Abstract
Context:
TSH provokes expression of inflammatory genes in CD34+ fibrocytes. These cells appear to infiltrate the orbit in Graves' disease (GD), where they putatively become the CD34+ orbital fibroblast subset (GD-OF). This may have importance in solving the pathogenesis of thyroid-associated ophthalmopathy. The IL-1 family is targeted by TSH in fibrocytes and OFs by inducing secreted IL-1 receptor antagonist (IL-1RA) and intracellular IL-1RA in a cell-specific pattern. Phosphoinositide 3-kinase (PI3K) mediates several TSH actions in thyroid. This pathway is modulated by phosphatase and tensin homolog deleted on chromosome 10 (PTEN). Vanishingly little is known currently about TSHR signaling to IL-1RA expression in nonthyroidal cells. Furthermore, factors modulating TSH action in these cells are largely unexplored.
Objectives:
To characterize intermediate signaling between TSHR and IL-1RA in fibrocytes and GD-OFs and to begin to identify the proximate regulators of TSHR signaling in nonepithelial, extrathyroidal cells as a strategy for developing therapies for thyroid-associated ophthalmopathy.
Design/Setting/Participants:
Fibrocytes and GD-OFs were collected and analyzed from healthy individuals and those with GD in an academic clinical practice.
Main Outcome Measures:
Real-time PCR, Western blot analysis, cell transfections, and chromatin immunoprecipitation analysis.
Results:
TSH induces IL-1RA in fibrocytes and GD-OFs by activating the PI3K/AKT pathway. Interrupting either PI3K or AKT with small molecule inhibitors or by knocking down their expression with targeting small interfering RNA attenuates the actions of TSH. OFs exhibit greater basal PTEN activity and lower constitutive AKT phosphorylation than do fibrocytes. Patterns of PTEN induction diverge in the two cell types.
Conclusions:
The current findings identify the PI3K/AKT pathway as critical to the induction by TSH of IL-1RA in fibrocytes and GD-OFs. Furthermore, PTEN modulates the amplitude of the induction. In GD-OFs, relatively high basal PTEN levels prevent secreted IL-1RA expression or release. Knocking down PTEN allows GD-OFs to exhibit a pattern of IL-1RA expression resembling fibrocytes.
Graves' disease (GD) is an autoimmune syndrome (1) sometimes accompanied by its ocular manifestation, also known as thyroid-associated ophthalmopathy (TAO) (2). TAO is an inflammatory process characterized by connective tissue remodeling, which can culminate in fibrosis. Orbital fibroblasts (OFs) are currently thought to orchestrate the tissue pathology that is initiated by the recruitment of lymphocytes. They comprise a mixture of CD34+ and CD34− cells in which the CD34+ fibroblast subset appears to derive from circulating CD34+ monocyte lineage fibrocytes (3). The first description of fibrocytes by Bucala et al (4) identified a cell type that plays an integral role in tissue remodeling. The phenotype of fibrocytes resembles that of both fibroblasts and monocytes on the basis of cell surface markers (5). In several experimental models of injury, they infiltrate affected tissue and appear to interact with residential fibroblasts and with other mononuclear cells recruited from the circulation (6, 7). In response to cytokines such as CD154 and IL-1β, fibrocytes express IL-8, IL-6, IL-1α, IL-1β, IL-12, and TNF-α (3, 8–10). We reported recently that fibrocytes express TSH receptor (TSHR), a G protein-coupled cell surface protein (3, 9). Activation of TSHR displayed on these cells leads to increased inflammatory gene expression (3, 9, 10). A major consequence of IL-1β and TSH-initiated signaling in fibrocytes is the induction of secreted and intracellular IL-1 receptor antagonists (sIL-1RA and icIL-1RA, respectively) (11, 12). Furthermore, fibrocytes exhibit a different pattern of IL-1RA induction compared to GD-OFs, despite the apparent derivation of the CD34+ subset from these cells (11–14). This is particularly important because IL-1RA serves a critical function as the endogenous modulator of the entire IL-1 pathway. In so doing, it serves as a critical governor of the intensity and duration of the inflammatory response.
TSHR signaling is complex in thyroid epithelium and is mediated through the activation of adenylate cyclase and generation of cAMP as well as the phosphoinositide 3-kinase (PI3K) pathway (15–18). This then leads to activation of downstream kinases such as protein kinase C (PKC) and AKT (PKB). The PI3K/AKT pathway plays an important role in cellular functions regulating host defense and immune response, including cell migration, phagocytosis, and apoptosis. Unlike GD-OFs, fibrocytes do not express detectable adenylate cyclase and thus fail to generate cAMP in response to TSH (19, 20). Furthermore, neither Gq nor Gs is consistently expressed in these cells. In contrast, PKCβII, a conventional PKC isozyme, and AKT become phosphorylated in fibrocytes, promote the activation of transcription factors CREB and NF-κB, and lead to the expression of IL-6 (19).
The PI3K/phosphatase and tensin homolog deleted on chromosome 10 (PTEN) signaling pathway has been shown to regulate innate immune reactions and thus requires tight modulation. PTEN represents an important tumor suppressor gene that acts as a negative regulator of the PI3K pathway by depleting levels of phosphatidylinositol-3,4,5-trisphosphate (21, 22). PTEN is essential for regulation of both basal and growth factor-stimulated PI3K-mediated signaling (23). It generally inactivates the PI3K/AKT pathway and is therefore an important gatekeeper for many growth factor-dependent actions. PTEN is itself regulated tightly through phosphorylation, ubiquitination, and the X-linked inhibitor of apoptosis protein, which govern the availability of PTEN and thus influence its phosphatase activity (24, 25). PTEN represents an essential determinant of apoptosis by virtue of its modulation of PI3K/AKT/FRAP/mTOR (26). Disruption of its activity can lead to malignant cell growth and survival (27). The potential role of PTEN in the regulation of TSH-dependent signaling has yet to be examined, but evidence supports the importance of PI3K as an effector of TSHR signaling within the thyroid (18) as well as outside (19).
In this study, we demonstrate that TSH induction of IL-1RA in GD-OFs and fibrocytes is mediated through PI3K/AKT. Blocking this pathway using small molecule inhibitors or targeting small interfering RNAs (siRNAs) attenuates the induction. TSH treatment increases phosphorylated AKT (pAKT) levels in fibrocytes considerably more than that observed in GD-OFs. Divergent patterns of PTEN activity in the two cell types may account for the differences in IL-1RA induction. Knocking down PTEN in GD-OFs increases the amplitude of TSH-dependent IL-1RA induction. In contrast, overexpressing PTEN in fibrocytes attenuates the induction by TSH of IL-1RA. The actions of PTEN on IL-1RA are mediated through alterations in gene transcription and mRNA stability. Taken together, these results indicate the importance of the PI3K/AKT/PTEN pathway in determining the patterns of IL-1RA response to TSH in GD-OFs and fibrocytes. They may provide potential therapeutic targets for knocking down inflammatory signaling in TAO.
