Abstract
The placenta plays a key role in regulation of fetal growth and development and in mediating in utero developmental programming. Obesity, which is associated with chronic inflammation and mitochondrial dysfunction in many tissues, exerts a programming effect in pregnancy. We determined the effect of increasing maternal adiposity and of fetal sex on placental ATP generation, mitochondrial biogenesis, expression of electron transport chain subunits, and mitochondrial function in isolated trophoblasts. Placental tissue was collected from women with prepregnancy BMI ranging from 18.5 to 45 following C-section at term with no labor. Increasing maternal adiposity was associated with excessive production of reactive oxygen species and a significant reduction in placental ATP levels in placentae with male and female fetuses. To explore the potential mechanism of placental mitochondrial dysfunction, levels of transcription factors regulating the expression of genes involved in electron transport and mitochondrial biogenesis were measured. Our in vitro studies showed significant reduction in mitochondrial respiration in cultured primary trophoblasts with increasing maternal obesity along with an abnormal metabolic flexibility of these cells. This reduction in placental mitochondrial respiration in pregnancies complicated by maternal obesity could compromise placental function and potentially underlie the increased susceptibility of these pregnancies to fetal demise in late gestation and to developmental programming.
Keywords: obesity, placenta, mitochondria
the rate of maternal obesity continues to increase, with up to 60% of the pregnant population in the US being overweight and 25% being obese (BMI >30) (13, 15). Maternal obesity is associated with several adverse perinatal outcomes, including hypertensive disorders, gestational diabetes, fetal macrosomia, and perinatal death. Furthermore, the male fetus is at greater risk for an adverse outcome than the female fetus (10), with a growing body of evidence showing sexual dimorphism in placental function, including basal placental gene expression (53), and changes in gene expression in response to maternal inflammatory status (7, 41, 43) or change in maternal diet (29).
Maternal obesity also programs the offspring for disease in later life, including obesity, cardiovascular disease, metabolic syndrome, and diabetes (2, 6, 12). Much of the evidence supporting a fetal programming effect of obesity in humans is based on epidemiological data, and it is only recently that attempts have been made to identify molecular mechanisms underlying programming effects. The placenta functions as a key regulator of fetal growth by facilitating nutrient supply to and waste removal from the fetus, with alterations in placental function having the ability to mediate fetal programming (9, 64). Mitochondrial oxidative phosphorylation is a key energy source for placental function (3); however, the changes in placental mitochondrial function with various pregnancy complications (e.g., diabetes and obesity) remain understudied.
Mitochondrial dysfunction associated with obesity has been studied predominantly in highly metabolic tissues, including adipose, heart, liver, and skeletal muscle. An increase in reactive oxygen species (ROS) and reduction in the oxidative capacity of brown adipocytes results in impaired thermogenesis and has been linked to diet-induced obesity (11). Wilson-Fritch et al. (63) demonstrated downregulation of approximately 50% of gene transcripts encoding mitochondrial proteins in adipose tissue in a rodent model with the onset of obesity. Several studies have shown that the increasing metabolic activity of placental mitochondria results in excessive production of ROS leading to oxidative stress, which may be exaggerated in pregnancies complicated by preeclampsia, intrauterine growth restriction (IUGR), and maternal obesity (24, 27, 59, 61, 62). However, besides being a major source of ROS and oxidative stress, mitochondria also appear to be highly susceptible to ROS attack (57). Proteins, lipids, and nucleic acids can be altered by ROS, resulting in covalent changes that affect mitochondrial structure and function (52). Thus, mitochondrial abnormalities and ROS formation could be part of a vicious cycle and represent a central mechanism of placental dysfunction in disease states.
In this study, we addressed the hypothesis that increasing maternal adiposity and fetal sex difference affect mitochondrial function in human placenta.
MATERIALS AND METHODS
Ethical Approval and Study Participants
Placentae were collected from the Labor and Delivery Unit at University Hospital San Antonio under a protocol approved by the Institutional Review Board of the University of Texas Health Science Center San Antonio, with informed consent from the patients.
Materials
Oligomycin, FCCP [4-(trifluoromethoxy) phenylhydrazone], rotenone, and antimycin A were obtained from Sigma and dissolved in DMSO as 2.5-mM stock solutions. The human oxidative phosphorylation (OXPHOS) antibody cocktail (MS601) was purchased from Abcam.
Collection of Placental Tissue
Placentae were collected immediately following delivery by cesarean section at term without labor from otherwise uncomplicated pregnancies in women with a range of prepregnancy BMI from 18.5 to 44.7, grouped as lean (LN; BMI 18.5–24.9), overweight (OW; BMI 25–29.9), and obese (OB; BMI >30). Villous tissue was randomly sampled from five sites in the placenta, as described previously (31), flash-frozen in liquid nitrogen, and stored at −80°C.
