Abstract
Context:
Brown adipose tissue (BAT) generates heat during adaptive thermogenesis in response to cold temperature. Thyroid hormone (TH) receptors, type 2 deiodinase, and TSH receptors are present on brown adipocytes, indicating that the thyroid axis regulates BAT. It is unknown whether absent TH in humans would down-regulate development of BAT and its thermogenic function.
Objective:
The objective of the study was to examine BAT by magnetic resonance imaging (MRI) and infrared thermal imaging (IRT) in a pediatric patient with severe primary hypothyroidism before and after TH treatment.
Design/Setting:
This study was a case report with longitudinal follow-up in a tertiary center.
Main Outcome Measures:
BAT fat fraction (FF) by MRI and skin temperature by IRT were measured.
Results:
An 11.5-year-old female was severely hypothyroid (TSH, 989 μIU/mL; free T4, 0.10 ng/dL; low thyroglobulin, 3.0 ng/mL). Low MRI measures of FF (56.1% ± 3.7%) indicated that BAT was abundantly present in the supraclavicular fossa. IRT showed higher supraclavicular temperature (36.0°C ±0.16°C) than the suprasternal area (34.3°C ± 0.19°C). After 2 months of TH replacement, she was euthyroid (TSH, 4.3 μIU/mL; free T4, 1.49 ng/dL; T3, 102 ng/dL) at which time supraclavicular BAT decreased (increased FF 60.7% ± 3.8%). IRT showed a higher, more homogeneous skin temperature throughout the upper thorax (supraclavicular, 37.1°C ± 0.23°C; suprasternal, 36.4°C ± 0.13°C). The overall size of the supraclavicular fat depot decreased from 84.79 cm3 to 41.21 cm3.
Conclusions:
These findings document the presence of BAT and thermogenesis in profound hypothyroidism and suggest a role for TSH and/or TRH as a potential regulator of BAT.
Brown adipose tissue (BAT) is a specialized fat that produces energy in the form of heat to tolerate exposure to cold. Studies suggest that effective adaptive thermogenesis in BAT requires not only adrenergic stimulation and uncoupling protein 1 (UCP1) expression but also thyroid hormone (TH). The current view of the complex interactions between TH and BAT is based almost exclusively on murine molecular and cell culture investigations. To our knowledge, only two studies have examined the relationship between TH and BAT thermogenesis in humans, using positron emission tomography/computed tomography. One study showed an association between TH therapy and volume of activated BAT in a woman after thyroidectomy (1), and the other study found increased glucose uptake in BAT in hyperthyroid patients vs healthy controls (2). However, to date, the necessity of TH for BAT thermogenesis has not been formally shown.
The importance of TH in BAT physiology would be most noticeable in extreme deficiencies of TH, such as severe hypothyroidism. Because BAT is most prevalent in children, we assessed for the presence or absence of BAT in a pediatric patient with severe, acquired primary hypothyroidism using magnetic resonance imaging (MRI) techniques that have been developed to provide accurate and reliable measures of BAT (3–6). We also used infrared thermal imaging (IRT), a noninvasive technique that has been validated in older children (7), to assess for BAT thermogenesis by measuring skin temperature overlying the supraclavicular region. We hypothesized that the severely hypothyroid patient would have diminished BAT volume and function due to profound hypothyroidism and that both would increase following TH replacement.
Study participant and methods
The Children's Hospital Los Angeles Committee on Clinical Investigations (Institutional Review Board) approved this study. Written informed consent was obtained from the parents and written assent obtained from the patient, who was recruited from the pediatric endocrinology clinic.
An 11.5-year-old female with short stature had a markedly elevated TSH level of 989 μIU/mL (0.45–4.50) and very low free T4 of 0.10 ng/dL (0.8–2.0). She complained of constipation, cold intolerance, pale and dry skin, puffiness of the face, and mild weight gain over the prior year. She did not have a family history of thyroid or autoimmune conditions.