Materials and Methods
Materials
DMEM containing 4.5 g/L d-glucose and l-glutamine was purchased from Life Technologies (catalog no. 11965-092). Fetal bovine serum (FBS) was from Life Technologies (catalog no. 16000-044). 5,6-Dichlorobenzimidazole (DRB) came from Cayman Chemical Co (catalog no. 10010302). LY294002 (catalog no. 440202) and AKT inhibitor IV (catalog no. 124011) were obtained from Calbiochem/EMD Biosciences. Bovine TSH (bTSH) was from Calbiochem (catalog no. 609385). M22 was purchased from Kronus Inc. PTEN inhibitor SF1670 was from Cellagen Technology (catalog no. C7316-2s), and VO-OHpic trihydrate was from Santa Cruz Biotechnology (catalog no. sc-216061). siRNA oligonucleotides targeting PI3K (catalog no. SR303516), AKT (catalog no. SR300143), and PTEN (catalog no. SR303860) were from OriGene. An ELISA kit for human IL-1RA was purchased from R&D Systems. Anti-PI3K (catalog no. 4249S), anti-pAKT (catalog no. 4058S), anti-AKT (catalog no. 9272S), anti-phospho-PTEN (catalog no. 9549S), and anti-PTEN (catalog no. 9188S) antibodies came from Cell Signaling. A chromatin immunoprecipitation (ChIP) assay kit was purchased from Millipore (catalog no. 17-925). Anti-RNA Pol II antibody (catalog no. GAH-111) and two quantitative PCR primers for amplifying 1-kb regions of icIL-1RA and sIL-1RA gene promoters were from SABioscience.
Fibroblast and fibrocyte cultivation
Orbital tissue was obtained as surgical waste generated during orbital decompressions for severe TAO, derived from healthy tissues of the deep orbit or from periorbital tissues from individuals undergoing procedures to remove distant tumors or the globe, or were from healthy tissues removed during oculoplastic cosmetic surgery. Donors were uniformly euthyroid at the time of study participation. These activities have been approved by the Institutional Review Board of the University of Michigan Medical Center. OFs were allowed to proliferate as previously described (28), and monolayers were covered with DMEM containing 10% FBS, 2 mm glutamine, sodium pyruvate (110 mg/mL), penicillin/streptomycin (100 U/mL), and 4.5% glucose. They were maintained in a 37°C, humidified, 5% CO2 environment. Culture strains were utilized between the fifth and 12th passages, an interval during which we have determined that cell phenotypes remain constant. Medium was changed every 4 days.
Human fibrocytes (n = 30) were isolated from human peripheral blood by Ficoll density centrifugation as described (14). Typically, 107 peripheral blood mononuclear cells were inoculated into each well of a six-well plate and then cultured in DMEM supplemented with 10% FBS, penicillin/streptomycin, and glutamine. Unattached cells were discarded after 7 days, whereas the remaining monolayers were incubated for an additional 7–10 days before experimental manipulations. Greater than 90% of the adherent cells carried the CD45+CD34+TSHR+CXCR4+ phenotype by flow cytometric analysis (29) .
RNA isolation and quantitative RT-PCR
Confluent six-well plates were shifted to medium containing 1% FBS for 16 hours before treatment with bTSH (5 mIU/L) or the other test agents indicated. RNA was extracted using the Aurum Total RNA Mini Kit (Bio-Rad; catalog no. 732-6820). Purified RNA was used to generate cDNA by reverse transcription using oligo(dt) and SuperScript III reverse transcriptase (Invitrogen Inc; catalog no. 205311). Real-time PCR was performed using iQ SYBR Green Supermix (Bio-Rad; catalog no. 170-8882) iTaq DNA polymerase, dNTPs, and SYBRGreen I, fluorescein on a CFX96 Real-Time PCR system (Bio-Rad). Each sample was analyzed in triplicate with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as reference. Primer sequences were: icIL-1RA forward, 5′-CTATCAGGCCCTCCCCATGGC-3′, and reverse, 5′-CAACTAGTTGGTTGTTCCTCC-3′; sIL-1RA forward, 5′-CTGCAGTCACAGAATGGAAATC-3′, and reverse, 5′-CAACTAGTTGGTTGTTCCTCC-3′; IL-6 forward, 5′-CAGGAGCCCAGTATAACT-3′, and reverse, 5′-GAATGCCCATGCTACATTT-3′; GAPDH forward, 5′-TTGCCATCAATGACCCCTTCA-3′, and reverse, 5′-CGCCCCACTTGATTTTGGA-3′. Reactions were performed at 95°C for 5 minutes, and 40 cycles at 95°C for 10 seconds and 60°C for 30 seconds.
Western blot analysis
Cell lysates were prepared as previously described (29). Protein concentration was determined with the Microprotein assay kit (Bio-Rad), separated by SDS-PAGE, and transferred to polyvinylidene difluoride membrane. These were blocked in 7.5% nonfat milk and probed with primary and secondary antibody in 5% milk, and bands were detected using SuperSignal (Thermo Scientific).
siRNA knockdown
Specific targeting siRNA oligonucleotides and scramble controls were transfected into fibroblasts using Lipofectamine RNAiMAX (Invitrogen; catalog no. 13778-075). Briefly, approximately 80% confluent cultures in six-well plates were transfected with either 200 nmol targeting siRNAs or 200 nmol scramble siRNA. After 48 hours, cells were treated without or with bTSH for 12 hours, and RNA was harvested and subjected to real-time RT-PCR. All transfections were performed in triplicate and repeated three times. Transfection of fibrocytes utilized Amaxa Nucleofector Technology (Lonza). Detached cells were rinsed and centrifuged, and cell pellets were resuspended in 100 μL buffer solution with 200 nmol of targeting siRNA or 200 nmol scramble siRNA. Transfection was performed using program U23 (29). Cells were recultured for 48 hours and then treated with nothing or with bTSH. Triplicate samples were subjected to quantitative RT-PCR. Results were normalized to GAPDH.
IL-1RA ELISA
Cell-associated IL-1RA protein was quantified in lysis buffer containing 0.5% Nonidet P-40, 50 mm Tris-HCl (pH 8.0), 1 mm EDTA, and 10 μg phenylmethanesulfonyl fluoride. One hundred microliter medium and 50 μg cell protein were subjected to ELISA. Assays were performed in triplicate.
mRNA stability assay
Confluent OFs and fibrocytes were shifted to medium with 1% FBS for 16 hours and pretreated with bTSH for 12 hours. DRB (50 μm) was added without or with bTSH (5 mIU/L) at time = 0, and RNA was harvested at times indicated (30). Samples were subjected to real-time PCR; t1/2 values were calculated using the comparative critical threshold method. Normalized data for time = 0 were arbitrarily set at 100%. Data were graphed as a best-fit line.