Visualization and Quantification of ROS Generation and Hydrogen Peroxide Content in Placental Villous Tissue
Flash-frozen villous tissue sections (7 μm) from six placentas in each group were incubated with 5 μM 2′,7′-dichlorofluorescein diacetate (Invitrogen) for 30 min at 37°C, and staining was performed as described (33). Dichlorofluorescein (DCF) staining was quantified using ImageJ (National Institutes of Health). Hydrogen peroxide levels were assayed using the Amplex Red kit (Invitrogen) in whole cell lysates from placental villous extracts according to the manufacturers' protocol.
ATP Levels
ATP levels were determined in villous tissue lysates from placenta of male and female fetuses (n = 6 for each sex/BMI group) using the Enliten ATP assay (Promega) according to the manufacturer's protocol.
Western Blots
Mitochondrial fractions for Western blotting were obtained from villous tissue using a standard differential centrifugation protocol (40) and suspended in isolation medium (0.25 M sucrose and 1 mM EDTA, pH 7.4) supplemented with a protease and phosphatase inhibitor cocktail (Sigma). Total protein concentrations in the fraction were determined using Bradford's reagent (Bio-Rad). Mitochondrial fraction proteins (10 μg) were separated on 4–20% precast linear gradient gels (Bio-Rad), transferred to nitrocellulose membranes, and blocked with 5% (wt/vol) nonfat milk in Tris-buffered saline with 0.1% Tween 20 (TBST) for 1 h. Membranes were incubated overnight at 4°C with primary antibody diluted in 1% nonfat milk (wt/vol) in TBST and detected using an appropriate peroxidase-conjugated secondary antibody. Products were visualized by ECL chemiluminescence (Millipore). Band intensities were measured using the G-box system (Syngene).
Mitochondrial Biogenesis
Mitochondrial DNA copy number.
Total genomic DNA was isolated from villous tissue from placentae of males and female fetuses (n = 6 from each sex/maternal BMI group) using the GenElute Mammalian Genomic DNA Miniprep Kit (Sigma-Aldrich). Mitochondrial DNA copy number was determined using real-time PCR, using primers for mitochondrial 16S rRNA and nuclear β2-microglobulin as described (60).
Citrate synthase activity.
The activity was measured in the villous tissue using a commercial kit from Sigma (CS0720–1KT) according to the manufacturer's protocol. Placental tissue was homogenized using lysis reagent (C3228; Sigma).
Isolation and Culture of Primary Trophoblasts
Villous cytotrophoblasts (CTs) were isolated from placental tissue of women with a range of adiposity using trypsin/DNAse digestion and the percoll gradient purification method, as we have described previously (28). CT cells were plated in Seahorse XF24 plates, cultured in the DMEM containing glucose (17 mM), and allowed to syncytialize for a period of 72 h. For the last 24 h, medium was switched to contain either 17 mM glucose or galactose (10 mM). The choice of 10 mM galactose was based on previously published studies on other cell lines (30, 48).
Assessment of Mitochondrial Function
Mitochondrial function of the cultured syncytiotrophoblasts was measured using a Seahorse XF24 analyzer (Seahorse Biosciences) as described (28). Oxygen consumption rates (OCR) were normalized to total cellular protein (Bradford method). Basal respiration was calculated from four baseline OCR readings. ATP coupled, maximum respiration, spare capacity, proton leak, and nonmitochondrial respiration was calculated from OCR readings following the injection of oligomycin (1 μM), FCCP (1 μM), and a mixture of rotenone (3 μM) and antimycin A (1.5 μM).
Statistical Analysis
Data are reported as means ± SE. Comparisons between two groups were performed using Student's t-test. One or two-way analysis of variance (ANOVA) and Bonferroni post hoc test were used to compare data sets with more than two groups. P < 0.05 was considered significant. The OCR parameters were analyzed by regression and correlation analysis against maternal BMI using Excel and GraphPad (version 5.0).
RESULTS
Clinical Characteristics of Study Patients
There were no significant differences in maternal or gestational age at delivery and placental weight between the women of differing BMI (Table 1). Maternal weight gain was significantly lower in OB women with female fetuses (P < 0.05). Most births were to Hispanic women (86.6%), followed by white (8%), African-American (4%) women, and Asian women (1.3%). By experimental design, maternal BMI differed significantly between the chosen groups (1-way ANOVA and Bonferroni analysis). In the LN group, the birth weights of female fetuses were significantly smaller than male fetuses (P < 0.05). In addition, female fetuses from OB mothers were significantly heavier compared with female fetuses from LN mothers (P < 0.05).
Table 1.
