Abstract
Background:
Ghrelin is a 28-amino acid peptide released from the stomach. Ghrelin is found in the circulation in two forms: acyl- and desacyl-ghrelin. Acyl- and desacyl-ghrelin concentrations increase at night, when cortisol concentrations are low. Acute ghrelin administration increases ACTH and cortisol concentrations and a feedback loop between the ghrelin and ACTH-cortisol axis has been postulated. A previous study showed that exogenously induced hypercortisolism for 5 days decreased plasma ghrelin concentrations.
Objective:
The objective of the study was to determine whether a 4-hour infusion of hydrocortisone given at a time of low endogenous cortisol concentrations (11:00 pm to 3:00 am) acutely suppresses acyl- and desacyl-ghrelin.
Methods:
Eight healthy young men aged (mean ± SD) 21.5 ± 2.7 years with a body mass index of 22.4 ± 2.5 kg/m2 were studied in a single-blind, placebo-controlled study during two separate overnight admissions on the Clinical Research Unit. The volunteers received either a 4-hour (11:00 pm to 3:00 am) infusion of hydrocortisone or a saline infusion. The hydrocortisone infusion rate was 0.3 mg/kg·h for the initial 3 minutes, 0.24 mg/kg·h for 9 minutes, and then 0.135 mg/kg·h until the end of the infusion. Plasma acyl- and desacyl-ghrelin concentrations (in-house two site sandwich assay) and ACTH, cortisol, insulin, GH, and glucose levels were measured every 10 minutes for 16 hours (5:00 pm to 9:00 am).
Results:
The mean differences (lower 95% limit; upper 95% limit) between the saline infusion and hydrocortisone infusion for acyl- and desacyl-ghrelin concentrations were not significantly different from zero. The infusion period (11:00 pm to 3:00 am) was as follows: acyl-ghrelin, 0.22 (−7.39; 7.83) (P = 1.00); desacyl-ghrelin, −3.36 (−17.66; 10.95) (P = 1.00). The postinfusion period (3:00–7:00 am) was as follows: acyl-ghrelin, 8.68 (1.07; 16.28); (P = .056); desacyl-ghrelin, 8.75 (−5.56; 23.05) (P = .403).
Conclusions:
A short-term increase in circulating cortisol concentrations by exogenous hydrocortisone infusion does not suppress circulating nocturnal acyl- or desacyl-ghrelin concentrations. Thus, it is likely that the diurnal pattern of ghrelin secretion is under circadian control and not directly regulated by cortisol.
Acyl-ghrelin is a 28-amino acid peptide released from the stomach (1, 2). The acylation of ghrelin is necessary for its orexigenic and GH-releasing effects (3). The mechanisms that regulate the secretion and acylation of ghrelin are yet to be elucidated. Both acyl- and desacyl-ghrelin increase at night when cortisol concentrations are low (4, 5). Studies in rodents and humans demonstrate an ACTH- and cortisol-releasing effect after acute administration of ghrelin and a feedback loop was postulated (6, 7). Otto et al (8) demonstrated that 5 days of exogenously induced glucocorticoid excess decreases total ghrelin concentrations. This study addresses the question of whether low endogenous cortisol concentrations are responsible for the change in nocturnal acyl- and desacyl-ghrelin concentrations. We tested whether exogenous cortisol can suppress circulating midnight acyl- and desacyl-ghrelin concentrations in healthy adults.
Subjects and Methods
The study was approved by the Institutional Review Board and General Clinical Research Center of the University of Virginia. Before enrollment, volunteers gave written informed consent. Screening included a medical history questionnaire, physical examination, and fasting blood profile. Exclusion criteria included smoking, acute illness, or medications known to affect ghrelin. Strenuous daily exercise was restricted to less than 1 hour per day.
Eight young men aged 21.5 ± 2.7 years (range 18–25 y) and body mass index of 22.4 ± 2.5 kg/m2 (range 19–27 kg/m2) were studied. Each subject was admitted to the Clinical Research Unit for two separate overnight stays at least 1 month apart. In a single-blind, randomized fashion, volunteers received either a 4-hour (11:00 pm to 3:00 am) hydrocortisone or saline infusion.
