Abstract
Objectives
Persons with bipolar disorder experience a disproportionate burden of medical conditions, notably cardiovascular disease (CVD), leading to impaired functioning and premature mortality. The study objective was to determine whether the Life Goals Collaborative Care (LGCC) intervention compared to enhanced usual care, reduced CVD risk factors and improved physical and mental health outcomes in VA patients with bipolar disorder.
Method
Patients with ICD-9 diagnosis of bipolar disorder and >=1 CVD risk factor (n=118) enrolled in the Self-Management Addressing Heart Risk Trial conducted April 2008–May 2010, were randomized to LGCC (N=58) or enhanced usual care (N=60). LGCC included four weekly self-management sessions followed by tailored contacts combining health behavior change strategies, medical care management, registry tracking, and provider guideline support. Enhanced usual care included quarterly wellness newsletters sent over a 12-month period in addition to standard treatment.
Results
Out of the 180 eligible patients identified for study participation, 134 were enrolled (74%) and 118 completed outcomes assessments (mean age = 53, 17% female, 5% African American). Mixed effects analyses comparing changes in 24-month outcomes among patients in LGCC (N=57) versus enhanced usual care (N=59) groups revealed that patients receiving LGCC had reduced systolic (Beta=−3.1, P=.04) and diastolic blood pressure (Beta=−2.1, P=.04) as well as reduced manic symptoms (Beta=−23.9, P=.01). LGCC had no significant impact on other primary outcomes (total cholesterol, physical health-related quality of life).
Conclusions
LGCC compared to enhanced usual care may lead to reduced CVD risk factors, notably through decreased blood pressure, as well as reduced manic symptoms.
Keywords: mental disorders, cardiovascular disease, collaborative care, health behaviors
INTRODUCTION
Bipolar disorder is a chronic mental illness that is associated with substantial functional impairment, morbidity, economic burden, and mortality.1,2 Individuals with bipolar disorders (I, II, NOS) also die younger than the general U.S. population,1,3 and a key driver of morbidity and subsequent premature mortality is cardiovascular disease (CVD).1,4,5 Some of the most common medical conditions (e.g., hypertension, obesity) that disproportionately burden patients with bipolar disorder5 are also the leading risk factors for CVD.
Bipolar disorder presents a unique challenge apart from other mental illnesses, and may require a customized intervention strategy to reduce CVD risk.6,7,8 The cyclical nature of this condition (alternating mood episodes) may exacerbate CVD risk factors which are further compounded by unhealthy behaviors,9,10 multiple medications,11 and fragmentation of health care.6 Yet few treatment models have been developed to address these multifaceted CVD risk factors in bipolar disorder, especially self-management of risk factors.12
Current treatment interventions that have focused on reducing CVD risk in persons with bipolar or other mental disorders chiefly relied on care management strategies (e.g., depression in primary care settings,13 psychotherapy,12 or intensive lifestyle interventions in mental health specialty settings.14,15) Life Goals Collaborative Care (LGCC) is based on the Chronic Care Model16,17 but places an emphasis on self-management through targeted health behavior change strategies to address the psychosocial origins of CVD risk factors.18 LGCC has been shown to improve overall mental health outcomes,19–21 and given its focus on multiple risk factors, has the potential to reduce CVD risk factors as well.22
The purpose of the Self-Management Addressing Heart Risk Trial (SMAHRT) randomized controlled effectiveness trial is to determine whether LGCC compared to enhanced usual care reduces CVD risk factors and improves overall outcomes among patients with bipolar disorder. We hypothesized that compared to those receiving enhanced usual care, patients with bipolar disorder receiving LGCC will have reduced CVD risk factors, including reductions in blood pressure, cholesterol, and improved physical health-related quality of life.
METHOD
The design and rationale for SMAHRT have been described elsewhere in a protocol paper.22 In brief, SMAHRT is a randomized controlled effectiveness trial comparing VA patients with bipolar disorder and at least one CVD risk factor receiving care in outpatient mental health or primary care clinics who were randomized to receive LGCC or enhanced usual care.
Setting and Participants
Patients were recruited from a mental health outpatient clinic in southeastern Michigan and a primary care outpatient clinic in northern Ohio. Patients diagnosed with bipolar disorder and a CVD risk factor who received care between fiscal year (FY) 2008 and FY 2009 were first identified based on a medical record review of patients with an International Classification of Diseases, Ninth Revision (ICD-9) diagnosis (codes 296.0–296.1 and 296.4–296.8). Patient inclusion criteria were: (1) adult patients who had a current diagnosis of bipolar disorder in the past 12 months (I, II, NOS, or schizoaffective disorder-bipolar subtype) based on clinician documentation of diagnosis or treatment plan; and (2) diagnosis of or receiving treatment for at least one of the following medical conditions associated with increased CVD risk within the past 12 months: hyperlipidemia or dyslipidemia (documented diagnosis, low-density lipoprotein ≥160 mg/dL, or receiving statin or other treatment), hypertension (documented diagnosis or blood pressure of ≥140/90 mmHg on 2 occasions), diabetes mellitus (documented diagnosis or HbA1C ≥7%, or receiving treatment), obesity (documented diagnosis or body mass index (BMI) >30), or current diagnosis of arteriosclerotic cardiovascular disease.
