Abstract
Context:
Thyroid-associated ophthalmopathy (TAO) is the component of Graves' disease characterized by orbital inflammation and connective tissue remodeling. The IGF-1 receptor (IGF-1R) and TSH receptor (TSHR) form a physical and functional complex in orbital fibroblasts. A subset of these fibroblasts is derived from infiltrating CD34+ fibrocytes. Teprotumumab (RV 001, R1507) is a human monoclonal anti-IGF-1R blocking antibody currently undergoing a phase 2 clinical trial in patients with active TAO.
Objective:
To determine whether teprotumumab inhibits the induction by TSH of IL-6 and IL-8 in fibrocytes.
Design:
Fibrocytes were treated without or with teprotumumab in combination with IGF-1 or TSH.
Main Outcome Measures:
IL-6 and IL-8 mRNA expression and protein production were analyzed by real-time PCR and Luminex, respectively. Phosphorylated Akt (S473) levels were analyzed by Western blot. TSHR and IGF-1R display was assessed by flow cytometry.
Results:
Fibrocyte display of IGF-1R and TSHR was reduced with teprotumumab, as were IGF-1- and TSH-dependent phosphorylated Akt levels. TSH induction of IL-6 and IL-8 mRNA and protein was also reduced by the monoclonal antibody.
Conclusions:
Teprotumumab attenuates the actions of both IGF-1 and TSH in fibrocytes. Specifically, it blocks the induction of proinflammatory cytokines by TSH. These results provide, at least in part, the molecular rationale for interrogating the therapeutic efficacy of this antibody in TAO.
Thyroid-associated ophthalmopathy (TAO) is the inflammatory orbital manifestation of Graves' disease (GD) (1). The molecular mechanisms underlying TAO remain obscure. At the heart of GD is the generation of activating antibodies directed against the TSH receptor (TSHR) (2). In addition, antibodies that activate the IGF-1 receptor (IGF-1R) have also been detected (3–6). TSHR and IGF-1R have been shown to form a physical and functional complex in orbital fibroblasts and thyroid epithelial cells (7). Moreover, blocking IGF-1R appears to attenuate TSH-dependent signaling (7). These findings have been confirmed recently by another laboratory group (8). They suggest that blocking IGF-1R using the monoclonal antibody (mAb) antagonist might reduce both TSHR- and IGF-1-dependent signaling and therefore interrupt pathological activities initiated through both receptors.
Monoclonal antibodies directed against IGF-1R have been developed and assessed as a therapeutic strategy for several types of solid tumors and lymphomas (9–11). Teprotumumab (RV 001, R1507) is a fully human mAb that binds to the ligand binding extracellular α-subunit domain of IGF-1R (12). This molecule is currently under phase 2 clinical investigation in patients with moderate to severe, active TAO. Its potential for attenuating the actions of TSH has not been investigated previously.
In TAO, the orbit appears to be infiltrated by fibrocytes, bone marrow-derived progenitor cells of the monocyte lineage (13). These cells express leukocyte and fibroblast surface antigens and remarkably high levels of functional TSHR (14, 15). Fibrocytes participate in wound healing and tissue remodeling and appear to be involved in the pathogenesis of pulmonary fibrosis and rheumatic arthritis (16, 17). Fibrocytes activated by TSH and IgGs from patients with GD express several proinflammatory cytokines, including IL-1β, IL-1 receptor antagonist, IL-6, IL-8, and TNFα (13, 15, 18). Besides the orbit, fibrocytes also infiltrate the thyroid gland in GD, providing a potential mechanistic link between affected tissues (19).
In this study, we report for the first time that teprotumumab decreases TSHR and IGF-1R display by fibrocytes and attenuates TSH-dependent IL-6 and IL-8 expression and Akt phosphorylation. These findings further help elucidate the interplay between TSHR and IGF-1R that may be critical to the pathogenesis of TAO and support the therapeutic rationale for blocking IGF-1R in this disease.
