Abstract
The purpose of this study was to examine the three-dimensional (3-D) expression and distribution of anion transporters pendrin (SLC26A4) and anion exchanger (AE)4 (SLC4A9) in β-intercalated cells (β-ICs) of the rabbit cortical collecting duct (CCD) to better characterize the adaptation to acid-base disturbances. Confocal analysis and 3-D reconstruction of β-ICs, using identifiers of the nucleus and zona occludens, permitted the specific orientation of cells from normal, acidotic, and recovering rabbits, so that adaptive changes could be quantified and compared. The pendrin cap likely mediates apical Cl−/HCO3− exchange, but it was also found beneath the zona occludens and in early endosomes, some of which may recycle back to the apical membrane via Rab11a+ vesicles. Acidosis reduced the size of the pendrin cap, observed as a large decrease in cap volume above and below the zona occludens, and the volume of the Rab11a+ apical recycling compartment. Correction of the acidosis over 12–18 h reversed these changes. Consistent with its proposed function in the basolateral exit of Na+ via Na+-HCO3− cotransport, AE4 was expressed as a barrel-like structure in the lateral membrane of β-ICs. Acidosis reduced AE4 expression in β-ICs, but this was rapidly reversed during the recovery from acidosis. The coordinate regulation of pendrin and AE4 during acidosis and recovery is likely to affect the magnitude of acid-base and possibly Na+ transport across the CCD. In conclusion, acidosis induces a downregulation of AE expression in β-ICs and a diminished presence of pendrin in apical recycling endosomes.
Keywords: acidosis, alkalosis, anion exchangers, anion exchanger 4, confocal microscopy, rabbit
final regulation of acid-base transport occurs in the distal nephron of the kidney, particularly in the cortical collecting duct (CCD). We have previously shown in rabbits that acidosis results in increased proton secretion and decreased HCO3− secretion, respectively, by α- and β-intercalated cells (ICs) from isolated perfused CCDs (23, 25, 26, 29). Some of these adaptive changes occur via a downregulation of the apical Cl−/HCO3− exchanger, which mediates HCO3− secretion (17, 26). This exchanger in β-ICs was subsequently shown to be pendrin (SLC26A4) (22); it appears as an apical cap and clearly plays a role in acid-base regulation. However, other studies have shown that pendrin expression is also dependent on urinary Cl− excretion (11, 34) and, if unregulated, could cause hypertension (34). A study (21) of pendrin expression during acid-base disturbances revealed that the expression, mRNA, protein abundance, and number of pendrin-positive cells in rabbit kidneys were decreased after 3 days of acidosis. Similar findings have been observed in acidotic rats (20). These changes were reversed in kidneys taken from acidotic rabbits that had then been alkali loaded over 12–18 h (21). The rapid reversibility of the changes in pendrin expression suggests that mechanisms other than changes in pendrin gene expression may play key roles in the acidosis-induced reduction in apical pendrin activity.
Anion exchanger (AE)4 (SCL4A9) is a member of the SLC4 superfamily that exhibits more sequence homology with Na+-HCO3− cotransporters than with prototypical Cl−/HCO3− exchangers (28). There have been conflicting reports as to the subcellular localization of AE4 and as to whether AE4 expression is restricted to α- or β-ICs in the collecting duct (13, 28). Acid-base disturbances failed to influence the subcellular localization of AE4 in rat kidneys (13). Moreover, there has been no systematic evaluation of AE4 message or protein abundance under different acid-base conditions. We have recently shown that AE4 is localized basolaterally in rabbit and mouse β-ICs and mediates basolateral Na+-dependent HCO3− flux (6). Together with pendrin and SLC4A8, AE4 may facilitate salt absorption in the CCD and thereby contribute to the control of fluid homeostasis and blood pressure.
It was the purpose of the present study to systematically examine the three-dimensional (3-D) expression and distribution of AEs in β-ICs of isolated whole mounted CCDs taken from normal, acidotic, and recovering rabbits. Our goal was to better characterize some details of the adaptation to acid-base disturbances. Confocal analysis and 3-D reconstruction of these β-ICs, using identifiers of the nucleus, early and apical recycling endosomes, and zona occludens, allowed us to specifically orient these cells under the different conditions, so that adaptive changes could be quantified and compared. The results indicate that the pendrin cap is not simply an apically expressed protein but is also expressed subapically and below the zona occludens; it is also found constitutively in early endosomes. Alterations in cap size and area as well as the amount of pendrin in the apical recycling endosomal compartment reflect changes in the amount of pendrin that is available for transport within β-ICs. We also monitored changes in rabbit AE4 expression with acid-base disturbances and found that both pendrin and AE4 were regulated similarly under these conditions.
