Abstract
Introduction
The epidemiology of tamoxifen and venous thromboembolism (VTE) is not well understood, and most data on tamoxifen toxicity are from adjuvant clinical trials. This study examined the relationship between the duration of tamoxifen use in female patients with breast cancer and the risk of VTE in a large population-based setting.
Materials and Methods
Retrospective electronic data extraction on tamoxifen utilization was undertaken among a cohort of 3572 women with breast cancer seen at Marshfield Clinic between January 1, 1994 and June 31, 2009. Observational follow-up extended until February, 2010.
Results
On initial exposure to tamoxifen, women had a clustering of VTE events. Cox proportional hazards regression, adjusting for multiple clinically-important covariates including age, body mass index, cancer stage, and concurrent diabetes, demonstrated that as use of tamoxifen continued in those without earlier VTE events, risk of subsequent VTE gradually increased, albeit at a lower rate (hazard ratio per year of tamoxifen duration=1.225, P <0.0001).
Conclusions
In our study population, initiating tamoxifen coincided with an initial clustering of VTE events, with risks due specifically to tamoxifen, increasing during continued exposure. Evidence suggested that the VTE clustering occurred in high risk individuals at initiation of tamoxifen therapy. Careful selection of patients for whom tamoxifen therapy is appropriate based on susceptibility to VTE is thus required prior to initiation of therapy.
Keywords: Breast cancer, DVT, Tamoxifen, VTE
Introduction
Tamoxifen is a selective estrogen receptor modulator approved by the US Food and Drug Administration in 1977, and it remains the most prescribed hormonal/endocrine therapy for treatment and prevention of breast cancer. Tamoxifen use improves mortality rates for those with estrogen receptor (ER) positive breast cancer and decreases breast cancer incidence rates in high-risk women [1–5]. A review of all breast cancer risk reduction trials estimated a 38% reduction in estrogen-positive breast cancer incidence [6]. Tamoxifen has been associated with significant side effects and complications, including increased risk of endometrial cancer, deep vein thrombosis (DVT), and pulmonary embolism (PE) [7–12].
An estimated 207,090 women were diagnosed with breast cancer in the United States in 2010, with 39,840 deaths attributed to the disease [13]. Despite the availability of newer aromatase inhibitors, and more recently generic anastrazole, tamoxifen remains widely used in pre-and post-menopausal women due to its ready availability, relatively low cost and well-demonstrated efficacy. Data on potential tamoxifen-related complications in non-clinical trial settings are scarce, despite more than three decades of tamoxifen use [14]. Previous findings from clinical trials are often invalidated as a consequence of reporting or publication bias. In some studies non-significant findings may result in an underestimation of absolute risk or an overestimation of risk due to unusually high incidence of a particular health outcome [15]. Generally, reporting bias may result in underestimation of risk when surveillance is limited due to an abbreviated follow-up period, which is characteristic of many clinical trials. If an adverse event is not targeted as a primary outcome of a study or has not been identified prior to the study, this may hinder accurate reporting of results. Accurate risk estimation from these trials may also be limited by the inability to identify and capture specific and important clinical characteristics.
A comprehensive study of tamoxifen use performed in a community practice setting may provide wider generalizability than clinical trials. Our study design, from a community health perspective, allowed us to identify patient risk characteristics associated with tamoxifen use relative to length of tamoxifen treatment. The goal of this research project was to better define the epidemiology of tamoxifen-related venous thromboembolism (VTE). This research applied an evidence-based approach in a clinical setting to address a critical clinical knowledge gap in the treatment and care of breast cancer patients.
Materials and methods
The study was approved by the Institutional Review Board of the Marshfield Clinic. Marshfield Clinic is a large multi-specialty, multi-site group practice with regional centers located throughout central and northern Wisconsin. In partnership with St. Joseph’s Hospital in the city of Marshfield, Marshfield Clinic has one of the oldest internally-developed combined electronic medical records (EMRs) in the United States [16], with coded diagnoses dating back to the early 1960’s and laboratory observations dating back to 1985. The EMR data collected for clinical care are transferred daily into the Marshfield Clinic Data Warehouse where they are available for approved research. The clinical EMR also provides access to over 60 million free-text electronic clinical documents dating back to 1988.