Patient exclusion criteria were minimal and designed to maximize the generalizability of LGCC, and included: (1) substance intoxication or withdrawal symptoms at the time of recruitment encounter, (2) current enrollment in an intensive case management or assertive community treatment program based on medical record review, or (3) unwilling or unable to provide informed consent or comply with study requirements at the time of the recruitment encounter (e.g., due to terminal medical illness or dementia).
Recruitment and Consent Procedures
A study clinical assessor with a background in nursing oversaw recruitment and clinical assessments. Using the list of potentially eligible patients based on medical record data, the clinical assessor approached patients at the time of their appointment. After confirming eligibility, patients provided informed consent and completed a clinical exam and a self-completed survey. Participants received renumeration of $10 for each assessment. Study recruitment, enrollment, and patient participation in the 12-month intervention phase occurred between April 2008 and May 2010. The last assessment of 24-month outcomes was completed in May 2012. The study was reviewed and approved by the VA Medical Center Institutional Review Board.
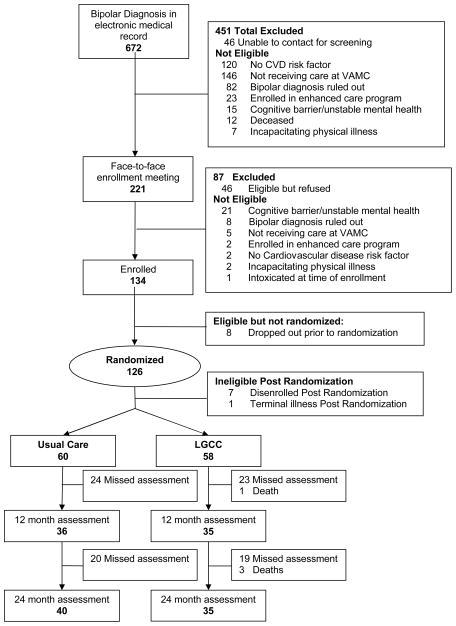
Out of the 180 eligible patients identified for study participation, 134 were initially enrolled after verification of diagnosis and suitability with providers. Eight dropped out pre-randomization after signing consent but did not complete any assessments, and hence, no data are available on these patients. An additional eight dropped out immediately after randomization (n=7) or due to sudden death (n=1), leaving a total of 118 with complete baseline and follow-up data for analysis (See Consort Figure 1).
Figure 1.
CONSORT Diagram for the Self-Management Addressing Heart Risk Trial (SMAHRT) Comparing Life Goals Collaborative Care to Enhanced Usual Care
Treatment Assignment and Intervention
Participants were randomized to the LGCC intervention or enhanced usual care (Table 1) in blocks of 15–20 people that were stratified by age, race, and diabetes diagnosis in order to ensure balance of these characteristics.
Table 1.
Life Goals Collaborative Care and Enhanced Usual Care Components
| LGCC | Enhanced Usual Care |
|---|---|
|
Self-management sessions by health specialist Four group sessions (90–120 minutes, approx. 8–10 individuals per group cohort) covering:
|
No self-management sessions or follow-up contacts by health specialist Wellness mailings, including summaries of topics unrelated to LGCC self-management content, including sleep, smoking cessation, and fatigue. |
Care management by Health Specialist:
Ongoing clinical management by assigned primary care and mental health provider team case management, psychotherapy, and group sessions |
No ongoing contacts or care management by health specialist or registry tracking provided Ongoing clinical management by assigned primary care and mental health provider team that included case management, psychotherapy, and group sessions |
Clinical registry tracking
|
No registry tracking |
Guideline support
|
Guideline Support
|
LGCC Intervention
The SMAHRT trial protocol for the LGCC intervention arm (Table 1), has been described in detail elsewhere,22 was implemented by a master’s level-trained health specialist, and included four of the six components of the original Chronic Care Model23 but enhanced using Social Cognitive Theory to focus on health behavior change.22
Following randomization, the health specialist initiated a pre-session assessment to promote treatment engagement and participation. During this time, the health specialist assessed patient preferences for communication, motivation for health changes, availability for group participation, and principal provider contact information for emergency situations. Participants were then scheduled to attend the group self-management sessions, described below.
Self-management support
The bulk of the LGCC intervention time was devoted to self-management, in part because many of the CVD risk factors were potentially mutable to health behavior change. The health specialist led four 2-hour group self-management sessions held weekly based on the Life Goals psychotherapy program24 enhanced to include discussion on the management of CVD risk factors within the context of bipolar disorder.18,21,22 The first session provided an overview of bipolar disorder and CVD risk, stigma issues, and a discussion of personal goals, values, and strategies to help in achieving positive health behavior change. The next two sessions focused on health behavior change strategies that also helped with bipolar symptom coping strategies, notably diet (e.g., avoiding over-eating when depressed) and exercise (e.g., walking to reduce stress), and the final session focused on enhancing communication with providers, the role of collaborative care management, and setting specific objectives in health behavior goals.
