Abstract
Context:
There is an inverse relationship between triglycerides and high-density lipoprotein cholesterol (HDL-C) in insulin resistance, such that improvement in insulin resistance decreases triglycerides and increases HDL-C. Patients with lipodystrophy have extreme insulin resistance with high triglycerides and low HDL-C. Leptin replacement in lipodystrophy leads to a marked decrease in triglycerides (∼60%).
Objective:
Our objective was to study the effects of metreleptin on triglycerides and HDL-C in lipodystrophy in contrast to changes in triglycerides and HDL-C in interventions for the obesity-associated metabolic syndrome.
Design, Setting, and Patients:
This open-label nonrandomized study at the National Institutes of Health included 82 patients with various forms of lipodystrophy.
Intervention:
Metreleptin (0.06–0.24 mg/kg/d) was administered for 24 months in lipodystrophy.
Main Outcome Measures:
Serum triglycerides and HDL-C were measured.
Results:
At baseline, lipodystrophy patients had low HDL-C (30 ± 1 mg/dL) and high triglycerides (961 ± 220 mg/dL) with an inverse relationship between the two (R = −0.37, P = .0006). There was no change in HDL-C with metreleptin despite major improvement in triglycerides, and individual changes in triglycerides only weakly predicted HDL-C change. On linear regression, in obesity, a decrease of 0.1 mg/dL in log(triglycerides) was associated with a 4.2 mg/dL rise in HDL-C, whereas in lipodystrophy, a decrease of 0.1 mg/dL in log(triglycerides) was associated with only a 0.6 mg/dL rise in HDL-C.
Conclusions:
The normal reciprocal relationship between triglyceride and HDL-C change seen in response to interventions for the obesity-associated metabolic syndrome is quantitatively different from that seen in lipodystrophy in response to metreleptin. Further work is needed to understand HDL-C regulation in this condition.
The lipodystrophies are a rare heterogeneous group of acquired or congenital disorders characterized by generalized or partial paucity of adipose tissue (1). A common feature in all forms of lipodystrophy is relative deficiency of leptin, resulting in metabolic abnormalities that include insulin resistance, diabetes, dyslipidemia, and nonalcoholic steatohepatitis (2). The lipid phenotype is characterized by moderate to extreme hypertriglyceridemia and low high-density lipoprotein cholesterol (HDL-C). Administration of metreleptin (recombinant human methionyl leptin) in lipodystrophy ameliorates many of these metabolic features, resulting in a 60% decrease in triglycerides. However, the very low HDL-C does not appear to change with metreleptin (3).
The metabolic features of lipodystrophy are similar to those seen in the obesity-associated metabolic syndrome (MetSyn) with high triglycerides and low HDL-C (4, 5). In MetSyn, there is a reciprocal relationship between low HDL-C and high triglycerides (6) such that when individuals transition from an insulin-resistant to a more insulin-sensitive state, there is lowering of triglycerides seen in conjunction with increased HDL-C (7).
In this study, we examine the relationship between HDL-C and triglycerides in lipodystrophic patients in the leptin-deficient state and after metreleptin replacement. We then compare the relationship between HDL-C and triglyceride change during metreleptin treatment in lipodystrophy with changes in HDL-C and triglycerides observed in MetSyn from published clinical trials.
Patients and Methods
Patients with lipodystrophy participated in an institutional review board-approved open-label study of metreleptin at the National Institutes of Health (NIH) (NCT00025883). Informed consent was obtained from patients or guardians and assent from children under 18 years. Inclusion criteria were lipodystrophy, low serum leptin (<8 and <12 ng/mL in males and females, respectively), and age >6 months plus one or more of the following: diabetes, fasting insulin ≥30 μU/mL (215 pmol/L), or fasting triglycerides >200 mg/dL (2.25 mmol/L). Exclusion criteria included pregnancy and HIV infection.
Metreleptin was self-administered sc at doses of 0.06 to 0.24 mg/kg/d, with doses adjusted based on metabolic control. A lipid panel and insulin, and hemoglobin A1c levels were obtained after a 12-hour fast and analyzed using standard methodology of the NIH Clinical Center. Leptin was measured by RIA using a commercial kit (Linco Research). Body fat was measured using whole-body dual-energy x-ray absorptiometry (Hologic QDR 4500; Hologic).