RNA Pol II ChIP assay
Recruitment of polymerase II to the IL-1RA gene promoters was quantified by the method of Bittencourt and Auboeuf (31), with the minor modification described previously (11).
Statistics
Significance was determined with a two-tailed Student's t test. All experiments were conducted at least three times.
Results
TSH induction of IL-1RA is mediated through the PI3K/AKT pathway in GD-OFs and fibrocytes
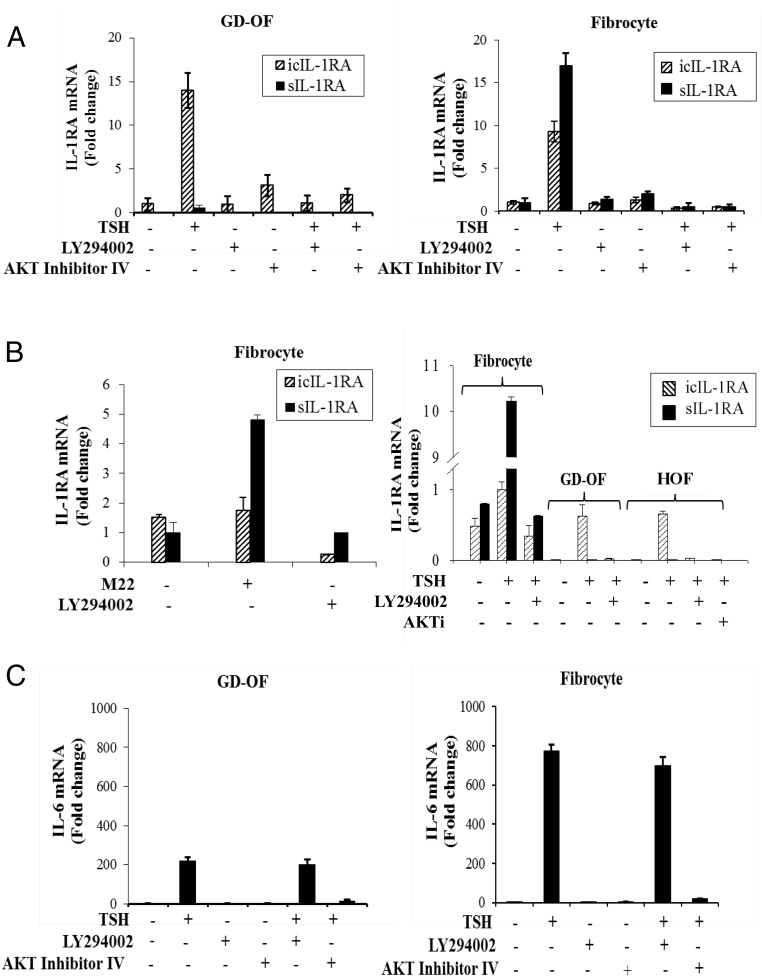
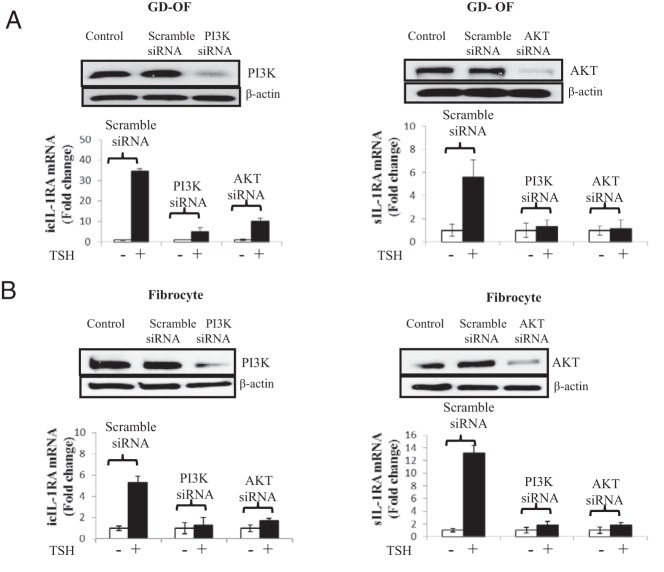
The impact of PI3K/AKT pathway inhibition on the induction of IL-1RA by bTSH was assessed. As the data in Figure 1A demonstrate, bTSH (5 mIU/mL) up-regulates steady-state icIL-1RA mRNA in GD-OFs and fibrocytes. The magnitude of the effects is 14.3 ± 2.1-fold and 9.8 ± 1.8-fold after 12 hours (mean ± SD) in the two respective cell types. sIL-1RA is minimally affected by bTSH treatment in GD-OFs but is induced 18.1 ± 3.5-fold in fibrocytes. Addition of either LY294002 (an inhibitor of PI3K, 10 μm) or AKT inhibitor IV (AKTI, 1 μm) abolished these effects in both cell types. M22 (1 μg/mL), a commercially available monoclonal activating antibody targeting TSHR, also induced IL-1RA in fibrocytes (Figure 1B, left). LY294002 (10 μm) could also block its effects after 12 hours. The magnitude of IL-1RA mRNA induction by TSH in OFs from healthy donors was similar to that in GD-OFs (Figure 1B, right), indicating that no signaling defects can be identified in GD-OFs. To substantiate the roles of PI3K and AKT in up-regulating IL-1RA that are implied by the results with these small molecule inhibitors, specific siRNAs targeting PI3K and AKT or their scrambled oligonucleotide counterparts were transfected into the cells. Although the scrambled controls failed to alter the TSH effects, those targeting the kinases attenuated the impact of bTSH on icIL-1RA and sIL-1RA in both OFs and fibrocytes (Figure 2, A and B). Responses to bTSH were similar in OFs obtained from patients with TAO and those from healthy donors. The same held true for fibrocytes (data not shown).
Figure 1.
Divergent PI3K/AKT pathway involvement in the induction by TSH of IL-1RA and IL-6. OFs from patients with GD (GD-OF) and fibrocytes were cultured in six-well plates and incubated without or with LY294002 (10 μm) or AKT inhibitor IV (1 μm) for 1 hour before the addition of nothing or bTSH (5 mIU/mL) for 12 hours. A, RNA was harvested and analyzed by real-time RT-PCR for icIL-1RA mRNA and sIL-1RA mRNA. B, M22 (left panel) and bTSH (right panel) were examined for their effects on sIL-1RA and icIL-1RA expression in fibrocytes, GD-OFs, or OFs from healthy donors (HOF). Cultures were treated with M22 (1 μg/mL) and bTSH (5 mIU/mL) for 12 hours without or with the inhibitors indicated. C, IL-6 mRNA was assessed after treatment of fibrocytes and GD-OFs without or with bTSH in the absence or presence of LY294002 or AKT Inhibitor IV. Data are expressed as mean ± SD of triplicates from three representative studies.
