Abstract
Context:
Metformin is considered first-line treatment for type 2 diabetes mellitus. However, little is known about its effects in African American individuals.
Objective:
The objective of the study was to assess whether metformin's effect on glycemic control differs by race-ethnicity
Design:
Electronic health records were used to identify adults who had a diagnosis of diabetes, two or more fills of metformin, and two or more glycated hemoglobin (HbA1c) measurements. Pharmacy claims were used to estimate metformin exposure based on fill frequency and dose dispensed. Regression analyses modeled the relationship between metformin exposure and HbA1c levels. Analyses were stratified by race-ethnicity and baseline HbA1c values.
Setting:
The study was conducted at a large health system in southeast Michigan.
Main Outcome Measure:
Differences in HbA1c levels while on metformin were measured.
Results:
We identified 19 672 patients with diabetes taking metformin; 7429 were African American and 8783 were European American. Baseline HbA1c values in these two groups were 7.81% (61.8 mmol/mol) and 7.38% (57.1 mmol/mol), respectively. Compared with no use, metformin was associated with a 0.62% (6.8 mmol/mol) reduction in HbA1c; however, there was a significant difference by race-ethnicity (P < .001). Among African American individuals, metformin use was associated with a 0.90% (9.8 mmol/mol) reduction in HbA1c levels, whereas among European Americans, metformin was associated with a 0.42% (4.6 mmol/mol) reduction. Irrespective of baseline HbA1c, metformin use was associated with lower HbA1c levels in African American individuals.
Conclusions:
African American individuals appear to have a better glycemic response to metformin when compared with European Americans. Further studies are needed to determine whether this translates to commensurate reductions in diabetes complications.
Diabetes affects a staggering number of Americans with approximately 26 million individuals or 11% of the general population 20 years of age or older with the disease (1). These numbers are even more discouraging for adult African American individuals, who are nearly twice as likely to be diagnosed with diabetes when compared with European Americans (1, 2). Similarly, among persons with diabetes, African American individuals have higher average glycated hemoglobin (HbA1c) levels when compared with European American individuals (3).
Metformin is considered to be first-line treatment for type 2 diabetes mellitus (4). Among individuals with diabetes, it has salutary effects on glycemia and macrovascular disease complications (5, 6). In 2010, metformin was the ninth most frequently prescribed medication in the United States (and the most commonly prescribed diabetes medication) (7). However, the relative effectiveness of metformin in treating African American individuals with diabetes as compared with European American individuals is not well known, which is due in part to the paucity of studies with sufficient numbers of African American individuals to perform subgroup analyses (8–10). Complicating matters further is the frequently described difference in medication adherence between these groups (3, 11–13), which may confound assessments of treatment response.
In this study, we estimated differences in metformin treatment response between African American and European American individuals enrolled in a large health system serving southeast Michigan and the greater Detroit metropolitan area. The treatment response was assessed via HbA1c levels, and the analysis accounted for individual variation in medication use over time.
Materials and Methods
Study subjects and setting
This study was approved by the Institutional Review Board of the Henry Ford Health System and was compliant with its Health Insurance Portability and Accountability Act policy. We identified adult individuals (age ≥ 18 y) with a recorded diagnosis of diabetes in the electronic medical record and two or more fills of metformin between January 1, 1997, and June 2, 2013. Patients were also required to have at least two HbA1c measurements separated by 4 months while on metformin (ie, between the first and last metformin prescription fills on record) and have prescription drug coverage as members of both the health system and its affiliated health insurance provider during the time of observation. Because we wanted to assess the relationship between metformin exposure and the changes in HbA1c, we did not want to ascribe to metformin those changes in HbA1c that predated treatment (with the exception of our last sensitivity analysis). Therefore, unless otherwise specified, the first HbA1c measurement after the treatment initiation was considered the baseline value. As described in further detail below, changes in the HbA1c levels could then be related to the measures of metformin exposure in the intervening time period while adjusting for the total time on treatment.
Assessment of medication exposure
As we have done previously, we used pharmacy records to estimate medication exposure (14–17). These data capture nearly all prescription fills for covered, health plan members (18). For the purposes of this study, we limited these calculations to metformin and other blood glucose-lowering agents. To calculate a continuous measure of medication exposure for metformin, we used the amount of medication dispensed at a prescription fill (ie, the pill number and strength) to estimate the average daily amount taken until the next prescription fill. The average daily amount was divided by the maximum recommended daily dose (ie, 2550 mg) to produce a value that ranged from 0 to 1 and that represented the proportion of the maximal dose taken daily. This value was assigned to each day between a given pair of prescription fills.