Frequent blood sampling
Volunteers were admitted at 3:00 am, and a standard meal of 20% protein, 30% fat, and 50% carbohydrates, with caloric content based on the Harris-Benedict equation, was served at 6:00 pm on each admission. Intravenous catheters were inserted into the forearm or hand vein of each arm at 4:00 pm. Blood was drawn every 10 minutes from 5:00 pm until 9:00 am the next morning. Saline was infused to keep lines patent from 7:00 pm to 9:00 am. Lights were turned off at 9:00 pm and subjects were encouraged to sleep. The overnight blood draws were performed while volunteers were sleeping. Every effort was made not to disturb their sleep, and the activity level was documented throughout the study.
Infusions
To rapidly achieve circulating cortisol concentrations that approximate endogenous early-morning peak cortisol concentrations of 30 μg/dL, the following hydrocortisone infusion paradigm [Debold et al (9)] was used: 0.3 mg/kg·h for the initial 3 minutes, 0.24 mg/kg·h for 9 minutes, and then 0.135 mg/kg·h until the end of the infusion (11:12 pm to 3:00 am). Infusions were delivered by a Medfusion 2010i pump. During the saline admission, saline was infused from 11:00 pm to 3:00 am.
Sample collection for ghrelin assays
Blood (1.3 mL) was added to chilled 3-mL EDTA Vacutainer tubes preloaded with 4-[2-aminoethyl benzene] sulfonylfluoride (Alexis Biochemicals) (4 mM final concentration) and stored on ice. Blood was centrifuged for 10 minutes at 2000 × g at 4°C within 1 hour of collection. After separation, 0.5 mL plasma was acidified with 100 μL of 1 N HCl. Samples were stored at −20°C until assay.
Ghrelin sandwich assays
Plasma acyl-ghrelin was measured with an in-house, two-site sandwich ELISA as described previously (4).
Cortisol, ACTH, GH, insulin, and glucose assays
Serum cortisol, ACTH, insulin, and GH concentrations were measured in singlicate by enzyme-amplified chemiluminescence assay on an Immulite 2000 analyzer (Siemens). The intraassay coefficient of variation (CV) for cortisol was 5% and the interassay CV was 6.8%. The intraassay CV for insulin was 2.2% and the interassay CV was 4.8%. The intraassay CV for ACTH was 2.6% and the interassay CV was 6.4%. Blood glucose was measured on a YSI instrument.
Statistical analysis
Per infusion, the subject-specific, 10-minute incremental measurements of total (acyl-ghrelin + desacyl-ghrelin), acyl- and desacyl ghrelin, cortisol and ACTH, GH, insulin, and glucose were summarized in 4-hour increments (ie, 7:00–11:00 pm, 11:00 pm to 3:00 am, and 3:00–7:00 am) by mean response during the 4-hour time interval. The 7:00–11:00 pm mean response measurements were then subtracted from the 11:00 pm to 3:00 am and 3:00–7:00 am mean response measurements to produce two sets of delta-values. One set represented the change in the 4-hour mean response from the 7:00–11:00 pm time interval to the 11:00 pm to 3:00 am time interval, whereas the other set represented the change in the 4-hour mean response from the 7:00–11:00 pm time interval to the 3:00–7:00 am time interval. The study was initially powered for 16 nonobese volunteers (eight men, eight women) and 16 obese volunteers (eight men, eight women) with a two-sided type 1 error rate of the test no greater than 0.05 and the power of the test being no less than 0.8. However, in view of these results, the study was stopped at the current number of men when our analysis showed that there is no difference between the saline and hydrocortisone admission.
With regard to the data analyses, the two sets of change scores of each response variable were analyzed as repeated measures via a linear mixed model. Infusion type (eg, saline) and the time interval (eg, 11:00 pm to 3:00 am) were the two sources of response variation that were examined by each linear mixed model, and each linear mixed model was specified so that interinfusion-related hypotheses could be tested.
With regard to hypothesis testing, the interinfusion-related hypotheses tested whether the impact on the mean change in the 4-hour mean response from the mean response during the 7:00–11:00 pm time interval was the same irrespective of infusion type. A Bonferroni correction type I error rate of 0.05 was used as the null hypothesis rejection rule. Because two interfusion comparisons were conducted per outcome, one comparison for the 7:00–11:00 pm time interval and one for the 11:00 pm to 3:00 am time interval, a two-sided Bonferroni correction type I error rate of 0.05 was used as the null hypothesis rejection rule.