Clinical characteristics of study patients
| LN |
OW |
OB |
||||
|---|---|---|---|---|---|---|
| Males (n = 17) | Females (n = 11) | Males (n = 11) | Females (n = 10) | Males (n = 10) | Females (n = 16) | |
| Maternal age, yr | 28.6 ± 1.25 | 28.5 ± 1.6 | 30.2 ± 1.9 | 28.8 ± 0.92 | 28.1 ± 1.0 | 27.5 ± 1.0 |
| Ethnicity (Hispanic/non-Hispanic) | 12/5 | 10/1 | 9/2 | 7/3 | 7/3 | 15/1 |
| Gestational age, wk | 39.1 ± 0.2 | 38.7 ± 0.2 | 38.7 ± 0.3 | 39.0 ± 0.3 | 38.6 ± 0.3 | 38.8 ± 0.3 |
| Prepregnancy BMI, kg/m2 | 22.4 ± 0.4 | 21.9 ± 0.6 | 27.5 ± 0.5* | 27.3 ± 0.4* | 33.4 ± 1.4* | 34.5 ± 0.9* |
| Weight gain, lbs. | 29.0 ± 3.0 | 26.4 ± 3.0 | 24.5 ± 1.8 | 26.8 ± 5.5 | 19.2 ± 4.4 | 13.4 ± 5.4* |
| Placental weight, g | 755 ± 39 | 702 ± 14 | 724 ± 40 | 785 ± 43 | 793 ± 61 | 718 ± 47 |
| Fetal weight, g | 3,388 ± 73 | 3,128 ± 106# | 3,488 ± 136 | 3,268 ± 1,433 | 3,360 ± 99 | 3,409 ± 77* |
Values are means ± SE. LN, lean; OW, overweight; OB, obese. Significant differences were determined using a 1-way ANOVA and Bonferroni post hoc testing for each sex relative to the LN group.
P < 0.05 vs. LN group;
P < 0.05 vs. males within the same group of adiposity. Sample sizes are given for males and females.
Production of ROS Production in Villous Tissue
Production of ROS assessed by DCF staining was six- and 14-fold higher (P < 0.05) in placental villous tissue from fetuses of OW and OB women, respectively, compared with LN women (Fig. 1, A and B). Since DCF reacts with a wide variety of ROS, we separated the samples by fetal sex and measured the level of hydrogen peroxide using the Amplex Red probe. Horseradish peroxidase-catalyzed resorufin fluorescence, an indicator of the presence of hydrogen peroxide, was increased significantly (P < 0.05) in placentas of male and female fetuses from both OW and OB women compared with lean women (Fig. 1C).
Fig. 1.
Increased oxidative stress in placentas with increasing maternal adiposity. Visualization (A) and quantification (B) of dichlorofluorescein (DCF) in cryosections of placentae and production of H2O2 (C) measured by Amplex Red from lean (LN), overweight (OW), and obese (OB) women. Values are means ± SE. *P < 0.05 vs. LN group; n = 6/sex/group. AU, arbitrary units.
Effects of Maternal BMI on Placental ATP Levels
Since mitochondria are the main sources of ROS, we subsequently investigated whether an increase in production of ROS is associated with mitochondrial dysfunction. We initially evaluated mitochondrial function by measuring the levels of ATP in placental tissue (Fig. 2A). The ATP content was twofold higher (P < 0.05) in the LN female placentas compared with males. However, a significant reduction in ATP levels was seen with increasing adiposity in placentas of both males and females.
Fig. 2.
Reduction in ATP level and mitochondrial biogenesis in placentas of males and females with maternal obesity. A: ATP levels were normalized to total protein level of villous tissue extract. B and C: mitochondrial biogenesis was estimated by both citrate synthase activity (B) and mitochondrial DNA (mtDNA) copy number (C). mtDNA copy number was normalized to nuclear DNA (nDNA) (β2-microglobuln) for each sample. Values are means ± SE. *P < 0.05 vs. LN group and #P < 0.05 vs. males within the same group of adiposity; n = 6/sex/group.
Effect of maternal adiposity on mitochondrial biogenesis.
To explore the mechanism that may account for the decline in placental ATP levels with increasing adiposity, we utilized a RT-PCR approach to measure changes in mitochondrial biogenesis (Fig. 2, B and C). Mitochondrial content estimated by citrate synthase activity (23) showed a significant reduction in male and female placentas of OB but not OW mothers (P < 0.05) compared with LN. However, mitochondrial DNA copy number was slightly but significantly decreased only in placentas of male fetuses from the OW group, whereas in OB mothers, placentas from both males and females showed a significant reduction in mitochondrial biogenesis (P < 0.05).
Effect of maternal adiposity on expression of electron transport chain complexes.
