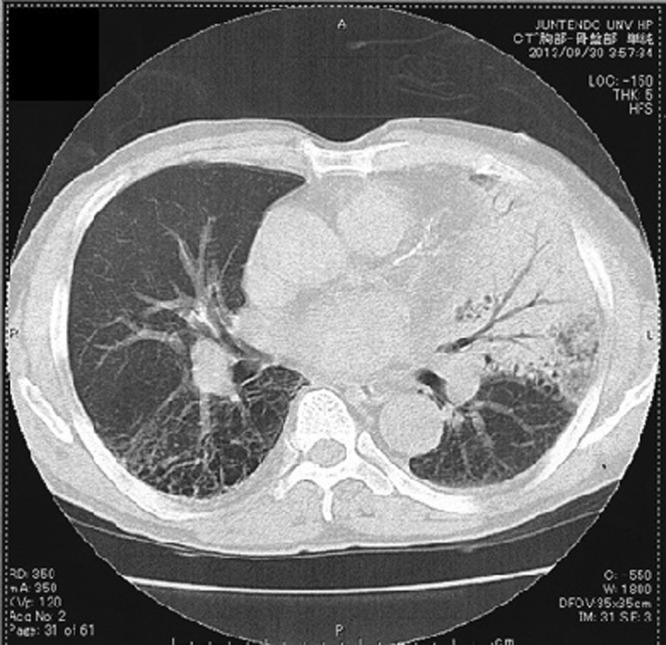
Thoracic CT on admission.
Clinical course
Acute decompensated heart failure complicated with streptococcal pneumonia was diagnosed. Intravenous nitroglycerine was administered for high blood pressure, and non-invasive positive airway pressure therapy was started in the emergency room. Atrial blood gas analysis revealed type 2 respiratory failure with a partial pressure of carbon dioxide of 70 mm Hg; therefore, the patient was immediately intubated and mechanical ventilation was started. In addition, an intravenous infusion of levofloxacin was administered, followed by ceftriaxone after obtaining blood, sputum and urine samples for Gram stain and culture. Continuous haemodialysis filtration was urgently performed for acute kidney injury. After these treatments were started, the patient developed hypotension with a systolic blood pressure of 80 mm Hg. Despite withdrawal of sedation and nitroglycerine, the hypotension did not improve. He was considered to have septic shock and was started on a continuous intravenous infusion of norepinephrine. Moreover, arginine vasopressin was given intravenously for resistant hypotension. Meanwhile, the purpura that was seen on his trunk on admission gradually expanded to the whole body, and resistant hypoglycaemia was also observed. Despite the systemic therapies, the patient's circulatory failure and metabolic disorder (acidaemia, hyperkalaemia and hypoglycaemia) did not improve. He developed cardiac arrest and died 18 h after admission. The results of the blood and sputum cultures, which were reported afterwards, were positive for S. pneumoniae.
Autopsy and pathological findings
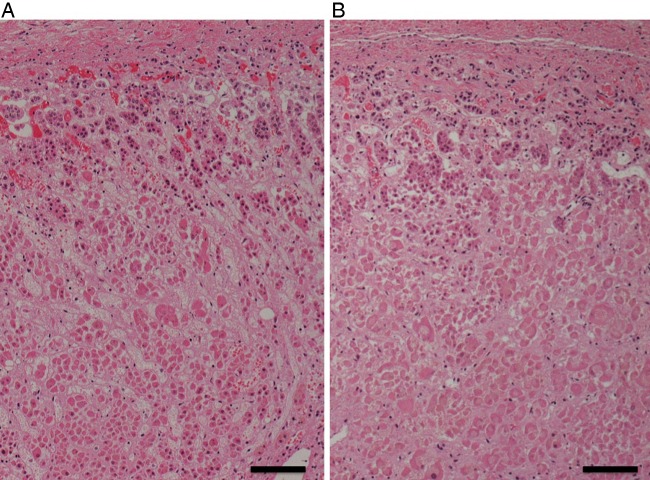
An autopsy was performed 13 h and 13 min after death. The patient's height was 180 cm, and his weight was 83 kg. On gross examination, systemic purpura (figure 3), haemorrhagic pneumonia (figure 4), pulmonary emphysema, old myocardial infarction and post coronary stent implantation status were evident. The microscopic findings were as follows.