For the current analysis, data were extracted from a subgroup of 82 patients who had both triglyceride and HDL-C measurements available at baseline and after 1 year of metreleptin. Additional lipids after 6, 18, and 24 months of metreleptin were included when available.
To contrast lipids in lipodystrophy to the more prevalent MetSyn, we extracted mean change in triglycerides and HDL-C from four published clinical trials in MetSyn before and after a lipid-lowering intervention (8–11). In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) (8) lipid study, the mean changes in HDL-C and triglycerides were extracted from the fenofibrate group at the end of the study. From the Action for Health in Diabetes study (Look AHEAD) (9) changes in triglycerides and HDL-C were extracted for both treatment arms (diabetes support and education and intensive lifestyle) at years 1 and 4. In the Swedish Obese Subjects study (SOS) study (10), changes in triglycerides and HDL-C 2 years after bariatric surgery were extracted from Tables 1 and 2. In the study, “Effects of weight loss, induced by gastric bypass surgery, on HDL-C remodeling in obese women” (11), the mean changes in triglycerides and HDL-C were extracted from Table 1.
Table 1.
Baseline Characteristics of Patients With Lipodystrophya
| Lipodystrophy Type | % Female | Ethnicity, % |
Age, y | Body Fat, % | Leptin, ng/mL | Triglyceride, mg/dL | HDL, mg/dL | Hemoglobin A1c (%) | Lipid-lowering Medication Use, % | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caucasian | African American | Hispanic | Other | |||||||||
| Congenital generalized lipodystrophy (n = 37) | 81.1 | 29 | 26 | 16 | 29 | 16 ± 1.6 | 9 ± 0.5 | 1.2 ± 0.2 | 747 ± 179 | 31 ± 1.6 | 8.2 ± 0.4 | 46 |
| Acquired generalized lipodystrophy (n = 16) | 62.5 | 75 | 25 | 23 ± 4.2 | 8 ± 0.5 | 1.1 ± 0.2 | 975 ± 462 | 24 ± 2 | 8.3 ± 0.6 | 37.5 | ||
| Familial partial lipodystrophy (n = 23) | 100 | 85 | 15 | 38 ± 3 | 23 ± 0.8 | 5.8 ± 0.6 | 816 ± 415 | 33 ± 2 | 7.4 ± 0.4 | 69.6 | ||
| Acquired partial lipodystrophy (n = 6) | 100 | 100 | 25 ± 3.7 | 20 ± 2.7 | 7.5 ± 2.8 | 2799 ± 1986 | 27 ± 2 | 10.4 ± 0.9 | 66.7 | |||
| All patients (n = 82) | 84.1 | 59 | 12 | 13 | 16 | 24 ± 1.7 | 15 ± 0.9 | 3 ± 0.4 | 961 ± 220 | 30 ± 1 | 8.2 ± 0.3 | 52 |
Data are mean ± SEM except as noted.
Statistical analysis
Statistical analyses were performed using SAS version 9.3 (SAS Institute), JMP version 8.0 (SAS Institute), and GraphPad Prism version 6.02. Data are shown mean ± SEM except as noted. P values <.05 were considered statistically significant. Triglycerides and insulin values were log transformed due to nonnormal distribution in lipodystrophic subjects. Linear regression was used to quantify the relationship between triglyceride and HDL-C change in response to interventions in lipodystrophy and MetSyn. Mixed models were used to analyze the trend of triglycerides and HDL-C over time in lipodystrophy. Spearman correlations were calculated to analyze the relationship between log(triglycerides) and HDL-C in lipodystrophy at baseline, and log(insulin) vs log(triglycerides) and HDL-C at all time points.
Results
Baseline characteristics
Baseline characteristics of lipodystrophic subjects are shown in Table 1. Triglycerides were markedly elevated at baseline (961 ± 220 mg/dL) compared with those in MetSyn from the published literature (range, 141–197 mg/dL). Baseline HDL-C was lower in lipodystrophy (30 ± 1 mg/dL) compared with MetSyn (range, 37–46 mg/dL). At baseline, subjects with lipodystrophy had an inverse relationship between triglycerides and HDL-C (Spearman R= −0.37, P = .0006).