We then assessed average daily metformin exposure for 120-day periods. This time period was selected so as to coincide with the average life span of red blood cells (ie, 120 d) (19); therefore, this time window was considered to be the drug exposure duration most likely to influence HbA1c levels. To derive this summary value, we summed the values assigned to each day, as described in the preceding paragraph, and divided by 120; this resulted in an estimate of the average daily amount of metformin taken over the preceding 120-period. Because of the 120-day exposure window, this process could be repeated for any day of follow-up (after the first 4 mo of treatment) while the patient was supposed to be taking metformin. The resulting summary values again represented the range of no use (0) to a daily use at the maximum recommended dose of 2550 mg (1) over any given 120-day exposure window. We used these time-updated, averaged values when assessing the relationship between metformin exposure and HbA1c levels. Alternatively, for the analysis assessing the relationship between metformin exposure and the change in HbA1c levels we measured metformin exposure as the average daily exposure (ie, the average daily exposure as a proportion of the maximal dose) for the period between the two HbA1c measurements. In all of these analyses, the estimated effect of metformin was the extrapolated effect of going from no exposure (0) to daily exposure at the maximum dose of 2550 mg (1) for the time period of interest.
We calculated exposure to other classes of oral hypoglycemic medications (ie, α-glucosidase inhibitors, dipeptidyl peptidase-4 inhibitors, meglitinides, pramlintide, sulfonylureas, and thiazolidinediones) in an analogous fashion to metformin. However, because there were often multiple medications within each class, we assessed exposure to each medication by first assigning each day between fills a value calculated as the ratio of days supplied to number of days between fills. In other words, each day's value represented the proportion of the prescribed dose taken. We calculated longitudinal drug exposure for each medication class by summing the daily values for all medications within a class and dividing by 120 (ie, the length of the exposure window). Therefore, we had 120-day medication exposure windows calculated for each day of follow-up for each oral hypoglycemic medication.
To account for amount of insulin used, we assessed the quantity (in units) of fast-acting (eg, aspart, glulisine, lyspro, and regular insulin), and long-acting insulin (eg, glargine, detemir, and neutral protamine Hagedorn insulin) dispensed at each prescription fill. These quantities were divided by the number of days between fills to obtain separate measures for the average daily amount of fast-acting and long-acting insulin used. To assess longitudinal use, we then averaged the sum of daily use over the preceding 120 days to obtain an average daily use over a 4-month period.
Additional study variables
In addition to information on medication usage, we also had demographic, anthropomorphic, and laboratory data on study individuals as a result of health plan membership. Many of these variables were collected and recorded in the electronic medical record as part of routine care. Race-ethnicity was available for most patients and was most often self-reported and collected at the time of initial patient registration into the health system. Patient weight and height were measured and charted at the time of clinic visits; these measures were used to calculate body mass index (BMI), defined as (weight in kilograms)/(height in meters).2 Glycated hemoglobin levels, HbA1c, were measured at a central lab within the health system and were available through the laboratory information system. However, HbA1c levels were ordered at the discretion of the treating clinician as part of diabetes screening and management. We considered baseline HbA1c to be the first HbA1c measurement while using metformin. Glycated hemoglobin levels are reported as percentages according to the National Glycohemoglobin Standardization Program (NGSP) units and in Système International units of millimoles of HbA1c per moles of hemoglobin (millimoles per mole) according to the International Federation of Clinical Chemistry (IFCC). The formula for this conversion is IFCC (millimoles per mole) = 10.931 × NGSP (percentage) − 23.524, and it is based on the work of Hoelzel et al (20). The conversions for the change or SD of the NGSP values (in the range of 0.01%–5%) to the IFCC units can be found at http://www.ngsp.org/convert2.asp.
Statistical analysis
The primary outcomes of this study were HbA1c levels and the change in HbA1c levels over time. All study patients by definition had at least two HbA1c levels separated by 120 days while being treated with metformin, the exposure variable of interest.
We compared the baseline patient characteristics of African American and European American study patients using a t test or χ2 statistics for continuous and categorical variables, respectively. A general estimating equation approach was used to estimate the relationship between metformin exposure and HbA1c levels using the 120-day metformin exposure window assessed at the time of the HbA1c measurement. This approach accounted for repeated outcome measures among study individuals. We assessed both the univariable and multivariable relationship between drug exposure and glycemic control (ie, HbA1c levels). Only HbA1c measurements after the initial measurement (ie, the baseline HbA1c) and after 4 months of metformin use were used as outcomes in these regression models. Multivariable regression models accounted for patient age, sex, race-ethnicity, duration of time on treatment, baseline HbA1c level, and contemporaneous use of other diabetes medications (ie, separate exposure measures for meglitinides, sulfonylureas, thiazolidinediones, fast acting insulin, and slow acting insulin). Because BMI was measured in considerably fewer individuals, we ran separate multivariable models including BMI.