Care management/registry tracking
The health specialist followed up with patients after the end of the self-management sessions on a monthly basis for up until 12 months to track symptoms, medical need, as well as progress towards health behavior change and personal goals. As part of ongoing care management, the health specialist used an electronic MS Access™ registry to track patient health behaviors and care needs over the course of the intervention.25 If patients were having elevated symptoms or needed medical care, the health specialist contacted their mental health and general medical providers to schedule a follow-up appointment. The health specialist also provided information to the patient’s general medical and mental health providers regarding the patient’s current CVD risk factor indicators (e.g., weight, blood pressure, labs) via consult notes available in the electronic medical record or secure email.
Guideline support
The health specialist disseminated treatment guidelines to mental health and primary care providers at participating sites. Guidelines included one-page tips for management of bipolar disorder (www.healthquality.va.gov) as well as summaries of the American Diabetes Association/American Psychiatric Association practice guidelines for CVD risk assessment.26
LGCC Fidelity
The health specialist underwent a 2-day training program developed by the investigators and followed a standardized set of protocols and intervention manual. Fidelity was measured using a combination of reviews of health specialist logs and direct observation of a random sample of Life Goals group sessions in which a content checklist was used to evaluate session delivery.24 Key fidelity indicators included number of group sessions completed by the patient and number of care management contacts.
Enhanced Usual Care
Enhanced usual care patients received regular mailings regarding wellness topics in addition to standard mental health and medical treatment (Table 1). They did not receive the other principal LGCC components (health specialist contact, self-management, or care management). Enhanced usual care patients’ general medical and mental health providers did receive the same practice guideline information at the beginning of the study as the providers for the LGCC patients, but the health specialist did not communicate with providers about their specific patients. Standard treatment at both sites included outpatient case management and group psychotherapy sessions specifically focused on mental health treatment that were provided by a mental health provider team, as well as access to a primary care provider.
Data Collection and Measures
Patients underwent a clinical exam (blood pressure, non-fasting blood draws, body mass index-BMI, and waist circumference) at baseline, 6, 12, and 24 months. The nurse clinical assessor measured the patient’s blood pressure based on two readings while he or she was sitting, measured height and weight, and conducted blood draws that were analyzed at on-site VA labs. Non-fasting total cholesterol was ascertained from blood draws because of the difficulty in obtaining fasting values (i.e., barriers for patients to come in the early morning for blood draws) and evidence suggesting that non-fasting total cholesterol is comparable to fasting values.27
Patients also completed a survey at the time of the clinical assessment to ascertain outcomes including health-related quality of life, functioning, symptoms, and other patient factors.19,28–31 The clinical assessor was blinded to randomization of patient assignment.
Primary and Secondary Outcomes
Primary outcomes were identified a priori to address the hypothesis that LGCC compared to enhanced usual care demonstrated reduced CVD risk factors within 12 months. Primary outcomes for which this study was powered (correcting for Bonferroni) included systolic and diastolic blood pressure, non-fasting total cholesterol, and physical health-related quality of life. Blood pressure and total cholesterol are considered key intermediate physiological measures of CVD-related morbidity and mortality.32 They also represent the two most common CVD risk factors observed among individuals diagnosed with bipolar disorder.5 Taking control of these risk factors can decrease CVD risk substantially.32 The 12-item Short Form Health Survey (SF-12) was used to assess physical health-related quality of life.33 Two composite scores for physical (PCS) and mental health (MHS) were generated based on the SF-12.
Secondary physical health outcomes included non-fasting high-density lipoprotein levels (HDLs), and direct low-density lipoprotein levels (LDLs), weight, including body mass index (BMI), and waist circumference. We also calculated the Framingham Risk score, which is an estimate of 10-year CVD risk based on age, gender, current cholesterol, blood pressure, diabetes diagnosis, and current smoking status.34
Additional secondary outcomes based on the patient survey included mental health-related quality of life based on the SF-12, functioning, and psychiatric symptoms. The World Health Organization’s Disability Assessment Scale is a 12-item survey assessing the degree of functional impairment experienced over the past month regarding self-care, mobility, cognition, social functioning, and role functioning.35 Psychiatric symptoms were ascertained using the Internal State Scale (ISS), a 16-item-item assessment of depressive and manic symptoms which was strongly correlated with clinician ratings of manic or depressive episodes.36
Analysis
Descriptive and bivariate analyses were conducted comparing treatment arms. Mixed effects analyses were also used to determine the effect of LGCC versus enhanced usual care on primary outcomes (blood pressure, total cholesterol, and physical health-related quality of life), which, based on previous research,21 were hypothesized to be most responsive to the intervention. Effect size estimates from our previous pilot work21 suggested a Cohen’s D for primary outcomes (blood pressure) to range from .40–.42, thereby a sample size of 108 was sufficient to detect significant effects in our primary outcomes. We used repeated measures mixed effects models to examine LGCC effect, which allow unequal observations per subject, i.e., missing observations on some measures for some subjects. The main outcomes models included the baseline value of the outcome measure (to account for differences in outcomes at baseline), baseline obesity (defined as BMI >=30), effect of LGCC, time (6, 12, 24 months), and the interaction of time and study group.
RESULTS
Of the 118 patients enrolled in the study, 58 were randomized to the LGCC group and 60 were randomized to enhanced usual care. Of the 118, there were two non-study related deaths, leaving a total of 116 with available data for 12-month analyses (Figure 1). Mean participant age was 52.8 (SD±9.9), 17% were women, 5% non-white, and the majority (62%) were diagnosed with bipolar II disorder. Based on the high prevalence of multiple CVD risk factors, the majority (59%) had a high 10-year risk for a serious CVD event based on a Framingham score of >=20% (Table 2). Post-hoc analyses of medication use showed that 97% of enrolled patients were prescribed an antihypertensive at baseline and 3% had started an antihypertensive medication during the follow-up period.