Patients and Methods
Patient samples
Patients with GD (n = 6) were recruited from the patient population of the Kellogg Eye Center at the University of Michigan. Informed consent was obtained in compliance with policies of the Institutional Review Board of the University of Michigan Health System. Research methods followed the tenets of the Declaration of Helsinki.
Fibrocyte cultures
Fibrocytes were generated from peripheral blood mononuclear cells isolated from leukocyte reduction filters provided by the American Red Cross or from blood of patients with GD and were cultured as described previously (13, 20). Briefly, peripheral blood mononuclear cells were isolated by centrifugation over Ficoll-PaquePlus (catalog no. 17–1440-03; GE Healthcare Bio-Science). After washing, cells were resuspended in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin mixture (catalog no. 15140–122; Life Technologies). Each culture well of a six-well plate was inoculated with 107 cells and incubated at 37°C in a 5% CO2 atmosphere. After 7 days, cultures were rinsed, and nonadherent cells were removed by gentle aspiration. Medium was changed every 3 days. After 10 to 14 days of cultivation, culture purity was verified to be >90% fibrocytes by flow cytometry. Twenty-four hours before experimental treatments, medium containing 1% FBS was substituted.
Flow cytometry
Cell surface IGF-1R and TSHR were assessed before and after treatment with teprotumumab for the times indicated along the abscissas in Figure 1B. Cells were collected by gentle scraping and washed with staining buffer consisting of Dulbecco's PBS, 2% FBS, and 0.1% sodium azide. The following antihuman fluorochrome-conjugated antibodies were used: mouse IgG1, κ isotype control-FITC (catalog no. 555748); mouse IgG1, κ isotype control-PE (catalog no. 554680); CD34 FITC (catalog no. 555821); and CD221-PE (catalog no. 555999), from BD Biosciences; CD45-PercP (catalog no. MHCD4531), from Life Technologies; and TSHR-PE (catalog no. 53542), from Santa Cruz Biotechnology. After 30-minute incubations, cells were washed and fixed with 1% paraformaldehyde. Analysis was performed using an LSR II flow cytometer (BD Biosciences). Greater than 105 events were collected. Mean fluorescence intensity (MFI) was calculated as a ratio of geometric mean fluorescence of sample and that of an isotype control.
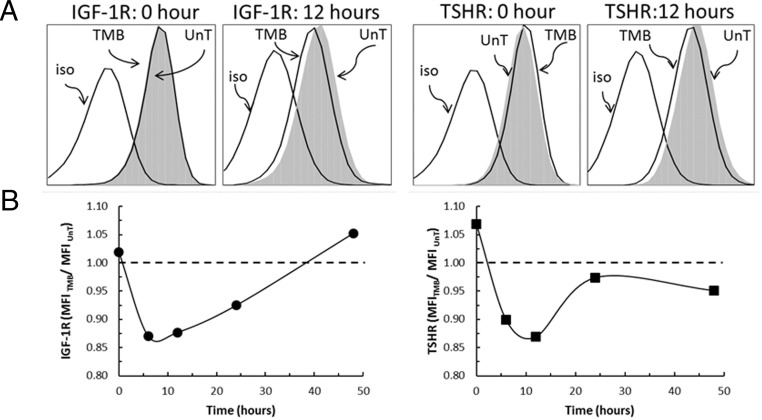
Figure 1.
Teprotumumab reduces fibrocyte cell surface expression of IGF-1R and TSHR. A, Fibrocytes express IGF-1R and TSHR (MFI, 3.01 and 3.97, respectively). They were treated with 50 μg/mL of teprotumumab (TMB) for 12 hours and then analyzed for IGF-1R and TSHR display by flow cytometry. IGF-1R and TSHR of TMB-treated cells were compared to those of untreated cells (UnT). B, A time course of teprotumumab treatment. Cells were treated as in panel A, harvested at the times indicated along the abscissa, and analyzed for receptor display by flow cytometry.
