Abstract
The adhesion and proliferation of human fetal osteoblasts, hFOB 1.19, on micro arc oxidized (MAO) gamma titanium aluminide (γTiAl) surfaces were examined in vitro. Cells were seeded on MAO treated γTiAl disks and incubated for 3 days at 33.5°C and subsequently for 7 days at 39.5°C. Samples were then analyzed by Scanning Electron Microscopy (SEM) and the Alkaline Phosphatase Assay (ALP) to evaluate cell adhesion and differentiation, respectively. Similar Ti-6Al-4V alloy samples were used for comparison. Untreated γTiAl and Ti-6Al-4V disks, to study the effect of micro arc oxidation and glass coverslips as cell growth controls were also incubated concurrently. The ALP Assay results, at 10 days post seeding, showed significant differences in cell differentiation, with p values < 0.05 between MAO γTiAl and MAO Ti-6Al-4V with respect to the corresponding untreated alloys. While SEM images showed that hFOB 1.19 cells adhered and proliferated on all MAO and untreated surfaces, as well as on glass coverslips at 10 days post seeding, cell differentiation, determined by the ALP assay, was significantly higher for the MAO alloys.
1 Introduction
It is well known that titanium and its alloys spontaneously form a thin biocompatible surface oxide layer, composed primarily of TiO2 when exposed to a biological environment. Nonetheless, when the titanium alloy is implanted in vivo, the oxide stability may be altered resulting in increased metal ion release and implant failure [1]. Several treatments have been developed to modify the surface oxides on Ti alloys that improve corrosion and wear resistance [1–3]. Corrosion may lead to the undesirable release of metal ions, which may potentially result in a localized or systemic cytotoxicity, genotoxicity or have carcinogenic effects in vivo [4,5]. Advanced surface modification methods such as plasma ion implantation, anodizing, ultrapassivation, nitriding, electrochemical and thermal oxidation has been applied to Ti-6Al-4V leading to the formation of stable superficial oxides [6–9]. Among the methods used to generate the oxide layer, micro arc oxidation (MAO) has been reported to be a beneficial method in that it provides a good combination of porous and thick oxide films with a well characterized biocompatible substrate containing Ca and P ions [10–13], that enhances corrosion resistance and bioactivity [16]. Thus, the surface modification produced by MAO results in a porous and firmly adherent TiO2 layer on Ti implants, concomitantly enhancing the fixation of the implants to the bone and improving their in vivo corrosion behavior. Different from mere anodic oxidation, MAO has specific characteristics, such as producing local high temperatures, and forming a ceramic coating. The type of electrolyte used and process conditions applied by MAO can affect the surface morphology, chemical composition, and crystalline structure of the oxide coatings formed.
The TiO2 layer generated by the MAO treatment was found to significantly improve the biological cellular activities on titanium alloys in vitro and the bone-implant bonding properties in vivo [15]. These improvements have been attributed to the increase in surface roughness and the incorporation of Ca and P within the oxide layer. The porous and rough morphology has been shown to increase cell attachment and mechanical interlocking between the tissue and implant [15]. Moreover, the Ca and P source, incorporated from the electrolyte solution into the oxide layer, improved osteoblast cell response and enhanced osseointegration [14,16].
Recently the potential of gamma titanium aluminide (γTiAl) as a biomaterial for orthopedic applications has been extensively researched and promising indicators have been observed [17–20]. It is believed that this potential biomaterial potential for implant applications can be further enhanced by modifying the surface of γTiAl by the MAO technique. The main objective of this research was to study in vitro cell attachment and cell differentiation of human fetal osteoblast cells, hFOB 1.19, cultured on MAO γTiAl, in order to determine its biocompatibility. Cell attachment was qualitatively observed by means of Scanning Electron Microscopy (SEM) while cell differentiation was measured using the Alkaline Phosphatase Assay. Alkaline phosphatase (ALP) plays a role in skeletal mineralization and is the most widely recognized biochemical marker for osteoblast activity. In all experimentation, Ti-6Al-4V was used as a parallel material for study. Untreated γTiAl and Ti-6Al-4V were utilized as a control, to understand the effects of the MAO process while glass coverslips were also used as a control to monitor cell growth during the study.
2 Materials and Methods
2.1 Preparation of titanium disks
γTiAl (Ti-48Al-2Cr-2Nb at%) and Ti-6Al-4V disks, 7mm in diameter and with an approximate thickness of 1 mm, were cut from machined γTiAl rods and wrought (α + β) annealed Ti-6Al-4V rods using a slow speed saw (Buehler™). The surfaces of these disks were prepared manually in an Ecomet 3 (Buehler™) by wet grinding with 240, 320, 600 and 1200 grit silicon carbide paper. These metal disks were ultrasonically cleaned in 0.8% Alconox (Fisher, Pittsburgh, Pennsylvania) and 70% ethanol for 10 minutes each, while rinsing with de-ionized water between each application. The metal disks were dried and then oxidized by the micro arc process at a constant temperature and current. Based on earlier results [21], applied current values of 200 mA and 225 mA (current density values of 520 mA/cm2 and 585 mA/cm2 respectively) and treatment times of 3 minutes and 4 minutes were selected for both alloys. The MAO alloys were then placed in 48-well culture plates (Corning, Corning, New York) and cells were seeded on these materials as described in Section 2.4.
2.2 Micro Arc Oxidized (MAO) coatings
The electrolyte used for MAO consisted of 0.025M Ca(H2PO4)2, 0.075M Ca(OOCCH3)2 and 0.12 M Na2(EDTA) dissolved in ultra-pure water [21]. The basic component of the electrolyte is calcium dihydrogen phosphate with a Ca/P ratio of 0.5. In order to increase this ratio in the solution, the chelating agent Na2(EDTA) was added, increasing the calcium ions to augment the Ca/P ratio in the coating. Hence, the electrolyte used consisted of an aqueous solution of phosphate (0.05 M) and calcium (0.1M), the latter in a chelated form due to the formation of a complex. The EDTA concentration in the electrolyte was 0.12 M. Each reactant (high purity grade) was dissolved separately in different containers until complete solubility was achieved, and later mixed together to form the electrolyte. NaOH pellets were finally added until the solution had a pH of 11.