On examination, she was alert with no appreciable delays in cognition, and in no apparent distress, but with a slow heart rate of 60 beats/min and a blood pressure of 93/58 mm Hg. Her height was 131.9 cm (less than the third percentile) and weight of 32.4 kg (33rd percentile). She had dry skin and hair, myxedema of the face and neck, and no palpable thyroid gland. She had Tanner III breast tissue and a normal-sized tongue. She did not have periorbital edema or hoarseness of the voice. She exhibited dyslipidemia with a total cholesterol level of 370 mg/dL (100–169), triglycerides of 104 mg/dL (0–89), high-density lipoprotein of 99 mg/dL (>39), very low-density lipoprotein of 21 mg/dL (5–40), and low-density lipoprotein of 250 mg/dL (0–109). Her electrocardiogram was normal. A hand/wrist radiograph revealed the skeletal maturation to be delayed by 2 years. Her antithyroid antibody titers were elevated: antithyroglobulin of 9.3 IU/mL (≤1.0) and antithyroperoxidase of 213 IU/mL (0–26). She had a low thyroglobulin level of 3.0 ng/mL (<40), and a MRI study revealed a small amount of eutopic thyroid tissue.
The patient was diagnosed with severe acquired hypothyroidism secondary to Hashimoto thyroiditis and was started on levothyroxine (LT4) replacement at 25 μg/d (0.8 μg/kg·d), increasing every 2 weeks by 25 μg to a final dose of 75 μg/d (2.4 μg/kg·d). Two months after the initiation of treatment, she returned to the clinic euthyroid (TSH, 4.3 μIU/mL; free T4, 1.49 ng/dL; and T3, 102 ng/dL), with improved height velocity (6.6 cm/y) and a 1.4-kg weight loss. Her heart rate was improved (78 beats/min) and her blood pressure was 102/64 mm Hg. Her dyslipidemia had also resolved.
Magnetic resonance imaging
Baseline and follow-up MRI examinations were performed on a 3 T human platform (Achieva, R3.2; Philips Healthcare) at room temperature of approximately 22°C, using a 16-channel torso array. A six-echo chemical-shift mDIXON water-fat pulse sequence was used in this study as previously described (5). Water-fat MRI techniques provide quantitative fat fraction (FF; range 0%-100%) measurements of the underlying tissue and have been demonstrated and validated in characterizing BAT in humans (5, 6). Image analysis was performed with SliceOmatic (version 5.0; Tomovision) segmentation software, including volume rendering of segmented BAT depots for visualization. The volume of the supraclavicular BAT depot was measured in cubic centimeters across MRI slices. For the purpose of this study, the supraclavicular depot was defined as adipose tissue bound by the trapezius muscle posteriorly, the sternocleidomastoid muscle medially, the level of the superior cornu of the thyroid cartilage superiorly, and the subclavian artery/vein inferiorly while the patient lay supine with both arms resting at her side. Within this volume, the mean FF and its SD were computed and used as a surrogate measure of the amount of resident BAT. A lower FF value denotes reduced intracellular fat content within brown adipocytes.
Infrared thermal imaging
Prior to the MRI scan, IRT examinations were performed using a thermal imaging camera (FLK-Ti32 60 Hz industrial-commercial thermal imager; Fluke Corporation). The IRT was obtained at room temperature (∼22°C) without the use of cold stimulation, at a distance of 1 foot from the subject. The emissivity setting was 0.95. At baseline and follow-up examinations, two images were obtained, before and after the MRI examination (∼ 30 min apart) by the same operator; the average of both measures were used in this study. Images assessed skin temperature differences between the supraclavicular depot and suprasternal area (2 × 8 cm area of the lower neck and upper thorax centered at the suprasternal notch). Intraobserver and interobserver variability for the measurements of temperature and thermal area in children and adults have been reported as 0.07%-1.17% (7).
Results
The main results of our study show MRI measures of low FF (56.1% ± 3.7%; Figure 1A) at diagnosis, indicating that BAT was abundantly present in the supraclavicular fossa. Two months after the TH replacement (Figure 1B), there was a decrease in the overall volume of the supraclavicular fat depot (84.4–41.2 cm3) along with an increase in mean FF at this site (60.7% ± 3.8%), suggesting a change in the intracellular fat content of the adipose tissue.