Quantitative RT-PCR
Fibrocytes were treated as indicated in the figure legends. RNA was isolated using Aurum Total RNA Mini Kit (Bio-Rad) and reverse-transcribed with a QuantiTec Reverse Transcription Kit (QIAGEN). Quantitative PCR was performed with SYBR Green technique (Bio-Rad) in a Bio-Rad CFX96 thermocycler. The following primers were used: IL-6—forward, 5′-TGAGAAAGGAGACATGTAACAAGAGT-3′, and reverse, 5′-TTGTTCCTCACTACTCTCAAATCTGT-3′; IL-8—forward, 5′-GGCAGCCTTCCTGATTTCTG-3′, and reverse, 5′- GGGTGGAAAGGTTTGGAGTATG-3′; IGF-1R—forward, 5′- TGGAGTGCTGTATGCCTCTG-3′, and reverse, 5′- CCCTTGGCAACTCCTTCATA-3′; and GAPDH—forward, 5′-TTGCCATCAATGACCCCTTCA-3′, and reverse, 5′-CGCCCCACTTGATTTTGGA-3′. TSHR primers were purchased from QIAGEN (catalog no. PPH02344C).
Cytokine Luminex assays
Fibrocytes were treated as indicated in the figure legends, and the media were subjected to IL-6 and IL-8 analysis using human Singleplex Bead kits (catalog no. LHC0061 and LHC0081; Life Technologies).
Measurement of Akt phosphorylation
Fibrocyte lysates from cultures treated without or with teprotumumab (50 μg/mL) and bovine TSH (bTSH; 5 mU/mL; Calbiochem EMD Biosciences) or recombinant human IGF-1 (100 ng/mL; catalog no. 291-G1–01M; R&D Systems) for 1 hour were subjected to Western blot analysis for phosphorylated Akt (pAkt) using an anti-pAkt (S473) antibody (catalog no. 4058) and anti-β-actin antibody (catalog no. 3700) from Cell Signaling.
Statistics
Experiments were conducted in triplicate and performed at least three times. Data are reported as the mean ± SD. Statistical analysis was performed using ANOVA with a confidence level greater than 95%.
Results
Teprotumumab reduces IGF-1R and TSHR surface display on fibrocytes
Consistent with previous reports, IGF-1R and TSHR expression on the surface of untreated fibrocytes was substantial (MFI, 3.01 and 3.97, respectively; Figure 1A) (13, 15). First, we tested whether there is interference of teprotumumab with the detection of IGF-1R or TSHR (data not shown). Briefly, fibrocytes were incubated with teprotumumab at graded concentrations (0, 5, 50, and 500 μg/mL) at 4°C for 2 hours. Receptor protein expression remained constant. These results indicate that teprotumumab does not interfere with the detection of either receptor on the cell surface.
We then investigated whether teprotumumab treatment alters IGF-1R expression after incubation at 37°C and found a significant decrease in surface IGF-1R levels after 12 hours (Figure 1, A and B, left panels). The levels were reduced as early as 6 hours and gradually returned to baseline by 48 hours. Because IGF-1R and TSHR form a physical complex (7), the effect of teprotumumab on TSHR display was also investigated. Similar to IGF-1R, TSHR levels declined after treatment with teprotumumab, reaching a nadir after 12 hours (Figure 1, A and B, right panels). Levels returned to baseline by 24 hours. Fibrocytes from patients with GD also showed significant reduction of surface IGF-1R and TSHR due to teprotumumab treatment (Supplemental Table 1). We also determined that other cell surface proteins—CD40, CD34, and CD45—were not altered as a result of teprotumumab treatment (Supplemental Figure 2). IGF-1R and TSHR mRNA abundance was unaffected by teprotumumab after 24 hours (data not shown).