Study population
The study population consisted of a cohort of 3572 female patients with breast cancer ≥18 years of age who were treated with tamoxifen at Marshfield Clinic. Eighteen percent of the population was deceased. Among patients who died, only 15 terminated tamoxifen at the time (n=3) or within one week of (n=12) of a VTE event (0.4%). Subjects were identified through the Marshfield Clinic/St. Joseph’s Hospital Cancer Registry using International Classification of Diseases for Oncology third revision (ICD-O-3) codes C50.0–C50.9 and/or through the Marshfield Clinic EMR using International Classification of Diseases ninth revision (ICD-9) codes 174.0–174.9, 175.0–175.9, 198.81, and 233.0. Dates of first tamoxifen treatment ranged between the years 1994 and 2009. Follow-up data were collected through interrogation of the EMR through February, 2010.
Data collection
The following data were extracted from the EMR as available for each subject: age, gender, height, weight, body mass index (BMI), diagnosis of diabetes, use of statins and hormone replacement therapy (HRT), blood pressure, lipid measures (low-density lipoprotein, high-density lipoprotein, triglycerides, total cholesterol), stage of cancer at diagnosis, treatment (tamoxifen dose, surgery, radiation, and chemotherapy), and dates of diagnoses for the events of interest. The pivotal temporal reference point was the date of tamoxifen initiation, and all subsequent patient measures were extracted from the EMR as close in time as possible to that date.
Validation
Manual record review was used to validate various aspects of the electronic coding. Data for 261 tamoxifen subjects had been manually abstracted in a pilot study, and 242 of these subjects (93%) were identified electronically for the current study. The remaining 19 subjects were excluded due to missing or uncertain tamoxifen coding in the electronic record. In addition, cancer staging was obtained manually for 1329 subjects since these data were not captured among registry data for a number of subjects.
Statistical analyses
Standard descriptive statistics were used to summarize important characteristics of the tamoxifen cohort. The primary study outcomes were PE and DVT (i.e., VTE) subsequent to tamoxifen treatment. The numbers of events were summarized by type, both prior and subsequent to treatment, but subjects with prior events were excluded from Cox proportional hazards time-to-event analyses since new incident events may be difficult to distinguish in the EMR, and since event risks are likely different for subjects with prevalent events.
In preliminary analyses, those without prevalent events were divided into quartiles (four equal-sized groups) based on the duration of tamoxifen use observed during the study period. By dividing patients into quartiles of duration of tamoxifen use, we were able to group our patients based on increasing length of exposure to tamoxifen, and for each duration group (quartile) time to VTE was measured from time of initiation of tamoxifen till end of follow-up. The numbers of events subsequent to tamoxifen initiation were summarized in each group for the first five years.
Proportional hazards (PH) analyses were also used to investigate potential confounding by age and other established clinical risk factors. We checked the PH assumption for our primary factor of interest (duration of tamoxifen) by dividing time into three intervals and found no significant interaction of duration and time period (P=0.14). However, time in our analyses is not “survival” per se, but rather time to the first venous event, with time being censored at the most recent EMR observation date in those not having events. While it may seem like the duration of tamoxifen and duration of follow-up are similar, in reality the duration of follow-up is usually much greater. Thus, a patient discontinuing tamoxifen in year 1 may still be followed up for 15 years. Since the relative risks for adverse events on a drug may change with duration of exposure [17], the methods described below were used to incorporate tamoxifen duration as a time-varying covariate in the time-to-event analyses. A covariate, “years of use”, was defined as the duration of use in years, which was 0 at initiation and increased until tamoxifen was stopped, at which time it equaled the total duration of use and remained at that value until the end of the patient’s observation time. The assumption of linearity in the effect of duration was investigated using restricted cubic splines [18], but the spline adjustments were not statistically significant, and we report only results for linear effects for age here. Results for the proportional hazards analyses were obtained with and without adjustment for age and other established clinical risk factors. BMI and hospitalizations were coded as categorical variables. For 19% of the cohort BMI was unavailable, so in those cases overall mean value was used in categorization. Results from the proportional hazards model were summarized with the estimated hazard ratio per year of tamoxifen duration (HR) and 95% confidence limits, and the significance of these comparisons was tested with Wald chi-square statistics.