Effect of metreleptin on triglycerides and HDL-C in lipodystrophy
Treatment with metreleptin was associated with a change in triglycerides of −467 ± 161 mg/dL (52% decrease, n = 65) after 12 months, and −827 ± 317 mg/dL (67% decrease, n = 45) after 24 months, with most of the change occurring in the first 6 months (P = .002 for change in log[triglycerides] over time; Supplemental Figure 1). In contrast, there was essentially no change in HDL-C, with a change of 0.2 ± 1.0 mg/dL after 12 months (0.6% increase, n = 64), and −0.3 ± 1.3 mg/dL after 24 months (0.9% decrease, n = 43) (P = .67; Supplemental Figure 1). A sensitivity analysis in patients with generalized lipodystrophy who had baseline triglycerides <500 mg/dL (n = 34) showed a significant reduction in log(triglycerides) (baseline triglycerides 266 ± 132 mg/dL; 171 ± 125 after 24 months, P = .0003) but no change in HDL-C (31.2 ± 10 mg/dL at baseline; 32 ± 9 after 24 months, P = .7). There was a significant positive correlation between log(triglycerides) and log(insulin) at all time points except at baseline and a significant negative correlation between HDL-C and log(insulin) at all time points except at 18 months. Use of lipid-lowering drugs declined from 52% at baseline to 40% at 24 months.
Comparison of triglyceride and HDL-C change in lipodystrophy vs MetSyn
In the 4 published clinical trials, interventions for MetSyn led to decreased triglycerides with a reciprocal increase in HDL-C (P = .003, R2 = 0.79; Figure 1); a decrease of 0.1 in log(triglycerides) was associated with a 4.2 mg/dL rise in HDL-C. In contrast, in lipodystrophy, a decrease of 0.1 in log(triglycerides) was associated with only a 0.6 mg/dL rise in HDL-C (P = .04, R2 = 0.1; Figure 1).
Figure 1.
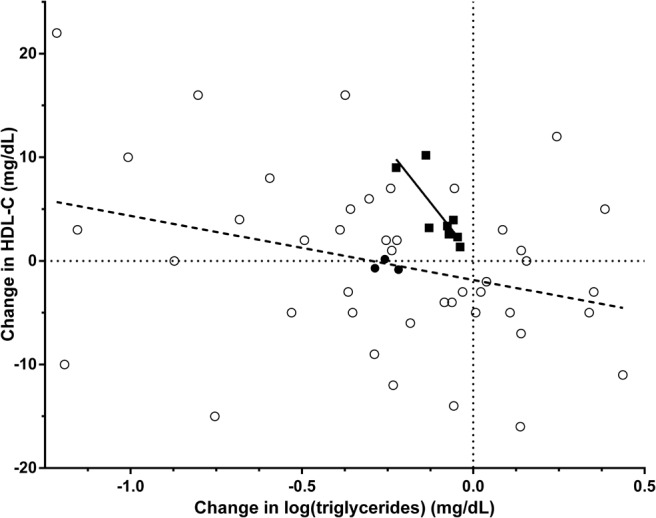
Change in log(triglycerides) and HDL-C in response to metreleptin in lipodystrophy compared with interventions for the obesity-associated MetSyn. Subject level data in lipodystrophy patients after 24 months of metreleptin is shown in open circles (linear regression as dashed line). Mean changes in log(triglyceride) vs HDL-C at 6, 12, and 18 months of metreleptin treatment in lipodystrophy are shown as filled circles, falling on the same regression line as that for individual subjects at 24 months. Mean changes in log(triglyceride) and HDL-C from clinical trials in the obesity associated metabolic syndrome are shown as black squares (linear regression in black line). There was a 7-fold difference in the HDL-C increase associated with a given log(triglyceride) decrease in lipodystrophy vs the obesity-associated MetSyn.
Discussion
In the present study, we observed that, at baseline, patients with lipodystrophy had a similar, albeit more extreme, pattern of lipid abnormalities compared with patients with MetSyn, with low HDL-C, elevated triglycerides, and an inverse relationship between the two. After treatment with metreleptin, lipodystrophy patients failed to exhibit significant improvement in HDL-C despite large and sustained improvement in triglycerides. A given decrease in triglycerides was associated with a 7-fold greater rise in HDL-C in MetSyn as compared with lipodystrophy patients treated with metreleptin. These findings suggest that there may be fundamental differences in lipid regulation in lipodystrophy as compared with MetSyn.