To accomplish the MAO treatment, a stainless steel beaker was used as the cathode, and the titanium alloy sample (either γTiAl or Ti-6Al-4V) was used as the anodic electrode. The sample was mounted in a titanium holder specially designed to allow complete exposure to the electrolyte [22]. A Hoeffer PS300-B high voltage power supply (300V; 500mA) was used to produce the MAO coatings on both alloys. The power supply was operated in a galvanostatic mode, in order to account for the increase in electric resistance of the oxide film on the titanium alloy surface with increasing thickness. Voltage changes as a function of time were set manually for each process condition. After treatment, samples were rinsed with distilled water and then dried with a blow dryer. The modified surfaces will be hereafter referred to as MAOGTi for γTiAl and MAOTiV for Ti-6Al-4V materials respectively. The modified surfaces were imaged by Scanning Electron Microscopy, JEOL-JSM-5410 LV SEM (JEOL, Japan). Image J® software was utilized to determine the average pore diameter from these images.
2.3 Cell line
Human osteoblast cells, cell line hFOB 1.19 (ATCC CRL-11372 , Manassas, Virginia) were obtained from a primary culture of fetal tissue and transfected with a gene that codes for a temperature-sensitive mutant (tsA58) of the SV40 large T antigen and a gene encoding for neomycin (G418) resistance [22]. The cell line hFOB 1.19 can be cultured at 33.5°C for up to passage 30 without problems, thereafter which (at passage 32–34) proliferation slows considerably. These cells were cultured in 90% Dulbecco’s Modified Eagle’s Medium Nutrient Mixture F-12 Ham (DMEM) (Sigma- Aldrich, St. Louis, Missouri) with 2.5 mM L-Glutamine and 15 mM Hepes, without phenol red, supplemented with 0.3 mg/mL G-418 (Calbiochem, San Diego, California) and 10% Fetal Bovine Serum (FBS) (Hyclone, Logan, Utah). Cells were grown in 25 cm² plastic culture flasks (Corning, Corning, New York) and incubated at 33.5°C until confluence. At approximately 95% confluency, cells were washed three times with Phosphate Buffer Saline (PBS) solution (137mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2HPO4) and harvested using 0.25% trypsin- 0.53mM EDTA (Gibco, Gaithersburg, Maryland) at 37°C for 5 min. Cells were then pelleted by low speed centrifugation (3,300 rpm) for 5 minutes and subcultured at a 1:3 ratio. At permissive temperatures or as subconfluent cultures, hFOB 1.19 cells exhibit rapid cell division whereas at restrictive temperatures or at confluence, cell division is reduced and differentiation increases. This particular behavior of the human fetal osteoblast cell line 1.19 (hFOB 1.19) is due to the presence in its genome, of a transfected gene coding for a temperature sensitive mutant (tsA58) of the SV40 T antigen [22]. Expression of the T antigen in human cells results in an increased rate of proliferation when it interacts with the retinoblastoma gene product Rb. Under conditional immortalization, the mutant T antigen is only active (immortalizes cells) at the permissive temperature of 39.5°C. Furthermore, the cell line hFOB (incubated at 39.5°C ) exhibits an increase of cAMP levels in response to 1–34 parathyroid hormone (PTH) treatment, an increase of osteocalcin and alkaline phosphatase activity in response to dihydroxyvitamin D3 treatment (1, 25 D3) and formation of mineralized nodules [29].Thus, the return to a non-immortalized state can be manipulated by changing the incubation temperature of the cells to a restrictive one .
The human osteoblast cells were seeded as described above. After the cells were seeded on the 48-well plates (Becton- Dickinson, Lincoln Park, NJ) at a density of 5 × 104 cells/cm² on MAO disks (7mm in diameter), the plate was incubated for 3 days at 33.5°C. After the 3 day incubation period, the wells were washed three times with PBS 1 X solution and then supplemented with 90% Dulbecco’s Modified Eagle’s Medium Nutrient Mixture F-12 Ham (DMEM), 0.3 mg/mL G-418 and 10% Fetal Bovine Serum (FBS). The wells were supplemented with cell medium every two days. The 48-well plate was then transferred to an incubator with the restrictive temperature of 39.5°C, for 7 days , allowing for cell differentiation. This allows for a slow proliferation of the cells and the formation of mineralization nodules.
2.4 Scanning Electron Microscopy (SEM)
Cell adhesion on γTiAl and Ti-6Al-4V surfaces were evaluated qualitatively by SEM. Cells were seeded on MAO disks in 48-well plates (Becton- Dickinson, Lincoln Park, NJ) at a density of 5 × 104 cells/cm². Samples were incubated for 3 days at 33.5°C and then for 7 days at 39.5°C to allow for osteoblast differentiation. Cells were also seeded as described above, on untreated γTiAl and Ti-6Al-4V disks to study the effect of the MAO treatment on cell proliferation and differentiation. Cells were also grown on glass coverslips as a control for cell growth. After the incubation period, samples were washed carefully with PBS and fixed overnight in 4% glutaraldehyde buffered in PBS at 4°C. After washing three times in PBS, the samples were dehydrated in graded alcohol ranging from 10% to 100% ethanol in intervals of 10 minutes each. After critical point drying (EMS 850) (Electron Microscopic Science, Washington), samples were mounted on stubs and sputtered coated with gold and palladium in EMS 550X (Electron Microscopic Science, Washington). Samples were then examined with a JEOL JSM-5410 LV SEM (JEOL, Japan) at 10 KV.
2.5 Alkaline Phosphatase Assay
The Alkaline Phosphatase Colorimetric Assay Kit (ab83369, Abcam®) was used to evaluate osteoblast differentiation quantitatively on MAO γTiAl and Ti-6Al-4V disks as well as corresponding untreated Ti alloys disks and glass coverslips. Cells were seeded in 48-well plates (Becton, Dickinson, Lincoln Park, NJ) at a density of 5 × 104 cells/cm² each on individual MAOGTi, MAOTiV, untreated γTiAl and untreated Ti-6Al-4V disks. Samples were incubated for 3 days at 33.5°C and then for 7 days at 39.5°C to allow osteoblast differentiation. Cells were grown on coverslips to be used as cell growth controls. To achieve a more efficient cell lysis modifications to the protocol were made, these included washing the samples carefully three times with PBS and homogenizing in 60 μL of the Assay Buffer. Another modification made was to use Triton X-100 (80 μL) to lyse the cells for an efficient measurement of intracellular ALP. Stop solution (20 μL) was added to terminate ALP activity in the sample. The solution in each well was transferred to a 96-well plate (Becton-Dickinson, Lincoln Park, NJ). pNPP Solution (50 μl) was added to each well containing the test samples and background controls. The reaction was incubated for 60 minutes at 25 °C, protected from light.