Results
Descriptive values for the study population are shown in Tables 1 and 2. Patient median age was 62; median BMI was 28.6 kg/m2 (which is considered “overweight”); median blood pressure was borderline hypertensive, and median lipid measures were consistently within normal or even optimal ranges. The latter finding, in conjunction with data indicating statin use by 37% of subjects, indicated that lipid levels represented were in part clinically normalized. Breast cancer stage was most frequently Stage 1 (45%); and 68.7% of subjects had undergone surgery as a treatment option. DVT or PE prior to tamoxifen use occurred in 2.5% (92/3527). Among the 3435 women without prior DVT or PE, 8.4% (289/3435) experienced DVT or PE after initiation of tamoxifen therapy. Among the 289 who had events after initiation of tamoxifen, the incidence in 25 women (8.7%) occurred within 5 days of last recorded tamoxifen exposure, while 136 women (47.1%) experienced thromboembolic events sometime later following tamoxifen discontinuation.
Table 1.
Patient characteristics.
| Characteristic | Classification | % (Number) |
|---|---|---|
| Age (Years) | 0–29 | 0.3 (9/3572) |
| (Median 62) | 30–49 | 21.0 (750/3572) |
| 50–59 | 23.8 (850/3572) | |
| 60–69 | 24.7 (882/3572) | |
| 70–79 | 21.0 (751/3572) | |
| 80+ | 9.2 (330/3572) | |
| Body mass index (kg/m2) (Median 28.6) | Underweight: < 18.5 | 1.2% (34/2866) |
| Normal: 18.5 – 24.9 | 25.4% (729/2866) | |
| Overweight: 25.0 – 29.9 | 31.6% (906/2866) | |
| Obese: 30.0 – 39.9 | 34.3% (983/2866) | |
| Extremely Obese: ≥ 40.0 | 7.5% (214/2866) | |
| Blood Pressure (mmHg) (Median 130/76) | Normal: SBP <120, DBP <80 | 23.4% (757/3237) |
| Prehypertension: SBP 120–139, DBP 80–89 | 41.5% (1344/3237) | |
| Stage 1 Hypertension: SBP 140–159, DBP 90–99 | 28.2% (913/3237) | |
| Stage 2 Hypertension: SBP >160, DBP>100 | 6.9% (223/3237) | |
| LDL (mg/dL) (Median 117) | Optimal: <100 | 31.3% (760/2427) |
| Near Optimal: 100–129 | 31.7% (770/2427) | |
| Borderline: 130–159 | 23.4% (567/2427) | |
| High: 160–189 | 9.8% (237/2427) | |
| Very High: >190 | 3.8% (93/2427) | |
| HDL (mg/dL) (Median 54) | Low: <40 | 14.3% (355/2480) |
| Optimal: 40–60 | 49.0% (1214/2480) | |
| High: >60 | 36.7% (911/2480) | |
| Total Cholesterol (mg/dL) (Median 196) | Desirable:<200 | 54.1% (1534/2835) |
| Borderline: 200–239 | 30.6% (867/2835) | |
| High: >240 | 15.3% (434/2835) | |
| Triglyceride (mg/dL) (Median 129) | Normal: <150 | 60.2% (1509/2505) |
| Borderline-high: 150–199 | 17.6% (442/2505) | |
| High: 200–499 | 20.6% (515/2505) | |
| Very high: ≥ 500 | 1.6% (39/2505) | |
| Statin Use | 37.0% (1322/3572) | |
| Diabetes | 13.8% (494/3572) | |
| Smoking | 41.1% (907/2207) | |
| Hormone Replacement Therapy | 17.9% (639/3572) |
SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein
Table 2.