Hypertriglyceridemia is thought to be the primary lipid abnormality in both lipodystrophy and MetSyn. Increased de novo lipogenesis is seen in insulin resistance primarily through upregulation of the transcription factor sterol response element binding protein-1c, which regulates most genes involved in fatty acid and triglyceride synthesis (12, 13). Increased flux of free fatty acids to the liver, especially in the postprandial state, leads to accumulation of triglycerides and increased hepatic secretion of very-low-density lipoprotein (14). In addition, there is increased secretion of apolipoprotein-B48–containing chylomicrons in insulin resistance, further exacerbating hypertriglyceridemia (15). Low HDL-C in insulin resistance results from decreased HDL-C production as well as increased HDL-C catabolism. Insulin resistance leads to decreased lipoprotein lipase activity, thus reducing breakdown of triglyceride-rich lipoproteins (eg, very-low-density lipoprotein), decreasing the availability of apolipoproteins needed for HDL-C synthesis. The increased catabolism of HDL-C results from increased exchange of cholesteryl ester for triglyceride by cholesteryl ester transfer protein. The resultant triglyceride-rich HDL-C is a good substrate for hydrolysis by hepatic lipase, which has increased activity in insulin resistance (16) and is cleared rapidly from the circulation (17, 18).
The positive correlation between insulin and triglycerides and the negative correlation between insulin and HDL-C in lipodystrophic patients when analyzed cross-sectionally is consistent with the conventional understanding of the role of hyperinsulinemia and insulin resistance to raise triglycerides and lower HDL-C. Despite this, treatment with metreleptin, which improves insulin sensitivity, failed to increase HDL-C. Although we could not demonstrate the mechanisms underlying the different relationship between triglyceride and HDL-C change in lipodystrophy vs MetSyn, several hypotheses have been proposed to explain this phenomenon. First, the extreme hypertriglyceridemia of lipodystrophy is not completely corrected even by a potent intervention such as metreleptin. Thus, although triglycerides improved markedly after metreleptin, triglyceride levels may still be above a threshold for HDL-C responsiveness. This hypothesis is supported by a study showing that an HDL-C rise with a given decrease in triglycerides was greater in patients with baseline triglycerides <200 mg/dL (7). We explored the possibility of a threshold effect in our patients by restricting our analysis to the subgroup of generalized lipodystrophy with less extreme triglyceride elevation at baseline (<500 mg/dL, mean 266 mg/dL). Although triglycerides decreased to 171 mg/dL in this subgroup (comparable to MetSyn), HDL-C did not increase, suggesting that the dissociation of triglycerides and HDL-C change in lipodystrophy may not be solely due to a threshold effect. Second, although metreleptin in lipodystrophy broadly targets manifestations of the metabolic syndrome, in a similar manner to caloric restriction in obesity, there may be additional direct effects of metreleptin to alter triglyceride, but not HDL-C, metabolism. Third, the enzymes involved in synthesis and clearance of triglycerides and HDL-C (eg, lipoprotein lipase and cholesteryl ester transfer protein) may be regulated differently in lipodystrophy vs MetSyn. Finally, the most obvious physiologic difference between these 2 groups of patients is the paucity of fat in lipodystrophy, vs excess fat in MetSyn, suggesting that differences in HDL-C regulation might be occurring at the level of the adipocyte.
Understanding lipid physiology is important in lipodystrophy because premature cardiovascular disease and accelerated atherosclerosis have been reported in Dunnigan-type familial partial lipodystrophy (19, 20). Because low HDL-C is an independent risk factor for coronary heart disease in the general population, it is desirable to understand the mechanism of low HDL-C in lipodystrophic patients to modify their cardiovascular risk.
Our study is limited by a small sample size and few men. However, lipodystrophy is a rare disorder and we have one of the largest cohorts of patients in the world. Our patients were on lipid-lowering medications; however, use of these medications declined slightly during the study, and their effects are minor compared with metreleptin.