Figure 2.
Knocking down of PI3K or AKT blocks the induction by TSH of IL-1RA in OFs, in this case from a donor with GD, and fibrocytes. Subconfluent (80%) cultures were transfected with either scrambled (control) oligonucleotides or specific siRNAs targeting PI3K or AKT. After 48 hours, monolayers were untreated or bTSH (5 mIU/mL) was added to medium for 12 hours. Monolayers were disrupted and cellular protein was subjected to Western blot analysis for PI3K or AKT. RNA was subjected to real-time RT-PCR for icIL-1RA or sIL-1RA in OFs (A) and fibrocytes (B). Data are expressed as mean ± SD of three independent replicates.
Induction of IL-6 by TSH is independent of PI3K
Although the induction of IL-1RA by bTSH is dependent on the activity of PI3K, that of IL-6 was unaffected by LY294002 (Figure 1C). On the other hand, AKTI blocked the effects of bTSH on IL-6 expression. Thus, it would appear that bTSH is acting through PI3K in its induction of both IL-1RA isoforms but is not required in TSH-dependent regulation of IL-6.
Divergent levels of constitutive PTEN and AKT phosphorylation in GD-OFs and fibrocytes
Because of the central role that PTEN plays in regulating the PI3K/AKT pathway in many cell types, constitutive levels of this factor were quantified in three strains each of OFs and fibrocytes, each from a different donor. As the Western blots in Supplemental Figure 1A indicate, phosphorylated PTEN (pPTEN) levels are higher in OFs than those found in fibrocytes despite equivalent levels of PTEN protein in the two cell types. When expressed as ratios, pPTEN/PTEN was greater in OFs than in fibrocytes (0.46 ± 0.11 vs 0.23 ± 0.06; P < .05). In contrast, levels of pAKT in fibrocytes greatly exceeded those in OFs. TSH treatment for graded intervals resulted in the reduction of pPTEN in OFs until at 2 hours they became undetectable. In contrast, bTSH (5 mIU/mL) provoked a substantial increase in pAKT at 30 minutes, which was sustained for the duration of the study (2 h; Supplemental Figure 1B). The effects of bTSH in fibrocytes were somewhat different. The low level of pPTEN in untreated cells was increased modestly by bTSH so that by 2 hours, levels had doubled (Supplemental Figure 1C). On the other hand, levels of pAKT increased very rapidly after exposure to bTSH and remained elevated for at least 2 hours.
Inhibiting PTEN or knocking down its expression enhances the induction by bTSH of IL-1RA in GD-OFs
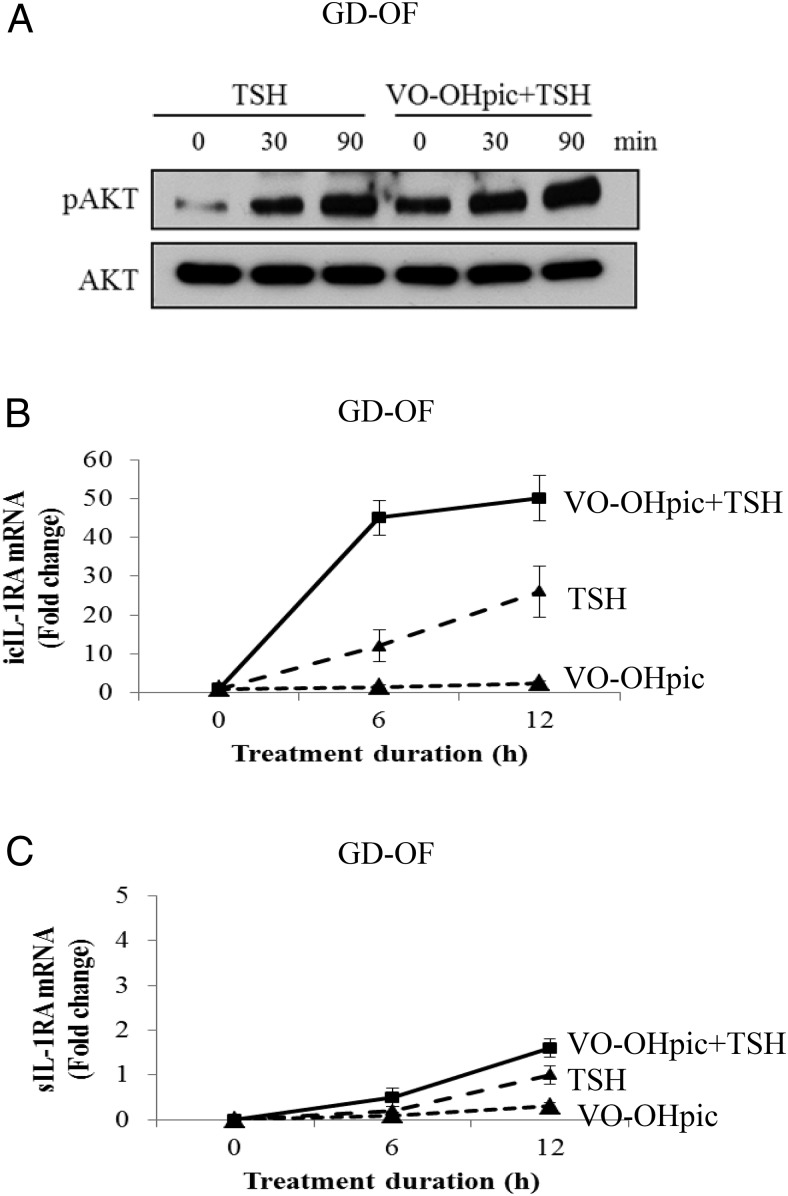
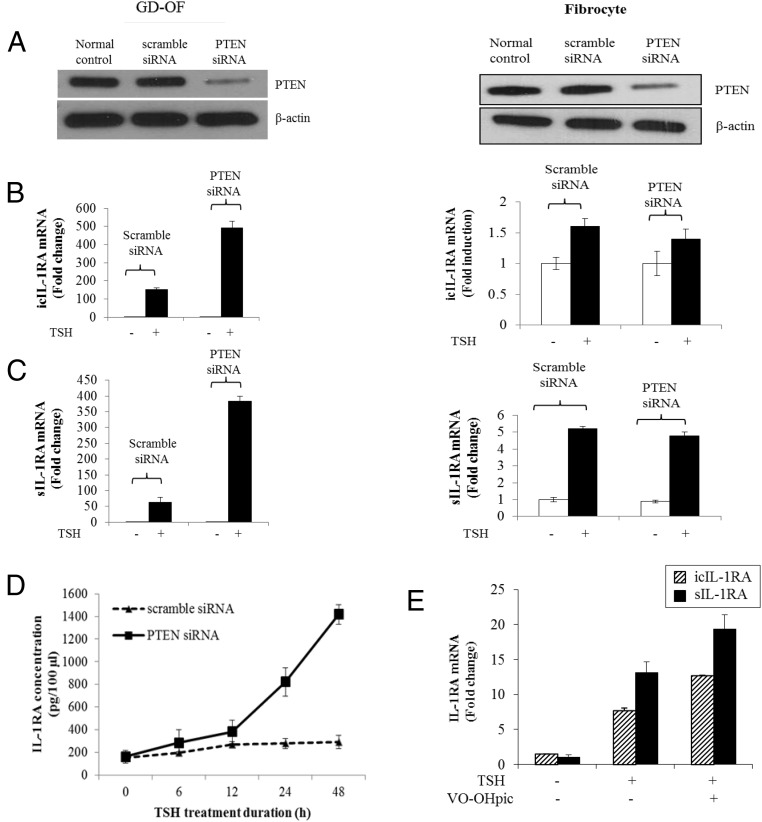
Different levels of pPTEN can be detected in OFs and fibrocytes (Supplemental Figure 1A). Higher levels of this phosphorylated protein in OFs could account for the divergent patterns of IL-1RA induction observed in these two cell types. To test this possibility, PTEN activity was reduced with the specific inhibitor, VO-OHpic (500 nm). This compound enhanced TSH-dependent AKT phosphorylation (Figure 3A). Furthermore, the steady-state icIL-1RA and sIL-1RA mRNA levels achieved after bTSH (5 mIU/mL) treatment were further increased in cultures receiving VO-OHpic (Figure 3, B and C). Next, siRNA targeting PTEN or its scrambled control were transfected into OFs and fibrocytes. In both cell types, PTEN protein was knocked down by the specific targeting siRNA (Figure 4A). Knocking down PTEN in OFs resulted in dramatic increases in the amplitude of inductions of icIL-1RA (Figure 4B) and sIL-1RA (Figure 4C) after treatment with bTSH (5 mIU/mL) for 12 hours (icIL-1RA, 456 ± 11.3 [mean ± SD], P < .01; sIL-1RA, 371 ± 9.2, P < .01). In contrast to this substantial impact of PTEN knockdown in OFs, the magnitude and pattern of IL-1RA induction was essentially unaffected in fibrocytes, suggesting that it exerts relatively little influence on TSH-dependent signaling in that cell type.