Breast cancer stage and treatment, including events of deep vein thrombosis (DVT) or pulmonary embolism (PE).
| Characteristic | Classification | % (Number) |
|---|---|---|
| Breast Cancer Stage | Stage 0 | 14.5% (446/3074) |
| Stage I | 45.4% (1396/3074) | |
| Stage II | 32.3% (994/3074) | |
| Stage III | 5.7% (174/3074) | |
| Stage IV | 2.1% (64/3074) | |
| Treatment (From Cancer Registry) | Chemotherapy | 26.5% (475/1794) |
| Surgery | 68.7% (1232/1794) | |
| Radiation | 53.2% (839/1794) | |
| Median (Number) | ||
| Duration of Tamoxifen by Quartile (years) (Overall Median 3.0) | Q1 | 0.5 (893/3572) |
| Q2 | 2.1 (893/3572) | |
| Q3 | 4.1 (893/3572) | |
| Q4 | 5.1 (893/3572) | |
| Pre-existing event | DVT | 2.4 (84/3572) |
| PE | 0.4 (15/3572) | |
| Either DVT or PE | 2.6 (92/3572) | |
| Post-tamoxifen initiation event | DVT | 6.8 (237/3572) |
| PE | 3.4 (117/3572) | |
| Either DVT or PE | 8.3 (289/3572) |
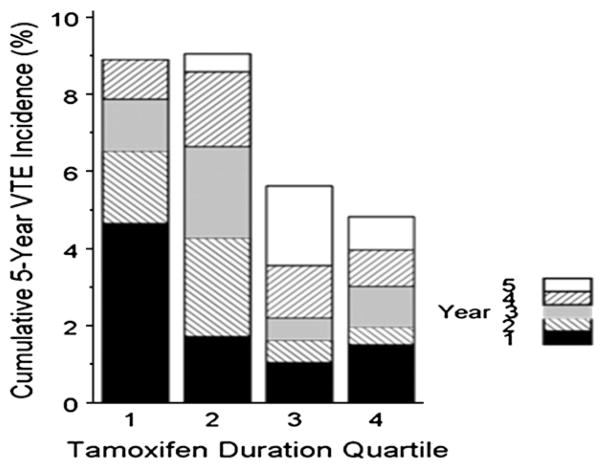
Median duration of tamoxifen use by quartile was as follows: 1st Quartile 0.5 years, 2nd Quartile 2.1 years, 3rd Quartile 4.1 years, and 4th Quartile 5.1 years. Fig. 1 shows the cumulative 5-year incidence of VTE by quartile of duration of tamoxifen. This plot demonstrates increased VTE risk at early time points in the first two quartiles compared to the last two quartiles of duration of tamoxifen use.
Fig. 1.
Cumulative incidence of VTE in the first five years by quartile of tamoxifen duration This plot demonstrates the early clustering of events associated with early discontinuation of tamoxifen.
If there is a lower incidence when a subject belongs to a longer duration group this could suggest that tamoxifen protects against VTE. We actually found this (Fig. 1), but we know, based on knowledge of the drug, that tamoxifen cannot possibly protect against VTE. Rather, we conclude that this represents discontinuation of the drug early in the two shorter duration groups because of clustering of VTE with onset of tamoxifen; thus, Fig. 1 does not tell the full story (and thus is not our primary analysis) but serves to illustrate an important point. We followed this up by an analysis that accounts for variability in duration of tamoxifen use over time. Table 3 reports results of analyses using time-varying covariates to model duration in the proportional hazards regression analysis of tamoxifen duration and VTE hazard. The hazard ratio implies that at any given time, the odds that an event will occur in those on longer duration of tamoxifen compared to another individual is significantly greater (P <0.0001). Hazard ratios and associated P values showed little variation with or without adjustment (all P values <0.0001). The reason for the apparent discrepancy between results from the latter analysis (Table 3) and the analysis in Fig. 1 is that a large subset of patients clustered around 0.5–2 years of tamoxifen treatment developed VTE early on (Quartiles 1 and 2), while events appeared more widely distributed over time for the remaining patients. In summary, overall early clustering following tamoxifen initiation led to the appearance of a relatively higher event rate in an early period following tamoxifen initiation (Fig. 1). Those that did not have an early event were then at increased risk for a later event albeit at a lower rate, but this risk increased over time and was not attenuated by the conventional risk factors for VTE.
Table 3.