Figure 1.
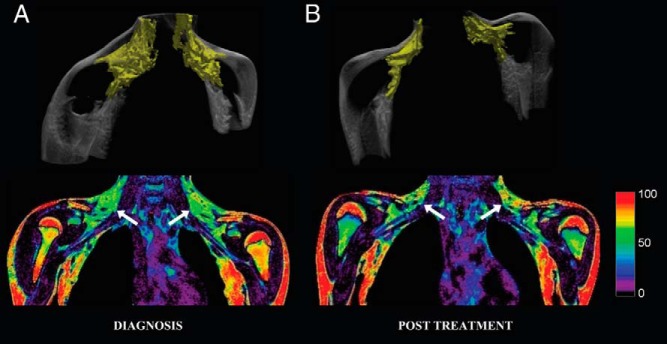
MRI volume rendering (top row) and FF (bottom row, coronal views) images of an 11.5-year-old female with severe, acquired hypothyroidism at diagnosis (A) and after 2 months of thyroid hormone (LT4) replacement therapy at which time she was euthyroid (B). Volume rendering depicts supraclavicular fat pad in yellow (white arrows). These images show an overall decrease in the volume of the supraclavicular fat depot (white arrows; 84.79–41.21 cm3) and a concomitant increase in mean FF at this site (56.1% ± 3.7% to 60.7% ± 3.8%). Scale to the right represents the FF (percentage).
An IRT showed higher skin temperature overlying the supraclavicular depot (36.0°C ± 0.16°C; Figure 2A) when compared with the suprasternal region at diagnosis (34.3°C ± 0.19°C). after more than 2 months of TH replacement, an IRT showed a higher, more homogeneous skin temperature throughout the upper thorax, with a smaller difference between the skin temperature overlying the supraclavicular depot (37.1°C ± 0.23°C; Figure 2B) and the suprasternal region (36.4°C ± 0.13°C).
Figure 2.
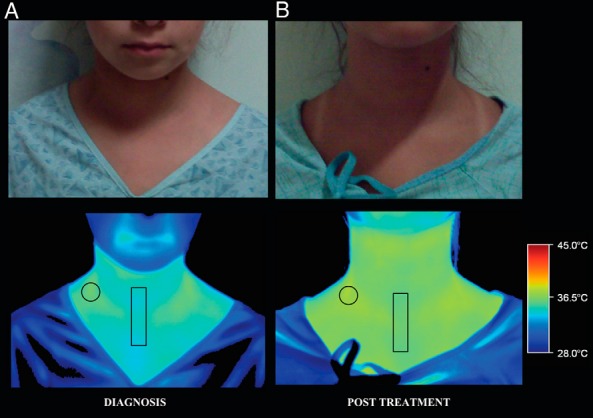
Digital (top row) and thermal (bottom row) images of an 11.5-year-old female with severe, acquired hypothyroidism at diagnosis (A) showing a higher skin temperature in the supraclavicular area (circle; 36.0°C ± 0.16°C) than the suprasternal area (rectangle; 34.3°C ± 0.19°C). After 2 months of thyroid hormone (LT4) replacement therapy (B), infrared thermography reveals a higher, more homogenous skin temperature throughout the upper thorax (supraclavicular area, 37.1°C ± 0.23°C; suprasternal area, 36.4°C ± 0.13°C). Scale to the right represents the temperature (degrees Celsius).
Discussion
We used two noninvasive techniques, MRI and IRT, to assess for the presence and function of BAT in an 11.5-year-old adolescent with severe acquired hypothyroidism. In contrast to our hypothesis, we found substantial amounts of BAT in the severely hypothyroid state. After 2 months of replacement with TH, the volume of adipose tissue in the supraclavicular depot decreased by approximately 50%, and the MRI fat fraction at this site increased, suggesting a decrease in the amount of BAT. The increased fat fraction suggests increased intracellular fat content of BAT and could potentially reflect white adipocyte infiltration in the depot. Because water-fat MRI cannot readily distinguish between BAT that is metabolically active and inactive, an IRT was used to assess for thermogenesis within the supraclavicular region. At diagnosis, an IRT depicted a localized increase in skin temperature overlying the supraclavicular area, which became less of a difference with the rest of the upper thorax after TH treatment. Altogether these findings provide unexpected evidence for the presence and function of BAT in a hypothyroid adolescent with little TH present.
