Abstract
During aging, chondrocytes become unresponsive to insulin-like growth factor-I (IGF-I). This study examined the role of Cdc42 (cell-division-cycle 42) in IGF-I signaling during aging. Experiments were performed using cartilage and chondrocytes isolated from horses ages 1 day – 25 years. Northern analysis was used to examine expression of the small GTPases Cdc42, Rac, and RhoA. Western analysis was utilized to assess total Cdc42 (GTP + GDP-bound); active, GTP-Cdc42 was assessed using a pull-down assay with western analysis. GTP-Cdc42 was also measured following IGF-I treatment. Gene expression for Cdc42 and Rac were decreased in mature samples, but there was no difference in total Cdc42 (GTP+GDP-bound) protein expression due to age. GTP-Cdc42 was significantly greater in prepubescent samples compared to other age groups. IGF-I diminished the GTP-bound state of Cdc42 in prepubescent chondrocytes, however, this effect was lost during aging. No differences in results were observed due to sample type; i.e. cartilage tissues vs. isolated chondrocytes. These studies suggest that loss of IGF-I – mediated regulation of Cdc42 activation may be a mechanism for the chondrocyte unresponsive state during aging. Further, the activation state of Cdc42, measured in native and IGF-I-treated cartilage tissue for the first time, is similar to that of isolated chondrocytes, indicating that the activation state of small G-proteins is not affected by isolation of chondrocytes from the extracellular matrix. Continued studies will identify the upstream regulators of Cdc42 which will further elucidate the molecular mechanism of IGF-I resistance during aging thereby providing insight into targeted strategies for age-related osteoarthritis.
Introduction
Aging is associated with progressive, diminished cellular function for which several fundamental mechanisms have been proposed and investigated. The effect of aging on the biochemical and histological composition of articular cartilage has been a source of investigations for over 50 years [1,2]. The molecular processes most commonly emphasized in aging research include oxidative damage to protein and DNA, accumulation of glycation end products, decreased cell proliferation through senescence or apoptosis, and mitochondrial damage [3–6]. Contemporary studies in articular cartilage are aimed at understanding the basic molecular processes underlying oxidative stress [7–10] and chondrocyte senescence [3,10,11].
Senescence is a stable, post-mitotic state of cells that is reached after a finite number of replications [6]. As they become senescent, cells secrete tissue-specific proteins into the surrounding matrix, and they display altered responsiveness to growth factors [6,12]. The culmination of these effects is diminished tissue maintenance and repair, and ultimately failure of structure and function. Increased chondrocyte senescence during aging has been documented in normal cartilage, providing possible molecular mechanisms for age-related decline in chondrocyte function and the development of osteoarthritis [3,11]. As previously stated, senescent cells have altered responses to growth factor stimulation. In aging articular chondrocytes, several studies have demonstrated a decreased responsiveness to insulin-like growth factor-I (IGF-I) [13–15] which is an important anabolic growth factor in the maintenance of articular chondrocyte phenotypic expression [16–21]. We have previously shown that in articular chondrocytes, IGF-I down-regulates the small GTPase protein Cdc42 (cell-division-cycle 42) thereby decreasing the GTP-bound, active pool of Cdc42 [22]. Combined with gain and loss-of-function studies of Cdc42 in chondrocytes, the theory of active, GTP-Cdc42 functioning as a de-differentiation factor was proposed [22]. This concept is supported by studies in which expression of dominant negative mutants of Cdc42 in articular chondrocytes attenuates the induction of c-Jun transcription activity by shear stress [23].
Cdc42 is a member of the Rho-subfamily of GTPases (RhoA, Rac, Cdc42) which regulate organization of the actin cytoskeleton and many other cellular activities such as gene transcription and cell cycle progression (see [24] for review). The purpose of these studies was to determine if the mRNA or protein expression status of Cdc42 was altered in articular chondrocytes or cartilage during aging, and if the activation state of Cdc42 was affected by isolation of chondrocytes from their native matrix. Additionally, we sought to determine if the previously observed decreased activation of Cdc42 in response to IGF-I treatment was altered during aging. Our hypothesis was that Cdc42 mRNA and protein expression would decrease during aging but the down-regulation of Cdc42 by IGF-I would remain unaffected.
Materials and methods
Cartilage / chondrocyte collection and evaluation
Normal articular cartilage was harvested from 33 horses, ages 1 day to 25 years, which were euthanized for reasons other than this study and with approval from our Institutional Animal Care and Use Committee. Horses were categorized as prepubescent (0–7.5 months), pubescent (7.5–15 months), post-pubescent (15–24 months), or fully mature with respect to articular cartilage as signified by formation of a tidemark occurring at ≥24 months of age [25]. No horse exhibited signs of joint disease at the time of euthanasia (no baseline or inducible lameness with manipulation tests and no synovial effusion), none had a previous history of arthritis, and all samples were grossly normal at the time of tissue collection. Osteochondral samples were obtained from the lateral trochlear ridge of the femora for histology, and full thickness cartilage samples were obtained for RNA analysis, western analysis, and chondrocyte isolation. Care was taken during dissection to avoid visibly apparent underlying bone or vascular tissue in young animals.
Osteochondral sections were fixed in 4% paraformaldehyde, decalcified in 10% EDTA, embedded in paraffin, and sectioned at 10 µm. The sections were stained with toluidine blue, examined for signs of cartilage fibrillation or loss of matrix metachromasia, and assigned a Mankin score [26]. Only cartilage samples with a Mankin score of 0 were used in these studies.
Chondrocyte culture
Chondrocytes were isolated by 0.075% collagenase digestion as described previously [27]. Isolated chondrocytes were cultured in medium consisting of Ham’s F-12 supplemented with 50 µg/ml ascorbic acid/ml, 30 µg/ml α-ketoglutaric acid/ml, 300 µg/ml L-glutamine, 10% FBS. Cultures were grown at 0.8 × 106 viable cells/cm2 (100% confluence) with medium exchange at 48 hours. Twelve hours after medium exchange, chondrocytes were treated +/− human recombinant IGF-I (Tercica Inc., San Francisco, CA; 200 ng/ml) for 30 minutes. Time and concentration of IGF-I treatment were chosen based on previous studies investigating Cdc42 activation [22]. After treatment, assays were performed as described below.