MATERIALS AND METHODS
Animals.
Female New Zealand White rabbits weighing 1.5–3.0 kg were maintained on standard rabbit chow and tap water (21). Acid loading was accomplished by providing 100 mM NH4Cl-7.5% sucrose solution for 3 days with food intake limited to 30 g rabbit chow/day. For rapid amelioration of acidosis (i.e., recovery), rabbits administered NH4Cl in the drinking water for 3 days were abruptly transitioned to 100 mM NaHCO3-7.5% sucrose for 12–18 h. The pH and serum HCO3− levels of blood samples taken from anaesthetized animals as well as the pH of urine collected directly from the bladder immediately after euthanasia were measured with a Radiometer blood gas analyzer (Copenhagen ABL5 model LB17493, Westlake, OH). The protocols for these animal experiments were submitted to and approved by the University Committee on Animal Rights of the University of Rochester Medical Center (UCAR-2005-199R).
Immunofluorescence staining of kidney tissue.
Rabbit kidneys were perfused with Dulbecco's PBS (dPBS; Life Technologies, Grand Island, NY) followed by periodate lysine paraformaldehyde fixation prepared as previously described (16). Kidney slices were cut perpendicular to the long axis (1–2 mm thickness) and immersion fixed in periodate lysine paraformaldehyde supplemented with 5% sucrose for 6–8 h at room temperature. Fixed tissues were embedded in paraffin, and 4- to 8-μm sections were baked onto positively charged glass slides by heating in an oven at 50–60°C for 30–60 min. After deparaffinization in Pro-Par clearant (Anatech, Battle Creek, MI), slides were rehydrated by passage through a decreasing ethanol series. Antigen retrieval through heating to 90 ± 5°C for 5–10 min in high-pH antigen unmasking solution (H-3301, Vector Labs, Burlingame, CA) was followed by blockade of nonspecific binding using 5% donkey serum (Jackson ImmunoResearch, Birmingham, AL) in PBS. Sections were then incubated with 1:50–1:100 dilutions of primary antibodies in PBS supplemented with 1% donkey serum. Primary antibodies included polyclonal rabbit anti-human SLC4A9 (Alpha Diagnostic, San Antonio, TX) and mouse monoclonal antibody IVF12 directed against AE1 (Iowa Hybridoma Bank, Iowa City, IA) (12). Sections were overlaid with antibody solution, covered with a small rectangle of parafilm, and incubated in a humidified chamber for ∼16 h at 4°C. Slides were then washed in PBS for 5–10 min with two buffer changes before incubation with a 1:1,000 dilution of secondary antibodies (donkey anti-mouse FITC, donkey anti-rabbit Dylight 649, Jackson ImmunoResearch) for 2–4 h at room temperature. Before being mounted, slides were washed in PBS for 10 min with two changes followed by a single rinse in water. After air drying, slides were mounted in fluorescence mounting medium (VectaShield, H-1000, Vector Labs). Slides were analyzed for green and far red fluorescence using a Nikon E400 fluorescent microscope and photographed at ×400 magnification using a Spot RT, model 7.0 monochrome camera and Spot RT software (Diagnostic Instruments, Sterling Heights, MI).
Immunofluorescence staining of microdissected CCDs.