To determine the concentration of ALP activity in the sample a standard curve was generated, 40 μl of the 5 mM pNPP solution was diluted with 160 μl Assay Buffer to generate a 1 mM pNPP standard. 0, 4, 8, 12, 16, 20 μl was added into 96-well plate in duplicate to generate 0, 4, 8, 12, 16, 20 nmol/well pNPP standard. The final volume was brought to 120 μl with Assay Buffer. ALP enzyme solution (10 μl) was added to each well containing the pNPP standard. The reaction was incubated for 60 minutes at 25°C and protected from light. All reactions were stopped by adding 20 μl of Stop Solution into each standard and sample reaction except the sample background control reaction (since 20 μl of the Stop Solution had been added to the background control when prepared previously). The Optical Density was measured at 405 nm in a micro plate reader. The background was corrected by subtracting the value derived from the 0 standards from all standards, samples and sample background control. The pNPP Standard Curve was plotted and the sample readings were applied to the standard curve to get the amount of pNPP generated. ALP activity of the test samples was calculated using the following equation:
where A is the amount of pNPP generated by samples (in μmol), V is the volume of sample added into the assay well (in ml) and T is the reaction time (in minutes)
2.6 Atomic Force Microscopy
Atomic Force Microscopy (AFM) was used to investigate the surface topography of the coatings obtained on both titanium alloys. A Veeco Model CP-II AFM was used for this purpose, under a non-contact mode for topography imaging and analysis of a 40 μm × 40 μm area. A large area (~90 μm) piezoelectric scanner was used to obtain the images of the topography of the MAO surfaces of both γTiAl and Ti-6Al-4V alloys. Average roughness data was extracted from these measurements.
2.7 Statistical Analysis
The alkaline phosphatase assay was performed in three independent experiments, each with three replicates, for a total of nine replicates per surface evaluated (micro arc oxidized MAOGTi, MAOTiV, untreated γTiAl, untreated Ti-6Al-4V and glass coverslips) for a 10 cell growth period. The data of the alkaline phosphatase assay is presented as the mean of the optical density of differentiated cells on the different surfaces and the amount of alkaline phosphatase detected. Each value represents the mean of the measurements (using three disks) of cell differentiation performed on a specific surface tested in one of the three independent experiments. A factorial analysis of variance (ANOVA) was used to assess the main effect and the significant interactions between the type of metal (γTiAl or Ti-6Al-4V), and the type of surface treatment (micro arc oxidization at 200mA, 3min., 200mA, 4min., 225mA, 3min. and 225mA, 4min.). When these interactions were found, they were graphically analyzed. In addition, a Randomized Block Design was performed to reduce the variance in the data. Furthermore, a contrast test was performed to compare the type of metal (γTiAl and Ti-6Al-4V) with the surface treatments. A LSD Fisher test was performed to check for the presence of significant differences in cell differentiation on the type of metal and surfaces tested. p values < 0.05 were considered to be statistically significant. All analyses were performed using Infostat® (Infostat Inc).
3 Results
3.1 Scanning Electron Microscopy
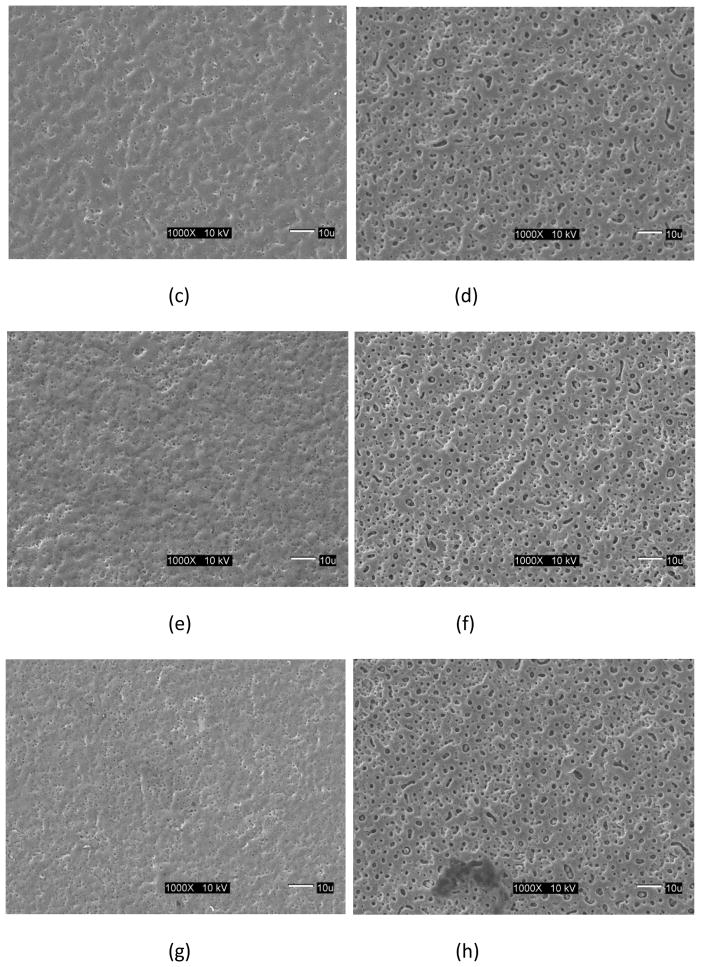
Surface images of the micro arc oxidized surface of the γTiAl and Ti-6Al-4V surfaces are shown in Figure 1 for the four treatment conditions used in this study. It is visually evident that these treated surfaces are typically porous although the average pore diameter is smaller for the γTiAl samples regardless of the treatment condition (see Table 1). While the larger pore size of the Ti-6Al-4V surfaces have been proposed to enhance bone ingrowth, resulting in increased fixation [23], submicron pore sizes observed in γTiAl have been reported to elicit cell growth and function at an increased level [24,25]. After 10 days of incubation, a confluent multilayer of cells was observed on all the MAO treated surfaces, untreated γTiAl and Ti-6Al-4V disks and glass coverslips. SEM images of the cells cultured on the Ti alloy surfaces, clearly indicate that hFOB 1.19 cells exhibited normal growth on the surfaces of both MAO γTiAl and Ti-6Al-4V disks, similar to earlier reports [20,26]. The cell multilayer observed, was constituted by elongated and polygonal cells. The polygonal elongated cell morphology corresponds to the start of the cell-surface interaction processes such as anchorage and adhesion of the cell [22]. Also, cellular boundaries were difficult to establish due to the close contact between neighboring cells. Some cells showed variable appearances (spherical, oval or polygonal) and some of the spherical cells grew over the elongated cells [20]. Also, cell to cell interactions were observed with the presence of cytoplasmic projections (mitotic like structures) and lamellipodia extending from the cells. This lamellipodia is typical of migratory cells and are also part of the cytoskeletal organization which involves transport phenomena, cell division and help extend the cell surface and improve the exchange of substances [22]. For this reason, the presence of cytoskeletal components on the cells indicates normal cellular activity. Cell attachment was similar for the MAO treated surfaces of both metals regardless of treatment conditions, osteoblast cells appeared to spread and anchor very well. Cells were clearly attached to the substrate surface and exhibited typical growth characteristics such as slender cytoplasmic projections (filopodia) extending from the cells in all directions. Furthermore, microspikes and even small round structures which probably correspond to mineralized nodules, indicative of osteoblast differentiation, were observed highlighting the bioactive nature of the MAO surfaces on γTiAl as well as on Ti-6Al-4V.