Teprotumumab inhibits TSH-induced IL-6 and IL-8
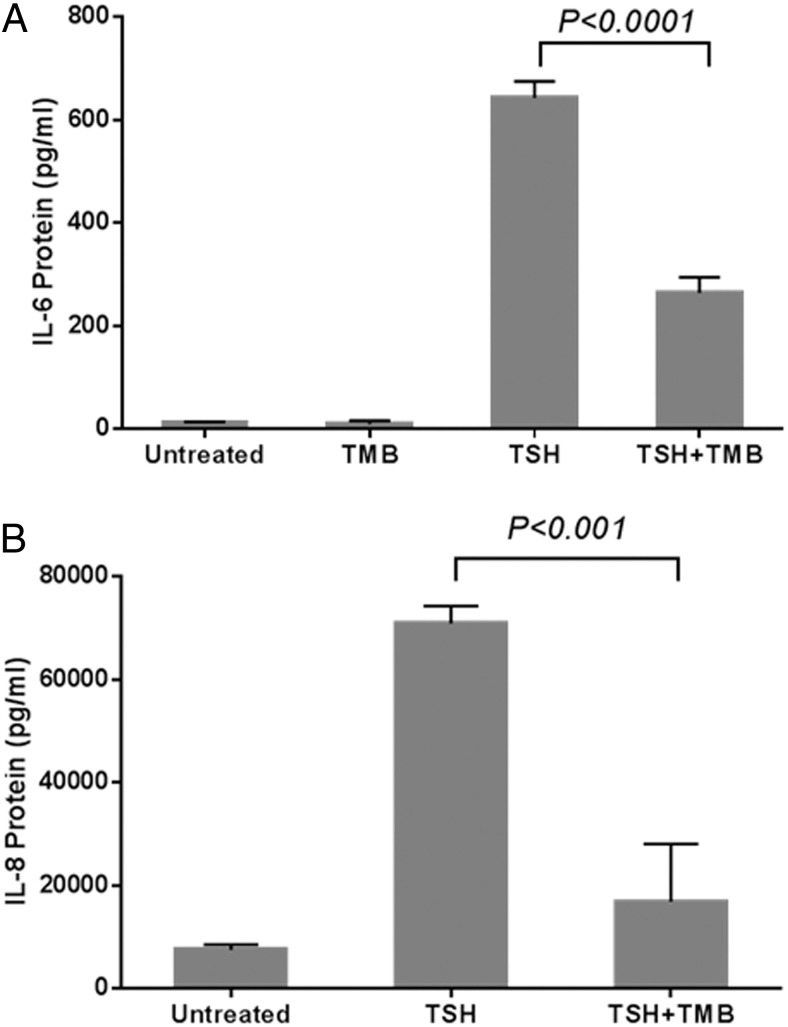
IL-6 and IL-8 may play roles in TAO and are induced by TSH in fibrocytes (13, 15). Untreated fibrocytes produce negligible IL-6. However, addition of bTSH increased the IL-6 concentration in culture medium by approximately 50-fold after 24 hours (13 ± 1 vs 643 ± 32 pg/mL; P < .0001; Figure 2A). Pretreatment with teprotumumab (24 hours) significantly reduced TSH-provoked IL-6 (643 ± 32 to 265 ± 30 pg/mL; P < .0001). Fibrocytes produce substantial IL-8 under basal (untreated) conditions. Like IL-6, IL-8 expression was also increased by bTSH (7595 ± 995 vs 70 911 ± 3429 pg/mL; P < .0001; Figure 2B). Teprotumumab significantly inhibited the induction by TSH of IL-8 (70 911 ± 3429 to 16 910 ± 11 199 pg/mL; P < .001).
Figure 2.
Teprotumumab inhibits TSH-stimulated IL-6 (A) and IL-8 (B) protein production by fibrocytes. Fibrocytes were pretreated with nothing or with 50 μg/mL of teprotumumab (TMB) for 24 hours before nothing (untreated) or bTSH (5 mU/mL) addition. Cultures were incubated with bTSH for 24 hours. Media were collected, and IL-6 and IL-8 concentrations were measured with Luminex technology. Data are presented as mean ± SD (n = 3). TSH significantly induced IL-6 (from 13 to 643 pg/mL; P < .0001) and IL-8 (from 7595 to 70 911 pg/mL; P < .0001). Teprotumumab (TMB) significantly inhibited the TSH-mediated IL-6 (P < .0001) and IL-8 (P < .001) production.