Table 2.
Baseline Demographic and Clinical Characteristics of Study Enrollees
| Total (N = 116) | LGCC (N=57) | Usual care (N =59) | ||||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | t or χ2 | p-value | |
| Demographics | ||||||||
| Age M ± (SD)a | 52.8 ± 9.9 | 53.1 ± 10.6 | 52.4 ± 9.2 | −.40 | .69 | |||
| Age breakdown | .48 | .79 | ||||||
| <50 years | 40 | 33.9 | 22 | 38.0 | 22 | 36.7 | ||
| 50–59 years | 44 | 37.3 | 18 | 31.0 | 22 | 36.7 | ||
| ≥60 years | 34 | 28.8 | 18 | 31.0 | 16 | 26.6 | ||
| Female | 20 | 17.0 | 10 | 17.2 | 10 | 16.7 | .01 | .93 |
| Non-white | 6 | 5.1 | 3 | 5.2 | 3 | 5.0 | .00 | 1.00 |
| Some college education | 80 | 68.4 | 42 | 73.7 | 38 | 63.3 | 1.45 | .23 |
| Lives alone | 35 | 31.8 | 18 | 32.1 | 17 | 31.5 | .01 | .94 |
| Current Smoker | 47 | 40.9 | 23 | 40.4 | 24 | 41.4 | .01 | .91 |
| Hazardous Drinkingb | 15 | 15.3 | 7 | 15.6 | 8 | 15.1 | .00 | .95 |
| Current Illicit Drug Use | 31 | 26.5 | 15 | 25.9 | 16 | 27.1 | .02 | .88 |
| Clinical Factors | ||||||||
| Current Bipolar Diagnosis | 3.68 | .30 | ||||||
| Bipolar I | 44 | 37.3 | 20 | 34.5 | 24 | 40.0 | ||
| Bipolar II | 26 | 22.0 | 14 | 24.1 | 12 | 20.0 | ||
| Bipolar NOS | 45 | 38.2 | 21 | 36.2 | 24 | 40.0 | ||
| Schizoaffective-Bipolar subtype | 3 | 2.5 | 3 | 5.2 | 0 | 0.0 | ||
| Psychotropic Medications | ||||||||
| Rx any atypical antipsychotic | 59 | 50.0 | 30 | 51.7 | 29 | 48.3 | .14 | .71 |
| Rx any mood stabilizer | 91 | 77.1 | 47 | 81.0 | 44 | 73.3 | .99 | 32 |
| Lithium | 20 | 17.0 | 11 | 19.0 | 9 | 15.0 | .33 | .57 |
| Lamotrigine | 28 | 23.7 | 14 | 24.1 | 14 | 23.3 | .01 | .92 |
| Valproate | 39 | 33.1 | 20 | 34.5 | 19 | 31.7 | .11 | .74 |
| Carbamazepine | 10 | 8.5 | 5 | 8.6 | 5 | 8.3 | .00 | 1.00 |
| CVD-Diagnoses | ||||||||
| Hypertension | 81 | 69.8 | 43 | 74.1 | 38 | 65.5 | 1.02 | .31 |
| Hyperlipidemia | 98 | 83.8 | 45 | 77.6 | 53 | 89.8 | 3.22 | .07 |
| Diabetes mellitus | 30 | 25.4 | 13 | 22.4 | 17 | 28.3 | .55 | .46 |
| Obesity (BMI ≥ 30) | 58 | 50.0 | 38 | 66.7 | 45 | 76.3 | 1.27 | .25 |
| Heart disease | 22 | 19.0 | 11 | 19.0 | 11 | 19.0 | .00 | 1.0 |
| Framingham Risk Scorec | 1.11 | .57 | ||||||
| < 10% | 48 | 40.7 | 26 | 44.8 | 22 | 36.7 | ||
| 10–20% | 48 | 40.7 | 23 | 39.7 | 25 | 41.7 | ||
| ≥ 20% | 22 | 18.6 | 9 | 15.5 | 13 | 21.6 |
Range = 31–80 years.
Current hazardous drinking was defined based on the Alcohol Use Disorders Identification Teat (AUDIT) question on whether the patient consumed 6 or more drinks during a single occasion.
Framingham Risk Scores is divided into 3 risk categories help estimate the 10-year risk for coronary heart disease: high risk (10-year risk ≥20%), moderately high risk (10-year risk, 10% to 20%), or lower to moderate risk (10-year risk <10%)
Baseline health-related quality of life scores were lower than U.S. population norms of 50 (Table 2). There were no significant differences in demographic or clinical characteristics between the two randomized arms except for baseline BMI.
Mixed effects analyses assessing changes in 24-month outcomes revealed that compared to enhanced usual care, LGCC reduced systolic (Beta=−3.1, P=.04, Cohen’s D=−0.22) and diastolic blood pressure (Beta=−2.1, P=.04, Cohen’s D =−0.23). There were no significant differences in the other primary outcomes (Table 3). After post-hoc Bonferoni adjustment for multiple comparisons, these findings were not statistically significant (P > 0.0125).