To determine whether changes in placental ATP levels are related to altered protein expression of the mitochondrial electron transport complexes, we performed Western blotting on mitochondrial fractions using an antibody cocktail, recognizing epitopes of purified subunits of complexes I (NDUFB8), II (SDHB), III (UQCRC2), IV (MTCOX1), and V (ATP5α) (Fig. 3A). There were no fetal sex-dependent changes in expression of any of the five mitochondrial complexes in LN, OW, or OB women. However, the expression levels of subunits of complexes I–V placentas were significantly reduced (P < 0.05) in placentas of males and females from OW and OB women compared with LN women (Fig. 3, B–F).
Fig. 3.
Expression of placental mitochondrial complexes with increasing maternal adiposity. A: representative Western blots for males (m) and females (f) are shown. B–F: quantification of expression of peptides from mitochondrial complexes I (CI), II (CII), III (CIII), IV (CIV), and V (CV) in placental mitochondrial fractions. Voltage-dependent anion channel (VDAC) was used as loading control. *P < 0.05 vs. LN group; n = 6/sex/group.
Placental Mitochondrial Energetics in Syncytiotrophoblast Culture
We next determined the effects of increasing maternal adiposity on mitochondrial function in vitro using cultured syncytiotrophoblasts (Fig. 4). Linear regression and correlation analysis revealed a significant decrease in basal respiration, ATP-coupled respiration, maximum respiration, spare capacity, and nonmitochondrial respiration with increasing maternal adiposity (Fig. 4).Proton leak was unaffected by increasing maternal BMI (Fig. 4). No statistically significant differences were observed between primary trophoblast cultures from male or female fetuses; therefore, all data are combined.
Fig. 4.
Effect of increasing maternal BMI on mitochondrial respiration. Mitochondrial respiratory parameters were measured in syncytiotrophoblast cultures from women of a range of adiposity. Data were fitted using linear regression analysis (solid line). The coefficient of determination (r2) and P values are shown. Correlation analysis yielded significant correlations for basal respiration, ATP-coupled respiration, maximum respiration, spare capacity, and nonmitochondrial respiration with maternal BMI. The correlation of proton leak and maternal BMI was not significant. Oxygen consumption rate (OCR) measurements are normalized to total cellular protein content (pmol O2/μg protein); n = 33 separate cultures from placentas of females (□) and males (■).
Metabolic Flexibility of Syncytiotrophoblasts is Compromised with Maternal Adiposity
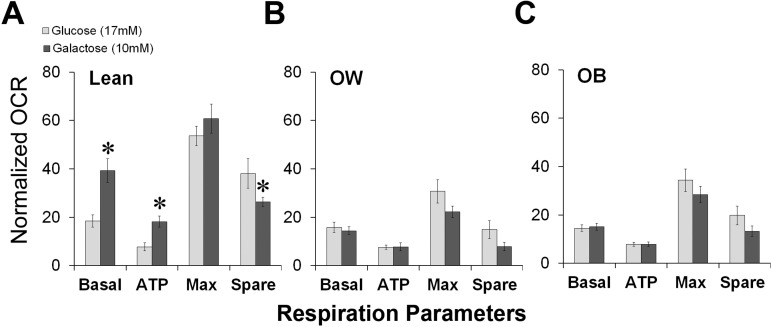
Culturing cells in galactose as the sole sugar source forces mammalian cells to rely on OXPHOS and is a strategy used previously to diagnose human mitochondrial disorders (47). Syncytiotrophoblasts from LN women cultured in galactose appeared to be metabolically flexible and demonstrated a twofold increase in basal mitochondrial oxygen consumption and ATP-coupled respiration rates compared with LN cells cultured in glucose (P < 0.05; Fig. 5A). Maximal respiration in these cells remained unchanged, leading to a 50% reduction in spare capacity. In contrast, syncytiotrophoblasts derived from OW and OB women, where respiratory parameters were already reduced, failed to increase their aerobic respiration when cultured in galactose-containing medium (Fig. 5, B and C), suggesting that they were compromised in oxidative metabolism. No differences were observed between syncytiotrophoblasts isolated from male and female placentas.
Fig. 5.
Impairment of metabolic adaptation and reliance on mitochondrial energy generation with increasing maternal adiposity. Cytotrophoblasts were isolated from placentae of males and females from LN, OW, and OB women and allowed to syncytialize for 72 h. The medium was changed in the final 24 h to contain glucose (17 mM) or galactose (10 mM) as a carbohydrate source, facilitating the reliance on either glycolysis and mitochondrial or exclusively mitochondrial respiration, respectively. Mitochondrial function was determined, and the OCR parameters were calculated. *P < 0.05 vs. glucose; n = 8/BMI group. Basal, basal respiration; ATP, ATP-coupled respiration; Max, maximal respiration; Spare, spare capacity.