Inhibition of cytokine production by teprotumumab is mediated at the pretranslational level
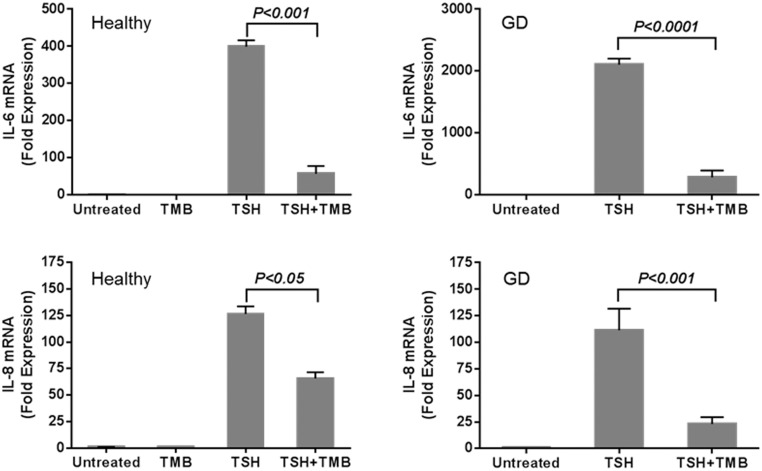
The molecular mechanism involved in the teprotumumab effect on bTSH-provoked cytokine induction was examined in fibrocytes from healthy donors and patients with GD (Figure 3). The mAb failed to alter basal steady-state IL-6 and IL-8 mRNA levels. TSH increased IL-6 transcripts in fibrocytes from healthy donors and patients with GD by 400- and 2000-fold, respectively, after 6 hours (P < .000). Teprotumumab significantly inhibited the induction by bTSH of IL-6 mRNA in fibrocytes from healthy donors and patients with GD (P < .001 and P < .0001, respectively). Similar results were observed when steady-state IL-8 mRNA was measured (Figure 3, lower panels). Addition of bTSH induced IL-8 mRNA in fibrocytes from both sources (P < .0001). Teprotumumab significantly reduced the bTSH-induced IL-8 mRNA in fibrocytes from patients with GD and healthy donors (P < .001 and P < .05, respectively).
Figure 3.
Teprotumumab inhibits TSH induction of IL-6 and IL-8 mRNA in fibrocytes from healthy donors (left panels) and from patients with GD (right panels). Fibrocytes were pretreated with nothing or with 50 μg/mL teprotumumab (TMB) for 24 hours. After pretreatment, cultures were treated without or with bTSH (5 mU/mL) for 6 hours. Cytokine mRNA levels were determined by real-time PCR. Teprotumumab significantly abrogates the induction by bTSH of IL-6 and IL-8 mRNA.
Teprotumumab blocks TSH-induced Akt phosphorylation in fibrocytes
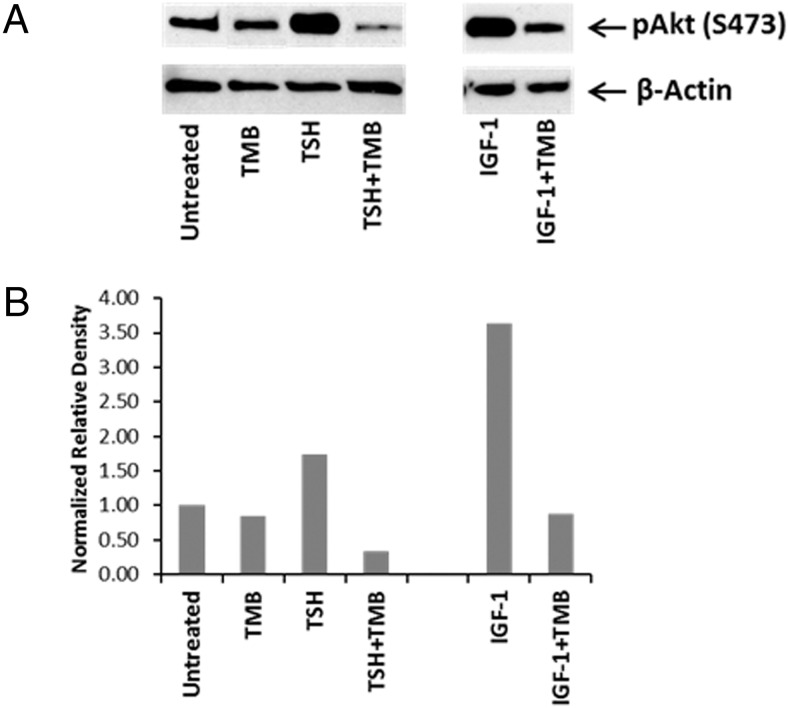
TSH-provoked IL-6 and IL-8 expression in fibrocytes is mediated in part through the Akt pathway (22). Fibrocytes were treated with teprotumumab for 1 hour and treated with bTSH, and Akt phosphorylation was assessed. Teprotumumab partially blocked the effects of TSH on pAkt (S473) (density normalized to β-actin, 1.73 to 0.33; Figure 4). The mAb also blocked the expected increase in pAkt (S473) provoked by IGF-1 (Figure 4).