Figure 3 .
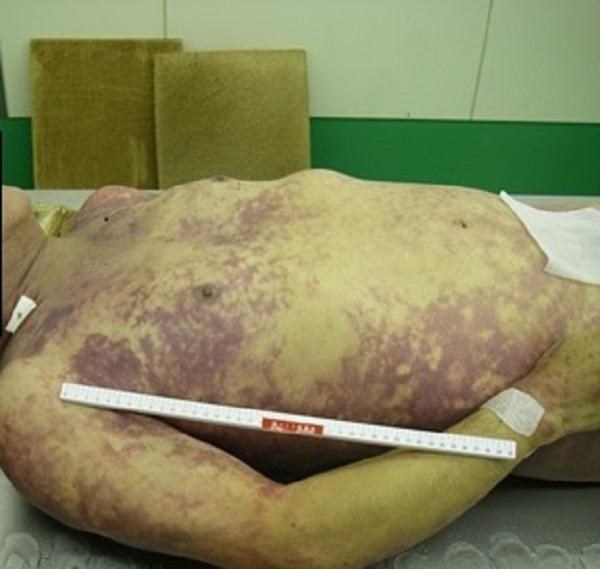
Appearance of the whole body.
Figure 4.
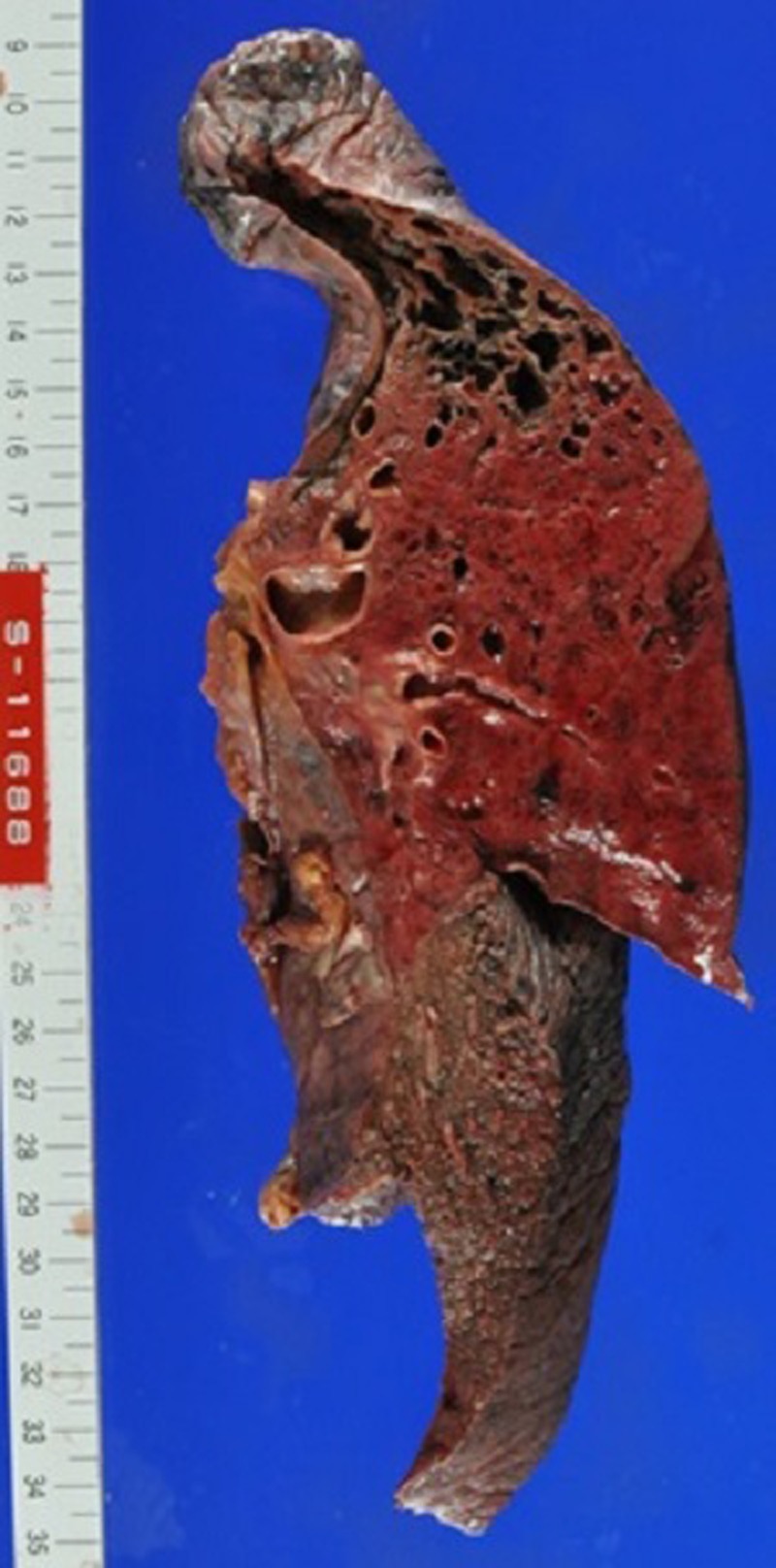
Macroscopic findings of the left lung.
Haemorrhagic pneumonia
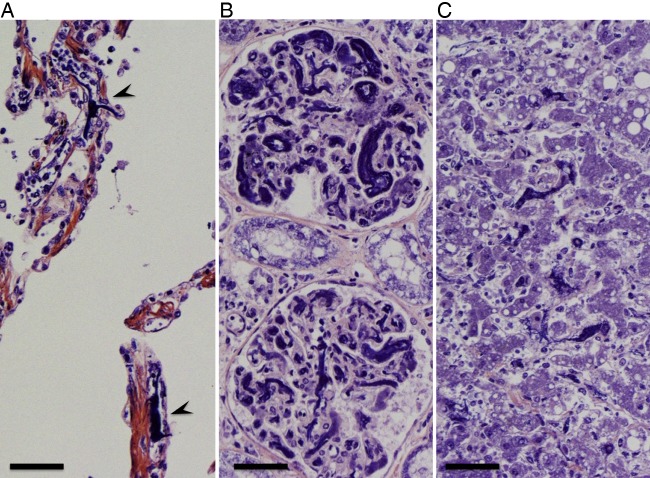
The left upper lung lobe was diffusely red coloured. The left lung was heavier than the right (left 1040 g, right 940 g), partly due to haemorrhage. Large amounts of