Hazard Ratios for venous events (DVT or PE) adjusting for potential confounders via multivariable Cox regression analysis.
| Hazard Ratio | 95% Confidence Interval
|
P-value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Tamoxifen duration (years)** | 1.215 | 1.105 | 1.336 | <.001 |
| Age linear (years) | 1.032 | 1.020 | 1.043 | <.001 |
| BMI 30+ vs, <25 (kg/m2) | 2.778 | 1.755 | 4.397 | <.001 |
| BMI 25–29 vs. 30+ (km/m2) | 2.320 | 1.479 | 3.637 | <.001 |
| Hospitalizations 4+ vs. none | 1.952 | 1.416 | 2.691 | <.001 |
| Hospitalizations 1–3 vs. none | 4.922 | 3.433 | 7.058 | <.001 |
| Breast Cancer Stage (0–4) | 1.383 | 1.196 | 1.601 | <.001 |
| Diabetes (Y/N) | 1.338 | 0.967 | 1.851 | 0.079 |
| HRT use (Y/N) | 0.960 | 0.693 | 1.330 | 0.806 |
| Statin use (Y/N) | 1.021 | 0.786 | 1.326 | 0.877 |
DVT, deep vein thrombosis; PE, pulmonary embolism; BMI, body mass index
There was no attenuation of effect as covariates were added. Also, a simple time-dependent indicator of being on (vs. off) tamoxifen revealed similar results
Discussion
The clinical association between VTE and cancer is well established, with an overall risk for VTE elevated 7-fold in patients with cancer. Notably, breast cancer is associated with one of the lowest risks [4]. Based on the experiences of patients exposed to tamoxifen during the past 30 years, epidemiological evidence suggests tamoxifen use is associated with an increased incidence of VTE. Adjuvant hormonal therapy with tamoxifen is thought to increase the risk for VTE, independent of the breast cancer diagnosis [4]. A recent population-based study suggests that the first 2 years after the initiation of tamoxifen therapy may be the most crucial time for monitoring VTE risk, particularly in older women [9], and studies of chemoprophylaxis in women without a breast cancer diagnosis also suggest an increased risk for PE and DVT with tamoxifen use [19]. The fact that some previous studies report a “decrease” in risk of VTE over time with sustained use [9] is consistent with this clustering effect, but this issue remains understudied, and existing published reports on this issue have not provided consistent conclusions.
Among our cohort of women exposed to tamoxifen, the rates of VTE over the same early window of follow-up were clearly higher amongst those in the lower quartiles of duration of tamoxifen use compared to those in the higher quartiles (Fig. 1). Simple analyses of incidence seem to suggest that something has changed at the individual level. However, it is in fact the population risk that has changed. The fact that many medication effects vary by duration is well known [17,20]. Clustering of estrogenic effect or other side effects within the first year or more of starting estrogen or tamoxifen, similar to our finding, has been reported by others [9,21]. For example, summary estimates of endometrial cancer risk among individuals on estrogen has also been reported to be higher within about a year or more of starting estrogen [21].
Early clustering of events may be the result of a combination of VTE effect of tamoxifen and conventional risk factors of VTE such as age, obesity, cancer stage, and immobilization due to hospitalization. The fact that these early events could have occurred in those who might have had more of such conventional risk factors of VTE is supported by data from the literature. There is evidence of a link between atherosclerosis and VTEs which suggests that tamoxifen triggers some of these common mechanistic pathways [2]. There is also evidence that common VTE risk factors tend to be associated with the earlier development of VTE on tamoxifen [7], similar to the effect reported for HRT [22]. It is therefore plausible that in those individuals with risk factors, emergence of VTE following tamoxifen exposure occurs early, while others without risk factors are at a considerably lower risk of VTE following tamoxifen exposure. This explains the possible clustering effect of venous events occurring in a subset of patients around the first 6 to 12 months on tamoxifen.
When duration of tamoxifen use was entered as a time-varying covariate in the proportional hazards model, there was an apparent reversal in the trend, and the hazard of VTE events was greater in those with persistent ongoing use over time. This discrepancy between the analyses simply suggests that longer duration quartiles would have been ‘depleted’ of patients at high risk for the VTE event, either through having the event itself and stopping tamoxifen or through having warning symptoms leading to discontinuation before the event occurred [17]. The effect works by changing the population at risk, and hence changing the estimate at a population level even though nothing may be changed at an individual level [17]. This is because the initial clustering leads to selection of a lower risk population on tamoxifen in the later years. This might suggest that those on tamoxifen had lower rates of VTE over time, but this conclusion would be erroneous, although such instances have occurred in the literature [9,23]. In our study, those with longer duration of tamoxifen utilization eventually showed higher event rates after adjusting for initial clustering that led to discontinuation of tamoxifen. Adjustments for other clinical or demographic factors had little effect on the results using duration as a variable length of exposure covariate, indicating that long-term tamoxifen therapy itself remains an important independent factor for increased thrombotic risk.