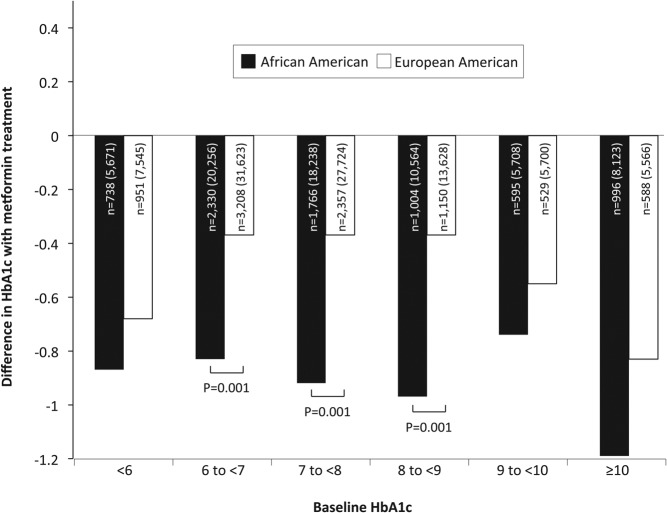
To assess for potential differences in metformin treatment response by race-ethnicity, we also performed separate multivariable analyses, which included an interaction term for these two variables. We also performed stratified analyses for African American and European American patients, and we further stratified these analyses by baseline HbA1c levels (Figure 1). To assess whether the differences in treatment response by baseline HbA1c levels shown in Figure 1 constituted a trend, we repeated the multivariable regression analysis with an interaction term between metformin exposure and an ordinal variable for baseline HbA1c level (ie, <6% = 0, 6% to <7% = 1, 7% to <8% = 2, 8% to <9% = 3, 9% to <10% = 4, and >10% = 5). We also assessed for differences in this trend for African American and European American individuals by including a three-way interaction term that included a variable for race-ethnicity.
Figure 1.
The estimated effect of metformin treatment on HbA1c levels stratified by race-ethnicity and baseline HbA1c levels. Larger negative values suggest a greater effect of metformin in reducing long-term glycemia. Differences in the estimated effect of metformin between African American and European American individuals that reach statistical significance are denoted with the corresponding P value. Also presented is the number of individuals contributing data to each category along with the total number of observations for those individuals shown in parentheses. Analyses accounted for repeated measures within individuals.
As an alternative method of assessing metformin response, we assessed the relationship between metformin exposure and the change in HbA1c levels (ie, in lieu of just adjusting for baseline HbA1c). For these analyses, we calculated the change in HbA1c levels for measurements separated by more than 120 days while on metformin treatment. Average daily metformin exposure for the time between HbA1c measurements was calculated in the previously described manner. We again used univariable and multivariable general estimating equation regression. The multivariable models accounted for age, sex, BMI, race-ethnicity, baseline HbA1c levels, the duration of time between HbA1c measurements, and use of other diabetes medications (ie, separate exposure measures for meglitinides, sulfonylureas, thiazolidinediones, fast acting insulin, and slow acting insulin). We assessed for differences in metformin treatment response by race-ethnicity with the use of an interaction term. Analyses were also stratified by race-ethnicity.
Lastly, we repeated the above assessment limiting our analysis to those HbA1c measurements that were separated by 4–5 months (ie, 120–155 d). This was done so that the time interval for both the assessment of medication exposure and HbA1c measurements was almost completely concordant and so that both exposure and outcome duration reflected the time period for which HbA1c represents blood glycemia (ie, ∼120 d). We adjusted these analyses for patient age, sex, the level of the first HbA1c measurement (ie, the first value in the paired measurements separated by 4–5 mo), total time on metformin treatment (ie, the duration of time between the first recorded prescription fill for metformin and the time of the second HbA1c measurement in the paired measurements), and contemporaneous use of other diabetes medications. As a final check, we restricted our analysis to de novo metformin monotherapy users with HbA1c measurements within 2 weeks of starting therapy and between 4 and 5 months after the treatment initiation. In this analysis, baseline HbA1c predated metformin initiation but was not greater than 2 weeks preceding treatment. African American and European American individuals were matched on baseline HbA1c [within 0.1% (1.1 mmol/mol)]. The regression analyses predicting a change in HbA1c between baseline and follow-up adjusted for patient age, sex, and baseline HbA1c levels.
Analyses were performed using statistical software, SAS version 9.2 (SAS Institute Inc) (21). The threshold for statistical significance was set at a type I error level of P = .05.
Results
The characteristics of the study participants are shown in Table 1. We identified 19 672 patients with diabetes taking metformin, among whom 7429 individuals self-identified as being African American and 8783 identified themselves as being European American. We compared the characteristics of these two groups and found that the African American patients being treated for diabetes were younger (mean age 55.1 vs 59.5 y, P = .001), were more likely to be female (58.6% vs 47.8%, P = .001), and had higher baseline HbA1c values [7.81% (61.8 mmol/mol) vs 7.38% (57.1 mmol/mol), P = .001] when compared with the European American patients. Over the course of the observation, African American individuals were also less likely to be treated with dipeptidyl peptidase-4 inhibitors (3.0% vs 4.1%, P = .001) and were more likely to receive long-acting insulin (26.7% vs 24.1%, P = .001). The average duration of time between metformin initiation and baseline HbA1c measurement was 171 days (±240 d, SD, median 107 d), and the average duration of follow-up was 5.18 years (±3.76 y, SD).
Table 1.