Northern blot analysis for Cdc42, Rac, and RhoA expression during aging
Snap-frozen cartilage samples from 27 animals (0–7.5 mo (n=8), 7.5–15 mo (n=6), 15–24 mo (n=6), >24 mo (n=7) were pulverized and RNA was isolated using the Trizol procedure (Invitrogen Corp., Carlsbad, CA). Isolated chondrocytes from the same animals were also used for Northern analysis to determine if RNA expression patterns were altered in response to the isolation and culture procedures. Northern analyses were performed to determine if there were changes in expression or mRNA splicing patterns of the small G-proteins, Cdc42, Rac, or RhoA due to age, and therefore no samples were treated with IGF-I prior to RNA extraction. Northern blot analyses were performed using 5µg RNA and equine-specific, 32P-labeled probes for Cdc42, Rac, RhoA with EF1-α serving as a house-keeping gene. Data was acquired on a phosphoimager using integrated pixel density calculations and expressed as relative pixel density of Cdc42, Rac, or RhoA to expression of EF1-α to correct for loading and transfer differences between samples.
Western blot analysis of Cdc42 and actin expression during aging
Prior to determining if the activation state of Cdc42 was altered during aging, we sought to determine if the expression of total Cdc42 (GTP and GDP-bound) or beta actin protein content changed during aging. These experiments were critical since total Cdc42 or actin content would be used to analyze relative changes in GTP-bound Cdc42 during aging. Pulverized cartilage samples (400 mgs) were homogenized in lysis buffer (1% Triton, 50mM Tris-HCl pH 8.0, 140mM NaCl, 1mM sodium orthovanadate, 1mM phenylmethylsulfonyl fluoride) and centrifuged to remove cellular debris. An aliquot of each clarified lysate was assayed for DNA content using the Hoechst method [28] to ensure that any detected protein change was not due to age-associated changes in cellularity of the cartilage tissue as previously reported [29]. Equal protein concentrations (5µg) from each clarified lysate were resolved by 12% SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and probed with primary antibodies to Cdc42 (Transduction Laboratories, San Jose, CA), or actin (Sigma-Aldrich, St. Louis, MO). Primary antibodies were detected with horseradish peroxidase-coupled sheep anti-mouse (Cdc42) or anti-rabbit (actin) antibody using enhanced chemiluminescence detection reagent. To determine if protein expression patterns were altered during isolation and culture of chondrocytes, similar western blot analyses were performed on chondrocyte monolayer cultures from the same animals. Cartilage samples from 23 animals (0–7.5 mo (n=6), 7.5–15 mo (n=5), 15–24 mo (n=6), >24 mo (n=6)) were used for these studies. Western blots were quantified using densitometry (Scion Image 4.02, Frederick, MD) and data was expressed as pixel intensity of Cdc42 or actin / µg DNA.
Cdc42 activation studies
The activation status of Cdc42 was determined in cartilage and isolated chondrocytes from various aged horses prior to evaluating the effect of IGF-I on Cdc42 activation. These were important foundation experiments to determine if Cdc42 activation was affected by isolation and subsequent culture of chondrocytes. No comparable data was available in other cell/tissue types to suggest what effect cell isolation from native matrix might have on GTPase activation status. Cartilage and chondrocytes were prepared as described above under Western blot analysis. To determine the GTP-bound pool of Cdc42, equal protein concentrations from each clarified lysate (10–20 µg for chondrocytes, 50–70 µg for cartilage) were incubated for 30 minutes with 20 µg of glutathione s-transferase - p-21 activated kinase binding domain fusion protein (GST-PBD) which was immobilized on agarose beads (PBD pull-down assay) [30,31]. The agarose beads were washed with lysis buffer and boiled in loading dye for Western analysis as described above. Western analyses were also performed on an aliquot of the whole cell lysates that were not incubated with GST-PBD to further ensure that equal amounts of total (active and inactive) endogenous Cdc42 were incubated with the GST-PBD beads. Western blots were quantified using densitometry. Cartilage and chondrocyte samples from 21 horses (0–7.5 mo (n=6), 7.5 – 15 mo (n=5), 15–24 mo (n=5), >24 mo (n=5)) were used for these studies. Data was expressed as GTP-Cdc42:total (GTP+GDP)-Cdc42 so that changes in the relative pool of active:total Cdc42 in cartilage and chondrocytes as a result of aging could be determined.
Effect of insulin-like growth factor-I on Cdc42 activation
Chondrocytes in monolayer were treated with +/− IGF-I, 200 ng/ml, 10 minutes. The cells were lysed and processed as described above for Cdc42 activation studies. Chondrocytes 22 animals (0–7.5 mo (n=6), 7.5 – 15 mo (n=6), 15–24 mo (n=5), >24 mo (n=5)) were used for these studies. Data was expressed as GTP-Cdc42:total Cdc42.
Statistical analyses
Gene expression data and content of GTP-Cdc42 in cartilage and chondrocytes were analyzed using an ANOVA with Tukey’s post hoc procedure to determine if there were differences due to age. A paired t-test was used to determine if IGF-I changed the relative amount of GTP-Cdc42 within each age/puberty-defined category. Statistics were performed using Statistix 8 (Analytical Software, Tallahassee, FL) with a p-value of <0.05 considered significant.