Table 3.
Repeated Measures Analysis: 24-Month Outcomes Comparing Life Goals Collaborative Care (LGCC) versus Enhanced Usual Care
| LGCC | Enhanced Usual Care | Repeated Measuresa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 month | 12 month | 24 month | Baseline | 6 month | 12 month | 24 month | Beta | 95% CI | T | P | |
| Blood Pressure | ||||||||||||
| SBP | 131.8±16.4 | 128.3±14.0 | 127.7±17.7 | 127.2±15.4 | 133.8±17.4 | 135.9±18.2 | 134.2±18.9 | 130.4±13.6 | −2.94 | −6.00 to .12 | −1.90 | .05 |
| DBP | 80.7±11.4 | 76.3±11.7 | 75.3±10.6 | 75.9±10.4 | 83.8±14.0 | 82.2±11.3 | 80.5±10.3 | 78.5±10.3 | −2.18 | −4.23 to −.12 | −2.10 | .03 |
| Total Cholesterol | 176.9±37.2 | 173.6±32.6 | 173.6±42.4 | 178.9±45.5 | 195.6±44.2 | 191.5±48.4 | 181.8±44.7 | 175.9±42.4 | 3.63 | −2.88 to 10.15 | 1.10 | .27 |
| HDL | 37.6±10.6 | 36.7±13.0 | 37.3±8.0 | 39.0±12.1 | 35.9±13.9 | 36.0±11.4 | 38.3±11.3 | 37.1±7.9 | .04 | −1.73 to 1.80 | .04 | .96 |
| LDL | 103.8±30.4 | 105.3±28.2 | 103.1±32.1 | 105.6±39.5 | 117.1±38.3 | 116.2±40.1 | 107.3±36.0 | 105.7±34.2 | 2.34 | −3.15 to 7.83 | .85 | .39 |
| Weight (BMI) | 32.0±6.2 | 33.3±6.9 | 32.3±5.9 | 31.3±5.8 | 34.5±9.1 | 33.1±5.1 | 34.0±5.7 | 33.4±6.1 | .11 | −1.01 to 1.23 | .19 | .84 |
| Waist circumference | 43.1±6.1 | 43.9±6.4 | 43.4±5.5 | 43.2±5.3 | 44.8±5.6 | 43.8±5.8 | 44.7±6.2 | 44.9±6.2 | .17 | −.43 to .77 | .56 | .57 |
| Framingham Score | 12.4±8.9 | 10.4±6.3 | 11.5±8.4 | 11.5±6.4 | 15.4±10.8 | 15.2±10.3 | 11.8±7.0 | 9.9±6.8 | −.34 | −1.48 to .79 | −.60 | .55 |
| SF-12 Quality of lifeb | ||||||||||||
| Mental health | 32.7±7.7 | 34.4±6.8 | 32.6±8.3 | 34.9±7.5 | 33.6±7.7 | 32.9±8.4 | 33.4±7.1 | 34.6±7.1 | .89 | −.51 to 2.29 | 1.26 | .21 |
| Physical health | 35.9±7.2 | 35.8±7.8 | 37.5±7.8 | 36.8±6.6 | 33.6±7.2 | 34.5±7.3 | 36.3±6.6 | 35.3±7.0 | .11 | −1.09 to 1.32 | .18 | .85 |
| Functioning (WHO-DAS)c | 16.6±9.4 | 15.9±8.0 | 15.4±8.9 | 15.0±10.9 | 17.9±9.9 | 19.1±10.4 | 17.0±9.5 | 16.5±10.7 | −.44 | −1.64 to 0.76 | −.73 | .46 |
| Mood Symptomsd | ||||||||||||
| Depressive symptoms | 64.1±53.2 | 52.3±43.4 | 67.7±55.8 | 50.6±46.4 | 84.3±53.5 | 75.5±53.9 | 70.0±62.8 | 60.3±55.9 | −3.91 | −14.18 to 6.36 | −.75 | .45 |
| Manic symptoms | 190.5±122.0 | 175.8±139.4 | 153.0±92.0 | 148.9±120.9 | 183.5±120.8 | 192.5±130.1 | 193.9±125.9 | 173.4±105.8 | −23.9 | −42.9 to −4.78 | −2.48 | .01 |
Repeated measures analyses models included the baseline value of the outcome measure (to account for differences in outcomes at baseline), baseline obesity (defined as BMI >=30), effect of LGCC, time (6, 12, 24 months), and the interaction of time and study group
The 12-item Short-Form Health Survey (SF-12) includes a mental health component score (MCS) and physical health component score (PCS). Possible scores range from 0 to 100, with higher scores indicating better health. For both summary scores, the population M ± SD is 50 ± 10.
World Health Organization Disability Assessment Scale; Possible scores range from 0 to 48; higher scores indicating worse functioning.
Symptom scores (0–10 points for each item) are based on the Internal State Scale (ISS). For depressive symptoms, possible scores range from 0 to 200, with higher scores indicating more severe depressive symptoms. For manic symptoms, possible scores range from 0 to 500, with a higher score indicating more severe manic symptoms.