Characteristics of Study Individuals With Diabetes Receiving Metformin Treatment Stratified by Race-Ethnicity
| All Patients (n = 19 672) | African American Individuals (n = 7429) | European American Individuals (n = 8783) | P Valuea | |
|---|---|---|---|---|
| Age, y, mean ± SD | 57.3 ± 12.7 | 55.1 ± 12.9 | 59.5 ± 12.3 | .001 |
| Female, n, % | 10 192 (51.8) | 4355 (58.6) | 4202 (47.8) | .001 |
| Race-ethnicity, n, %b | ||||
| African American | 7429 (37.8) | 7429 (100.0) | ||
| European American | 8783 (44.6) | 8783 (100.0) | ||
| Other | 3460 (17.6) | |||
| Baseline HbA1c level, mean % ± SD, mmol/mol | 7.56% ± 1.71% (59.1 ± 18.7) | 7.81% ± 1.96% (61.8 ± 21.4) | 7.38% ± 1.50% (57.1 ± 16.4) | .001 |
| Use of other diabetes medications, n, %c | ||||
| α-Glucosidase inhibitors | 162 (0.8) | 42 (0.6) | 75 (0.9) | .031 |
| Dipeptidyl peptidase-4 inhibitors | 753 (3.8) | 221 (3.0) | 363 (4.1) | .001 |
| Insulin (fast acting) | 1962 (10.0) | 707 (9.5) | 915 (10.4) | .057 |
| Insulin (long acting) | 4903 (24.9) | 1986 (26.7) | 2114 (24.1) | .001 |
| Meglitinides | 94 (0.5) | 24 (0.3) | 43 (0.5) | .100 |
| Pramlintide | 76 (0.4) | 22 (0.3) | 41 (0.5) | .082 |
| Sulfonylureas | 10 238 (52.0) | 3859 (52.0) | 4637 (52.8) | .280 |
| Thiazolidinediones | 2417 (12.3) | 843 (11.4) | 1117 (12.7) | .008 |
mmol/mol indicates millimoles of HbA1c per mole of hemoglobin.
P value for the comparison of African American and European American individuals.
Individuals were categorized based on self-reported race-ethnicity. The Other category included individuals whose race-ethnicity was not reported, was other than African American or European American, or consisted of multiple race-ethnic groups.
Use of these other diabetes medication occurred over the course of observation, and some individuals used these diabetes medications simultaneously.
In assessing the univariable relationship between metformin exposure and HbA1c levels, metformin use at the maximum dose was associated with 0.61% (6.7 mmol/mol) lower HbA1c values when compared with no metformin use (Table 2, model 1). After accounting for other potential confounders including patient age, sex, race-ethnicity, BMI, baseline HbA1c levels, total time on metformin, and exposure to other diabetes medications, metformin use was still associated with HbA1c values that were 0.62% (6.8 mmol/mol) lower than that of no metformin exposure (Table 2, model 3). Moreover, in every model assessed, we observed a significant interaction (P < .001) between race-ethnicity and metformin use on HbA1c levels, suggesting that the effect of metformin differed by race-ethnicity.
Table 2.
Relationship Between Metformin Exposure and Glycemic Control Stratified by Race-Ethnicity
| Modela | Strata | Number | Estimated Effect of Metformin on HbA1c Levels, mmol/molb | P Value | Interaction P Valuec |
|---|---|---|---|---|---|
| 1 | All individuals | 19 672 | −0.61% ± 0.02% (−6.7 ± 0.2) | .001 | .001 |
| African American individuals | 7429 | −0.93% ± 0.05% (−10.2 ± 0.5) | .001 | ||
| European American individuals | 8783 | −0.42% ± 0.03% (−4.6 ± 0.3) | .001 | ||
| 2 | All individuals | 19 672 | −0.62% ± 0.02% (−6.8 ± 0.3) | .001 | .001 |
| African American individuals | 7429 | −0.92% ± 0.05% (−10.1 ± 0.3) | .001 | ||
| European American individuals | 8783 | −0.44% ± 0.03% (−4.8 ± 0.3) | .001 | ||
| 3 | All individuals | 13 360 | −0.62% ± 0.03% (−6.8 ± 0.3) | .001 | .001 |
| African American individuals | 5184 | −0.90% ± 0.05% (−9.8 ± 0.5) | .001 | ||
| European American individuals | 5493 | −0.42% ± 0.03% (−4.6 ± 0.3) | .001 |
mmol/mol denotes millimoles of HbA1c per mole of hemoglobin.
Model 1 includes only the variable for metformin exposure. Model 2 includes separate variables for each of the following: age, sex, time from medication initiation, baseline HbA1c levels, and exposure to other diabetes medications (ie, separate exposure measures for meglitinides, sulfonylureas, thiazolidinediones, fast acting insulin, and long acting insulin). Model 3 includes a variable for body mass index in addition to all of the variables included for Model 2.