Figure 4.
Teprotumumab blocks TSH-induced Akt phosphorylation in fibrocytes. A, Fibrocytes were pretreated with nothing or teprotumumab (TMB) for 1 hour and then without or with bTSH (5 mU/mL) or IGF-1 (100 ng/mL) for 1 hour. Akt phosphorylation at S473 was analyzed by Western blot (A). Densitometric analysis for the results is shown in panel B.
Discussion
Direct involvement of either TSHR or IGF-1R in the pathogenesis of TAO remains uncertain (23, 24). TSHR is detectable on orbital fibroblasts, but the levels of this protein are vanishingly low until the cells are subjected to conditions favoring their differentiation into adipocytes (7, 25). In contrast, TSHR levels in fibrocytes approach or exceed those detected on thyroid epithelium (26–28). Antibodies directed against TSHR and/or IGF-1R may trigger signaling through these receptors and induce production of proinflammatory cytokines. Thus, targeting and blocking both proteins may constitute therapy for TAO. No definitive therapy for the disease currently exists; however, drugs targeting cytokines or B or T cells have been suggested as potential treatments (29, 30). Small molecule TSHR antagonists have been described recently (27, 31), as have TSHR-blocking antibodies (32). Teprotumumab reduced not only cell surface IGF-1R but also TSHR display on fibrocytes. Furthermore, it inhibited IGF-1- and TSH-induced Akt phosphorylation, indicating that the mAb interferes with both IGF-1R- and TSHR-mediated actions. These findings are congruent with those reported earlier by Tsui et al (7), who demonstrated that 1H7, another IGF-1R-blocking mAb, could attenuate TSHR-initiated signaling in TAO orbital fibroblasts. A recent study by Kumar et al (8) confirmed these findings. Coupled with the current findings, it appears that IGF-1R plays an important role in regulating a component of the signaling conventionally attributed to TSHR in thyroid epithelial cells, orbital fibroblasts, and fibrocytes.
IL-6 and IL-8 have been implicated in the pathogenesis of TAO (21, 33). Teprotumumab inhibits the induction by TSH of both cytokines in vitro. This further strengthens the therapeutic rational for testing this mAb in moderate to severe, active TAO patients. Teprotumumab is currently undergoing assessment in a multicenter, placebo-controlled, phase 2 clinical trial (http://clinicaltrials.gov/show/NCT01868997). This trial should inform more definitively the roles of these receptors in the pathogenesis of TAO.
Acknowledgments
This work was supported by National Institutes of Health Grants Eye Core Grants EY008976, EY011708, DK063121, EY021197, and EY007003; the Bell Charitable Foundation; and the following grants from Research to Prevent Blindness: Career Development Award, a Lew Wasserman Merit Award, and a Physician Scientist Award.
Teprotumumab was furnished as a gift by River Vision Development Corp.
Disclosure Summary: The authors have no proprietary or commercial interest in any material discussed in this article.