Figure 3.
Inhibiting PTEN enhances the induction of IL-1RA by TSH in OFs, in this instance from a donor with GD. Cells were cultured in six-well plates and pretreated without or with VO-OHpic (500 nm) for 1 hour before treatment with bTSH (5 mIU/mL) alone or in combination with VO-OHpic for the times indicated. A, Total cell protein was collected and subjected to Western blot analysis for pAKT and AKT. RNA was harvested and analyzed by real-time RT-PCR for icIL-1RA (B) and sIL-1RA (C). Data are expressed as mean ± SD of triplicates from one of three representative studies.
Figure 4.
Knocking PTEN down enhances the induction of IL-1RA by TSH in GD-OFs but not in fibrocytes. Eighty percent confluent cultured cells were transfected with specific siRNAs targeting PTEN. Scrambled oligonucleotides were used as the control. After 48 hours, monolayers were treated without or with bTSH (5 mIU/mL) for 12 hours. A, Total cellular protein was collected for Western blot analysis to confirm the knocking down of PTEN. B and C, RNA was harvested and subjected to real-time RT-PCR for icIL-1RA (B) or sIL-1RA (C). D, Time course of TSH (5 mIU/mL) action on IL-1RA protein production and release into the culture medium of GD-OFs transfected with scrambled (control) or siRNA targeting PTEN. Aliquots of medium were subjected to a cytokine-specific ELISA as described in Methods. E, Fibrocytes were treated with bTSH (5 mIU/mL) without or with VO-OHpic (500 nm) for 12 hours, and mRNA was harvested and subjected to RT-PCR for either icIL-1RA or sIL-1RA mRNA. Data are expressed as mean ± SD of three replicates.
A substantial point of divergence between the phenotypes of GD-OFs and fibrocytes relates to the absence of detectable IL-1RA protein release from the former after treatment with either IL-1β (11–14) or bTSH (5 mIU/mL) (Figure 4D). Because knocking down PTEN in OFs results in a substantial induction of sIL-1RA (Figure 4C), we assessed whether these cells could now release IL-1RA into the culture medium. As the result in Figure 4D demonstrates, interrupting PTEN expression allows a substantial increase in the medium content of IL-1RA after bTSH treatment when compared to OFs transfected with control siRNA. After 24 hours of exposure to bTSH, IL-1RA levels in cultures transfected with siRNA targeting PTEN were 4-fold (P < .05) above those in cultures receiving control siRNA. By 48 hours, the duration of the study, levels were 7-fold (P < .05) greater. Inhibiting PTEN activity in fibrocytes with VO-OHpic does fractionally increase the induction by bTSH of both IL-1RA isoform mRNAs (Figure 4E and Supplemental Figure 2), but the effects are generally less robust than those observed in OFs. These results indicate that PTEN exerts substantially more modulation in OFs than fibrocytes. Moreover, reducing PTEN levels in OFs allows a transition to a phenotype more closely resembling that of fibrocytes. Furthermore, PTEN appears to represent a potentially important fulcrum in determining the inflammatory characteristics of these cells.
Overexpression of PTEN attenuates the IL-1RA induction by TSH in fibrocytes
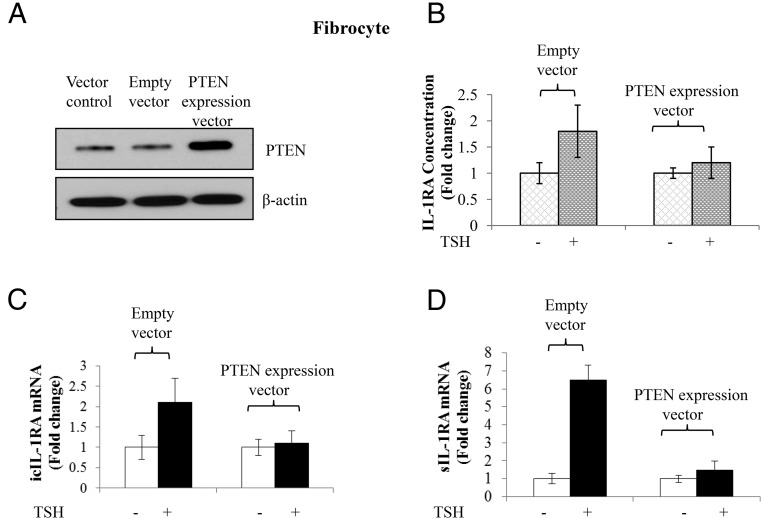
Knocking down PTEN expression in OFs results in augmentation of sIL-1RA induction and results in its release from the cell monolayer. The next studies examined whether a reciprocal relationship might exist on fibrocytes. PTEN was overexpressed in these cells by transfecting them with a PTEN expression plasmid containing its entire cDNA. As anticipated, levels of PTEN are increased compared to cells transfected with empty vector (Figure 5A). The increased PTEN blunts the induction by bTSH (5 mIU/mL) of IL-1RA release from the cell layer (Figure 5B) as well as the induction of icIL-1RA mRNA (Figure 5C) and sIL-1RA mRNA (Figure 5D).