Estimate of the relationship between metformin exposure and HbA1c levels. Metformin exposure is a continuous, time-updated measure of the amount of medication used over the preceding 120-day period. These estimates represent the measured effect on HbA1c levels in going from no use to daily use at the maximum recommended dose (ie, 2550 mg per day).
The interaction P value is derived from an amended model including both a variable for race-ethnicity and an interaction term between the metformin exposure and race-ethnicity. The value interaction term assesses for significant differences between the metformin effect estimate for African American and European American individuals.
This supposition was supported by the marked difference in metformin's estimated effect after stratifying by race-ethnicity. These adjusted, race-ethnicity stratified models (Table 2, model 3) showed that as compared with no use, metformin at the maximum dose was associated with HbA1c values that were 0.90% (9.8 mmol/mol) lower among African American individuals. In contrast, metformin use was associated with 0.42% (4.6 mmol/mol) lower HbA1c values among European American individuals. This apparent difference in treatment response was also seen when stratifying by both race-ethnicity and baseline HbA1c (Figure 1). At every level of baseline HbA1c examined, metformin appeared to have a larger effect on HbA1c levels among African American individuals when compared with European American individuals. These between-group differences were statistically significant for baseline HbA1c values ranging between 6% (42.1 mmol/mol) and 9% (74.9 mmol/mol). Tests for trend found both an overall increase in metformin treatment response with increasing baseline HbA1c levels (P < .001) and a significantly greater trend in treatment response among African American individuals when compared with European American individuals (P < .001).
We also measured the relationship between metformin use and intraindividual changes in HbA1c among tests obtained at least 120 days apart. Here we used a metformin exposure metric that estimated average daily use (as a proportion of the maximum dose) for the full duration between HbA1c measurements (in contrast to the 120 d exposure window used in the preceding analysis). The average duration of time between HbA1c measurements was 8.6 months (±4.2 mo, SD) for all patients, 9.1 months (±4.4 mo, SD) for African Americans, and 8.3 months (±4.1 mo, SD) for European Americans. The relationship between metformin use and the change in HbA1c was more modest when compared with the previous analysis. Among all individuals, metformin use at the maximum dose was associated with a 0.22% (2.4 mmol/mol) change in HbA1c as compared with no use (Table 3, model 1). After adjusting for multiple potential confounders, including age, sex, race-ethnicity, BMI, baseline HbA1c levels, duration of time between HbA1c measures, and exposure to other diabetes medications (Table 3, model 3), there was a similar association between metformin use and change in HbA1c for all individuals [ie, a drop in HbA1c of 0.19% (2.1 mmol/mol)]. We again observed significant interactions with race-ethnicity in all regression models (P < .001), suggesting a difference in treatment response between African American and European American individuals. After stratifying the sample by race-ethnicity, we observed that metformin use was associated with a 0.26% (2.8 mmol/mol) and a 0.17% (1.9 mmol/mol) change in HbA1c values among African American and European American individuals, respectively.
Table 3.
Relationship Between Metformin Exposure and Changes in HbA1c Levels Stratified by Race-Ethnicitya
| Modelb | Strata | Number | Estimated Effect of Metformin on HbA1c Levels, mmol/molc | P Value | Interaction P Valued |
|---|---|---|---|---|---|
| 1 | All individuals | 18 802 | −0.22% ± 0.01% (−2.4 ± 0.1) | .001 | .001 |
| African American individuals | 7072 | −0.29% ± 0.03 (−3.2 ± 0.3)% | .001 | ||
| European American individuals | 8430 | −0.19% ± 0.02% (−2.1 ± 0.2) | .001 | ||
| 2 | All individuals | 18 802 | −0.20% ± 0.01% (−2.2 ± 0.1) | .001 | .001 |
| African American individuals | 7072 | −0.29% ± 0.03% (−3.2 ± 0.3) | .001 | ||
| European American individuals | 8430 | −0.18% ± 0.01% (−2.0 ± 0.1) | .001 | ||
| 3 | All individuals | 12 840 | −0.19% ± 0.01% (−2.1 ± 0.1) | .001 | .001 |
| African American individuals | 4974 | −0.26% ± 0.03% (−2.8 ± 0.3) | .001 | ||
| European American individuals | 5297 | −0.17% ± 0.02% (−1.9 ± 0.2) | .001 |
mmol/mol denotes millimoles of HbA1c per mole of hemoglobin.
The outcome variable, change in HbA1c levels, measures the change in laboratory measurements separated by 120 days or more while being treated with metformin.
Model 1 includes only the variable for metformin exposure. Model 2 includes separate variables for each of the following: age, sex, baseline HbA1c levels, the duration of time between HbA1c measurements, and exposure to other diabetes medications (ie, separate exposure measures for meglitinides, sulfonylureas, thiazolidinediones, fast acting insulin, and long acting insulin). Model 3 includes a variable for body mass index in addition to all of the variables included for model 2.
Metformin exposure was measured as the average daily use between HbA1c measurements. The metformin exposure variable was adjusted to reflect the average daily amount as a proportion of the maximum daily amount (ie, 2550 mg). Therefore, each estimate represents the absolute change in HbA1c that would result in going from no metformin use to continuous use at the maximum daily amount.