Results
Expression of Cdc42 and Rac mRNA decline in cartilage during aging
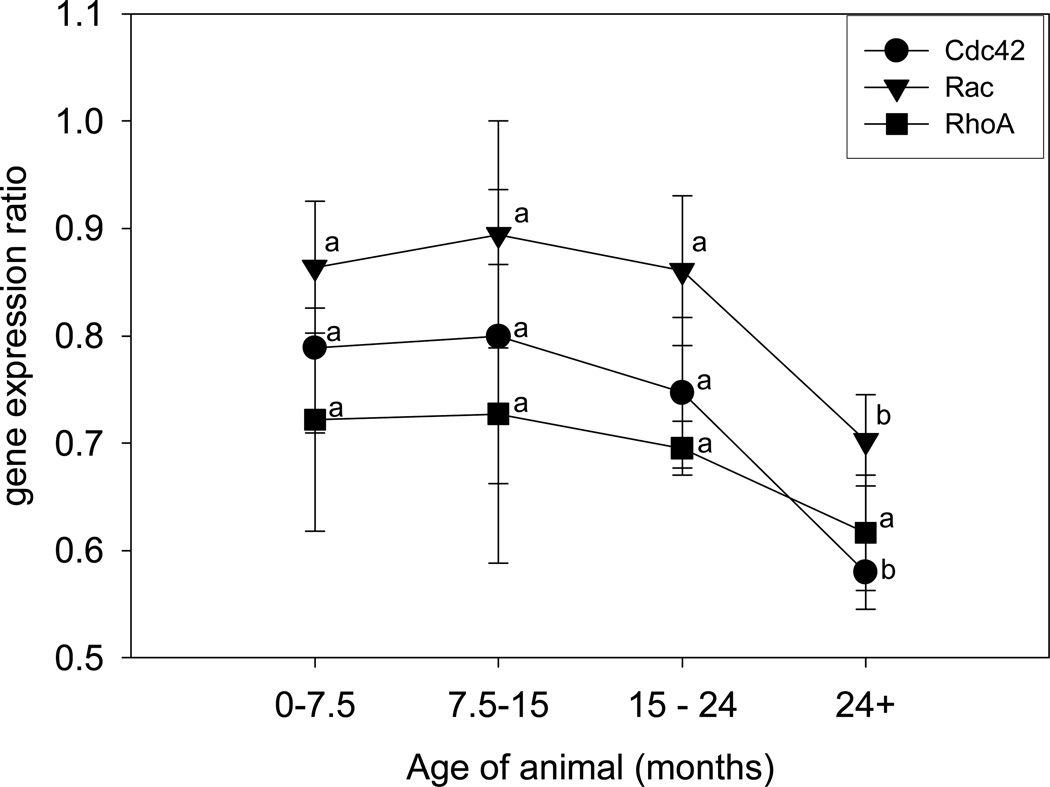
Gene expression for Cdc42 and Rac in articular cartilage tissues were significantly diminished in horses grouped as mature (≥24 months of age) compared to all groups of younger horses (Fig. 1; p=0.040). No significant changes in RhoA mRNA expression were detected between the different age-defined groups of horses. There were no observed changes in splice patterning for any of the three genes during aging. Similar results were obtained from chondrocytes which were isolated from the same horses as the cartilage tissues, and subsequently cultured in monolayer for three days (data not shown).
Fig. 1.
Gene expression of Cdc42, Rac, and RhoA in articular cartilage from horses of various ages. Samples were grouped according to ages representing prepuberty (0–7.5 mo; n=8), puberty (7.5–15 mo; n=6) post-puberty (15–24 mo; n=6) and full maturity in cartilage tissues (>24 mo; n=7). Expression of Cdc42 and Rac were significantly decreased in cartilage from horses with mature articular cartilage (>24 mo) compared to younger horses; p=0.040. There were no changes in RhoA expression due to age. Data are expressed as mean +/− SD with Tukey’s classification letters. Age-defined groups within a specific gene, and with different Tukey’s letters, are significantly different from each other; ANOVA.
Cdc42 protein content remains unchanged in horses during aging
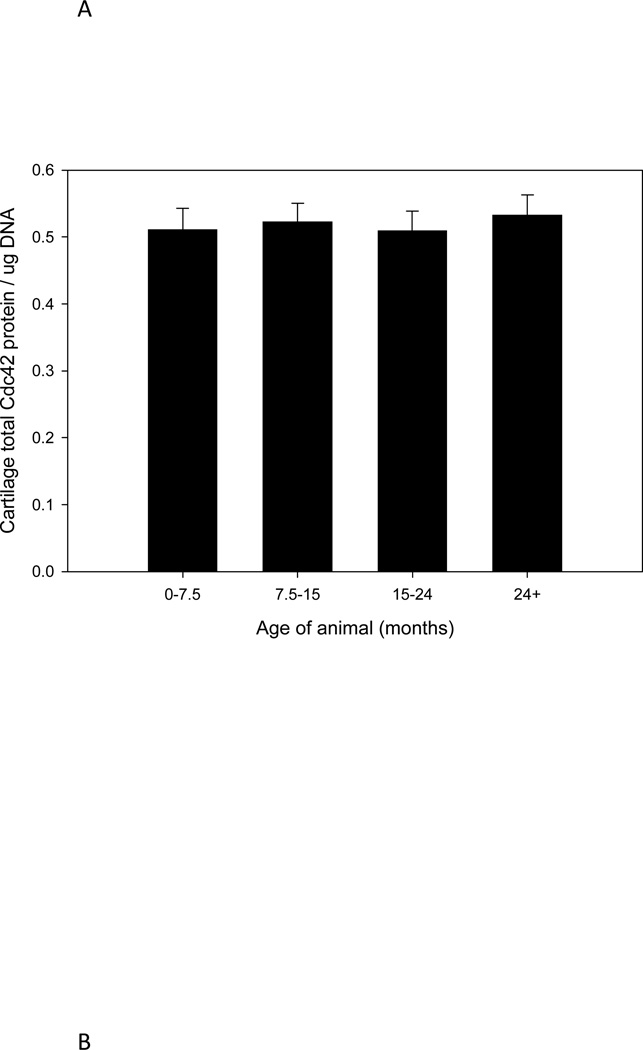
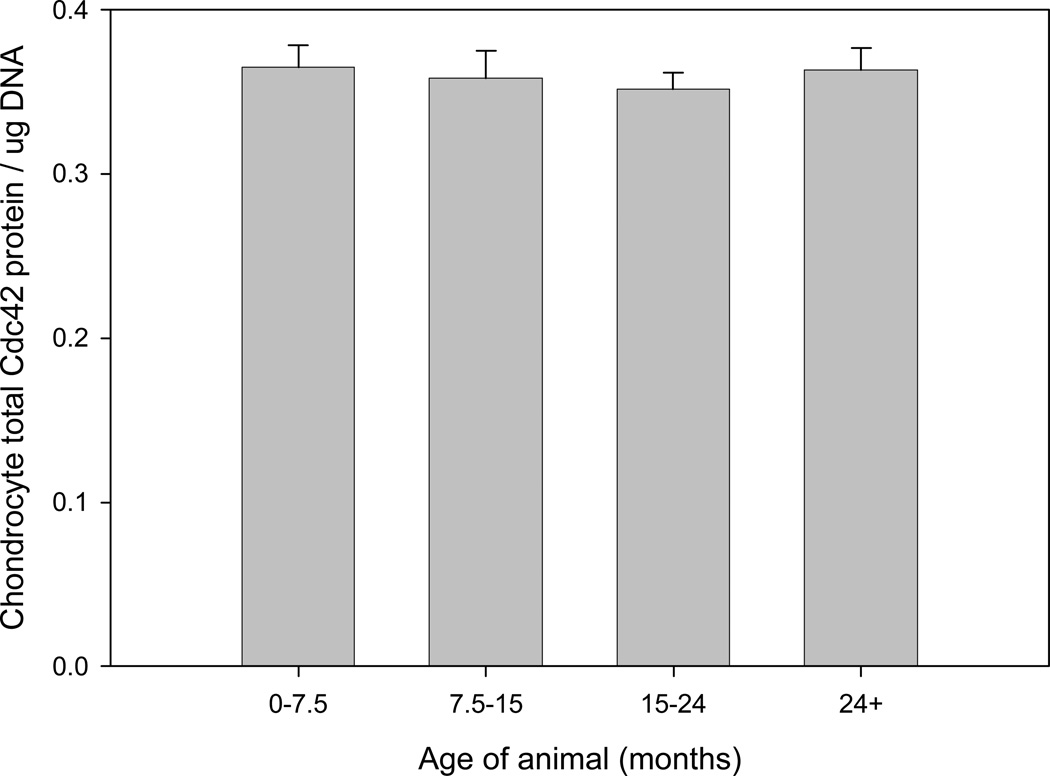