We also analyzed the difference between the mean glucose and insulin during both time periods (11:00 pm to 3:00 am and 3:00–7:00 am), without subtracting the preinfusion period, which included the postmeal insulin and glucose peak.
The MIXED procedure of the statistical software package SAS version 9.2 (SAS Institute Inc) was used to conduct the analyses.
Unless otherwise stated, data are expressed as mean ± SEM; P < .05 was considered statistically significant.
Results
The mean differences (lower 95% limit; upper 95% limit) between the saline infusion and hydrocortisone (HC) infusion for all variables during the study infusion period (11:00 pm to 3:00 am) and postinfusion period (3:00–7:00 am) are shown in Table 1.
Table 1.
Mean Differences Between Saline Infusion and hydrocortisone (HC) Infusion for the Infusion Period 2300–0300 h and the Post-infusion Period 0300–0700 h
| Variable | Saline vs HC Infusion (11:00 pm to 3:00 am) |
Saline vs HC Infusion (3:00–7:00 am) |
||||
|---|---|---|---|---|---|---|
| Mean Difference | Lower 95% CI; Upper 95% CI | HC vs Saline | Mean Difference | Lower 95% CI; Upper 95% CI | HC vs Saline | |
| Cortisol, μg/dL | −28.02 | −30.28; −25.76 | P < .001 | −12.13 | −14.87; −9.38 | P < .001 |
| ACTH, pg/mL | 1.62 | −0.86; 4.09 | P = .366 | 9.85 | 5.21; 14.49 | P = .001 |
| Acyl-ghrelin, pg/mL | 0.22 | −7.39; 7.83 | P = 1.00 | 8.68 | 1.07; 16.28 | P = .056 |
| Desacyl-ghrelin, pg/mL | −3.36 | −17.66; 10.95 | P = 1.00 | 8.75 | −5.56; 23.05 | P = .403 |
| Total ghrelin, pg/mL | −3.13 | −23.90; 17.63 | P = 1.00 | 17.42 | −3.35; 38.19 | P = .184 |
| Insulin, μIU/mLa | −0.2 | −4.55; 4.15 | P = 1.00 | −1.33 | −5.22; 2.55 | P = .95 |
| Glucose, mg/dLa | −6.90 | −12.17; −1.63 | P = .028 | −16.70 | −22.05; 11.36 | P = < .001 |
| GH, μg/L | −1.34 | −7.48; 4.80 | P = 1.00 | −2.86 | −6.46; 0.74 | P = .213 |
Without subtraction of the preinfusion period from the infusion and postinfusion periods because of the postdinner insulin and glucose peaks.
Cortisol and ACTH levels
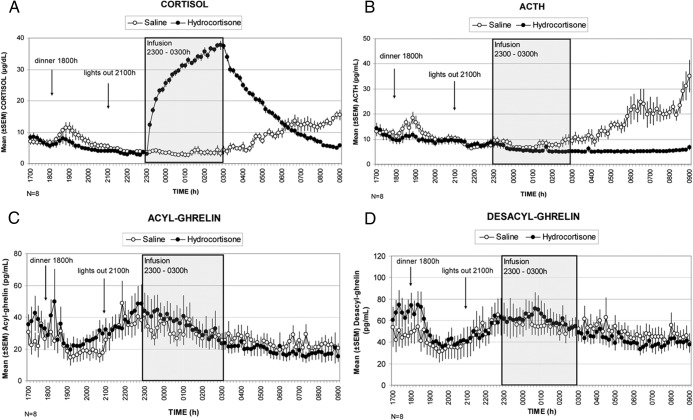
Cortisol concentrations were significantly higher during and after the hydrocortisone infusion compared with the saline infusion (11:00 pm to 7:00 am, P < .001). ACTH concentrations were suppressed after 2 hours of hydrocortisone infusion and were significantly suppressed postinfusion (3:00–7:00 am, P = .001) until the end of the study (Figure 1, A and B, and Table 1).
Figure 1.
The mean (±SEM) 16-hour concentration profiles (5:00 pm to 9:00 am) of serum cortisol (A) and plasma ACTH (B) and acyl-ghrelin (C) and desacyl-ghrelin (D) during the saline (open circles) and hydrocortisone (closed circles) admissions.