Fig 1.
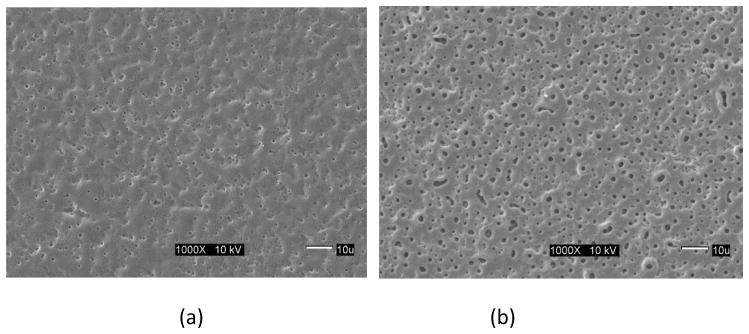
SEM images of the Micro Arc Oxidation surfaces for different process conditions: (a) γTiAl, 200 mA, 3 min, (b) Ti-6Al-4V, 200 mA, 3 min, (c) γTiAl, 200 mA, 4 min, (d) Ti-6Al-4V, 200 mA, 4 min, (e) γTiAl, 225 mA, 3 min, (f) Ti-6Al-4V, 225 mA, 3 min, (g) γTiAl, 225 mA, 4 min, (b) Ti-6Al-4V, 225 mA, 4 min
Table 1.
Average pore diameter obtained from measurements made with Image J® software.
| Material | Treatment conditions | Average pore diameter, μm |
|---|---|---|
| γTiAl | 200mA,3min. | 0.49 |
| 200mA,4min. | 0.52 | |
| 225mA,3min. | 0.37 | |
| 225mA,4min. | 0.44 | |
| Ti-6Al-4V | 200mA,3min. | 1.59 |
| 200mA,4min. | 1.80 | |
| 225mA,3min. | 1.48 | |
| 225mA,4min. | 1.52 |
The ECM was more fibrillar on the MAO Ti-6Al-4V and γ-TiAl disks with the highest current density (225mA with 4 minutes of treatment) exhibiting a ruffled surface and many filopodia in multiple directions.
3.2 Atomic Force Microscopy
Topographical features of the coatings obtained in γTiAl and Ti-6Al-4V alloys were analyzed using Atomic Force Microscopy (AFM). The increase in surface area for the coatings obtained by the MAO method is noticeable on both alloys (Figure 3). This is a desirable feature, particularly in hard tissue and structural applications. Rough surfaces and highly porous coatings can enable osseointegration by producing lasting mechanical interlocking between the implant and the bone, thus increasing service life of the implant similar to results on commercially pure titanium samples subjected to MAO in different electrolytes [27]. For the Ti-6Al-4V surfaces processed using MAO, the surface area appears to be greater when compared to γTiAl surfaces processed under the same conditions. Average roughness values of the MAO surfaces obtained from the AFM data, shown in Table 2, indicate that the average surface roughness was greater for the highest current density and time of treatment (225mA, 4 minutes of treatment).
Fig 3.
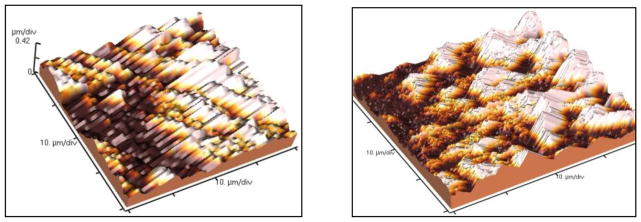
Morphological characterization of micro oxidized γTiAl and Ti-6Al-4V alloys by Atomic Force Microscopy. (a) γTiAl, 200 mA, 3 min, (b) Ti-6Al-4V, 200 mA, 3 min, (c) γTiAl, 200 mA, 4 min, (d) Ti-6Al-4V, 200 mA, 4 min, (e) γTiAl, 225 mA, 3 min, (f) Ti-6Al-4V, 225 mA, 3 min, (g) γTiAl, 225 mA, 4 min, (b) Ti-6Al-4V, 225 mA, 4 min
Table 2.