Total Cdc42 protein content (GTP and GDP-bound) in cartilage was not significantly affected by age (Fig. 2A). In experiments utilizing cartilage tissue, Cdc42 protein content was expressed with respect to DNA content to account for anticipated decreases in cellularity during aging [29]. Isolation of chondrocytes from the surrounding matrix did not affect the pattern of Cdc42 protein expression (Fig. 2B). Beta actin protein expression patterns in cartilage and isolated chondrocytes were also not affected by age (data not shown). These results indicated that any detected change in active, GTP-bound Cdc42 could be attributed to altered Cdc42 activation and not changes in intracellular protein concentration. Further, since neither total Cdc42 nor actin concentration were altered during aging, then either protein in whole cell lysate could be used to normalize measurements of GTP-Cdc42.
Fig. 2.
Graphic representation of Cdc42 protein content in cartilage tissues (A) and isolated chondrocytes (B) detected by Western blot analysis. Cell lysates were prepared from cartilage tissue or chondroytes in monolayer obtained from horses (n=23) of various ages. Western analysis was performed to determine the relative content of total Cdc42 (GTP and GDP-bound). When grouped according to stages of puberty/aging, there were no changes in cartilage Cdc42 protein expression as a result of aging (A); p=0.27. Isolation of chondrocytes from the native extracellular matrix did not alter total protein expression patterns (B) and there was also no effect due to aging, p=0.19.