Figure 5.
Overexpression of PTEN in fibrocytes attenuates the induction of IL-1RA by TSH. Between 70 and 80% of confluent cultures, in this instance from a healthy donor, were transfected with an expression vector for PTEN driven by the CMV promoter. Empty expression vector served as the control. After 48 hours, monolayers were treated without or with bTSH (5 mIU/mL) for 12 hours. A, Total cellular protein were collected for Western blot to confirm PTEN overexpression. B, Cell lysates were collected and analyzed by specific ELISA for IL-1RA. C and D, RNA was harvested and subjected to real-time RT-PCR for icIL-1RA (C) and sIL-1RA (D) mRNA. Data are expressed as mean ± SD of three independent replicates.
PTEN exerts dual actions on IL-1RA expression in OFs
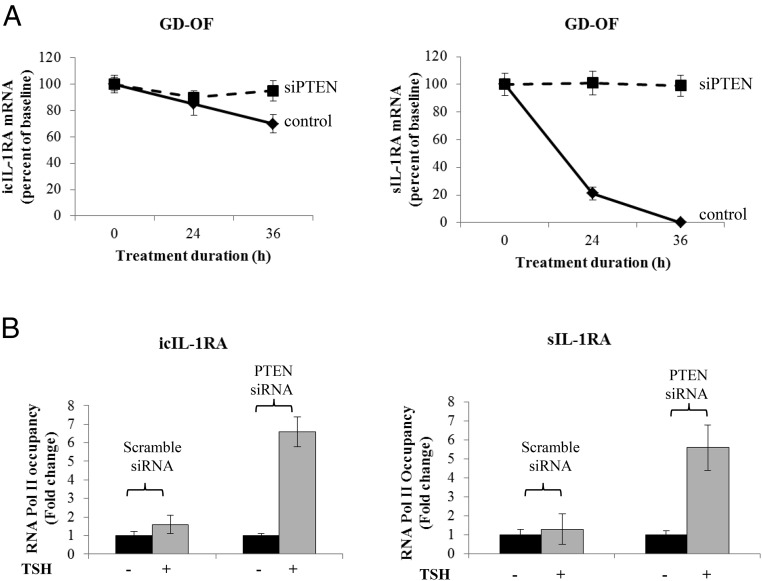
The next studies were designed to determine the mechanism(s) involved in the modulation by PTEN of IL-1RA induction. icIL-1RA mRNA is very stable in OFs (Figure 6A). Its stability was enhanced only slightly after 36 hours following PTEN knockdown. In contrast, sIL-1RA mRNA, which is extremely short-lived in OFs, was dramatically rescued after PTEN knockdown at 36 hours, the duration of the study. On the other hand, PTEN knockdown resulted in substantial increases in TSH-dependent Pol II occupancy of both icIL-1RA and sIL-1RA gene promoters (Figure 6B). These results indicate that PTEN regulates both IL-1RA gene transcription and transcript stability in OFs.
Figure 6.
Effect of knocking down PTEN on icIL-1RA and sIL-1RA mRNA stability and gene transcription in OFs. Eighty percent of confluent cultured cells were transfected with specific siRNAs targeting PTEN or scrambled oligonucleotide control. After 48 hours, monolayers were untreated or treated with bTSH (5 mIU/mL) for 12 hours. A, Stability of icIL-1RA and sIL-1RA mRNA was determined and analyzed by real-time RT-PCR as described under Materials and Methods. All cultures were pretreated with bTSH for 12 hours, and then at time = 0 DRB (50 μg/mL) was added to the cultures. Half the wells were then treated with bTSH for the times indicated along the abscissa while the others did not receive TSH (control). B, ChIP assays were performed using formaldehyde cross-linked samples from untreated cultures and those treated with bTSH. Promoter DNA for either icIL-1RA or sIL-1RA was generated by RT-PCR, and RNA Pol II occupancy was normalized to GAPDH and was expressed as mean ± SD of fold-change of three independent experiments. Data are expressed as mean ± SD of three replicates.
Discussion
PTEN has been shown to exert substantial control over several cellular and metabolic processes (32, 33). For instance, nuclear PTEN plays an important role in DNA repair (34). The regulation by PTEN of TSH signaling has apparently not been explored previously, yet from the current findings, its influence seems substantial. A single report by Tell et al (35) demonstrated that in FRTL-5 cells, a short duration of exposure to TSH (30–60 min) resulted in decreased PTEN levels, effects mediated through a cAMP-dependent mechanism. Longer treatment (days) resulted in the up-regulation of PTEN protein expression. Despite the paucity of information directly linking TSHR and PTEN, the studies we report here may provide an alternative concept surrounding Cowden syndrome (36). In that disease, germline PTEN mutations predispose to benign and malignant thyroid nodules (36, 37). Recent evidence has implicated loss of function PTEN mutations with increased nuclear translocation of PI3K and AKT (33). Those findings are consistent with a propensity for tumor development in the thyroid and other tissues. However, the current studies demonstrate that down-regulating PTEN might allow an exaggerated response in target cells to TSH mediated through PI3K/AKT. The well-established role of TSH-dependent thyroid stimulation in promoting rodent thyroid tumor formation may thus offer an alternative mechanism underlying increased incidence of tumors in individuals harboring loss of function mutations in PTEN.