CCDs were microdissected from the kidneys of normal, acid-loaded, and 16-h recovery rabbits and fixed for 15 min in a 1:4 dilution of Prefer concentrate in dPBS. After a wash in dPBS supplemented with 0.1% BSA, tubules were incubated overnight at 4°C with primary antibodies including polyclonal goat anti-pendrin (G-19, SC-23779, Santa Cruz Biotechnology, Santa Cruz, CA), IVF12 (AE1), mouse monoclonal antibodies to early endosomal antigen (EEA)-1 (BD Transduction Laboratories), Rab11a (clone 3D2F8, Abcam, Cambridge, MA), and polyclonal anti-AE4 (ADI) diluted in dPBS and supplemented with 5% donkey serum and 0.1% Triton-X-100. After a brief wash in dPBS-0.1% BSA, CCDs were incubated 2–4 h with secondary antibodies including donkey anti-rabbit, anti-mouse, or anti-goat conjugated with either far red (Dylight 649 or Alexa 647) or green (FITC or Alexa 488) fluorochromes (Jackson ImmunoResearch or Life Technologies). Lectin [peanut agglutinin (PNA)-FITC; Vector Labs] or zonula occludens (ZO)-1 (Alexa 488-conjugated mouse monoclonal antibody clone 1A12, Molecular Probes) staining was accomplished with a tertiary incubation for 0.5–1 h at 4°C in dPBS supplemented with 0.5% BSA and 0.1% Triton-X-100. CCDs were briefly washed and then mounted on slides by pipetting into mounting media (Prolong Gold antifade reagent with 4′,6-diamidino-2-phenylindole for staining nuclei, Molecular Probes, Life Technologies). Secure seal spacers (13-mm diameter × 0.12 mm thick, Electron Microscopy Sciences, Hatfield, PA) prevented the compression of CCDs under glass coverslips.
Confocal microscopy and image analysis.
Z-stack images of immunofluorescence-stained CCDs were obtained by collecting 0.4- to 1-μm optical sections using the ×60 objective of an Olympus FV1000 laser scanning confocal microscope (Center Valley, PA). The secure seal spacers increased the mounting media depth and caused some variation in the working distance, causing variable fluorescence quenching by the mounting media. Therefore, laser transmission and photomultiplier tube voltages were adjusted using the Ctrl-H function of Fluoview software so that the fluorescence was just below saturation to maintain consistency of the morphometric analyses. As a result, fluorescent intensity measurements could not be directly compared. 3-D reconstructions of individual ICs were performed in Fluoview FV1000 software. Imaris 7.4.2 (Bitplane, South Windsor, CT) was used to measure pendrin cap fluorescent intensities and volume (in μm3) as well as identifying EEA-1+ or Rab11a+ vesicular surfaces and measuring the vesicular volume and pendrin staining intensities within vesicular structures. This analysis was performed in consultation with Imaris technical support and did not include algorithm-based quantitification of colocalization. Since the identification of surface area involved establishing signal thresholds, confocal images were coded by an independent party, and thus analyses were performed without knowledge of the experimental condition. The size (in μm2) and length (in μm) of structures in digital images were also measured with the polygon line functions in ImageJ (National Institutes of Health). ImageJ size units were converted to square micrometers (areas) by determining the corresponding ImageJ unit area of rectangles stamped in images with Fluoview. The analytic functions of Fluoview software were used for measurements of AE4 and PNA morphometrics.
Analysis of steady-state mRNA levels by quantitative real-time RT-PCR.
Total RNA was isolated from 20–30 mg of the rabbit kidney cortex using the RNeasy Mini kit (Qiagen, Valencia, CA) with RNA-free DNase digestion according to protocols recommended by the manufacturer. cDNA was synthesized from 0.5–2 μg total RNA using the SuperScript first-strand synthesis system for RT-PCR, the Superscript III reverse transcriptase enzyme (Invitrogen, Carlsbad, CA), and random primers according to the manufacturer's recommended protocol. Specific primer sets and fluorogenic probes (TaqMan, fluorogenic 5′-nuclease chemistry) for rabbit pendrin and B1-V-ATPase were as previously described (21). The primer-probe set (forward primer: 5′-TGCCTTCTGCAGAGATTACAGCCT-3′, reverse primer: 5′-ATGAGGGCACAGAAACCTTCCTCA-3′, and probe: 5′-TGCTGGTGCGTTACTTCACCCGCTT-3′) specific for rabbit SLC4A9 (Accession No. NM_001082005) (28) was designed using algorithms provided by Integrated DNA Technologies (Coralville, IA) and synthesized by Sigma-Aldrich (St. Louis, MO). The relative abundance of pendrin (SLC26A4), AE4 (SLC4A9), and B1-subunit mRNAs was determined by quantitative real-time RT-PCR using the appropriate primer/probe sets and the Sequence Detection Systems 7500 instrument and software (Applied Biosystems, Carlsbad, CA). Serial dilutions of linearized plasmid DNA containing the gene-specific sequence were used to generate a standard curve from which the relative mRNA copy number was calculated.