For secondary outcomes, participants randomized to LGCC compared to those randomized to enhanced usual care experienced reduced manic symptoms over the 24-month period (Beta=−23.9, P=.01). There were no significant differences in the other secondary outcomes including HDL, LDL, BMI, depressive symptoms, or functioning.
Fidelity assessment to LGCC indicated that the majority (68%) completed at least three of the four self-management sessions and an adequate number of follow-up contacts over the 12 month intervention phase (mean=4.6, SD=3.6). Interventionist registry data indicated that the health specialist had a mean number of 1.2 (SD=1.0) and 0.3 (SD=0.6) contacts per patient with their mental health and primary care providers, respectively. To further determine the role of care management on patient outcomes, we conducted a post-hoc multivariate analysis to determine whether variation in health specialist-provider care management contacts might have explained changes in outcomes over time. We added to the main outcomes models for systolic and diastolic blood pressure the total number of mental health or primary care management contacts made by the health specialist with the patient’s providers among the LGCC intervention group. Number of care management contacts was not associated with changes in systolic (SBP) or diastolic blood pressure (DBP); respectively, Beta for number of care management contacts-SBP Beta = −0.61, P=0.44, DBP Beta = −0.10, P=0.85).
DISCUSSION
Compared to usual care, LGCC reduced cardiovascular disease risk, notably through systolic and diastolic blood pressure. Our observed changes in blood pressure (e.g., 4-point drop) can be associated with reduced stroke mortality by 14%, CVD mortality by 9%, and total mortality by 7%.37 Moreover, LGCC significantly improved blood pressure control despite 97% of the study cohort receiving prescriptions for anti-hypertensive medications at baseline. LGCC compared to enhanced usual care was also associated with reduced manic symptoms over time, a finding that is consistent with previous trials of the Chronic Care Model for bipolar disorder.19,20 LGCC was not associated with reductions in other CVD risk factors, including cholesterol or BMI, or changes in physical health-related quality of life. Still, health-related quality of life scores in our sample were almost 20 points below national norms, which was consistent with similar studies of VA patients with bipolar disorder.21,38 Fidelity to LGCC was on par compared to similar studies.19–21
To our knowledge this is the first study to demonstrate reductions in CVD risk factors for persons with bipolar disorder seen in primary or mental health specialty care settings. There is a paucity of treatment models specifically designed to address the unique characteristics of bipolar disorder. As one of the most costly mental disorders, evidence suggests that up to 70% of the costs of bipolar disorder are attributed to general medical care,39 and CVD is the most common cause of mortality.5 Hence, interventions that address gaps in physical as well as mental health outcomes are paramount for this group.
This is also one of the few Chronic Care Model-based studies to demonstrate clinically significant changes in physical outcomes for patients with mental disorders.23 Recent research has found that the Chronic Care Model may reduce CVD risk factors for patients with unipolar depression in primary care,40 and it primarily involved nurse care management. In contrast, our study is the first to demonstrate clinically significant effects on CVD risk factors based on a version of the Chronic Care Model that chiefly relied on patient-self-management. Notably, post-hoc analyses revealed that less than 3% (N=3) of enrolled patients started antihypertensive medications after study enrollment. Moreover, the number of provider care manager contacts was considerably less than self-management contacts by the health specialist, thus the effects of LGCC may not have been due to increased care management. Self-management is an important component of the Chronic Care Model, and LGCC takes advantage of similar approaches involving health behavior change and symptom coping strategies that are similar to existing treatment modalities such as group psychotherapy or wellness management,22 thus making it potentially more scalable to implement by existing providers in routine practice than care management.
Limitations of this study included the relatively small sample size which precluded adequate power to determine the effects of LGCC on secondary outcomes, and study involvement was limited to a couple of sites. We were also unable to obtain fasting lab values for lipids, in part because scheduling a time for blood draws prior to eating (e.g., in the early morning) was difficult for patients, as many of them were unable to make it into the clinic at that time. Study protocols also did not assess changes in either adherence or dose of medications used to treat cardiometabolic conditions.. Even though most of the LGCC intervention involved health specialist-patient contact, there was a possibility that health specialist contact with VA providers who cared for both LGCC and enhanced usual care patients may have affected care for both groups of patients . As the study was designed to mimic as closely as possible real-world clinical care settings, we did not use a formal diagnostic assessment for bipolar disorder but rather relied on diagnostic codes and verification from provider notes. Finally, the VA has a number of unique system characteristics (e.g. almost all patients have a primary care provider) and patient characteristics (e.g. preponderance of males) that may have affected generalizability.
Nonetheless, as a relatively brief intervention, LGCC may improve physical and mental health outcomes in patients with bipolar disorder, notably by reducing blood pressure and manic symptoms. In light of funding limitations and the implementation of new models of care (e.g. medical home models in the VA and elsewhere), LGCC is a potentially scalable model that can facilitate improved outcomes for persons with bipolar or other mental disorders. Additional research on whether existing providers available in community-based settings can implement LGCC in a similar fashion is warranted, especially given the potential scarcity of clinicians in smaller practices.
CLINICAL POINTS.
Bipolar disorder requires is a complex chronic condition that requires integrated medical and psychiatric care to produce optimal outcomes.
Collaborative chronic care models are an evidenced-based strategy that has the potential to efficiently improve the delivery of integrated services.