neutrophils and red blood cells were infiltrated in the alveoli of the left upper lobe (figure 5). Intravascular fibrin formation and vessel occlusion were also observed in the alveolar walls (figure 6A). The Gram stain was negative in the lung tissue. Emphysematous changes were found in bilateral pulmonary apices.
Figure 5.
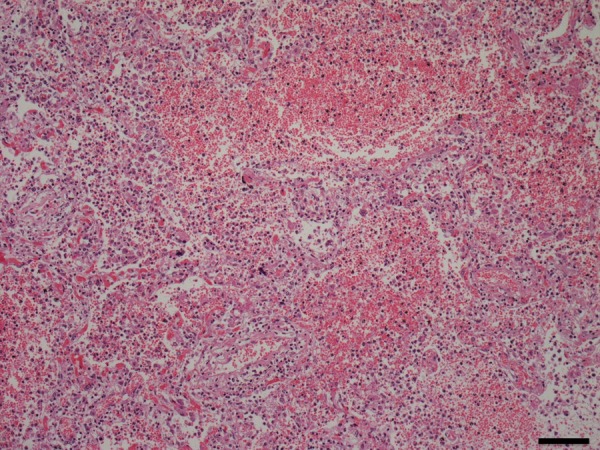
Pathological findings of the left lung.