DISCUSSION
The placenta is an extremely metabolically active fetal tissue that produces large amounts of peptides and steroid hormones that both influence the mother's metabolism to supply substrates to the fetus and also regulate fetal growth and development (65). Despite the utility of animal models (e.g., overnutrition) in examining the impact of maternal obesity on placental function (5), there is a paucity of data on the effect of maternal obesity on mitochondrial function in the human placenta. Reduction in the oxidative enzyme activity and the expression of mitochondrial respiratory complexes in skeletal muscle is reported in obese men and women (21) and as a risk factor for weight gain (56). Reduced expression of mitochondrial subunits was also found in white adipose tissue in response to genetic and nutritional obesity (8), short-term high-fat feeding (54), and type 2 diabetes (32, 42). We hypothesized that placental mitochondrial function would be compromised in response to increasing maternal adiposity.
Mitochondria are the main source of endogenous ROS in most mammalian cell types (26). Of the oxygen consumed by mitochondria, up to 5% is converted to ROS as byproducts of oxidation-reduction reactions in the respiratory chain. Excessive ROS in the placenta is thought to play a central role in the pathogenesis of preeclampsia and IUGR (37). Our data confirm that maternal obesity creates a state of increased oxidative stress within the placenta, suggesting that mitochondria may be functionally impaired with increasing maternal adiposity.
Sex-specific adaptation of the placenta may be central to the differences in fetal growth and survival. Male fetuses reputedly try to maximize growth in utero, a strategy that places them at risk in an adverse environment and may lead to increased incidence of adverse perinatal outcomes, including preterm birth, placenta previa, and premature lung development; in contrast, females were shown to be more sensitive to maternal asthma than males (35, 36, 55). Females may adapt to the adverse intrauterine environment in an attempt to survive further maternal insults and ensure survival. Differences in placental cytokine expression, insulin-like growth factor pathways, and the placental response to cortisol in relation to an adverse maternal condition (asthma) during pregnancy may regulate the sexual dimorphic survival responses (17, 53). Recently, we have demonstrated a sexual dimorphism in proinflammatory cytokine production and apoptosis in the placentas from pregnancies complicated by preeclampsia (34). We also found an increase in the expression and DNA binding activity of NF-κB transcription factor in the preeclamptic placentas compared with normotensive placentas with much higher levels in placentas of males compared with females (34). The sexual dimorphic responses in placental mitochondrial energetics and function are not well defined. However, in other tissues (e.g., liver, heart, and astrocytes), differences in mitochondrial dynamics (i.e., fission and fusion), energy metabolism, biogenesis, and regulation of complex subunits by phosphorylation were postulated to be regulated by sex hormones (19, 22, 49). Further studies are required to understand the mechanism(s) underlying the sexual dimorphism in placental metabolism.
Measurement of ATP content in villous tissue revealed a sexually dimorphic response. In LN women, placentas of female fetuses showed significantly higher ATP content compared with male fetuses. However, as maternal adiposity increased, female and male placentas from OW and OB women failed to maintain ATP levels, indicating a mitochondrial dysfunction. Interestingly, a decrease in mitochondrial DNA copy number was also fetal sex dependent, whereas citrate synthase activity, a marker of mitochondrial content, was reduced in placentas of both males and females from OB but not OW mothers. Mitochondrial “deficiencies” in the settings of obesity have been observed previously in the adipose tissue and skeletal muscle of obese rodents (39, 54, 58) and in the skeletal muscle of obese individuals (16, 45, 46).
To determine what affects ATP production in the placenta with maternal obesity, the expression of subunits encoding the complexes of electron transport chain was measured. All five mitochondrial complexes showed a tendency to decrease with increased adiposity, and there were no differences between male and female fetuses.
To further address the effect of maternal obesity on trophoblast respiration, we utilized an in vitro model of syncytiotrophoblast culture. Importantly, our data suggest that isolated trophoblasts can retain their in vivo phenotype in culture. Consistent with studies in other tissues (20), we found a decrease in mitochondrial function with increasing adiposity. The reduction in maximum respiration and spare respiratory capacity indicates that syncytiotrophoblasts from placentae of OW and OB women have an impaired cellular ability to meet energetic needs. In other cell types, such a reduction in energetic capacity renders them more susceptible to stressors (4). Similar findings of depressed mitochondrial oxygen consumption and decreased electron transport complex subunit mRNA expression have been observed in primary neurons of rodent models and skeletal muscle biopsies from obese and diabetic patients (20).