Behavioral interventions that support patient self-management, brief care management and registry monitoring can reduce bipolar symptoms and cardiovascular risks,
Acknowledgments
Funding Sources: AMK and MSB are authors of the workbook, “Overcoming Bipolar Disorder: A Comprehensive Workbook for Managing Your Symptoms & Achieving Your Life Goals” (New Harbinger Publications, Inc., 2008) which was the basis for many of the current study intervention materials and, receive publication royalties.
This work was supported by the Department of Veterans Affairs, Veterans Health Administration, Clinical Sciences Research and Development [CSRD S06], the VA Health Services Research and Development Center for Organization, Leadership, and Management Research (COLMR), and the National Institute of Mental Health [R34MH74509].
Footnotes
Indications of previous presentations: Study baseline data were presented at the VA Health Services Research and Development Annual Meeting in Washington, D.C. on July 18, 2012
Disclaimer statements: The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
The other authors declare no conflict of interest.
Trial Registration Number: ClinicalTrials.gov, NCT00499096; URL: http://clinicaltrials.gov/ct2/show/NCT00499096
References
- 1.Kilbourne AM, Ignacio RV, Kim HM, Blow FC. Datapoints: are VA patients with serious mental illness dying younger? Psychiatric Services. 2009;60:589. doi: 10.1176/ps.2009.60.5.589. [DOI] [PubMed] [Google Scholar]
- 2.Wells KB, Miranda J, Gonzalez JJ. Overcoming barriers and creating opportunities to reduce burden of affective disorders: a new research agenda. Ment Health Serv Res. 2002;4:175–178. doi: 10.1023/a:1020947829820. [DOI] [PubMed] [Google Scholar]
- 3.Roshanaei-Moghaddam B, Katon W. Premature mortality from general medical illnesses among persons with bipolar disorder: a review. Psych Serv. 2009;60:147–156. doi: 10.1176/ps.2009.60.2.147. [DOI] [PubMed] [Google Scholar]
- 4.Kilbourne AM, Morden NE, Austin K, et al. Excess heart-disease-related mortality in a national study of patients with mental disorders: identifying modifiable risk factors. Gen Hosp Psychiatry. 2009;31(6):555–563. doi: 10.1016/j.genhosppsych.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiner M, Warren L, Fiedorowicz JG. Cardiovascular morbidity and mortality in bipolar disorder. Ann Clin Psychiatry. 2011;23(1):40–47. [PMC free article] [PubMed] [Google Scholar]
- 6.Horvitz-Lennon M, Kilbourne AM, Pincus HA. From silos to bridges: meeting the general health care needs of adults with severe mental illnesses. Health Aff (Millwood) 2006 May;25(3):659–669. doi: 10.1377/hlthaff.25.3.659. [DOI] [PubMed] [Google Scholar]
- 7.Zeber JE, McCarthy JF, Bauer MS, Kilbourne AM. Self-Reported Access to General Medical and Psychiatric Care Among Veterans With Bipolar Disorder. Psychiatric Services. 2007;58(6):740. doi: 10.1176/ps.2007.58.6.740. [DOI] [PubMed] [Google Scholar]
- 8.Judd LL, Akiskal HS. The prevalence and disability of bipolar spectrum disorders in the US population: re-analysis of the ECA database taking into account subthreshold cases. J Affect Disord. 2003;73(1–2):123–131. doi: 10.1016/s0165-0327(02)00332-4. [DOI] [PubMed] [Google Scholar]
- 9.Kilbourne AM, Rofey DL, McCarthy JF, Post EP, Welsh D, Blow FC. Nutrition and exercise behavior among patients with bipolar disorder. Bipolar Disord. 2007 Aug;9(5):443–452. doi: 10.1111/j.1399-5618.2007.00386.x. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein BI, Kemp DE, Soczynska JK, McIntyre RS. Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: a systematic review of the literature. J Clin Psychiatry. 2009;70(8):1078–1090. doi: 10.4088/JCP.08r04505. [DOI] [PubMed] [Google Scholar]
- 11.Kupfer DJ. The increasing medical burden in bipolar disorder. Jama. 2005 May 25;293(20):2528–2530. doi: 10.1001/jama.293.20.2528. [DOI] [PubMed] [Google Scholar]
- 12.Druss BG, von Esenwein SA, Compton MT, Rask KJ, Zhao L, Parker RM. A randomized trial of medical care management for community mental health settings: the Primary Care Access, Referral, and Evaluation (PCARE) study. Am J Psychiatry. 2010;167(2):151–159. doi: 10.1176/appi.ajp.2009.09050691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katon W, Guico-Pabia CJ. Improving quality of depression care using organized systems of care: a review of the literature. The primary care companion to CNS disorders. 2011;13(1) doi: 10.4088/PCC.10r01019blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Citters AD, Pratt SI, Jue K, et al. A pilot evaluation of the In SHAPE individualized health promotion intervention for adults with mental illness. Community Mental Health Journal. 2010;46:540–552. doi: 10.1007/s10597-009-9272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daumit GL, Dalcin AT, Jerome GJ, et al. A behavioral weight-loss intervention for persons with serious mental illness in psychiatric rehabilitation centers. Int J Obes (Lond) 2011;35(8):1114–1123. doi: 10.1038/ijo.2010.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74(4):511–544. [PubMed] [Google Scholar]