Acyl- and desacyl-ghrelin and total ghrelin concentrations
Acyl-, desacyl-, and total ghrelin responses during and after the hydrocortisone infusion were not significantly different vs saline (Figure 1, C and D, and Table 1). There was a trend to lower postinfusion acyl-ghrelin concentrations (3:00–7:00 am, P = .056).
Insulin and glucose concentrations
There was no significant difference in insulin concentrations with hydrocortisone vs saline infusion (Supplemental Figure 1 and Table 1). Glucose concentrations were significantly higher during the hydrocortisone infusion (P = .016), when analyzed without subtracting the preinfusion period (Supplemental Figure 1 and Table 1).
Growth hormone
GH responses during and after the hydrocortisone infusion were not significantly different vs saline (Table 1).
Discussion
A short-term increase of circulating cortisol concentrations caused by an exogenous hydrocortisone infusion does not suppress circulating nocturnal total, acyl-, or desacyl-ghrelin concentrations, albeit there was a trend toward lower delta-acyl-ghrelin concentrations in the postinfusion period. The cortisol concentrations reached during the hydrocortisone infusion were in the supraphysiological range for morning cortisol and the biological effectiveness of the infusion is demonstrated by the suppression of ACTH.
Systemic administration of ghrelin has been shown to stimulate ACTH and cortisol (6, 7), although not all studies have demonstrated a significant increase (10). An increase in endogenous cortisol in patients with Cushing's syndrome and 5 days of the administration of prednisolone resulted in a decrease in ghrelin (8), suggesting a negative feedback loop between the hypothalamic-pituitary-adrenal axis and ghrelin. Our results suggest that if present, this feedback is not acute. This is further supported by the lack of a decrease in circulating acyl-ghrelin as a result of the physiological rise in morning cortisol during the saline admission. However, there was a nonsignificant trend toward lower acyl-ghrelin concentrations after the infusion period. Glucose as well as insulin has been shown to decrease circulating ghrelin (11, 12); therefore, this could be an indirect effect of hydrocortisone.
Separate analysis of the changes in total ghrelin did not show an effect of hydrocortisone infusion on ghrelin acylation. Similarly, GH concentrations were not different between the hydrocortisone and saline infusions. The discrepancy between our results and the study by Otto et al (8) is possibly explained by the fact that we studied a short- rather than a long-term effect of glucocorticoid increase. It is also possible that ghrelin cannot be suppressed when it is already rising between 9:00 and 11:00 pm. This might occur if cortisol influences only the synthesis and not the secretion of ghrelin, occurring before the hydrocortisone infusion. However, the detected suppression of ACTH shows a fast-acting biological response to hydrocortisone, but ACTH and ghrelin could be differentially regulated. Sleep deprivation has been shown to be associated with an increase in ghrelin concentrations (13), and although we have not performed sleep electroencephalograms, the fact that cortisol levels and ACTH levels were suppressed during the saline admission suggest that the volunteers were resting appropriately.
We cannot exclude that an increase in cortisol could decrease the biological activity of ghrelin. The decreased GH response to ghrelin during glucocorticoid excess shown by Giordano et al (14) supports this notion. Interestingly, data by Kageyama et al (15) in rat hypothalamic cells suggest that dexamethasone increases expression of ghrelin mRNA and ghrelin receptor expression, which favors a stimulatory effect of glucocorticoids. An intracellular interaction between cortisol and ghrelin in the hypothalamus also seems possible based on the findings of Muller et al (16). A link between the glucocorticoid and ghrelin systems at the gastric ghrelin cell has not been demonstrated. Data by LeSauter et al (17) show that oxyntic ghrelin cells in the rodent stomach coexpress ghrelin and the circadian clock proteins period (PER)-1 and PER2 and that ghrelin and PER expression are synchronized to prior feeding and not photic schedules. In addition, several targets of circadian clock proteins are present in the promoter region of the ghrelin gene (18). These findings support the concept of a circadian control of ghrelin secretion.
Our results support that the midnight rise in acyl- and desacyl-ghrelin is independent of the midnight decline in cortisol; thus, the nocturnal increase in ghrelin is independent of cortisol and likely under circadian control.
Acknowledgments
We thank the University of Virginia Clinical Research Unit nursing staff for their excellent support when conducting the study as well as the former General Clinical Research Center Core Laboratory staff and our volunteers who made this work possible. We also thank Dr David Orth for advising us about the dosing of hydrocortisone for this study.