There are several limitations to the present study. First, there was no truly unexposed control group for comparison; all cohort members were “exposed” to tamoxifen for varying lengths of time. This lack of a control group limited our ability to determine how the event risk may change over time in the absence of tamoxifen. Secondly, data were not available on the reason for tamoxifen discontinuation or precisely when discontinuation occurred, since our data defaults to the last clinic verification of a prescription for tamoxifen. Therefore, although there was not an absolute temporal sequence from VTE to tamoxifen discontinuation in every case, the sequence was close enough to suggest that it was generally the thromboembolic event itself that limited the duration of therapy, and the clustering we demonstrated suggests that this is indeed the case. Furthermore, occurrence of approximately a quarter of the events in the first year of follow-up supports our conclusions.
Conclusions
In our study population, tamoxifen use appeared to lead to a clustering of the VTE events at the start of therapy. Notably, a persistent effect on VTE events for women with sustained exposure to tamoxifen over time was also noted. We believe these results suggest that the initial clustering seen in the first year of use may represent other pre-existing risk factors in addition to risk contributed by tamoxifen. VTE is a serious life threatening condition with significant morbidity and mortality [24,25]. Observations from this study, therefore, have implications for breast cancer treatment, since enhanced ability to identify determinants which contribute to early VTE events in women on tamoxifen would support individualization of therapy with less stringent follow-up requirements for women at lower risk, thereby decreasing frequency of tamoxifen-induced occurrence of this potentially life-threatening complication in those with breast cancer.
Acknowledgments
Role of funding source
This study was supported by disease specific grant funds from the Marshfield Clinic Research Foundation.
The authors acknowledge the Office of Scientific Writing and Publication at the Marshfield Clinic Research Foundation for editorial assistance in the preparation of this manuscript.
Abbreviations
- BMI
body mass index
- DVT
deep vein thrombosis
- EMR
electronic medical record
- ER
estrogen receptor
- HRT
hormone replacement therapy
- PE
pulmonary embolism
- VTE
venous thromboembolism
Footnotes
Author contributions
A.A. Onitilo is the principal investigator, provided the original concept, provided study oversight, and wrote the manuscript. S.A.R. Doi, J.M. Engel, I. Glurich, J. Johnson, and R. Berg, assisted with the manuscript and contributed to the planning and conduct of the study. Statistical analysis and interpretation of the data was conducted by S.A.R. Doi and R. Berg. All authors have contributed to, reviewed and approve this manuscript.
Conflict of interest
The authors report no real or potential conflicts of interest.