Our results in a hypothyroid human differ from most, but not all, prior animal investigations. Hypothyroid rodents are unable to survive cold stress due to impaired adaptive thermogenesis in BAT; their brown adipocytes show decreased UCP1 levels and generate substantially less cAMP in response to adrenergic stimuli (8). The lack of a thyroid gland in newborn lambs is also associated with lower BAT activity and volume and a decreased response of BAT to sympathetic stimulation (9). In these hypothyroid animals, the physiological replacement of thyroid hormone (T4) rapidly restores adrenergic responsiveness, UCP1 gene expression, and BAT thermogenesis. In contrast, other studies have found that cold-acclimated, thyroidectomized rats show no difference in BAT weight, mitochondrial proteins, UCP1 mRNA expression, or oxygen consumption compared with normal animals (10), and mice with ablated TH receptors have substantial BAT, with high levels of UCP1 expression (11).
Several issues regarding MRI and infrared thermal imaging must be considered for the appropriate interpretation of the current results. The morphological differences between BAT and white adipose tissue (WAT) give rise to unique signatures that can reliably be detected and quantified by chemical-shift water-fat MRI (4, 5). With these techniques, a high FF is indicative of triglyceride-rich WAT, whereas lower FF values denote greater water content and the presence of brown adipocytes. However, FF measurements are subject to partial volume effects and can have difficulty differentiating between a depot of brown adipocytes and a cluster of brown and white adipocytes. After treatment, the patient lost weight concomitantly with both a decrease in the volume of the supraclavicular depot and an increase in the FF at this site. These findings could be the result of a decrease in the amount of triglycerides in the brown adipocytes as a consequence of its activation and could also reflect an increased proportion of WAT. Lastly, severe hypothyroidism can be associated with an increase in total body water, resulting from water retention by hydrophilic tissues (12). Because the FF values for sc WAT in the upper extremities did not change after LT4 treatment, it is unlikely that our results are a reflection of changes in water balance.
Our patient did not undergo a positron emission tomography/computed tomography study, the current gold-standard to detect BAT activity in humans, to avoid the high radiation exposure associated with this examination. Instead, we used IRT, a noninvasive technique, to assess for thermogenesis within the supraclavicular region (13). Previous studies have shown that BAT activity in the supraclavicular region of children and adults is closely related to the skin temperature overlying this fat depot (7, 13). Due to the risk associated with cooling a hypothyroid patient, the examination was performed without cold stimulation. However, the prevalence of functional BAT in children, even in a thermoneutral environment, is very high, approximately 10 times greater than that of adults.
The basis for the differences in results among previous studies is not known. However, two types of brown adipocytes with distinct developmental origins have been described in mice: Myf5-derived, preexisting classical brown adipocytes in the interscapular BAT and non-Myf5-derived beige adipocytes that emerge in the sc WAT in response to chronic cold exposure or peroxisome proliferator-activated receptor-γ agonists (14). In humans, with the exception of interscapular BAT in infants, nearly all thermogenic adipocytes abundantly express beige adipocyte-selective genes, whereas the expression of classical brown adipocyte-selective genes was nearly undetectable (14). It has recently been shown that two cytokines, irisin (an exercise induced myokine) and fibroblast growth factor 21 (FGF21), a brown adipokine, are collaboratively involved in the browning of human WAT. Cold exposure increased circulating irisin and FGF 21, whereas treatment with irisin precursors and/or FGF 21 regulated adipocyte BAT gene/protein expression and thermogenesis (15). This cytokine-mediated white fat browning could be operative in hypothyroid humans, but this remains to be examined. Studies are also needed to examine whether the thermogenic adipocytes in hypothyroid patients resemble brown or beige fat and whether murine and human adipocytes respond differently to thyroid hormones.