Statistics.
Statistical significance of changes in acid-base transporter mRNA abundance and transporter staining was assessed by Student's t-test (two tails, two samples with equal variance). When three comparisons were performed, statistical significance was established as P ≤ 0.017 using the Bonferroni correction for the 95% confidence interval (35). A Mann-Whitney U-test was used to establish the confidence interval for changes in vesicular volumes and transporter staining within vesicular surfaces, since vesicular parameters frequency exhibited a non-normal distribution.
RESULTS
The pendrin cap extends beyond the apical region of β-ICs.
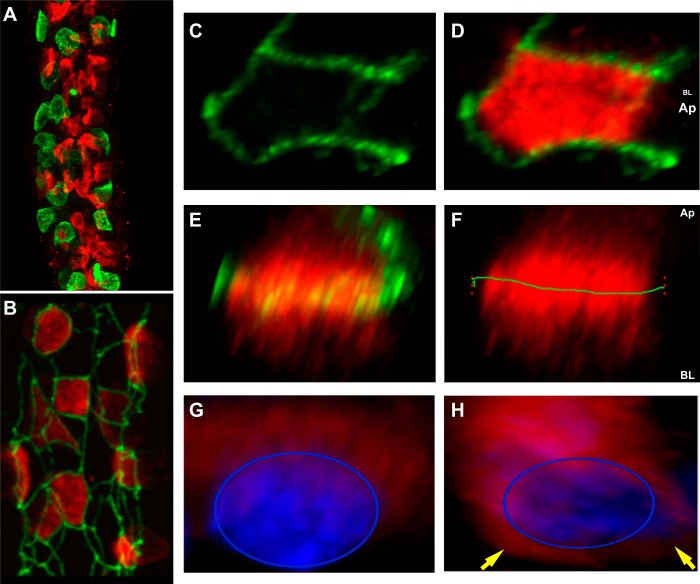
The apical Cl−/HCO3− exchange activity of β-ICs is mediated by pendrin (SLC26A4). In the rabbit CCD, pendrin expression is observed as a brightly stained crescent shape on the apical surface of β-ICs, designated as the pendrin cap. Previous studies from our laboratory and others have shown that pendrin expression at the mRNA and protein levels is regulated by changes in acid-base status (20, 21). In the present study, we examined the 3-D structure of the pendrin cap and quantified acid-base-induced changes in cap morphology and distribution to better understand the mechanisms through which pendrin activity is regulated. As shown in Fig. 1A, confocal microscopic analysis of microdissected CCDs stained for pendrin (red) and AE1 (green) revealed that β-ICs are the predominant IC subtype in the rabbit CCD, outnumbering AE1-positive α-ICs by at least 2:1. To characterize the pendrin cap distribution in β-ICs, microdissected CCDs were colabeled with antibody to ZO-1, a tight junction protein that delineates the apical domain from the basolateral domain of epithelial cells. Note that ZO-1 staining (green) of the rabbit CCD revealed a lattice-like network encircling pendrin caps (red; Fig. 1B). A look down on a 3-D reconstruction of individual β-ICs from Z-stack images showed ZO-1 bordering the pendrin cap (Fig. 1, C and D). When β-ICs were rotated to show a lateral perspective (apical surface up; Fig. 1E), the pendrin cap extended well below ZO-1 into the subapical region (Fig. 1F; the green line denotes ZO-1). Morphometric analysis of 98 cells in normal rabbit CCDs demonstrated that pendrin staining was equally distributed above and below ZO-1 (see Fig. 2). Additional evidence that pendrin staining is not restricted to the apical region is provided by CCDs stained for pendrin and nuclei, showing transporter distribution extending into the cytoplasmic space and overlapping with the nuclear staining (Fig. 1G; the blue ring delineates the nucleus). Occasionally, pendrin staining was also observed as a perinuclear ring pattern, as indicated by the yellow arrows in Fig. 1H, which may reflect altered trafficking as part of a synthetic or degradative pathway.