The Cdc42-GTP content of chondrocytes declines during aging
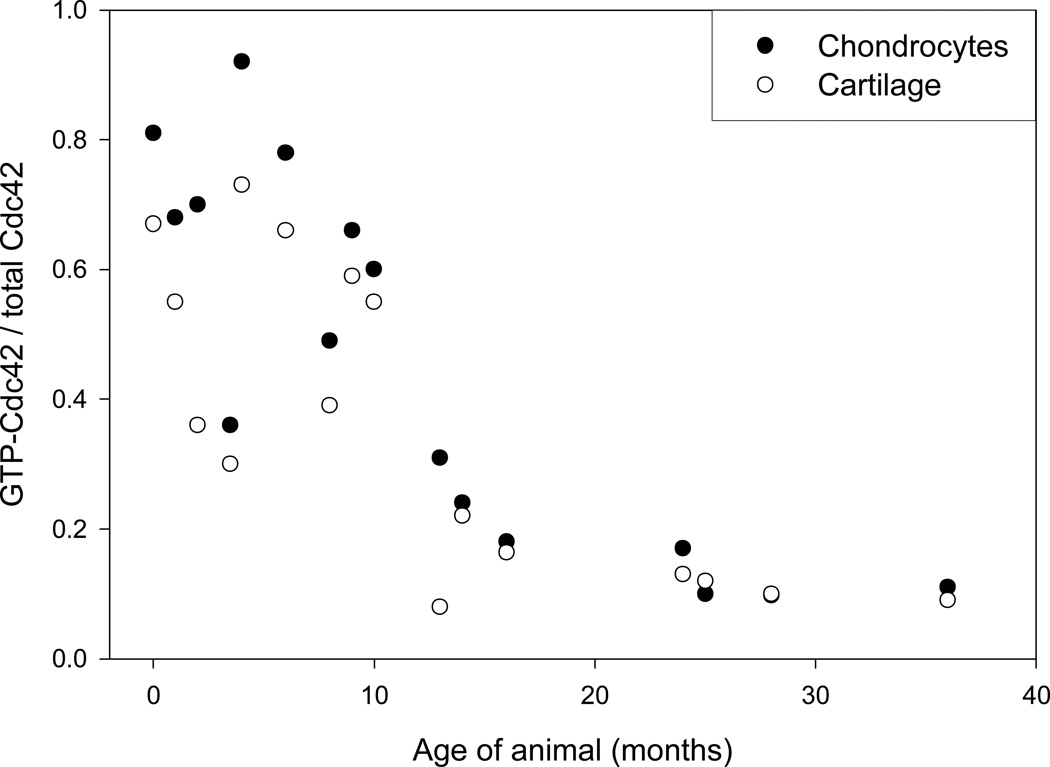
The amount of GTP-Cdc42 in cartilage and chondrocytes decreased during aging (Fig. 3). When grouped by age-related categories, prepubescent horses had significantly greater GTP-Cdc42 than pubescent, post-pubescent, or mature horses (p=0.021). There were no differences in GTP-Cdc42 between pubescent, post-pubescent, or fully mature samples. Summary statistics for each age-group are presented in Table 1. This pattern was statistically significant for both cartilage and chondrocyte samples, and there was no differences between cartilage and chondrocyte samples within an age-group. Although low levels were present in several mature horse samples, GTP-Cdc42 was detectable in all samples. GTP-Cdc42 was expressed as a ratio to total Cdc42 (GTP and GDP-bound) to control for unequal protein content in cell lysates, but the loss of GTP-Cdc42 cannot be attributed to a decrease in total Cdc42 protein expression during aging as previously demonstrated in Figs. 2A and 2B.
Fig. 3.
GTP-Cdc42 content in cartilage and chondrocytes. Cell lysates prepared from cartilage and chondrocytes obtained from prepubescent horses (<7.5 months of age) had significantly more GTP-Cdc42 than samples acquired from pubescent, post-pubescent, or fully mature animals (ANOVA, p=0.021). There were no differences in GTP-Cdc42 content between pubescent, post-pubescent, or fully mature chondrocyte or cartilage lysates.
Table 1.
Summary statistics of individual data, presented in Figure 3, documenting GTP-Cdc42 in chondrocyte and cartilage samples.
| Number of animals in each age-group | GTP-Cdc42 in chondrocytes mean (SD) |
GTP-Cdc42 in cartilage mean (SD) |
|---|---|---|
| Prepubescent (0 – 7.5 mo.); n=6 | 0.655 (0.201) | 0.498 (0.175) |
| Pubescent (7.5 – 15 mo.); n=5 | 0.405 (0.183) | 0.329 (0.206) |
| Post-pubescent (15– 24 mo.); n=5 | 0.174 (0.045) | 0.148 (0.040) |
| Mature (>24 mo.); n=5 | 0.140 (0.043) | 0.119 (0.038) |
IGF-I - induced decreased Cdc42 activation status is lost in aged chondrocytes
Insulin-like growth factor-I treatment of prepubescent chondrocytes resulted in a significant decrease in the activation state of Cdc42 (Fig. 4; p=0.029). There was a mean 65% less GTP-Cdc42 in chondrocytes treated with IGF-I than in no treatment controls. There were no significant differences in GTP-Cdc42 due to IGF-I treatment in pubescent, post-pubescent, or fully mature chondrocytes (p>0.12). The decrease of GTP-Cdc42 in pubescent chondrocytes after IGF-I treatment was highly variable, ranging from 0–60%. In post-pubescent chondrocytes, the decrease in GTP-Cdc42 was again highly variable, but was generally less than in pubescent samples and ranged from 0–25%. Data from individual animals is presented in Table 2.
Fig. 4.
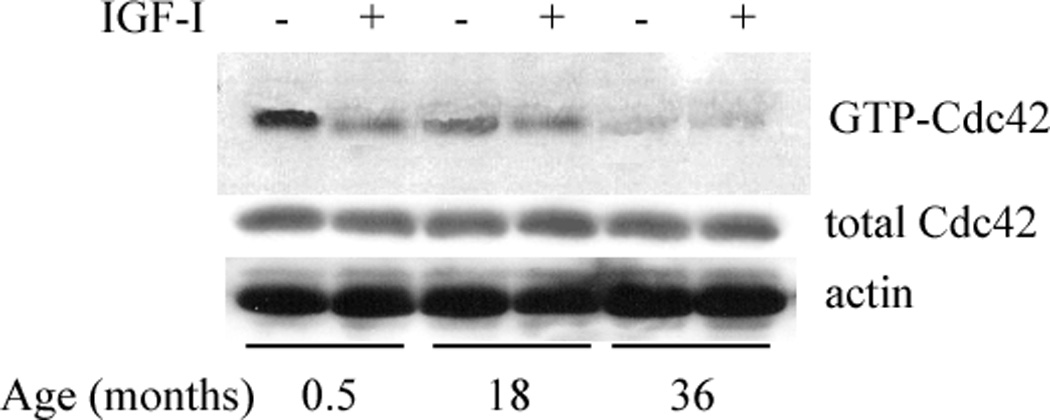
Western blot analysis of GTP-Cdc42 and total Cdc42 (GTP + GDP) in chondrocyte lysates from horses of various ages in response to IGF-I treatment. In prepubescent (<7.5 months old) chondrocytes, GTP-Cdc42 was significantly diminished following IGF-I treatment (paired t-test; p=0.029). There were no significant decrease in the GTP-bound pool of Cdc42 in pubescent, post-pubescent, or fully mature samples (p>0.12). Results are representative of at least five individual experiments. Data from individual samples is presented in Table 2.