Figure 6 .
Pathological findings of the pulmonary alveolar capillaries (A), renal glomeruli (B) and hepatic sinusoids (C).
Sepsis and disseminated intravascular coagulation
The spleen and hepatic sinusoids were infiltrated with neutrophils. Fibrin thrombi were found in microvessels of alveolar walls, glomeruli and hepatic sinusoids (figure 6A–C). Moreover, gastrointestinal haemorrhage and subcutaneous haemorrhage were seen. Taken together, these phenomena were considered to have originated from sepsis and disseminated intravascular coagulation (DIC) caused by fulminant pneumonia.
Old myocardial infarction
A white-coloured scar was observed in the posterior wall of the left ventricle, in which myocardium was replaced with collagenous fibre. The right and left coronary arteries had 70–80% stenosis in each vessel. Stent implantation was observed in the right coronary artery and the left circumflex artery. The vessels with stent placement and the distal portions of the vessels were patent.
Other findings
The weight of the liver was increased with congestion (1900 g). Kidney weight was 230 g for the right and 250 g for the left. Fibrin thrombi were found in glomerular capillaries (figure 6B). The adrenal glands (right 7.4 g, left 5.9 g) had multifocal necrosis (figure 7A, B). In summary, the autopsy findings suggested that the cause of death was multiple organ failure due to DIC.
Figure 7.
(A and B) Pathological findings of the bilateral adrenal glands.
Discussion
We experienced a case of fulminant streptococcal pneumonia presenting with a rapidly progressive clinical course, which was resistant to antibiotics that had been proven effective on the basis of antimicrobial susceptibility testing (figure 8) and systemic therapies. Previous reports described similar cases that had a severe and rapid clinical course.1 2 Fulminant pneumococcal infection is considered to be a pneumococcal infection complicated with sepsis and purpura fulminans, which tends to occur in immunocompromised hosts such as patients who are asplenic or on immunosuppressants.1 However, fulminant pneumococcal infection can occur in non-immunocompromised individuals,3 4 as in the present case. The autopsy findings of this case showed multiple organ failure, DIC and sepsis. To date, the underlying mechanisms that explain why pneumococcal infection causes fulminant pneumonia with DIC have not been fully clarified. A previous report suggested that pneumococcal capsular polysaccharides (PCPs) were responsible for initiating DIC through inflammation induced by PCPs, per se, or an antigen–antibody reaction.5 Other reports suggested that certain serotypes of S. pneumoniae (serotypes 1, 5 and 7) were associated with severe pneumococcal diseases,6 7 whereas there were also data that did not support this association.8 In the present case, the diagnosis of streptococcal infection was based on the positive result of the urinary antigen test for S. pneumoniae (Binax NOW Streptococcus pneumoniae). This test is known for its high specificity (97–98.8%); 9 10 however, it was incapable of quantifying the antigens and was unable to differentiate specific serotypes, and thus the serotype of this streptococcal infection could not be identified.
Figure 8.
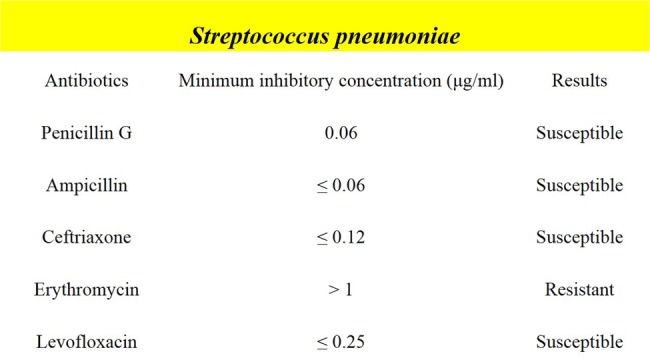
Results of antimicrobial susceptibility testing.