Footnotes
- bTSH
- bovine TSH
- FBS
- fetal bovine serum
- GD
- Graves' disease
- IGF-1R
- IGF-1 receptor
- mAb
- monoclonal antibody
- MFI
- mean fluorescence intensity
- pAkt
- phosphorylated Akt
- TAO
- thyroid-associated ophthalmopathy
- TSHR
- TSH receptor.
References
- 1. Gianoukakis AG, Khadavi N, Smith TJ. Cytokines, Graves' disease, and thyroid-associated ophthalmopathy. Thyroid. 2008;18:953–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khoo TK, Bahn RS. Pathogenesis of Graves' ophthalmopathy: the role of autoantibodies. Thyroid. 2007;17:1013–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pritchard J, Han R, Horst N, Cruikshank WW, Smith TJ. Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves' disease is mediated through the insulin-like growth factor I receptor pathway. J Immunol. 2003;170:6348–6354 [DOI] [PubMed] [Google Scholar]
- 4. Pritchard J, Horst N, Cruikshank W, Smith TJ. Igs from patients with Graves' disease induce the expression of T cell chemoattractants in their fibroblasts. J Immunol. 2002;168:942–950 [DOI] [PubMed] [Google Scholar]
- 5. Smith TJ, Hoa N. Immunoglobulins from patients with Graves' disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-I receptor. J Clin Endocrinol Metab. 2004;89:5076–5080 [DOI] [PubMed] [Google Scholar]
- 6. Varewijck AJ, Boelen A, Lamberts SW, et al. Circulating IgGs may modulate IGF-I receptor stimulating activity in a subset of patients with Graves' ophthalmopathy. J Clin Endocrinol Metab. 2013;98:769–776 [DOI] [PubMed] [Google Scholar]
- 7. Tsui S, Naik V, Hoa N, et al. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves' disease. J Immunol. 2008;181:4397–4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kumar S, Iyer S, Bauer H, Coenen M, Bahn RS. A stimulatory thyrotropin receptor antibody enhances hyaluronic acid synthesis in Graves' orbital fibroblasts: inhibition by an IGF-I receptor blocking antibody. J Clin Endocrinol Metab. 2012;97:1681–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burtrum D, Zhu Z, Lu D, et al. A fully human monoclonal antibody to the insulin-like growth factor I receptor blocks ligand-dependent signaling and inhibits human tumor growth in vivo. Cancer Res. 2003;63:8912–8921 [PubMed] [Google Scholar]
- 10. Ferté C, Loriot Y, Clémenson C, et al. IGF-1R targeting increases the antitumor effects of DNA-damaging agents in SCLC model: an opportunity to increase the efficacy of standard therapy. Mol Cancer Ther. 2013;12:1213–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang HJ, Angelo LS, Rodon J, et al. R1507, an anti-insulin-like growth factor-1 receptor (IGF-1R) antibody, and EWS/FLI-1 siRNA in Ewing's sarcoma: convergence at the IGF/IGFR/Akt axis. PloS One. 2011;6:e26060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schnitzer T, Kuenkele KP, Rebers F, et al. Characterization of a recombinant, fully human monoclonal antibody directed against the human insulin-like growth factor-1 receptor. Eur J Cancer Suppl. 2006;4:66–67 [Google Scholar]
- 13. Douglas RS, Afifiyan NF, Hwang CJ, et al. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2010;95:430–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87:858–870 [DOI] [PubMed] [Google Scholar]
- 15. Gillespie EF, Papageorgiou KI, Fernando R, et al. Increased expression of TSH receptor by fibrocytes in thyroid-associated ophthalmopathy leads to chemokine production. J Clin Endocrinol Metab. 2012;97:E740–E746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Borie R, Quesnel C, Phin S, et al. Detection of alveolar fibrocytes in idiopathic pulmonary fibrosis and systemic sclerosis. PloS One. 2013;8:e53736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Galligan CL, Fish EN. Circulating fibrocytes contribute to the pathogenesis of collagen antibody-induced arthritis. Arthritis Rheum. 2012;64:3583–3593 [DOI] [PubMed] [Google Scholar]