References
- 1.Cuzick J, Forbes JF, Sestak I, Cawthorn S, Hamed H, Holli K, et al. International Breast Cancer Intervention Study Investigators. Long-term results of tamoxifen prophylaxis for breast cancer-96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;99:272–82. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- 2.Decensi A, Maisonneuve P, Rotmensz N, Bettega D, Cost A, Sacchini V, et al. Effect of tamoxifen on venous thromboembolic events in a breast cancer prevention trial. Circulation. 2005;111:650–6. doi: 10.1161/01.CIR.0000154545.84124.AC. [DOI] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 4.Noble S, Pasi J. Epidemiology and pathophysiology of cancer-associated thrombosis. Br J Cancer. 2010;102(Suppl 1):S2–9. doi: 10.1038/sj.bjc.6605599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ragaz J, Coldman A. Survival impact of adjuvant tamoxifen on competing causes of mortality in breast cancer survivors, with analysis of mortality from contralateral breast cancer, cardiovascular events, endometrial cancer, and thromboembolic episodes. J Clin Oncol. 1998;16:2018–24. doi: 10.1200/JCO.1998.16.6.2018. [DOI] [PubMed] [Google Scholar]
- 6.Cuzick J, Powles T, Veronesi U, Forbes J, Edwards R, Ashley S, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361:296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 7.Duggan C, Marriott K, Edwards R, Cuzick J. Inherited and acquired risk factors for venous thromboembolic disease among women taking tamoxifen to prevent breast cancer. J Clin Oncol. 2003;21:3588–93. doi: 10.1200/JCO.2003.10.111. [DOI] [PubMed] [Google Scholar]
- 8.Goldhaber SZ. Tamoxifen: preventing breast cancer and placing the risk of deep vein thrombosis in perspective. Circulation. 2005;111:539–41. doi: 10.1161/01.CIR.0000156099.83394.A7. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez RK, Sorensen HT, Pedersen L, Jacobsen J, Lash TL. Tamoxifen treatment and risk of deep venous thrombosis and pulmonary embolism: a Danish population-based cohort study. Cancer. 2009;115:4442–9. doi: 10.1002/cncr.24508. [DOI] [PubMed] [Google Scholar]
- 10.Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation. 2003;107:I17–21. doi: 10.1161/01.CIR.0000078466.72504.AC. [DOI] [PubMed] [Google Scholar]
- 11.Love RR, Wiebe DA, Feyzi JM, Newcomb PA, Chappell RJ. Effects of tamoxifen on cardiovascular risk factors in postmenopausal women after 5 years of treatment. J Natl Cancer Inst. 1994;86:1534–9. doi: 10.1093/jnci/86.20.1534. [DOI] [PubMed] [Google Scholar]
- 12.Prandoni P, Falanga A, Piccioli A. Cancer and venous thromboembolism. Lancet Oncol. 2005;6:401–10. doi: 10.1016/S1470-2045(05)70207-2. [DOI] [PubMed] [Google Scholar]
- 13.American Cancer Society. [May 7, 2011 Accessed];Breast Cancer Facts & Figures. 2009–2010 Available at: http://www.cancer.org/research/cancerfactsfigures/breastcancerfactsfigures/breast-cancer-facts-figures-2009-2010.
- 14.Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;351:1451–67. No authors listed. [PubMed] [Google Scholar]
- 15.Braithwaite RS, Chlebowski RT, Lau J, George S, Hess R, Col NF. Meta-analysis of vascular and neoplastic events associated with tamoxifen. J Gen Intern Med. 2003;18:937–47. doi: 10.1046/j.1525-1497.2003.20724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sirohi E, Peissig P. Study of effect of drug lexicons on medication extraction from electronic medical records. Pac Symp Biocomput. 2005:308–18. doi: 10.1142/9789812702456_0029. [DOI] [PubMed] [Google Scholar]
- 17.Guess HA. Exposure-time-varying hazard function ratios in case–control studies of drug effects. Pharmacoepidemiol Drug Saf. 2006;15:81–92. doi: 10.1002/pds.1164. [DOI] [PubMed] [Google Scholar]
- 18.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–61. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 19.Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–62. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 20.Guess HA. Behavior of the exposure odds ratio in a case–control study when the hazard function is not constant over time. J Clin Epidemiol. 1989;42:1179–84. doi: 10.1016/0895-4356(89)90116-9. [DOI] [PubMed] [Google Scholar]
- 21.Grady D, Ernster VL. Endometrial Cancer. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. 2. New York, NY: Oxford University Press; 1996. pp. 1058–89. [Google Scholar]
- 22.Miller J, Chan BK, Nelson HD. Postmenopausal estrogen replacement and risk for venous thromboembolism: a systematic review and meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;136:680–90. doi: 10.7326/0003-4819-136-9-200205070-00011. [DOI] [PubMed] [Google Scholar]
- 23.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–13. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 24.Falanga A, Zacharski L. Deep vein thrombosis in cancer: the scale of the problem and approaches to management. Ann Oncol. 2005;16:696–701. doi: 10.1093/annonc/mdi165. [DOI] [PubMed] [Google Scholar]
- 25.Saphner T, Tormey DC, Gray R. Venous and arterial thrombosis in patients who receive adjuvant therapy for breast cancer. J Clin Oncol. 1991;9:286–94. doi: 10.1200/JCO.1991.9.2.286. [DOI] [PubMed] [Google Scholar]