Average roughness values obtained from Atomic Force Microscopy of MAO surfaces.
| Material | Treatment conditions | Average Roughness, Ra, nm |
|---|---|---|
| γTiAl | 200mA,3min. | 174.5 |
| 200mA,4min. | 185.9 | |
| 225mA,3min. | 137.5 | |
| 225mA,4min. | 189.8 | |
| Ti-6Al-4V | 200mA,3min. | 246.3 |
| 200mA,4min. | 247.6 | |
| 225mA,3min. | 213.3 | |
| 225mA,4min. | 301.7 |
3.3 Alkaline Phosphatase Assay
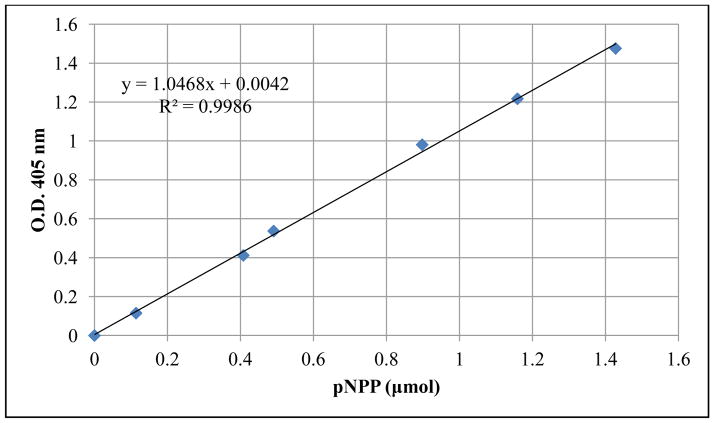
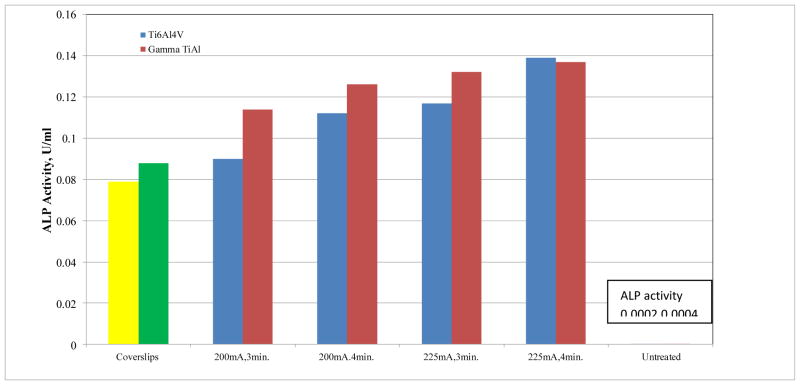
A linear relationship was observed between the alkaline phosphatase activity in the three standard curves (r2= 0.9986, 0.9788, and 0.9895) for the three experimental replicates respectively. For the sake of brevity, only one calibration curve is shown in Figure 4. Based on visual observation of the SEM images, no significant differences in the number of osteoblast cells attached on the MAO and untreated γTiAl and Ti-6Al-4V surfaces were observed. However, the alkaline phosphatase activity was significantly higher on MAO surfaces compared to the untreated γTiAl and Ti-6Al-4V surfaces as well as that from glass coverslips. The alkaline phosphatase activity of osteoblast cells attached on all the surfaces tested is shown in Figure 5. The interaction between type of metal (γTiAl or Ti-6Al-4V) and surface treatment (MAO at 200mA,3min, 200mA,4min, 225mA,3min and 225mA,4min) showed significant differences (p < 0.05) in cell differentiation among the surfaces studied after 10 days of incubation at 33.5°C and later at 39.5°C. There was a drastic increase in the ALP activity on MAO treated γTiAl or Ti-6Al-4V surfaces compared to the untreated surfaces. On the glass coverslips, the ALP activity was intermediate.
Fig 4.
pNPP Standard curve used to extrapolate pNPP concentration to determine ALP activity
Fig 5.
Alkaline phosphatase activity (n=12) on micro arc oxidized γTiAl and Ti-6Al-4V alloys for different treatment conditions. Note ALP data for glass coverslips and untreated γTiAl and Ti-6Al-4V are plotted for comparison
The factorial ANOVA carried out with the optical density and alkaline phosphatase activity data showed that the interaction between the two factors (type of metal and type of treatment) resulted in significant differences. In addition, alkaline phosphatase activity increased in treatments that were exposed longer to higher current densities and for longer time periods. A contrast test was performed to compare the type of metal (γTiAl and Ti-6Al-4V) with the surface treatments. Additionally, to check for the presence of significant differences in cell differentiation on the type of metal and surfaces tested, a LSD Fisher test was performed. The LSD Fisher test is used to examine the significance of the association between two kinds of classification. In our experimentation, this represents the interaction between the metal alloys (γTiAl and Ti-6Al-4V) and the treatment applied (MAOGTi and MAOTiV). The p values < 0.05 were considered to be statistically significant. The Fisher test clearly demonstrated that the interaction between alloy and treatment was significantly relevant to the cell behavior observed on these alloys. The p value obtained for this interaction was 0.009. This correlates with the results obtained with the Alkaline Phosphatase assay in which cell differentiation for the glass coverslips is much lower.
Collectively, there were no significant differences between γTiAl and Ti-6Al-4V MAO alloys for the same treatment conditions. Subsequently, it was observed that, with an increase in time of the treatment and application of a higher amperage to the surface, more ALP activity was observed regardless of the alloy type. There were significant differences between the glass coverslips and the MAO alloys. ALP activity on the untreated alloy surfaces was drastically lower, based on the data from Figure 5. This suggests that a highly porous and rough substrate resulting from the MAO process is definitely influential in the response of the hFOB cells to their macro-environment. Figure 5 shows that when more amperage and time of MAO treatment is applied to the alloys, more cell differentiation is observed. Also, the MAO γTiAl and Ti-6Al-4V alloys treated for 4 minutes at 225mA showed the highest degree of cell differentiation in comparison to the other treatments which indicates that the macro environment may be directly correlated to the rate of incorporation of calcium and phosphate ions on the substrate. On the other hand, ALP activity on both untreated γTiAl and Ti-6Al-4V surfaces was extremely low implying poor cell differentiation.