At diagnosis, our patient presented with very high serum concentrations of TSH, without negative feedback of TH on either TSH or TRH. After TH replacement, TSH normalized, accompanied by substantial decreases in volume of the supraclavicular fat depot and the amount of BAT at this site. These clinical observations raise the possibility that TSH, and possibly TRH, stimulates BAT thermogenesis to protect a further decrease in body temperature in the hypothyroid state.
Almost 40 years ago, it was suggested that TSH stimulates BAT as a thermoregulatory organ in addition to the thyroid gland (16). Brown adipocytes are now known to have TSH receptors, in addition to TH receptors and type 2 deiodinase (17). Interestingly, TSH can up-regulate UCP1 and type 2 deiodinase in brown adipocytes, similar to TH, and the deletion of TSH receptors results in impaired BAT thermogenesis in mice (18). Additionally, TSH and a functional TSH receptor could be required for normal temperature regulation. TSH receptor-deficient, hypothyroid mice become hypothermic in cold conditions despite T4 administration but markedly improve core temperature with transfection of TSH receptors into their BAT (19). In addition, cooling of the preoptic anterior hypothalamus in goats increases TSH, suggesting that TSH helps regulate thermogenesis via thermosensitive neurons in the hypothalamus (20). Alternatively, increased TRH (as also occurs with primary hypothyroidism) could indirectly affect thermogenesis by way of norepinephrine signaling (21). However, TRH may not be as important a modulator as TSH because BAT responses to norepinephrine are known to be blunted in hypothyroid states (8).
The recent discovery that humans have significant amounts of BAT has led to an increased interest in the factors involved in BAT regulation and its potential role as a target in the treatment of obesity. Although many symptoms of hypothyroidism, such as weight gain and decreased cold tolerance, correspond to the expected metabolic effects of decreased BAT activity, our study provides evidence for the presence and function of BAT when there is little to no TH available. Current imaging techniques can provide reliable BAT measures and serve as a platform to investigate BAT modulation by the hypothalamic-pituitary-thyroid axis, from infancy through young adulthood, in patients with pediatric thyroid disorders.
Acknowledgments
We thank Norma Castaneda and the Children's Hospital Los Angeles Children's Hospital Imaging Research Program for coordinating support, and Thomas G. Perkins, PhD, and Jonathan M. Chia, MS, (Philips Healthcare) for technical support.
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
This work was supported by Southern California Clinical and Translational Science Institute (National Institutes of Health/National Center for Research Resources/National Center for Advancing Translational Sciences) Grant KL2TR000131 (to M.S.K.); National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant K25DK087931 (to H.H.H.); National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant R21DK090778 (to V.G.); and The Saban Research Institute Innovative Pilot Award.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BAT
- brown adipose tissue
- FF
- fat fraction
- FGF 21
- fibroblast growth factor 21
- IRT
- infrared thermal imaging
- LT4
- levothyroxine
- MRI
- magnetic resonance imaging
- TH
- thyroid hormone
- UCP1
- uncoupling protein 1
- WAT
- white adipose tissue.