- 18. Kahaly GJ. The thyrocyte-fibrocyte link: closing the loop in the pathogenesis of Graves' disease? J Clin Endocrinol Metab. 2010;95:62–65 [DOI] [PubMed] [Google Scholar]
- 19. Smith TJ, Padovani-Claudio DA, Lu Y, et al. Fibroblasts expressing the thyrotropin receptor overarch thyroid and orbit in Graves' disease. J Clin Endocrinol Metab. 2011;96:3827–3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81 [PMC free article] [PubMed] [Google Scholar]
- 21. Ujhelyi B, Gogolak P, Erdei A, et al. Graves' orbitopathy results in profound changes in tear composition: a study of plasminogen activator inhibitor-1 and seven cytokines. Thyroid. 2012;22:407–414 [DOI] [PubMed] [Google Scholar]
- 22. Raychaudhuri N, Fernando R, Smith TJ. Thyrotropin regulates IL-6 expression in CD34(+) fibrocytes: clear delineation of its cAMP-independent actions. PLoS One. 2013;8:e75100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smith TJ. Orbital fibroblasts exhibit a novel pattern of responses to proinflammatory cytokines: Potential basis for the pathogenesis of thyroid-associated ophthalmopathy. Thyroid. 2002;12:197–203 [DOI] [PubMed] [Google Scholar]
- 24. Smith TJ, Tsai CC, Shih MJ, et al. Unique attributes of orbital fibroblasts and global alterations in IGF-1 receptor signaling could explain thyroid-associated ophthalmopathy. Thyroid. 2008;18:983–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Valyasevi RW, Erickson DZ, Harteneck DA, et al. Differentiation of human orbital preadipocyte fibroblasts induces expression of functional thyrotropin receptor. J Clin Endocrinol Metab. 1999;84:2557–2562 [DOI] [PubMed] [Google Scholar]
- 26. Douglas RS, Gianoukakis AG, Kamat S, Smith TJ. Aberrant expression of the insulin-like growth factor-1 receptor by T cells from patients with Graves' disease may carry functional consequences for disease pathogenesis. J Immunol. 2007;178:3281–3287 [DOI] [PubMed] [Google Scholar]
- 27. Turcu AF, Kumar S, Neumann S, et al. A small molecule antagonist inhibits thyrotropin receptor antibody-induced orbital fibroblast functions involved in the pathogenesis of Graves ophthalmopathy. J Clin Endocrinol Metab. 2013;98:2153–2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wiersinga WM. Autoimmunity in Graves' ophthalmopathy: the result of an unfortunate marriage between TSH receptors and IGF-1 receptors? J Clin Endocrinol Metab. 2011;96:2386–2394 [DOI] [PubMed] [Google Scholar]
- 29. Cawood TJ, Moriarty P, O'Farrelly C, O'Shea D. The effects of tumour necrosis factor-α and interleukin1 on an in vitro model of thyroid-associated ophthalmopathy; contrasting effects on adipogenesis. Eur J Endocrinol. 2006;155:395–403 [DOI] [PubMed] [Google Scholar]
- 30. El Fassi D, Nielsen CH, Hasselbalch HC, Hegedüs L. Treatment-resistant severe, active Graves' ophthalmopathy successfully treated with B lymphocyte depletion. Thyroid. 2006;16:709–710 [DOI] [PubMed] [Google Scholar]
- 31. Neumann S, Eliseeva E, McCoy JG, et al. A new small-molecule antagonist inhibits Graves' disease antibody activation of the TSH receptor. J Clin Endocrinol Metab. 2011;96:548–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Núñez Miguel R, Sanders J, Sanders P, et al. Similarities and differences in interactions of thyroid stimulating and blocking autoantibodies with the TSH receptor. J Mol Endocrinol. 2012;49:137–151 [DOI] [PubMed] [Google Scholar]
- 33. Bossowski A, Urban M. Serum levels of cytokines in children and adolescents with Graves' disease and non-toxic nodular goiter. J Pediatr Endocrinol Metab. 2001;14:741–747 [DOI] [PubMed] [Google Scholar]