Cell culture medium containing galactose is often used to study the effect of mitochondrial toxins (14) and has been used to examine mitochondrial dysfunction in primary myotubes derived from diabetic patients (1). We found that galactose was able to increase the oxidative metabolism of primary trophoblasts derived from LN women. In contrast, trophoblasts derived from OW and OB patients were not able to increase their oxygen consumption rate when cultured in galactose, suggesting a reduction in metabolic flexibility of trophoblasts in response to maternal obesity.
Maternal obesity creates a unique in utero environment characterized by a failure to adequately store excess fatty acids, resulting in a chronic elevation of circulating fatty acids that can become cytotoxic (lipotoxicity) (18, 44, 50). Previously, free fatty acids were shown to affect mitochondrial respiration by increasing the production of ROS (51) and mitochondrial proton conductance (uncoupling) (38). Therefore, it is not surprising that mitochondrial abnormalities have been observed in the placentas of obese and overweight women. The functional consequences of mitochondrial deficiency and metabolic inflexibility on placental and fetal health remain to be clarified, but work is underway to study this important phenomenon. We speculate that mitochondrial dysfunction and decreased ATP content will lead to abnormal placental function contributing to perinatal complications and programming for metabolic disease in later life. Previously, it has been proposed that placental mitochondrial dysfunction may be critical in fetal programming of atherosclerosis (25).
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-075297 (L. Myatt) and a Clinical and Translational Science Award (UL1RR025767) from the Institute for Integration of Medicine and Science at the University of Texas Health Science Center San Antonio (A. Maloyan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.M., S.M., A.M., and L.M. conception and design of research; J.M. and S.M. performed experiments; J.M., S.M., A.M., and L.M. analyzed data; J.M., S.M., A.M., and L.M. interpreted results of experiments; J.M., S.M., and A.M. prepared figures; J.M. and A.M. drafted manuscript; J.M., S.M., A.M., and L.M. edited and revised manuscript; A.M. and L.M. approved final version of manuscript.
REFERENCES
- 1.Aguer C, Gambarotta D, Mailloux RJ, Moffat C, Dent R, McPherson R, Harper ME. Galactose enhances oxidative metabolism and reveals mitochondrial dysfunction in human primary muscle cells. PLoS One 6: e28536, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker DJ. The fetal and infant origins of adult disease. BMJ 301: 1111, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bax BE, Bloxam DL. Energy metabolism and glycolysis in human placental trophoblast cells during differentiation. Biochim Biophys Acta 1319: 283–292, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J 435: 297–312, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bullock GC, Delehanty LL, Talbot AL, Gonias SL, Tong WH, Rouault TA, Dewar B, Macdonald JM, Chruma JJ, Goldfarb AN. Iron control of erythroid development by a novel aconitase-associated regulatory pathway. Blood 116: 97–108, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catalano PM. Obesity and pregnancy—the propagation of a viscous cycle? J Clin Endocrinol Metab 88: 3505–3506, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Clifton VL. Sexually dimorphic effects of maternal asthma during pregnancy on placental glucocorticoid metabolism and fetal growth. Cell Tissue Res 322: 63–71, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Crunkhorn S, Dearie F, Mantzoros C, Gami H, da Silva WS, Espinoza D, Faucette R, Barry K, Bianco AC, Patti ME. Peroxisome proliferator activator receptor gamma coactivator-1 expression is reduced in obesity: potential pathogenic role of saturated fatty acids and p38 mitogen-activated protein kinase activation. J Biol Chem 282: 15439–15450, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Cui XL, Brockman D, Campos B, Myatt L. Expression of NADPH oxidase isoform 1 (Nox1) in human placenta: involvement in preeclampsia. Placenta 27: 422–431, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJ. Boys live dangerously in the womb. Am J Hum Biol 22: 330–335, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 9: 203–209, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Forsén T, Eriksson JG, Tuomilehto J, Teramo K, Osmond C, Barker DJ. Mother's weight in pregnancy and coronary heart disease in a cohort of Finnish men: follow up study. BMJ 315: 837–840, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galtier-Dereure F, Boegner C, Bringer J. Obesity and pregnancy: complications and cost. Am J Clin Nutr 71: 1242S–1248S, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Gohil VM, Sheth SA, Nilsson R, Wojtovich AP, Lee JH, Perocchi F, Chen W, Clish CB, Ayata C, Brookes PS, Mootha VK. Nutrient-sensitized screening for drugs that shift energy metabolism from mitochondrial respiration to glycolysis. Nat Biotechnol 28: 