Table 2.
Data from individual samples specifying the percent decrease in GTP-Cdc42 relative to total Cdc42 in chondrocytes from different age-groups animals due to IGF-I treatment. Percent decrease in GTP-Cdc42 was calculated from scanned western blots as: (pixel intensity of GTP-Cdc42 / pixel intensity of total Cdc42) × 100.
| Prepubescent (0 – 7.5 months) | Pubescent (7.5 – 15 months) | Post-pubescent (15– 24 months) | Mature (>24 months) | ||||
|---|---|---|---|---|---|---|---|
| Age (months) |
% Decrease in GTP-Cdc42 |
Age (months) |
% Decrease in GTP-Cdc42 |
Age (months) | % Decrease in GTP- Cdc42 |
Age (months) |
% Decrease in GTP-Cdc42 |
| 0.5 | 80.0 | 8.1 | 60.1 | 16.5 | 9.87 | 24.5 | 0.44 |
| 2.0 | 54.0 | 8.8 | 13.4 | 18.0 | 24.9 | 26.5 | 13.8 |
| 3.4 | 66.4 | 10.0 | 0.21 | 18.0 | 13.0 | 36.0 | 8.77 |
| 4.5 | 49.8 | 12.5 | 2.84 | 21.5 | 0.60 | 36.0 | 24.81 |
| 5.0 | 75.5 | 12.9 | 8.44 | 22.0 | 11.1 | 40.0 | 10.4 |
| 6.9 | 68.4 | 14.0 | 23.3 | ||||
| Mean (SD) | 65.7 (11.8) | 19.72 (21.16) | 11.89 (8.70) | 11.64 (8.85) | |||
Discussion
The role of IGF-I as an anabolic growth factor for articular cartilage has been extensively studied [16–21]. Decreased responsiveness of normal cartilage to IGF-I due to aging has been documented in the early postnatal period [14] with a continued decline in older adults [15]. Osteoarthritic cartilage exhibits a similar IGF-I non-responsive state [13]. Here, we identify a possible intracellular signaling-based mechanism for IGF-I resistance in aging cartilage. Our data demonstrates that regulation of the small GTPase Cdc42 during aging of articular chondrocytes is primarily at the level of activation and not mRNA or protein expression. Although mRNA levels of Cdc42 were lower in the fully mature animals compared to the other age-groups, changes in the level of GTP-Cdc42 during aging were observed during the early stages of cartilage maturation, between prepubescent and pubescent animals, and then no further changes were observed. Further, we show that IGF-I decreases the active, GTP-bound pool of Cdc42 in chondrocytes from pre-pubescent horses (0–7.5 months of age), but this effect is lost in older chondrocytes.
Regulation of Cdc42 activity has been shown to be important in maintenance of chondrocyte phenotypic expression. Shear stress – induced expression of c-Jun transcription factor has been show to be attenuated by over-expression of dominant negative mutants of Cdc42 (T19N-Cdc42) [23]. Over-expression of T19N-Cdc42 also results in enhanced mRNA expression of collagen type II (Col2A1) and diminished expression of matrix metalloproteinase-3 (MMP-3), and treatment of T19N-Cdc42 expressing chondrocytes with IGF-I leads to a further increase in Col2A1 and MMP-3 gene expression [22]. Together, these results suggest that keeping the activation status of Cdc42 at a diminished level aids in preserving the normal chondrocyte phenotype. Previous studies demonstrated that IGF-I–induced GTP-Cdc42 repression is at the level of increased GAP activity [22]. Although the precise GAP has not yet been identified, and therefore neither have potential upstream regulators of GAP activity, the idea that an IGF-I regulated GAP might be exploited to reverse or slow loss of chondrocyte phenotype and preserve IGF-I responsiveness is attractive. There are over 70 Rho-GAP domain containing proteins, constituting the 31st largest protein family in the human genome [32,33]. In vitro activity for Cdc42 has been demonstrated for 16 of these GAPs with 6 showing in vivo activity [34]. Even though there are several Cdc42 GAPs, studies suggest that each GAP influences distinctly different biological functions [33,35]. Therefore, identification of the specific IGF-I responsive GAP would be a first step toward elucidating the role of GAP activation in cartilage homeostasis during aging.
Chondrocyte senescence during aging has been investigated as a molecular mechanism responsible for loss of cartilage function and the development of osteoarthritis [3,11]. There is accumulating evidence implicating small GTPases, including Cdc42, in the initiation of senescence in various cell types. In the human osteosarcoma cell line SAOS-2, repressed Rac activity was shown to be required for cellular senescence [36]. Senescent cells displayed an enlarged, flattened phenotype that was accompanied by an increase in polymerized actin cytoskeletal filaments, further establishing a link between senescent and small GTPases which regulate the organization of the actin cytoskeleton [24]. In human skin fibroblasts, an age-related decline in GTP-Cdc42 expression in association with retarded intracellular lipid transport has been reported, suggesting a role for Cdc42 in senescence-related characteristics of cells in atherosclerotic lesions [37]. And in aging rats, decreased activity of Ras has been associated with diminished kidney function [38]. Collectively, these studies provide strong evidence that the small GTPases are involved in aging and senescence of several cell types.
To date, no studies have demonstrated IGF-I resistance, senescence, and diminished Cdc42 activity in the same pool of cells. However, each of these molecular processes is individually linked to aging and the data of this study would suggest that they are all linked together in the continuum of aging in articular cartilage. Alterations in IGF-I ligand, IGF-I receptor, and IGF-binding proteins have been investigated, but have not been conclusively recognized as initiators of the IGF-I resistant state [16,39–43]. We propose that one mechanism for IGF-I resistance during aging is a post-ligand-binding receptor defect which in part results in a decreased pool of GTP-Cdc42 and an inability of IGF-I to further diminish GTP-Cdc42 in order to maintain a normal chondrocyte phenotype. While a diminished pool of GTP-Cdc42 in aged chondrocytes may serve a protective function, the evidence linking diminished activity of the small GTPases to initiation of cellular senescence, and the inability of the aged chondrocytes to respond to IGF-I and regulate Cdc42 activity, suggests that the loss of ability to regulate levels of GTP-Cdc42 might contribute to an overall loss of chondrocyte phenotype and cartilage function. Further studies will elucidate the mechanisms which regulate Cdc42 in disease states such as osteoarthritis.