4 Discussion
It is now well accepted that a highly porous and rough substrate affects cell behavior. St Pierre and colleagues [28] demonstrated that the pore size of titanium scaffolds influences proliferation, although the processes of differentiation and mineralization are not affected. As established earlier, by compositional and morphological analysis of the MAO coatings, the properties of the oxide coating are strongly dependent on the process parameters used, and are also material specific for each titanium alloy. In vitro studies on cell attachment (demonstrated qualitatively with SEM), proliferation and differentiation (demonstrated quantitatively with ALP) study the influence of surface topography and composition. The effects of surface porosity on bone ingrowth in porous metals have been modeled using in vivo models [29]. While a range of pore sizes has been identified within which bone ingrowth occurs, no consensus on optimal pore size exists due to the range of materials researched and their respective microstructural impacts. Similar in vitro studies indicate that pore size is an important microstructural parameter in the design of three-dimensional osteoconductive scaffolds [23–25,28]. Therefore, the roughness and porosity of the surface is of great significance. Cells are able to discriminate among subtle differences in surface roughness [15]. Osteoblast-like cells can discriminate not only between surfaces of different roughness but also between surfaces with comparable roughness but different topographies [30]. However, factors and mechanisms underlying the response of cells in contact with Ti alloys are poorly understood. For example, cells cultured on cpTi and Ti alloys display differential response, although both surfaces are covered with TiO2 [26]. Thus, these differences could be attributed to the microstructure, crystallinity or chemistry of the substrate. Early-phase cell differentiation activity, as monitored by ALP activity, is also positively impacted by cell interactions with the three dimensional scaffolds.
Deligianni et al [15] demonstrated the effect of surface roughness of Ti-6Al-4V on the short- and long-term response of human bone marrow cells in vitro and on protein adsorption. Cell attachment proteins (fibronectin and vitronectin) probably adhere to the surface depending on the amount of calcium ions present on the titanium substrate. Calcium and phosphate ions are suggested to enhance any initial response in vitro due to their high content in bone [31], which could imply that calcium and phosphate ions in the MAO surface may lead to a stronger ability of this surface to absorb proteins and osteoblast ligands. Thus the initial cell adhesion is considered chemical rather than physical. In addition, calcium ions mediate cell-cell communication via gap junctions. The controlled dissolution of a surface containing increased calcium ion concentration might also be beneficial to cell adhesion and greater cell activity. Kim et al [32] reported that the formation of a bone like structure on titanium alloys was a multi-step process involving absorption of calcium ions, followed by the absorption of phosphate ions, and subsequently the formation of amorphous calcium phosphate, and the final transformation into the apatite layer which is considered to have a major impact on the osseointegration of titanium alloys.
The expression of the alkaline phosphatase enzyme (ALP) is commensurate with cell differentiation, which is essential for enhancing osseointegration. ALP is among the first functional genes expressed in the process of calcification. It is therefore likely that at least one of its roles in the mineralization process occurs at an early step. ALP is a cell surface glycoprotein that is involved with mineralization as are also osteopontin and bone sialoprotein (BSP), which bind cell surface integrin receptors and regulate mineralization. Osteocalcin, a matrix protein that regulates osteoclast activity and biglycan, a small leucine-rich proteoglycan which binds to various extracellular matrix components have roles in mineralization. Expression of each of these markers is detected at a specific stage of osteogenic maturation. For instance, the expression of ALP is acquired during early differentiation. ALP activity studied by Marom et al [33], showed an increase in activity with an increase in cell density and time in culture. The importance of these matrix proteins in matrix mineralization has been elaborated in another study [34].
The incorporation of calcium and phosphate ions in the substrate will also affect cell behavior. Liu et al [35] demonstrated the effect of extracellular calcium ion (Ca2+) and inorganic phosphate (Pi) concentrations on the growth and differentiation of bone-marrow-derived mesenchymal stem cells. In addition, they also reported that incorporating calcium phosphate into a substrate in which calcium phosphate is gradually dissolved away and replaced by bone, while the dissolution products are readily assimilated by the human body was very beneficial. It is therefore important to take into consideration the effect of Ca2+ and Pi on bone cells, when designing or constructing scaffolds for bone tissue engineering. Also, calcium phosphate surfaces are capable of driving osteoblasts into differentiation in the absence of osteogenic differentiation supplements in the medium. Thus, surface roughness, porosity and ions incorporated into the surface structure might influence the molecular mechanisms which promote osteogenic differentiation in a mutually symbiotic manner.
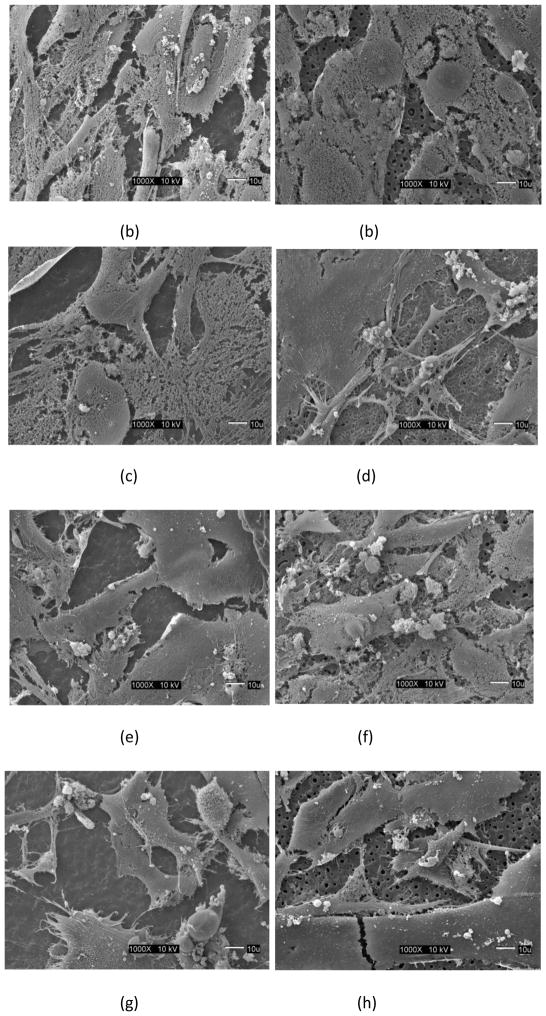
While SEM imaging provides an excellent opportunity in evaluating the quality of cell attachment and growth for assessing the biocompatibility of implant materials, the degree of cell differentiation is clearly not evident from the SEM images, although the presence of mineral nodules may imply the occurrence of this process. There is no evident variation in the present study, in the osteoblast cell appearance when cultured on the surface containing large micron-sized pores (Ti-6Al-4V) in comparison to the MAO layer with smaller sub-micron pores (γTiAl). Cells cultured on the surfaces of both γTiAl and Ti-6Al-4V (see Figure 2) have similar, if not nearly identical appearance to that of cells grown on glass coverslips, in forming a continuous and confluent cell layer. ALP results also suggest that although the cells initially attached and proliferated equally on all the surfaces, cell differentiation was clearly favored on MAO alloys when compared to the untreated alloy surfaces.