This work was supported by National Institutes of Health Grants K23RR018770 (to R.N.) and 1R01DK076037 (to M.O.T.), MO1 RR00847 (to the General Clinical Research Center at the University of Virginia), and R01DK082805 (to L.S.F.).
Disclosure Statement: M.O.T.is the founder of Ammonett Pharma; has received grant support from Novo Nordisk; is an advisory group member of Pfizer, Inc, Ipsen, and Chaisma. The other authors have nothing to disclose.
Footnotes
- CV
- coefficient of variation
- HC
- hydrocortisone
- PER
- period.
References
- 1. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660 [DOI] [PubMed] [Google Scholar]
- 2. Tschoep M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913 [DOI] [PubMed] [Google Scholar]
- 3. Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci USA. 2004;101:4679–4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu J, Prudom CE, Nass R, et al. Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J Clin Endocrinol Metab. 2008;93:1980–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nass R, Farhy LS, Liu J, et al. Evidence for acyl-ghrelin modulation of growth hormone release in the fed state. J Clin Endocrinol Metab. 2008;93:1988–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Broglio F, Benso A, Castiglioni C, et al. The endocrine response to ghrelin as a function of gender in humans in young and elderly subjects. J Clin Endocrinol Metab. 2003;88:1537–1542 [DOI] [PubMed] [Google Scholar]
- 7. Wren AM, Small CJ, Ward HL, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328 [DOI] [PubMed] [Google Scholar]
- 8. Otto B, Tschop M, Heldwein W, Pfeiffer AF, Diederich S. Endogenous and exogenous glucocorticoids decrease plasma ghrelin in humans. Eur J Endocrinol. 2004;151:113–117 [DOI] [PubMed] [Google Scholar]
- 9. Debold CR, Jackson RV, Kamilaris TC, et al. Effects of ovine corticotropin-releasing hormone on adrenocorticotropin secretion in the absence of glucocorticoid feedback inhibition in man. J Clin Endocrinol Metab. 1989;68:431–437 [DOI] [PubMed] [Google Scholar]
- 10. Vestergaard ET, Gormsen LC, Jessen N, et al. Ghrelin infusion in humans induces acute insulin resistance and lipolysis independent of growth hormone signaling. Diabetes. 2008;57:3205–3210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nass R, Liu J, Pezzoli SS, et al. Dose-dependent inhibition of acyl- and desacyl-ghrelin release in healthy young men during a euglycemic hyperinsulinemic clamp. Possible role of ghrelin in growth hormone (GH) regulation. Endocr Rev. 2013;34 [Google Scholar]
- 12. Flanagan DE, Evans ML, Monsod TP, et al. The influence of insulin on circulating ghrelin. Am J Physiol Endocrinol Metab. 2001; 284:E 313–E316 [DOI] [PubMed] [Google Scholar]
- 13. Schmidt SM, Hallschmid M, Jauch-Chara K, Born J, Schultes B. A single night of sleep deprivation increases ghrelin levels and feelings of hunger in normal-weight healthy men. J Sleep Res. 2008;17:331–334 [DOI] [PubMed] [Google Scholar]
- 14. Giordano R, Picu A, Broglio F, et al. Ghrelin, hypothalamus-pituitary-adrenal (HPA) axis and Cushing's syndrome. Pituitary. 2004;7:243–248 [DOI] [PubMed] [Google Scholar]
- 15. Kageyama K, Hanada K, Suda T. Differential regulation and roles of urocortins in human adrenal H295R cells. Regul Pept. 2010;162:18–25 [DOI] [PubMed] [Google Scholar]
- 16. Muller TD, Muller A, Yi CX, et al. The orphan receptor Gpr83 regulates systemic energy metabolism via ghrelin-dependent and ghrelin-independent mechanisms. Nat Commun. 2013;4:1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. LeSauter J, Hoque N, Weintraub M, Pfaff DW, Silver R. Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proc Natl Acad Sci USA. 2009;106:13582–13587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kanamoto N, Akamizu T, Tagami T, et al. Genomic structure and characterization of the 5′-flanking region of the human ghrelin gene. Endocrinology. 2004;145:4144–4153 [DOI] [PubMed] [Google Scholar]