Acknowledgements
This work was supported by the National Institutes of Health Grant AG00905 (to L.A.F.)
References
- 1.Shetlar MR, Masters YF. Effect of age on polysaccharide composition of cartilage. Proc Soc Exp Biol Med. 1955;90:31–33. doi: 10.3181/00379727-90-21931. [DOI] [PubMed] [Google Scholar]
- 2.Eichelberger L, Roma M. Effects of age on the histochemical characterization of costal cartilage. Am J Physiol. 1954;178:296–304. doi: 10.1152/ajplegacy.1954.178.2.296. [DOI] [PubMed] [Google Scholar]
- 3.Carrington JL. Aging bone and cartilage: cross-cutting issues. Biochem Biophys Res Commun. 2005;328:700–708. doi: 10.1016/j.bbrc.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 4.Hadley EC, Lakatta EG, Morrison-Bogorad M, et al. The future of aging therapies. Cell. 2005;120:557–567. doi: 10.1016/j.cell.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 5.Lombard DB, Chua KF, Mostoslavsky R, et al. DNA repair, genome stability, and aging. Cell. 2005;120:497–512. doi: 10.1016/j.cell.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 6.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Carlo MD, Jr, Loeser RF. Increased oxidative stress with aging reduces chondrocyte survival: correlation with intracellular glutathione levels. Arthritis Rheum. 2003;48:3419–3430. doi: 10.1002/art.11338. [DOI] [PubMed] [Google Scholar]
- 8.Matsushita T, Fukuda K, Yamazaki K, et al. Hypoxia-induced nitric oxide protects chondrocytes from damage by hydrogen peroxide. Inflamm Res. 2004;53:344–350. doi: 10.1007/s00011-004-1267-z. [DOI] [PubMed] [Google Scholar]
- 9.Yudoh K, Nguyen T, Nakamura H, et al. Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Res Ther. 2005;7:R380–R391. doi: 10.1186/ar1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin JA, Klingelhutz AJ, Moussavi-Harami F, et al. Effects of oxidative damage and telomerase activity on human articular cartilage chondrocyte senescence. J Gerontol A Biol Sci Med Sci. 2004;59:324–337. doi: 10.1093/gerona/59.4.b324. [DOI] [PubMed] [Google Scholar]
- 11.Martin JA, Buckwalter JA. The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair. J Bone Joint Surg Am. 2003;85-A(Suppl 2):106–110. doi: 10.2106/00004623-200300002-00014. [DOI] [PubMed] [Google Scholar]
- 12.Campisi J. Cellular senescence and apoptosis: how cellular responses might influence aging phenotypes. Exp Gerontol. 2003;38:5–11. doi: 10.1016/s0531-5565(02)00152-3. [DOI] [PubMed] [Google Scholar]
- 13.Ashton IK, Matheson JA. Change in response with age of human articular cartilage to plasma somatomedin activity. Calcif Tiss Int. 1979;29:89–94. doi: 10.1007/BF02408062. [DOI] [PubMed] [Google Scholar]
- 14.Martin JS, Ellerbrock SM, Buckwalter JA. Age-related decline in chondrocyte response to insulin-like growth factor-I: the role of growth factor binding proteins. J Orthop Res. 1997;15:491–498. doi: 10.1002/jor.1100150403. [DOI] [PubMed] [Google Scholar]
- 15.Loeser RF, Shanker G, Carlson CS, et al. Reduction in the chondrocyte response to insulin-like growth factor 1 in aging and osteoarthritis: studies in a non-human primate model of naturally occurring disease. Arthritis Rheum. 2000;43:2110–2120. doi: 10.1002/1529-0131(200009)43:9<2110::AID-ANR23>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 16.Middleton J, Manthey A, Tyler J. Insulin-like growth factor (IGF) receptor, IGF-I, interleukin-1β (IL-1β), and IL-6 mRNA expression in osteoarthritic and normal human cartilage. J Histochem Cytochem. 1996;44:133–141. doi: 10.1177/44.2.8609369. [DOI] [PubMed] [Google Scholar]
- 17.McQuillan DJ, Handley CJ, Campbell MA, et al. Stimulation of proteoglycan biosynthesis by serum and insulin-like growth factor-1 in cultured bovine articular cartilage. Biochem J. 1986;240:423–430. doi: 10.1042/bj2400423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tyler JA. Chondrocyte-mediated depletion of articular cartilage proteoglycans in vitro. Biochem J. 1985;225:493–507. doi: 10.1042/bj2250493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denko CW, Boja B, Moskowitz RW. Growth promoting peptides in osteoarthritis and diffuse idiopathic skeletal hyperostosis -- insulin, insulin-like growth factor-I, growth hormone. J Rheumatol. 1994;21:1725–1730. [PubMed] [Google Scholar]
- 20.Wang E, Wang J, Chin E, et al. Cellular patterns of insulin-like growth factor system gene expression in murine chondrogenesis and osteogenesis. Endocrin. 1995;136:2741–2751. doi: 10.1210/endo.136.6.7750499. [DOI] [PubMed] [Google Scholar]
- 21.Posever J, Phillips FM, Pottenger LA. Effects of basic fibroblast growth factor, transforming growth factor-B1, insulin-like growth factor-1, and insulin on human osteoarthritic articular cartilage explants. J Orthop Res. 1995;13:832–837. doi: 10.1002/jor.1100130605. [DOI] [PubMed] [Google Scholar]