The interaction P value is derived from an amended model including both a variable for race-ethnicity and an interaction term between the metformin exposure and race-ethnicity. The interaction term assesses for significant differences in metformin's effect on HbA1c levels between African American and European American individuals.
We limited our assessment to only those HbA1c measurements that were separated within a range of 4–5 months (ie, 120–155 d). This was done so that the 120-day metformin exposure measure nearly completely overlapped the HbA1c measurements, resulting in changes in glycated hemoglobin levels that were most representative of the concurrent metformin exposure. For this analysis we had a total of 11 155 observations: 5272 observations for European American individuals and 3917 observations for African American individuals. In both the unadjusted and adjusted models, metformin use was associated with a larger response in African American individuals as compared with European American individuals, a 0.78% (8.5 mmol/mol) and 0.37% (4.0 mmol/mol) drop in the adjusted models, respectively (P = .001 for the difference).
As a final sensitivity analysis, we restricted our analysis to individuals with the following characteristics: de novo treatment for diabetes with metformin monotherapy (ie, no prior or concordant treatment with another diabetes medication, including insulin), a baseline HbA1c measured within the 2 weeks preceding treatment initiation, and a follow-up HbA1c measured between 4 and 5 months after the metformin initiation. This resulted in a sample size of 354 African American individuals and 502 European American individuals. Baseline HbA1c levels were significantly different between these groups [9.60% (81 mmol/mol) and 8.64% (71 mmol/mol), respectively (P = .001)]. We then matched individuals from each group by baseline HbA1c [within ±0.1% (1.1 mmol/mol)], resulting in a sample size of 568 individuals: 284 African Americans and 284 European Americans. Mean baseline HbA1c after matching was 9.1% in both groups. The overall change in HbA1c associated with metformin monotherapy initiation was −1.23% (13.4 mmol/mol) ± 0.30% (3.3 mmol/mol) SD. We again observed a larger drop in HbA1c levels among African American individuals [−1.66% (18.1 mmol/mol) ± 0.46% (5.0 mmol/mol) SD] when compared with European Americans [−0.56% (6.1 mmol/mol) ± 0.38% (4.2 mmol/mol) SD], although the between-group difference was no longer statistically significant in this much smaller sample (P = .334).
Discussion
This study suggests that the glycemic response to metformin therapy is greater among African American individuals when compared with European American individuals. Given the disproportionate burden of diabetes complications faced by African American individuals, it is heartening to know that metformin appears to be efficacious in this group.
Our estimates of metformin's effect on HbA1c levels were based on observational data collected as part of routine care. As a result, we cannot state with absolute certainty that the observed relationships are not confounded. Nevertheless, there are a number of factors that support the veracity of our findings. First, we have previously demonstrated that among health plan members, our data systems capture nearly all medication prescription fills, including information on medication strength and quantity dispensed (18). Although we could not confirm actual medication consumption, our group and others have previously shown these estimates of medication use to be consistently correlated with clinical outcomes in the expected direction and magnitude (14, 15, 22–26). Therefore, our estimates of medication exposure likely represent a good approximation of actual use. Second, we estimated metformin exposure for the time period most relevant to each HbA1c measurement (ie, the preceding 120 d period). In this way, metformin use was paired with corresponding glycated hemoglobin measurements, ensuring the proper temporal relationship between exposure and outcome. Third, our findings of a difference in metformin response by race-ethnicity were robust to varying methods in which we measured drug exposure and outcomes. Differences in methodology undoubtedly accounted for variation in the estimated absolute effect of metformin. Nevertheless, our key finding of a relatively greater metformin response among African American individuals when compared with European American individuals was consistent in all of our analyses.
In general, the magnitude of metformin's effect on HbA1c changes reported here is consistent with the findings of double-blind, placebo-controlled trials reported to be of high quality (27–29). Nevertheless, the absolute difference in HbA1c of 0.62 (6.8 mmol/mol) that we observed for all patients is less than that reported by Goldstein et al (30), who observed a 1.3% (14.2 mmol/mol) decrease in HbA1c after treatment with 2000 mg of metformin daily. Individuals in the latter trial were treated for a longer time period (ie, 26 wk as compared with our 4 mo exposure window), had higher baseline HbA1c levels [ie, 8.8% (73 mmol/mol)], and underwent a 6- to 10-week drug washout period prior to treatment. Nevertheless, the findings of Goldstein et al are similar to our restricted analysis of metformin monotherapy initiation. However, even in this much smaller analytic sample, we observed a larger (albeit nonsignificant) reduction in HbA1c levels among African American individuals when compared with European Americans.