PTEN expression and activity diverge in OFs and fibrocytes. These differences impact the regulation by TSH of icIL-1RA and sIL-1RA and are manifested by cell-specific patterns of TSH-dependent gene induction. TSH effects on IL-1RA, like those on IL-6 (19), are mediated at levels of both gene transcription and mRNA stability (Figures 5 and 6). From the current studies, PI3K, AKT, and PTEN emerge as important determinants of TSH actions in nonthyroid cells. PI3K and AKT appear to be indispensable mediators of TSH-initiated actions, whereas PTEN exerts an important negative influence, especially in OFs. Thus, these pathways comprise important functional fulcrums for the biological impact exerted by TSH, the balance of which determines the magnitude and duration of TSHR-dependent induction of IL-1RA. Furthermore, they could join other factors influencing the actions of TSH in extrathyroidal cells. These include the IGF-1 receptor (IGF-1R) (38). TSHR and IGF-1R form a physical and functional complex, and the latter protein appears to play an indispensable role in determining the TSH-dependent activation of ERK. An important aspect of the transition from circulating fibrocytes to OFs as they infiltrate the orbit in TAO may involve the rebalancing of this relationship and result in up-regulation of PTEN activity in OFs. By implication, the interplay between PTEN and the PI3K/AKT pathway may determine multiple aspects of the fibrocyte phenotype and potentially how it changes during transition to CD34+ OFs. An important departure in TSHR signaling common to both OFs and fibrocytes relates to the participation of PI3K in the induction of IL-1RA, but its apparent irrelevance in the induction of IL-6 (Figure 1). This finding suggests that the interface downstream from TSHR relating to these two cytokines must differ. Whether these relate to peculiarities in gene transcription or mRNA stability will require additional studies.
The relationship between PI3K and PTEN is well conserved and in aggregate influences a diverse array of cellular functions, including metabolism, proliferation, and apoptosis (39). This pathway mediates signaling of many growth factors and cytokines by generating the second messenger phosphatidylinositol-3,4.5 triphosphate (40). PTEN, a tumor suppressor gene product, serves as the governing brake for PI3K activities. This is accomplished through its promotion of phosphatidylinositol-3,4,5-trisphosphate dephosphorylation to phosphatidylinositol-4,5-bisphosphate. Imbalances between PI3K and PTEN can have catastrophic consequences, such as the abnormalities associated with cancer development.
Kumar et al (41) reported previously that LY294002 could reduce levels of leptin and adiponectin mRNA in GD-OFs undergoing adipogenesis in the presence of bTSH and/or M22. They also found that the inhibitor could lessen the accumulation of hyaluronan in OFs (42). Both studies relied on relatively lengthy treatment with LY294002 (10 d and 48 h, respectively). Adipogenesis and hyaluronan generation are extremely complex processes requiring numerous biological reactions. Thus, the reliance on treatment with a single small molecule inhibitor, such as LY294002, for prolonged exposures as the only evidence supporting involvement of PI3K in these cellular responses to TSH makes interpretation of the results in either study difficult.
The current findings identify additional molecular pathways that mediate the actions of TSH in OFs and fibrocytes and modulate their biological consequences. It is thus possible that TSHR actions are regulated by IGF-1R and PTEN and in so doing influence the pathogenic role that the receptor may play in TAO. Levels of TSHR are dramatically higher in fibrocytes than in their derivative GD-OFs (3, 9). Yet bTSH induces cytokines such as IL-6 in both cell types, albeit at a lower amplitude in OFs than fibrocytes (19). An important divergence between TSH action in the two cell types relates to the absence of adenylate cyclase expression or cAMP generation in response to TSH in fibrocytes (19). In OFs, bTSH provokes modest but detectable generation of the cyclic nucleotide, whereas in fibrocytes, none can be detected (19). Furthermore, adenylate cyclase is expressed in the former but is completely undetectable in the latter (19). It would appear therefore that fibrocytes might represent an ideal cell type in which to interrogate noncyclic AMP-related TSHR signaling. Thus, the effects of TSH on IL-1RA in these cells are most likely also independent of the G protein-coupling functions that dominate TSH action in the thyroid. The current studies also suggest an important phenotypic transition as fibrocytes infiltrate the orbit and become OFs. They imply that levels of PTEN activity increase substantially and in so doing, lower levels of both icIL-1RA and sIL-1RA and their responses to bTSH. We have reported previously that native (nonfibrocyte-derived) CD34− fibroblasts impose a substantial influence on CD34+ OFs (29). Clearly, the molecular interplay between CD34+ OFs, ie, those that putatively derive from circulating fibrocytes, and CD34− OFs must be more fully investigated before the phenotypic divergence between OFs and fibrocytes can be completely understood. In any event, the current findings provide new and potentially important insights into the regulation of the inflammatory characteristics of nonthyroid cells by TSH.
Acknowledgments
The authors are indebted to Ms Roshini Fernando for performing several of the experiments.
This work was supported by National Institutes of Health Grants EY008976, EY011708, and DK063121; Center for Vision Grant EY007003 from the National Eye Institute; an unrestricted grant from Research to Prevent Blindness; and the Bell Charitable Foundation.
Disclosure Summary: All authors have nothing to declare.
Footnotes
- bTSH
- bovine TSH
- ChIP
- chromatin immunoprecipitation
- DRB
- 5,6-dichlorobenzimidazole
- FBS
- fetal bovine serum
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GD
- Graves' disease
- icIL-1RA
- intracellular IL-1RA
- IGF-1R
- IGF-1 receptor
- IL-1RA
- IL-1 receptor antagonist
- OF
- orbital fibroblast
- pAKT
- phosphorylated AKT
- PI3K
- phosphoinositide 3-kinase
- PKC
- protein kinase C
- pPTEN
- phosphorylated PTEN
- PTEN
- phosphatase and tensin homolog deleted on chromosome 10
- sIL-1RA
- secreted IL-1RA
- siRNA
- small interfering RNA
- TAO
- thyroid-associated ophthalmopathy
- TSHR
- TSH receptor.