249–255, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guelinckx I, Devlieger R, Beckers K, Vansant G. Maternal obesity: pregnancy complications, gestational weight gain and nutrition. Obes Rev 9: 140–150, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Guillet C, Delcourt I, Rance M, Giraudet C, Walrand S, Bedu M, Duche P, Boirie Y. Changes in basal and insulin and amino acid response of whole body and skeletal muscle proteins in obese men. J Clin Endocrinol Metab 94: 3044–3050, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Huang X, Ding L, Bennewith KL, Tong RT, Welford SM, Ang KK, Story M, Le QT, Giaccia AJ. Hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation. Mol Cell 35: 856–867, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarvie E, Hauguel-de-Mouzon S, Nelson SM, Sattar N, Catalano PM, Freeman DJ. Lipotoxicity in obese pregnancy and its potential role in adverse pregnancy outcome and obesity in the offspring. Clin Sci (Lond) 119: 123–129, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Justo R, Boada J, Frontera M, Oliver J, Bermúdez J, Gianotti M. Gender dimorphism in rat liver mitochondrial oxidative metabolism and biogenesis. Am J Physiol Cell Physiol 289: C372–C378, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Kalichman MW, Powell HC, Mizisin AP. Reactive, degenerative, and proliferative Schwann cell responses in experimental galactose and human diabetic neuropathy. Acta Neuropathol 95: 47–56, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51: 2944–2950, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Lagranha CJ, Deschamps A, Aponte A, Steenbergen C, Murphy E. Sex differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circ Res 106: 1681–1691, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsen S, Stride N, Hey-Mogensen M, Hansen CN, Bang LE, Bundgaard H, Nielsen LB, Helge JW, Dela F. Simvastatin effects on skeletal muscle: relation to decreased mitochondrial function and glucose intolerance. J Am Coll Cardiol 61: 44–53, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Lattuada D, Colleoni F, Martinelli A, Garretto A, Magni R, Radaelli T, Cetin I. Higher mitochondrial DNA content in human IUGR placenta. Placenta 29: 1029–1033, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Leduc L, Levy E, Bouity-Voubou M, Delvin E. Fetal programming of atherosclerosis: possible role of the mitochondria. Eur J Obstet Gynecol Reprod Biol 149: 127–130, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Lee HC, Wei YH. Oxidative stress, mitochondrial DNA mutation, and apoptosis in aging. Exp Biol Med (Maywood) 232: 592–606, 2007 [PubMed] [Google Scholar]
- 27.Llurba E, Gratacós E, Martín-Gallán P, Cabero L, Dominguez C. A comprehensive study of oxidative stress and antioxidant status in preeclampsia and normal pregnancy. Free Radic Biol Med 37: 557–570, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Maloyan A, Mele J, Muralimanohara B, Myatt L. Measurement of mitochondrial respiration in trophoblast culture. Placenta 33: 456–458, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao J, Zhang X, Sieli PT, Falduto MT, Torres KE, Rosenfeld CS. Contrasting effects of different maternal diets on sexually dimorphic gene expression in the murine placenta. Proc Natl Acad Sci USA 107: 5557–5562, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marroquin LD, Hynes J, Dykens JA, Jamieson JD, Will Y. Circumventing the Crabtree effect: replacing media glucose with galactose increases susceptibility of HepG2 cells to mitochondrial toxicants. Toxicol Sci 97: 539–547, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Mayhew TM. Taking tissue samples from the placenta: an illustration of principles and strategies. Placenta 29: 1–14, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstråle M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34: 267–273, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Muralimanoharan S, Maloyan A, Mele J, Guo C, Myatt LG, Myatt L. MIR-210 modulates mitochondrial respiration in placenta with preeclampsia. Placenta 33: 816–823, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muralimanoharan S, Maloyan A, Myatt L. Evidence of sexual dimorphism in the placental function with severe preeclampsia. Placenta 34: 1183–1189, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy VE, Gibson P, Talbot PI, Clifton VL. Severe asthma exacerbations during pregnancy. Obstet Gynecol 106: 1046–1054, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Murphy VE, Gibson PG, Giles WB, Zakar T, Smith R, Bisits AM, Kessell CG, Clifton VL. Maternal asthma is associated with reduced female fetal growth. Am J Respir Crit Care Med 168: 1317–1323, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Myatt L, Cui X. Oxidative stress in the placenta. Histochem Cell Biol 122: 369–382, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Nakamura MT, Yudell BE, Loor JJ. Regulation of energy metabolism by long-chain fatty acids. Prog Lipid Res 53: 124–144, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Noland RC, Koves TR, Seiler SE, Lum H, Lust RM, Ilkayeva O, Stevens RD, Hegardt FG, Muoio DM. Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J Biol Chem 284: 22840–22852, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olivera AA, Meigs RA. Mitochondria from human term placenta. I. Isolation and assay conditions for oxidative phosphorylation. Biochim Biophys Acta 376: 426–435, 1975 [DOI] [PubMed] [Google Scholar]