In conclusion, there is a quantitative difference in the relationship between triglyceride and HDL-C change in response to interventions that alter insulin resistance in lipodystrophy vs MetSyn. Elucidating the mechanism underlying the dissociation between triglyceride and HDL-C change in lipodystrophy may provide insights into HDL-C regulation that could be applicable both to lipodystrophy and the common MetSyn.
Acknowledgments
We acknowledge the patients with lipodystrophy, clinical fellows, and the nursing staff at the NIH Clinical Center involved in the care of these patients and Bristol Myers Squibb/Astra Zeneca for the metreleptin used in this study.
This work was supported by the Intramural Research Program of the NIH, the National Institute of Diabetes and Digestive and Kidney Diseases, and the National Heart Lung and Blood Institute.
Disclosure Summary: All authors state that they have no conflicts of interest.
Footnotes
- HDL-C
- high-density lipoprotein cholesterol
- MetSyn
- metabolic syndrome.
References
- 1. Javor ED, Cochran EK, Musso C, Young JR, Depaoli AM, Gorden P. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes. 2005;54:1994–2002 [DOI] [PubMed] [Google Scholar]
- 2. Garg A. Lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab. 2011;96:3313–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chong AY, Lupsa BC, Cochran EK, Gorden P. Efficacy of leptin therapy in the different forms of human lipodystrophy. Diabetologia. 2010;53:27–35 [DOI] [PubMed] [Google Scholar]
- 4. Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C; American Heart Association, National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438 [DOI] [PubMed] [Google Scholar]
- 5. Gorden P, Lupsa BC, Chong AY, Lungu AO. Is there a human model for the ‘metabolic syndrome’ with a defined aetiology? Diabetologia. 2010;53:1534–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chan DC, Watts GF, Barrett PH, Mamo JC, Redgrave TG. Markers of triglyceride-rich lipoprotein remnant metabolism in visceral obesity. Clin Chem. 2002;48:278–283 [PubMed] [Google Scholar]
- 7. Miller M, Langenberg P, Havas S. Impact of lowering triglycerides on raising HDL-C in hypertriglyceridemic and non-hypertriglyceridemic subjects. Int J Cardiol. 2007;119:192–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. ACCORD Study Group; Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Look AHEAD Research Group, Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170:1566–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sjöström L, Lindroos AK, Peltonen M; Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693 [DOI] [PubMed] [Google Scholar]
- 11. Asztalos BF, Swarbrick MM, Schaefer EJ, et al. Effects of weight loss, induced by gastric bypass surgery, on HDL remodeling in obese women. J Lipid Res. 2010;51:2405–2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J Biol Chem. 1999;274:30028–30032 [DOI] [PubMed] [Google Scholar]
- 14. Kissebah AH, Alfarsi S, Adams PW, Wynn V. Role of insulin resistance in adipose tissue and liver in the pathogenesis of endogenous hypertriglyceridaemia in man. Diabetologia. 1976;12:563–571 [DOI] [PubMed] [Google Scholar]
- 15. Duez H, Lamarche B, Uffelman KD, Valero R, Cohn JS, Lewis GF. Hyperinsulinemia is associated with increased production rate of intestinal apolipoprotein B-48-containing lipoproteins in humans. Arterioscler Thromb Vasc Biol. 2006;26:1357–1363 [DOI] [PubMed] [Google Scholar]
- 16. Tan CE, Foster L, Caslake MJ, et al. Relations between plasma lipids and postheparin plasma lipases and VLDL and LDL subfraction patterns in normolipemic men and women. Arterioscler Thromb Vasc Biol. 1995;15:1839–1848 [DOI] [PubMed] [Google Scholar]
- 17. Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ Res. 2005;96:1221–1232 [DOI] [PubMed] [Google Scholar]
- 18. Mann CJ, Yen FT, Grant AM, Bihain BE. Mechanism of plasma cholesteryl ester transfer in hypertriglyceridemia. J Clin Invest. 1991;88:2059–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hegele RA. Premature atherosclerosis associated with monogenic insulin resistance. Circulation. 2001;103:2225–2229 [DOI] [PubMed] [Google Scholar]
- 20. Weterings AA, van Rijsingen IA, Plomp AS, et al. A novel lamin A/C mutation in a Dutch family with premature atherosclerosis. Atherosclerosis. 2013;229:169–173 [DOI] [PubMed] [Google Scholar]