References
- 1. Brent GA. Clinical practice. Graves' disease. N Engl J Med. 2008;358:2594–2605 [DOI] [PubMed] [Google Scholar]
- 2. Bahn RS. Graves' ophthalmopathy. N Engl J Med. 2010;362:726–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Douglas RS, Afifiyan NF, Hwang CJ, et al. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2010;95:430–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81 [PMC free article] [PubMed] [Google Scholar]
- 5. Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PloS One. 2009;4:e7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562 [DOI] [PubMed] [Google Scholar]
- 7. Phillips RJ, Burdick MD, Hong K, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160:419–425 [PubMed] [Google Scholar]
- 9. Gillespie EF, Papageorgiou KI, Fernando R, et al. Increased expression of TSH receptor by fibrocytes in thyroid-associated ophthalmopathy leads to chemokine production. J Clin Endocrinol Metab. 2012;97:E740–E746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gillespie EF, Raychaudhuri N, Papageorgiou KI, et al. Interleukin-6 production in CD40-engaged fibrocytes in thyroid-associated ophthalmopathy: involvement of Akt and NF-κB. Invest Ophthalmol Vis Sci. 2012;53:7746–7753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li B, Smith TJ. Divergent expression of IL-1 receptor antagonists in CD34+ fibrocytes and orbital fibroblasts in thyroid-associated ophthalmopathy: contribution of fibrocytes to orbital inflammation. J Clin Endocrinol Metab. 2013;98:2783–2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li B, Smith TJ. Regulation of IL-1 receptor antagonist by TSH in fibrocytes and orbital fibroblasts. J Clin Endocrinol Metab. 2014;99:E625–E633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cao HJ, Smith TJ. Leukoregulin upregulation of prostaglandin endoperoxide H synthase-2 expression in human orbital fibroblasts. Am J Physiol. 1999;277:C1075–C1085 [DOI] [PubMed] [Google Scholar]
- 14. Cao HJ, Han R, Smith TJ. Robust induction of PGHS-2 by IL-1 in orbital fibroblasts results from low levels of IL-1 receptor antagonist expression. Am J Physiol Cell Physiol. 2003;284:C1429–C1437 [DOI] [PubMed] [Google Scholar]
- 15. Tsygankova OM, Saavedra A, Rebhun JF, Quilliam LA, Meinkoth JL. Coordinated regulation of Rap1 and thyroid differentiation by cyclic AMP and protein kinase A. Mol Cell Biol. 2001;21:1921–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tsygankova OM, Feshchenko E, Klein PS, Meinkoth JL. Thyroid-stimulating hormone/cAMP and glycogen synthase kinase 3β elicit opposing effects on Rap1GAP stability. J Biol Chem. 2004;279:5501–5507 [DOI] [PubMed] [Google Scholar]
- 17. Porcellini A, Messina S, De Gregorio G, et al. The expression of the thyroid-stimulating hormone (TSH) receptor and the cAMP-dependent protein kinase RII β regulatory subunit confers TSH-cAMP-dependent growth to mouse fibroblasts. J Biol Chem. 2003;278:40621–40630 [DOI] [PubMed] [Google Scholar]
- 18. Suh JM, Song JH, Kim DW, et al. Regulation of the phosphatidylinositol 3-kinase, Akt/protein kinase B, FRAP/mammalian target of rapamycin, and ribosomal S6 kinase 1 signaling pathways by thyroid-stimulating hormone (TSH) and stimulating type TSH receptor antibodies in the thyroid gland. J Biol Chem. 2003;278:21960–21971 [DOI] [PubMed] [Google Scholar]
- 19. Raychaudhuri N, Fernando R, Smith TJ. Thyrotropin regulates IL-6 expression in CD34+ fibrocytes: clear delineation of its cAMP-independent actions. PloS One. 2013;8:e75100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Raychaudhuri N, Douglas RS, Smith TJ. PGE2 induces IL-6 in orbital fibroblasts through EP2 receptors and increased gene promoter activity: implications to thyroid-associated ophthalmopathy. PloS One. 2010;5:e15296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378 [DOI] [PubMed] [Google Scholar]
- 22. Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947 [DOI] [PubMed] [Google Scholar]
- 23. Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. PTEN is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–355 [DOI] [PubMed] [Google Scholar]
- 24. Van Themsche C, Leblanc V, Parent S, Asselin E. X-linked inhibitor of apoptosis protein (XIAP) regulates PTEN ubiquitination, content, and compartmentalization. J Biol Chem. 2009;284:20462–20466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maccario H, Perera NM, Gray A, Downes CP, Leslie NR. Ubiquitination of PTEN (phosphatase and tensin homolog) inhibits phosphatase activity and is enhanced by membrane targeting and hyperosmotic stress. J Biol Chem. 2010;285:12620–12628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Steck PA, Pershouse MA, Jasser SA, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–362 [DOI] [PubMed] [Google Scholar]
- 28. Smith TJ, Koumas L, Gagnon A, et al. Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2002;87:385–392 [DOI] [PubMed] [Google Scholar]
- 29. Fernando R, Atkins S, Raychaudhuri N, et al. Human fibrocytes coexpress thyroglobulin and thyrotropin receptor. Proc Natl Acad Sci USA. 2012;109:7427–7432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cao HJ, Wang HS, Zhang Y, Lin HY, Phipps RP, Smith TJ. Activation of human orbital fibroblasts through CD40 engagement results in a dramatic induction of hyaluronan synthesis and prostaglandin endoperoxide H synthase-2 expression. Insights into potential pathogenic mechanisms of thyroid-associated ophthalmopathy. J Biol Chem. 1998;273:29615–29625 [DOI] [PubMed] [Google Scholar]
- 31. Bittencourt D, Auboeuf D. Analysis of co-transcriptional RNA processing by RNA-ChIP assay. Methods Mol Biol. 2012;809:563–577 [DOI] [PubMed] [Google Scholar]
- 32. Li Y, He L, Zeng N, et al. Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) signaling regulates mitochondrial biogenesis and respiration via estrogen-related receptor α (ERRα). J Biol Chem. 2013;288:25007–25024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lian Z, Di Cristofano A. Class reunion: PTEN joins the nuclear crew. Oncogene 2005;24:7394–7400 [DOI] [PubMed] [Google Scholar]
- 34. Bassi C, Ho J, Srikumar T, et al. Nuclear PTEN controls DNA repair and sensitivity to genotoxic stress. Science. 2013;341:395–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tell G, Pines A, Arturi F, et al. Control of phosphatase and tensin homolog (PTEN) gene expression in normal and neoplastic thyroid cells. Endocrinology. 2004;145:4660–4666 [DOI] [PubMed] [Google Scholar]
- 36. Farooq A, Walker LJ, Bowling J, Audisio RA. Cowden syndrome. Cancer Treat Rev. 2010;36:577–583 [DOI] [PubMed] [Google Scholar]
- 37. He X, Saji M, Radhakrishnan D, et al. PTEN lipid phosphatase activity and proper subcellular localization are necessary and sufficient for down-regulating AKT phosphorylation in the nucleus in Cowden syndrome. J Clin Endocrinol Metab. 2012;97:E2179–E2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tsui S, Naik V, Hoa N, et al. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves' disease. J Immunol. 2008;181:4397–4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leslie NR, Downes CP. PTEN: the down side of PI 3-kinase signalling. Cell Signal. 2002;14:285–295 [DOI] [PubMed] [Google Scholar]
- 40. Divecha N, Irvine RF. Phospholipid signaling. Cell. 1995;80:269–278 [DOI] [PubMed] [Google Scholar]
- 41. Kumar S, Nadeem S, Stan MN, Coenen M, Bahn RS. A stimulatory TSH receptor antibody enhances adipogenesis via phosphoinositide 3-kinase activation in orbital preadipocytes from patients with Graves' ophthalmopathy. J Mol Endocrinol. 2011;46:155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kumar S, Iyer S, Bauer H, Coenen M, Bahn RS. A stimulatory thyrotropin receptor antibody enhances hyaluronic acid synthesis in graves' orbital fibroblasts: inhibition by an IGF-I receptor blocking antibody. J Clin Endocrinol Metab. 2012;97:1681–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]