References
- 1. Skarulis MC, Celi FS, Mueller E, et al. Thyroid hormone induced brown adipose tissue and amelioration of diabetes in a patient with extreme insulin resistance. J Clin Endocrinol Metab. 2010;95:256–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lahesmaa M, Orava J, Schalin-Jantti C, et al. Hyperthyroidism increases brown fat metabolism in humans. J Clin Endocrinol Metab. 2014;99(1):E28–E35 [DOI] [PubMed] [Google Scholar]
- 3. Hamilton G, Smith DL, Jr, Bydder M, Nayak KS, Hu HH. MR properties of brown and white adipose tissues. J Magn Reson Imaging. 2011;34:468–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hu HH, Tovar JP, Pavlova Z, Smith ML, Gilsanz V. Unequivocal identification of brown adipose tissue in a human infant. J Magn Reson Imaging. 2012;35:938–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hu HH, Yin L, Aggabao PC, Perkins TG, Chia JM, Gilsanz V. Comparison of brown and white adipose tissues in infants and children with chemical-shift-encoded water-fat MRI. J Magn Reson Imaging. 2013;38:885–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rasmussen JM, Entringer S, Nguyen A, et al. Brown adipose tissue quantification in human neonates using water-fat separated MRI. PLoS One. 2013;8:e77907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Symonds ME, Henderson K, Elvidge L, et al. Thermal imaging to assess age-related changes of skin temperature within the supraclavicular region co-locating with brown adipose tissue in healthy children. J Pediatr. 2012;161:892–898 [DOI] [PubMed] [Google Scholar]
- 8. Rubio A, Raasmaja A, Maia AL, Kim KR, Silva JE. Effects of thyroid hormone on norepinephrine signaling in brown adipose tissue. I. β1- and β2-Adrenergic receptors and cyclic adenosine 3′,5′-monophosphate generation. Endocrinology. 1995;136:3267–3276 [DOI] [PubMed] [Google Scholar]
- 9. Polk DH, Callegari CC, Newnham J, et al. Effect of fetal thyroidectomy on newborn thermogenesis in lambs. Pediatr Res. 1987;21:453–457 [DOI] [PubMed] [Google Scholar]
- 10. Zaninovich AA, Raices M, Rebagliati I, Ricci C, Hagmuller K. Brown fat thermogenesis in cold-acclimated rats is not abolished by the suppression of thyroid function. Am J Physiol Endocrinol Metab. 2002;283:E496–E502 [DOI] [PubMed] [Google Scholar]
- 11. Golozoubova V, Gullberg H, Matthias A, Cannon B, Vennstrom B, Nedergaard J. Depressed thermogenesis but competent brown adipose tissue recruitment in mice devoid of all hormone-binding thyroid hormone receptors. Mol Endocrinol. 2004;18:384–401 [DOI] [PubMed] [Google Scholar]
- 12. Brent GA, Larsen PR, Davies TF. Hypothyroidism and thyroiditis. In: Kronenberg HM, Shlomo M, Polonsky KS, Larsen PR, eds. Williams Textbook of Endocrinology. 11th ed Philadelphia: Saunders, an imprint of Elsevier Inc; 2008:382 [Google Scholar]
- 13. Lee P, Ho KK, Greenfield JR. Hot fat in a cool man: infrared thermography and brown adipose tissue. Diabetes Obes Metab. 2011;13:92–93 [DOI] [PubMed] [Google Scholar]
- 14. Wu J, Bostrom P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee P, Linderman JD, Smith S, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19:302–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doniach D. Possible stimulation of thermogenesis in brown adipose tissue by thyroid-stimulating hormone. Lancet. 1975;2:160–161 [DOI] [PubMed] [Google Scholar]
- 17. Murakami M, Kamiya Y, Morimura T, et al. Thyrotropin receptors in brown adipose tissue: thyrotropin stimulates type II iodothyronine deiodinase and uncoupling protein-1 in brown adipocytes. Endocrinology. 2001;142:1195–1201 [DOI] [PubMed] [Google Scholar]
- 18. de Jesus LA, Carvalho SD, Ribeiro MO, et al. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest. 2001;108:1379–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Endo T, Kobayashi T. Thyroid-stimulating hormone receptor in brown adipose tissue is involved in the regulation of thermogenesis. Am J Physiol Endocrinol Metab. 2008;295:E514–E518 [DOI] [PubMed] [Google Scholar]
- 20. Marques PR, Illner P, Williams DD, et al. Hypothalamic control of endocrine thermogenesis. Am J Physiol. 1981;241:E420–E427 [DOI] [PubMed] [Google Scholar]
- 21. Morley JE, Tuck ML, Mayes DM, Rosenblatt S, Hershman JM. Thyrotropin-releasing hormone increases plasma norepinephrine in man. Horm Res. 1981;14:18–23 [DOI] [PubMed] [Google Scholar]