- 41.Osei-Kumah A, Smith R, Jurisica I, Caniggia I, Clifton VL. Sex-specific differences in placental global gene expression in pregnancies complicated by asthma. Placenta 32: 570–578, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100: 8466–8471, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prescott SL, Clifton V. Asthma and pregnancy: emerging evidence of epigenetic interactions in utero. Curr Opin Allergy Clin Immunol 9: 417–426, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Pruis MG, van Ewijk PA, Schrauwen-Hinderling VB, Plösch T. Lipotoxicity and the role of maternal nutrition. Acta Physiol (Oxf). In press [DOI] [PubMed] [Google Scholar]
- 45.Ritov VB, Menshikova EV, Azuma K, Wood R, Toledo FG, Goodpaster BH, Ruderman NB, Kelley DE. Deficiency of electron transport chain in human skeletal muscle mitochondria in type 2 diabetes mellitus and obesity. Am J Physiol Endocrinol Metab 298: E49–E58, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 54: 8–14, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Robinson BH, Petrova-Benedict R, Buncic JR, Wallace DC. Nonviability of cells with oxidative defects in galactose medium: a screening test for affected patient fibroblasts. Biochem Med Metab Biol 48: 122–126, 1992 [DOI] [PubMed] [Google Scholar]
- 48.Rossignol R, Gilkerson R, Aggeler R, Yamagata K, Remington SJ, Capaldi RA. Energy substrate modulates mitochondrial structure and oxidative capacity in cancer cells. Cancer Res 64: 985–993, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Sanz A, Hiona A, Kujoth GC, Seo AY, Hofer T, Kouwenhoven E, Kalani R, Prolla TA, Barja G, Leeuwenburgh C. Evaluation of sex differences on mitochondrial bioenergetics and apoptosis in mice. Exp Gerontol 42: 173–182, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmatz M, Madan J, Marino T, Davis J. Maternal obesity: the interplay between inflammation, mother and fetus. J Perinatol 30: 441–446, 2010 [DOI] [PubMed] [Google Scholar]
- 51.Schonfeld P, Wojtczak L. Fatty acids as modulators of the cellular production of reactive oxygen species. Free Radic Biol Med 45: 231–241, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell 48: 158–167, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sood R, Zehnder JL, Druzin ML, Brown PO. Gene expression patterns in human placenta. Proc Natl Acad Sci USA 103: 5478–5483, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA, Smith SR. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes 54: 1926–1933, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Stark MJ, Clifton VL, Wright IMR. Sex-specific differences in peripheral microvascular blood flow in preterm infants. Pediatr Res 63: 415–419, 2008 [DOI] [PubMed] [Google Scholar]
- 56.Sun G, Ukkola O, Rankinen T, Joanisse DR, Bouchard C. Skeletal muscle characteristics predict body fat gain in response to overfeeding in never-obese young men. Metabolism 51: 451–456, 2002 [DOI] [PubMed] [Google Scholar]
- 57.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol 552: 335–344, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Valerio A, Cardile A, Cozzi V, Bracale R, Tedesco L, Pisconti A, Palomba L, Cantoni O, Clementi E, Moncada S, Carruba MO, Nisoli E. TNF-alpha downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J Clin Invest 116: 2791–2798, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valsamakis G, Kanaka-Gantenbein C, Malamitsi-Puchner A, Mastorakos G. Causes of intrauterine growth restriction and the postnatal development of the metabolic syndrome. Ann NY Acad Sci 1092: 138–147, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Venegas V, Wang J, Dimmock D, Wong LJ. Real-time quantitative PCR analysis of mitochondrial DNA content. Curr Protoc Hum Genet Chapter 19: Unit 19.7, 2011 [DOI] [PubMed] [Google Scholar]
- 61.Wang Y, Walsh SW. Placental mitochondria as a source of oxidative stress in pre-eclampsia. Placenta 19: 581–586, 1998 [DOI] [PubMed] [Google Scholar]
- 62.Watson AL, Skepper JN, Jauniaux E, Burton GJ. Susceptibility of human placental syncytiotrophoblastic mitochondria to oxygen-mediated damage in relation to gestational age. J Clin Endocrinol Metab 83: 1697–1705, 1998 [DOI] [PubMed] [Google Scholar]
- 63.Wilson-Fritch L, Nicoloro S, Chouinard M, Lazar MA, Chui PC, Leszyk J, Straubhaar J, Czech MP, Corvera S. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest 114: 1281–1289, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu G, Imhoff-Kunsch B, Girard AW. Biological mechanisms for nutritional regulation of maternal health and fetal development. Paediatr Perinat Epidemiol 26, Suppl 1: 4–26, 2012 [DOI] [PubMed] [Google Scholar]
- 65.Yen SS. The placenta as the third brain. J Reprod Med 39: 277–280, 1994 [PubMed] [Google Scholar]