As demonstrated by highly publicized, postmarket studies of rosiglitazone (31, 32), medications that lower blood glucose levels may not necessarily have beneficial vascular effects. Although a number of studies suggest that metformin has a long-term protective effect on macrovascular disease (6, 10, 33, 34), to our knowledge, there have not been any studies of metformin with sufficient power to assess these hard clinical end points in African American individuals (35). Gosmanova et al (36) assessed the association of metformin-containing diabetes regimens on all-cause mortality among patients who received care at one Veterans Affairs medical center. In this study African American patients treated with metformin had a lower risk of all-cause death when compared with European American patients (hazard ratio 0.89), but this difference was not statistically significant (P < .29).
In this study we use HbA1c as a proxy measure for metformin's effect on metabolic and glycemic control. Although HbA1c may be an imperfect proxy measure, Maruthur et al (37) showed that genetic ancestry explains only a small proportion of the variation in HbA1c levels among African Americans, and Selvin et al (38) showed that HbA1c is similarly predictive of cardiovascular events among African American and European American individuals. Therefore, the HbA1c reductions observed here may reflect true differences in metformin's effect (rather than genetic differences in the expression of the proxy measure), and this, in turn, may translate to greater improvements in hard clinical outcomes among African American individuals.
Studies looking at the prophylactic effect of metformin on diabetes development have been similarly underpowered to detect differences by race-ethnicity. For example, in the Diabetes Prevention Program, African American individuals treated with metformin were less likely to develop diabetes when compared with European American individuals (the reduction in disease incidence was 44% vs 24%, respectively); however, these differences were not statistically significant (39).
In summary, this study suggests that the glycemic response to metformin may be greater among African American individuals when compared with European American individuals. These differences persisted after accounting for patients' age, sex, BMI, use of other diabetes medications, baseline HbA1c levels, time on treatment, and amount of metformin used (ie, our metformin exposure metric accounted for both dose strength and patient adherence). Additional studies are needed both to confirm these findings and to examine whether differences in glycemic response mirror changes in the risk of hard clinical end points, such as vascular disease and death. Moreover, pharmacogenomics studies assessing the effect of genetic ancestry (40), rather than self-reported race-ethnicity, may help clarify whether there is a heritable component to population group differences in metformin response.
Acknowledgments
The funding organizations did not influence the study design, collection or analysis of results, interpretation of findings, or the decision to publish.
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (Grant R01DK064695), the National Institute of Allergy and Infectious Diseases (Grant R01AI079139), the National Heart, Lung, and Blood Institute (Grants R01HL118267, R01HL079055, R01HL103871, and K23HL085124) of the National Institutes of Health, and the Fund for Henry Ford Hospital.
Disclosure Summary: The authors have nothing to disclose.
For editorial see page 3125
- BMI
- body mass index
- HbA1c
- glycated hemoglobin
- IFCC
- International Federation of Clinical Chemistry
- NGSP
- National Glycohemoglobin Standardization Program.
References
- 1. Centers for Disease Control and Prevention. National Diabetes Fact Sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: Centers for Disease Control and Prevention; 2011 [Google Scholar]
- 2. Beckles GL, Zhu J, Moonesinghe R. Diabetes—United States, 2004 and 2008. MMWR Surveill Summ. 2011;60(suppl):90–93 [PubMed] [Google Scholar]
- 3. Heisler M, Faul JD, Hayward RA, Langa KM, Blaum C, Weir D. Mechanisms for racial and ethnic disparities in glycemic control in middle-aged and older Americans in the health and retirement study. Arch Intern Med. 2007;167:1853–1860 [DOI] [PubMed] [Google Scholar]
- 4. Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853 [PubMed] [Google Scholar]
- 6. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 7. IMS Institute for Healthcare Informatics. The Use of Medicines in the United States: Review of 2010. Parsippany, NJ: IMS Institute for Healthcare Informatics; 2011 [Google Scholar]
- 8. Karter AJ, Moffet HH, Liu J, et al. Glycemic response to newly initiated diabetes therapies. Am J Manag Care. 2007;13:598–606 [PMC free article] [PubMed] [Google Scholar]
- 9. Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 10. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–865 [PubMed] [Google Scholar]
- 11. Schectman JM, Nadkarni MM, Voss JD. The association between diabetes metabolic control and drug adherence in an indigent population. Diabetes Care. 2002;25:1015–1021 [DOI] [PubMed] [Google Scholar]
- 12. Shenolikar RA, Balkrishnan R, Camacho FT, Whitmire JT, Anderson RT. Race and medication adherence in Medicaid enrollees with type 2 diabetes. J Natl Med Assoc. 2006;98:1071–1077 [PMC free article] [PubMed] [Google Scholar]
- 13. Williams LK, Joseph CL, Peterson EL, et al. Race-ethnicity, crime, and other factors associated with adherence to inhaled corticosteroids. J Allergy Clin Immunol. 2007;119:168–175 [DOI] [PubMed] [Google Scholar]