- 17.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. Jama. 2002 Oct 9;288(14):1775–1779. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]
- 18.Kilbourne AM, Post EP, Nossek A, et al. Service delivery in older patients with bipolar disorder: a review and development of a medical care model. Bipolar Disord. 2008;10(6):672–683. doi: 10.1111/j.1399-5618.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 19.Bauer MS, McBride L, Williford WO, et al. Collaborative care for bipolar disorder: Part II. Impact on clinical outcome, function, and costs. Psychiatric Services. 2006;57(7):937–945. doi: 10.1176/ps.2006.57.7.937. [DOI] [PubMed] [Google Scholar]
- 20.Simon GE, Ludman EJ, Bauer MS, Unutzer J, Operskalski B. Long-term effectiveness and cost of a systematic care program for bipolar disorder. Arch Gen Psychiatry. 2006 May;63(5):500–508. doi: 10.1001/archpsyc.63.5.500. [DOI] [PubMed] [Google Scholar]
- 21.Kilbourne AM, Post EP, Nossek A, Drill L, Cooley S, Bauer MS. Improving medical and psychiatric outcomes among individuals with bipolar disorder: a randomized controlled trial. Psychiatric Services. 2008;59(7):760–768. doi: 10.1176/ps.2008.59.7.760. [DOI] [PubMed] [Google Scholar]
- 22.Goodrich DE, Kilbourne AM, Lai Z, et al. Design and rationale of a randomized controlled trial to reduce cardiovascular disease risk for patients with bipolar disorder. Contemp Clin Trials. 2012;33(4):666–678. doi: 10.1016/j.cct.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Woltmann E, Grogan-Kaylor A, Perron B, Georges H, Kilbourne AM, Bauer MS. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. Am J Psychiatry. 2012;169(8):790–804. doi: 10.1176/appi.ajp.2012.11111616. [DOI] [PubMed] [Google Scholar]
- 24.Bauer MS, McBride L, Williford WO, et al. Collaborative care for bipolar disorder: part I. Intervention and implementation in a randomized effectiveness trial. Psychiatric Services. 2006;57(7):927–936. doi: 10.1176/ps.2006.57.7.927. [DOI] [PubMed] [Google Scholar]
- 25.Kilbourne AM, McGinnis GF, Belnap BH, Klinkman M, Thomas M. The role of clinical information technology in depression care management. Adm Policy Ment Health. 2006;33(1):54–64. doi: 10.1007/s10488-005-4236-0. [DOI] [PubMed] [Google Scholar]
- 26.American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. . Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27:596–601. doi: 10.2337/diacare.27.2.596. [DOI] [PubMed] [Google Scholar]
- 27.Craig SR, Amin RV, Russell DW, Paradise NF. Blood cholesterol screening influence of fasting state on cholesterol results and management decisions. J Gen Intern Med. 2000 Jun;15(6):395–399. doi: 10.1046/j.1525-1497.2000.03509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon AJ, Maisto SA, McNeil M, et al. Three questions can detect hazardous drinkers. J Fam Pract. 2001 Apr;50(4):313–320. [PubMed] [Google Scholar]
- 29.Kessler RC, Andrews G, Mroczek D, Üstün TB, Wittchen HU. The World Health Organization Composite International Diagnostic Interview Short Form (CIDI-SF) Int J Methods Psychiatr Res. 1998;7:171–185. [Google Scholar]
- 30.Kilbourne AM, Farmer Teh C, Welsh D, et al. Implementing composite quality metrics for bipolar disorder: towards a more comprehensive approach to quality measurement. Gen Hosp Psychiatry. 2010 Nov-Dec;32(6):636–643. doi: 10.1016/j.genhosppsych.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chyba MM, Washington LR. Questionnaires from the National Health Interview Survey, 1985–89. Vital Health Stat 1. 1993;(31):1–412. [PubMed] [Google Scholar]
- 32.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 33.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 35.Ustun TB, Chisholm D. Global “burden of disease”-study for psychiatric disorders. Psychiatr Prax. 2001;28 (Suppl 1):S7–11. doi: 10.1055/s-2001-15381. [DOI] [PubMed] [Google Scholar]
- 36.Glick HA, McBride L, Bauer MS. A manic-depressive symptom self-report in optical scanable format. Bipolar Disord. 2003;5(5):366–369. doi: 10.1034/j.1399-5618.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- 37.Whelton PK, He J, Appel LJ, et al. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. Jama. 2002;288:1882–1888. doi: 10.1001/jama.288.15.1882. [DOI] [PubMed] [Google Scholar]
- 38.Kilbourne AMPB, Mezuk B, Welsh D, Ilgen M, Baurer MS. Co-occurring Conditions and Health-Related Quality of Life in Patients With Bipolar Disorder. Psychosomatic Medicine. 2009;71:894–900. doi: 10.1097/PSY.0b013e3181b49948. [DOI] [PubMed] [Google Scholar]
- 39.Bryant-Comstock L, Stender M, Devercelli G. Health care utilization and costs among privately insured patients with bipolar I disorder. Bipolar Disord. 2002;4:398–405. doi: 10.1034/j.1399-5618.2002.01148.x. [DOI] [PubMed] [Google Scholar]
- 40.Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363(27):2611–2620. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]