Fig 2.
SEM images of hFOB1.19 cell attachment on MAO surfaces for different process conditions: (a) γTiAl, 200 mA, 3 min, (b) Ti-6Al-4V, 200 mA, 3 min, (c) γTiAl, 200 mA, 4 min, (d) Ti-6Al-4V, 200 mA, 4 min, (e) γTiAl, 225 mA, 3 min, (f) Ti-6Al-4V, 225 mA, 3 min, (g) γTiAl, 225 mA, 4 min, (b) Ti-6Al-4V, 225 mA, 4 min
It is clear from the present study that MAO treatment can be successfully applied on γTiAl alloys to generate porous, thick, multilayered coatings, which are bioactive. An optimal porosity on the bioactive oxide layer which favors bone osseointegration by producing lasting mechanical interlocking between the implant and bone, as well as inducing extracellular matrix production will increase service life of the implant. The results obtained confirm that micro arc oxidized alloy surfaces demonstrated most ALP activity. This indicates that MAO may significantly enhance biocompatibility in γTiAl. Taken together, cell adhesion and differentiation studies indicated that hFOB 1.19 cells were able to attach similarly on the different MAO treated surfaces tested, for the time evaluated (10 days). These results suggest that all tested surfaces are biocompatible. MAO surfaces (at 200mA and 225mA at 3 and 4 minutes, respectively) allowed hFOB 1.19 cell adhesion and differentiation. No apparent cytotoxic effects were observed on hFOB 1.19 cells, suggesting that these surfaces have the potential to be used as implant materials.
5 Conclusions
The use of MAO on gamma titanium aluminide has the benefits of a thicker and more bioactive oxide layer compared to that resulting from simple anodic oxidation. The type of electrolyte used and process conditions applied can be altered to affect the surface morphology, chemical composition, including Ca and P incorporation, and crystalline structure of the oxide coatings formed by MAO. While MAO may not result in a Ca-P composition similar to hydroxyapatite, bioactivity of MAO titanium alloys is definitely enhanced. The results of the present study, supported by the SEM analysis of cell adhesion and the ALP assay which indicates excellent cell attachment and differentiation, suggest that MAO surfaces of gamma titanium aluminide are clearly biocompatible and have the potential for being used as implant materials. An animal model will be the next step in further evaluating the osseointegration properties of MAO treated gamma titanium aluminide for application as a biomaterial.
Acknowledgments
This project was supported by the grant SO6GM-08103 from the National Institutes of Health (NIH), National Institute of General Medical Sciences (NIGMS)/MBRS-SCORE, University of Puerto Rico, Mayagüez Campus. The authors would also like to acknowledge Boris Renteria from the General Engineering Department at UPRM for help with the AFM and Jose Almodóvar from the Biology Department for help with the SEM imaging.
References
- 1.Fukumoto S, Tsubakino H, Inoue S, Liu L, Terasawa M, Mitamura T. Surface modification of titanium by nitrogen ion implantation. Mater Sci Eng A. 1999;263:205–9. [Google Scholar]
- 2.Liu X, Chu PK, Ding CX. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater Sci Eng R. 2004;47:49–121. [Google Scholar]
- 3.Alves C, Jr, Guerra Neto CLB, Morais GHS, da Silva CF, Hajek V. Nitriding of titanium disks and industrial dental implants using hollow cathode discharge. Surf Coat Tech. 2005;194:196–204. [Google Scholar]
- 4.Hallab NJ, Anderson S, Stafford T, Glant T, Jacobs JJ. Lymphocyte responses in patients with total hip arthroplasty. J Orthop Res. 2005;23:384–91. doi: 10.1016/j.orthres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Mercado J, Roldan E, Altamirano M. Genotoxic effects of vanadium in human peripheral blood cells. Toxicol Lett. 2003;144:359–369. doi: 10.1016/s0378-4274(03)00255-8. [DOI] [PubMed] [Google Scholar]
- 6.Hanawa T. In vivo metallic biomaterials and surface modification. Mater Sci Eng A. 1999;267:260–6. [Google Scholar]
- 7.Yoshinari M, Hayakawa T, Wolke JGC, Nemoto K, Jansen JA. Influence of rapid heating with infrared radiation on RF magnetron-sputtered calcium phosphate coating. J Biomed Mater Res A. 1997;37:60–7. doi: 10.1002/(sici)1097-4636(199710)37:1<60::aid-jbm8>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 8.Wang CK, Chern JH, Ju CP, Ong HC, Chang RPH. Structural characterization of pulsed laser-deposited hydroxyapatite film on titanium substrate. Biomaterials. 1997;18:1331–8. doi: 10.1016/s0142-9612(97)00024-0. [DOI] [PubMed] [Google Scholar]
- 9.Cui FZ, Luo ZS, Feng QL. Highly adhesive hydroxyapatite coatings on titanium alloy formed by ion beam assisted deposition. J Mater Sci-Mater M. 1997;8:403–5. doi: 10.1023/a:1018597320022. [DOI] [PubMed] [Google Scholar]
- 10.Yerokhin AL, Nie X, Leyland A, Matthews A, Dowey SJ. Plasma electrolysis for surface engineering. Surf Coat Tech. 1999;122:73–93. [Google Scholar]
- 11.Mécuson FJ, Czerwiec T, Henrion G, Belmonte T, Dujardin L, Viola A, Beauvir J. Tailored aluminum oxide layers by bipolar current adjustment in the PEO process. Surf Coat Tech. 2007;201:8677–82. [Google Scholar]
- 12.Wei D, Zhou Y, Jia D, Wang Y. Chemical treatment of TiO2 based coatings formed by plasma electrolytic oxidation in electrolyte containing nano HA, calcium salts and phosphates for biomedical applications. App Surf Sci. 2008;254:1775–82. [Google Scholar]