- 22.Fortier LA, Deak MM, Semevolos SA, et al. Insulin-like Growth Factor-I Diminishes the Activation Status and Expression of the Small GTPase Cdc42 in Articular Chondrocytes. J Orthop Res. 2004;22:436–445. doi: 10.1016/j.orthres.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 23.Jin G, Sah RL, Li Y-S, et al. Biomechanical Regulation of Matrix Metalloproteinase-9 in Cultured Chondrocytes. J Orthop Res. 2000;18:899–908. doi: 10.1002/jor.1100180608. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz M. Rho signalling at a glance. J Cell Sci. 2004;117:5457–5458. doi: 10.1242/jcs.01582. [DOI] [PubMed] [Google Scholar]
- 25.Fortier LA, Kornatowski MA, Mohammed HO, et al. Age related changes in serum insulin-like growth factor-I, insulin-like growth factor binding protein-3, and articular cartilage structure in Thoroughbred horses. Equine Vet J. 2005;37:37–42. doi: 10.2746/0425164054406838. [DOI] [PubMed] [Google Scholar]
- 26.Mankin HJ. The effect of aging on articular cartilage. Bull N Y Acad Med. 1968;44:545–552. [PMC free article] [PubMed] [Google Scholar]
- 27.Nixon AJ, Lust G, Vernier-Singer M. Isolation, propagation and cryopreservation of equine articular chondrocytes. Am J Vet Res. 1992;53:2364–2370. [PubMed] [Google Scholar]
- 28.Rao J, Otto WR. Fluorimetric DNA assay for cell growth estimation. Anal Biochem. 1992;207:186–192. doi: 10.1016/0003-2697(92)90521-8. [DOI] [PubMed] [Google Scholar]
- 29.Bobacz K, Erlacher L, Smolen J, et al. Chondrocyte number and proteoglycan synthesis in the aging and osteoarthritic human articular cartilage. Ann Rheum Dis. 2004;63:1618–1622. doi: 10.1136/ard.2002.002162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor SJ, Shalloway D. Current Biology. 1996;6:1621–1627. doi: 10.1016/s0960-9822(02)70785-9. [DOI] [PubMed] [Google Scholar]
- 31.Bernard V, Bohl BP, Bokoch GM. Characterization of Rac and Cdc42 Activation in Chemoattractant-stimulated Human Neutrophils Using a Novel Assay for Active GTPases. J Biological Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- 32.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 33.Bernards A. GAPs galore! A survey of putative Ras superfamily GTPase activating proteins in man and Drosophila. Biochim Biophys Acta. 2003;1603:47–82. doi: 10.1016/s0304-419x(02)00082-3. [DOI] [PubMed] [Google Scholar]
- 34.Moon SY, Zheng Y. Rho GTPase-activating proteins in cell regulation. Trends Cell Biol. 2003;13:13–22. doi: 10.1016/s0962-8924(02)00004-1. [DOI] [PubMed] [Google Scholar]
- 35.Smith GR, Givan SA, Cullen P, et al. GTPase-activating proteins for Cdc42. Eukaryot Cell. 2002;1:469–480. doi: 10.1128/EC.1.3.469-480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alexander K, Yang HS, Hinds PW. Cellular senescence requires CDK5 repression of Rac1 activity. Mol Cell Biol. 2004;24:2808–2819. doi: 10.1128/MCB.24.7.2808-2819.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsukamoto K, Hirano K, Yamashita S, et al. Retarded intracellular lipid transport associated with reduced expression of Cdc42, a member of Rho-GTPases, in human aged skin fibroblasts: a possible function of Cdc42 in mediating intracellular lipid transport. Arterioscler Thromb Vasc Biol. 2002;22:1899–1904. doi: 10.1161/01.atv.0000036080.42391.33. [DOI] [PubMed] [Google Scholar]
- 38.Parekh VV, Falcone JC, Wills-Frank LA, et al. Protein kinase B, P34cdc2 kinase, and p21 ras GTP-binding in kidneys of aging rats. Exp Biol Med. 2004;229:850–856. doi: 10.1177/153537020422900819. [DOI] [PubMed] [Google Scholar]
- 39.Tavera C, Abribat T, Reboul P, et al. IGF and IGF-binding protein system in the synovial fluid of osteoarthritic and rheumatoid arthritic patients. Osteoarthritis Cart. 1996;4:263–274. doi: 10.1016/s1063-4584(05)80104-9. [DOI] [PubMed] [Google Scholar]
- 40.Olney RC, Tsuchiya K, Wilson DM, et al. Chondrocytes from osteoarthritic cartilage have increased expression of insulin-like growth factor I (IGF-I) and IGF-binding protein-3 (IGFBP-3) and -5, but not IGF-II or IGFBP-4. J Clin Endocr Metab. 1996;81:1096–1103. doi: 10.1210/jcem.81.3.8772582. [DOI] [PubMed] [Google Scholar]
- 41.Dore S, Pelletier J, DiBattista JA, et al. Human osteoarthritic chondrocytes possess an increased number of insulin-like growth factor 1 binding sites but are unresponsive to its stimulation: possible role of IGF-1 binding proteins. Arthritis Rheum. 1994;37:253–263. doi: 10.1002/art.1780370215. [DOI] [PubMed] [Google Scholar]
- 42.Chevalier X, Tyler JA. Production of binding proteins and role of the insulin-like growth factor I binding protein 3 in human articular cartilage explants. Br J Rheumatol. 1996;35:515–522. doi: 10.1093/rheumatology/35.6.515. [DOI] [PubMed] [Google Scholar]
- 43.Verschure PJ, Van Noorden CJF, van Marle J, et al. Articular cartilage destruction in experimental inflammatory arthritis: insulin-like growth factor-1 regulation of proteoglycan metabolism in chondrocytes. Histochem J. 1996;28:835–857. doi: 10.1007/BF02331388. [DOI] [PubMed] [Google Scholar]