The issue to be considered in this case is how we should manage and what we could do for this rapidly progressive life-threatening condition. Early diagnosis and definite recognition of the severity of the disease were the first steps, followed by aggressive cardiopulmonary support and administration of appropriate or wide-spectrum antibiotics and specific complementary therapies, such as renal replacement therapy, if required. This case demonstrated resistant hypotension, despite the use of multiple vasopressors, and resistant hypoglycaemia, which suggested the existence of acute adrenal failure. Pathophysiological findings showed multifocal necrosis in bilateral adrenal glands, which supported this hypothesis. Thus, systemic administration of corticosteroids might have been effective.
Learning points.
Fulminant pneumococcal infection is a life-threatening disease that is resistant to multidisciplinary treatment.
Fulminant pneumococcal infection tends to occur in immunocompromised hosts. However, fulminant pneumococcal infection can also occur in non-immunocompromised individuals.
Early diagnosis, understanding the severity of the disease and prompt responses to rapidly exacerbating conditions including disseminated intravascular coagulation and multiple organ failure are required to improve the survival rate.
Footnotes
Competing interests: None.
Patient consent: Not obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Lutwick LI. Infections in asplenic patients. In: Mandell GL, Bennett JE, Dolin R, eds Principles and practice of infectious diseases. 6th edn Elsevier Churchill Livingstone, 2005:3524–32 [Google Scholar]
- 2.Rosch JW, Boyd AR, Hinojosa E, et al. Statins protect against fulminant pneumococcal infection and cytolysin toxicity in a mouse model of sickle cell disease. J Clin Invest 2010;120:627–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakamura H, Saitou M, Kinjo S, et al. Overwhelming pneumococcal bacteremia revealed by a peripheral blood smear in a 74-year-old healthy woman. Intern Med 2007;46:303–6 [DOI] [PubMed] [Google Scholar]
- 4.Murph RC, Matulis WS, Hernandez JE. Rapidly fatal pneumococcal sepsis in a healthy adult. Clin Infect Dis 1996;22:375–6 [DOI] [PubMed] [Google Scholar]
- 5.Rytel MW, Dee TH, Ferstenfeld JE, et al. Possible pathogenetic role of capslar antigens in fulminant pneumococcal disease with disseminated intravascular coagulation (DIC). Am J Med 1974;57:889–96 [DOI] [PubMed] [Google Scholar]
- 6.Brueggemann AB, Peto TE, Crook DW, et al. Temporal and geographic stability of the serogroup-specific invasive disease potential of Streptococcus pneumoniae in children. J Infect Dis 2004;190:1203–11 [DOI] [PubMed] [Google Scholar]
- 7.Hausddorff WP, Feikin DR, Klugman KP. Epidemiological differences among pneumococcal serotypes. Lancet Infect Dis 2005;5:83–93 [DOI] [PubMed] [Google Scholar]
- 8.Yu VL, Chiou CC, Feldman C, et al. An international prospective study of pneumococcal bacteremia: correlation with in vitro resistance, antibiotics administration, and clinical outcome. Clin Infect Dis 2003;37:230–7 [DOI] [PubMed] [Google Scholar]
- 9.Farina C, Arosio M, Vailati F, et al. Urinary detection of Streptococcus pneumoniae antigen for diagnosis of pneumonia. New Microbiol 2002;25:259–63 [PubMed] [Google Scholar]
- 10.Smith MD, Derrington P, Evans R, et al. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol 2003;41:2810–13 [DOI] [PMC free article] [PubMed] [Google Scholar]