- 13.Chen JZ, Shi YL, Wang L, Yan FY, Zhang FQ. Preparation of HA containing titania coating by micro arc oxidation. Mater Lett. 2006;60:2538–2543. [Google Scholar]
- 14.Li LH, Kong YM, Kim HW, Kim YW, Kim HE, Heo SJ, Koak JY. Improved biological performance of Ti implants due to surface modification by microarc oxidation. Biomaterials. 2004;25:2867–75. doi: 10.1016/j.biomaterials.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 15.Deligianni DD, Katsala ND, Koutsoukos PG, Missirlis YF. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials. 2001;22:87–96. doi: 10.1016/s0142-9612(00)00174-5. [DOI] [PubMed] [Google Scholar]
- 16.Hu HJ, Liu XY, Ding CX. Preparation and in vitro evaluation of nanostructured TiO2/TCP composite coating by plasma electrolytic oxidation. J Alloy Compd. 2010;498:172–8. [Google Scholar]
- 17.Rivera-Denizard O, Diffoot-Carlo N, Navas V, Sundaram PA. Biocompatibility studies of human osteoblast cells cultured on gamma titanium aluminide. J Mater Sci-Mater M. 2008;19:153–8. doi: 10.1007/s10856-006-0039-4. [DOI] [PubMed] [Google Scholar]
- 18.Castañeda-Muñoz DF, Sundaram PA, Ramirez N. Bone tissue reaction to Ti-48Al-2Cr-2Nb (at %) in a rodent model: a preliminary SEM study. J Mater Sci-Mater M. 2007;18:1433–8. doi: 10.1007/s10856-006-0095-9. [DOI] [PubMed] [Google Scholar]
- 19.Delgado C, Sundaram PA. Corrosion evaluation of Ti-48Al-2Cr-2Nb (at%) in Ringer’s solution. Acta Biomater. 2006;2:701–8. doi: 10.1016/j.actbio.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Bello SA, De Jesus-Maldonado I, Rosim-Facchini E, Sundaram PA, Diffoot-Carlo N. In vitro evaluation of human osteoblast adhesion to a thermally oxidized γTiAl intermetallic alloy of composition Ti-48Al-2Cr-2Nb (at %) J Mater Sci-Mater M. 2010;21:1739–50. doi: 10.1007/s10856-010-4016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lara Rodriguez L, Sundaram PA, Rosim-Fachini E, Padovani AM, Diffoot-Carlo N. Plasma electrolytic oxidation coatings on γTiAl alloy for potential biomedical applications. J Biomed Mater Res. doi: 10.1002/jbm.b.33079. [DOI] [PubMed] [Google Scholar]
- 22.Harris SA, Enger R, Riggs L, Spelberg T. Development and characterization of a conditionally immortalized human fetal osteoblastic cell line. J Bone Miner Res. 1995;10:178–86. doi: 10.1002/jbmr.5650100203. [DOI] [PubMed] [Google Scholar]
- 23.Jones JR, Hench LL. Regeneration of trabecular bone using porous ceramics. Curr Opin Solid St M. 2003;7:301–7. [Google Scholar]
- 24.Khang D, Lu J, Yao C, Haberstroh KM, Webster TJ. The role of nanometer and sub-micron surface features on vascular and bone-cell adhesion on titanium. Biomaterials. 2008;29:970–83. doi: 10.1016/j.biomaterials.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Jäger M, Zilkens C, Zanger K, Krauspe R. Significance of nano- and microtopography for cell-surface interactions in orthopaedic implants. J Biomed Biotechnol. 2007;8:69036. doi: 10.1155/2007/69036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anselme K. Osteoblast adhesion on biomaterials. Biomaterials. 2000;21:667–81. doi: 10.1016/s0142-9612(99)00242-2. [DOI] [PubMed] [Google Scholar]
- 27.Park IS, Woo TG, Jeon WY, Park HH, Lee MH, Bae TS, Seol KW. Surface characteristics of titanium anodized in the four different types of electrolytes. Electrochim Acta. 2007;53:863–70. [Google Scholar]
- 28.St-Pierre JP, Gauthier M, Lefebvre LP, Tabrizian M. Three-dimensional growth of differentiating MC3T3-E1 pre-osteoblasts on porous titanium scaffolds. Biomaterials. 2005;26:7319–28. doi: 10.1016/j.biomaterials.2005.05.046. [DOI] [PubMed] [Google Scholar]
- 29.Subramaniam M, Jalal SM, Rickard DJ, Harris SA, Bolander ME, Spelsberg TC. Further characterization of human fetal osteoblastic hFOB 1. 19 and hFOB/ER cells: bone formation in vivo and karyotype analysis using multicolor fluorescent in situ hybridization. J Cell Biochem. 2002;87:9–15. doi: 10.1002/jcb.10259. [DOI] [PubMed] [Google Scholar]
- 30.Curtis AS, Varde M. Control of cell behaviour: topological factors. J Natl Cancer Inst. 1964;33:15–26. [PubMed] [Google Scholar]
- 31.Ma PX. Scaffolds for tissue fabrication. 2004 Materials Today. 2004;7:30–40. [Google Scholar]
- 32.Kim MS, Ryu JJ, Sung YM. One-step approach for nano-crystalline hydroxyapatite coating on titanium via micro-arc oxidation. Electrochem Commun. 2007;9:1886–91. [Google Scholar]
- 33.Marom R, Shur I, Solomon R, Benayahi D. Characterization of adhesion and differentiation markers of osteogenic marrow stromal cells. J of Cell Physiol. 2005;202:41–8. doi: 10.1002/jcp.20109. [DOI] [PubMed] [Google Scholar]
- 34.Groeneveld EH, van den Bergh JP, Holzmann P, ten Bruggenkate CM, Tuinzing DB, Burger EH. Mineralization processes in demineralized bone matrix grafts in human maxillary sinus floor elevations. J Biomed Mater Res A. 1999;48:393–402. doi: 10.1002/(sici)1097-4636(1999)48:4<393::aid-jbm1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 35.Liu YK, Lu QZ, Pei R, Ji HJ, Zhou GS, Zhao XL, Tang RK, Zhang M. The effect of extracellular calcium and inorganic phosphate on the growth and osteogenic differentiation of mesenchymal stem cells in vitro: implication for bone tissue engineering. Biomed Mater. 2009;4:025004. doi: 10.1088/1748-6041/4/2/025004. [DOI] [PubMed] [Google Scholar]