- 14. Habib ZA, Tzogias L, Havstad SL, et al. Relationship between thiazolidinedione use and cardiovascular outcomes and all-cause mortality among patients with diabetes: a time-updated propensity analysis. Pharmacoepidemiol Drug Saf. 2009;18:437–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hayek S, Canepa EF, Sattar A, et al. Effect of ezetimibe on major atherosclerotic disease events and all-cause mortality. Am J Cardiol. 2013;111:532–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wells KE, Peterson EL, Ahmedani BK, Severson RK, Gleason-Comstock J, Williams LK. The relationship between combination inhaled corticosteroid and long-acting β-agonist use and severe asthma exacerbations in a diverse population. J Allergy Clin Immunol. 2012;129:1274–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Habib ZA, Havstad SL, Wells K, Divine G, Pladevall M, Williams LK. Thiazolidinedione use and the longitudinal risk of fractures in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010;95:592–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williams LK, Joseph CL, Peterson EL, et al. Patients with asthma who do not fill their inhaled corticosteroids: a study of primary nonadherence. J Allergy Clin Immunol. 2007;120:1153–1159 [DOI] [PubMed] [Google Scholar]
- 19. Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the Diabetes Control and Complications Trial. Diabetes Care. 2002;25:275–278 [DOI] [PubMed] [Google Scholar]
- 20. Hoelzel W, Weykamp C, Jeppsson JO, et al. IFCC Working Group on HbA1c Standardization. IFCC reference system for measurement of hemoglobin A1c in human blood and the national standardization schemes in the United States, Japan, and Sweden: a method-comparison study. Clin Chem. 2004;50:166–174 [DOI] [PubMed] [Google Scholar]
- 21. SAS Institute Inc. SAS/STAT version 9.2 users guide. Cary, NC: SAS Institute Inc; 2008 [Google Scholar]
- 22. Pladevall M, Williams LK, Potts LA, Divine G, Xi H, Lafata JE. Clinical outcomes and adherence to medications measured by claims data in patients with diabetes. Diabetes Care. 2004;27:2800–2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Williams LK, Pladevall M, Xi H, et al. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. J Allergy Clin Immunol. 2004;114:1288–1293 [DOI] [PubMed] [Google Scholar]
- 24. Williams LK, Peterson EL, Wells K, et al. Quantifying the proportion of severe asthma exacerbations attributable to inhaled corticosteroid nonadherence. J Allergy Clin Immunol. 2011;128:1185–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ho PM, Magid DJ, Masoudi FA, McClure DL, Rumsfeld JS. Adherence to cardioprotective medications and mortality among patients with diabetes and ischemic heart disease. BMC Cardiovasc Disord. 2006;6:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ho PM, Magid DJ, Shetterly SM, et al. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J. 2008;155:772–779 [DOI] [PubMed] [Google Scholar]
- 27. Saenz A, Fernandez-Esteban I, Mataix A, Ausejo M, Roque M, Moher D. Metformin monotherapy for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005:CD002966. [DOI] [PubMed] [Google Scholar]
- 28. Horton ES, Clinkingbeard C, Gatlin M, Foley J, Mallows S, Shen S. Nateglinide alone and in combination with metformin improves glycemic control by reducing mealtime glucose levels in type 2 diabetes. Diabetes Care. 2000;23:1660–1665 [DOI] [PubMed] [Google Scholar]
- 29. Mather KJ, Verma S, Anderson TJ. Improved endothelial function with metformin in type 2 diabetes mellitus. J Am Coll Cardiol. 2001;37:1344–1350 [DOI] [PubMed] [Google Scholar]
- 30. Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE, Sitagliptin 036 Study Group. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care. 2007;30:1979–1987 [DOI] [PubMed] [Google Scholar]
- 31. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471 [DOI] [PubMed] [Google Scholar]
- 32. Graham DJ, Ouellet-Hellstrom R, MaCurdy TE, et al. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA. 2010;304:411–418 [DOI] [PubMed] [Google Scholar]
- 33. Kooy A, de Jager J, Lehert P, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med. 2009;169:616–625 [DOI] [PubMed] [Google Scholar]
- 34. Selvin E, Bolen S, Marinopoulos SS, et al. Cardiovascular outcomes in trials of oral diabetes medications: a systematic review. Arch Intern Med. 2008;168:2070–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boussageon R, Supper I, Bejan-Angoulvant T, et al. Reappraisal of metformin efficacy in the treatment of type 2 diabetes: a meta-analysis of randomised controlled trials. PLoS Med. 2012;9:e1001204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gosmanova EO, Canada RB, Mangold TA, Rawls WN, Wall BM. Effect of metformin-containing antidiabetic regimens on all-cause mortality in veterans with type 2 diabetes mellitus. Am J Med Sci. 2008;336:241–247 [DOI] [PubMed] [Google Scholar]
- 37. Maruthur NM, Kao WH, Clark JM, et al. Does genetic ancestry explain higher values of glycated hemoglobin in African Americans? Diabetes. 2011;60:2434–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Selvin E, Steffes MW, Matsushita K, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362:800–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang JJ, Cheng C, Devidas M, et al. Ancestry and pharmacogenomics of relapse in acute lymphoblastic leukemia. Nat Genet. 2011;43:237–241 [DOI] [PMC free article] [PubMed] [Google Scholar]