Fig. 1.
The pendrin cap extends beyond the apical region of β-intercalated cells (ICs). A and B: microdissected cortical collecting ducts (CCDs) from normal rabbit kidney stained for pendrin (red) and anion exchanger (AE)1 (green; A) or zonula occludens (ZO)-1 (green; B). Note that the intensely green dot in A is an adherent red blood cell. C and D: three-dimensional (3-D) reconstructions of an individual β-IC showing ZO-1 (green) bordering the pendrin cap (red). E and F: the same cell rotated such that ZO-1 demarcates the apical cell boundary, as illustrated by the green line across the pendrin cap (F). The green 1 refers to the region of interest in Fluoview software. G and H: 3-D reconstructions of individual β-IC viewed from the lateral perspective stained for pendrin (red) and nuclei (blue). Yellow arrows point to perinuclear pendrin staining extending beyond the pendrin cap region. AP, apical side; BL, basolateral side; BL/AP, apical surface in the foreground and basolateral surface in the background.
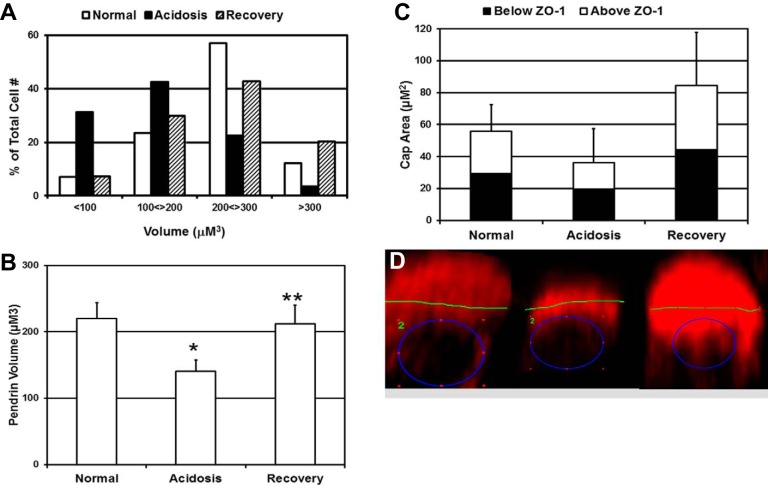
Fig. 2.
Acidosis reduces pendrin cap volume without changing distribution with respect to ZO-1. A and B: distribution (A) and means ± SE (B) for pendrin cap volumes measured in Z-stack images of microdissected CCDs obtained by confocal microscopy. Results presented are from 3 rabbits for each acid-base condition, a minimum of 3 microdissected CCDs from each rabbit, and a total of between 300 and 400 cells analyzed for each acid-base condition. C: mean ± SE values for pendrin cap area, above and below ZO-1, for the indicated acid-base condition. Results were from 2 rabbits per acid-base condition, 3 CCDs for each rabbit, and a total of 25–98 cells for each acid-base condition. *Normal → acidosis: P < 0.005; **acidosis → recovery: P < 0.005. D: ImageJ was used to measure pendrin cap areas (red) in two-dimensional images of individual β-ICs viewed from the lateral perspective. The blue circle defines the outer edge of nuclear staining, whereas the green line represents ZO-1 staining in the horizontal plane, delineating the apical surface. The green 2 represents the particular region of interest in Fluoview software. Left, cell from a normal rabbit; middle, cells from an acidotic rabbit; right, cell from a recovering rabbit. *Normal → acidosis: P < 0.005; **acidosis → recovery: P < 0.005.
Acidosis reduces pendrin cap volume without changing distribution with respect to ZO-1.
We have previously reported that reductions in pendrin mRNA and protein expression in response to acidosis were associated with a decrease in pendrin cap staining in rabbit kidney sections (21). In the present study, we confirmed and extended these results by examining the effect of acid-base status on the 3-D structure of the pendrin cap in β-ICs of microdissected CCDs. We compared the pendrin cap volume, measured with Imaris software, in CCDs from normal rabbits (normal: urine pH of 8.25 ± 0.24 and serum HCO3− of 27 ± 4 mM), rabbits administered NH4Cl for 3 days (acidosis: urine pH of 4.70 ± 0.4 and serum HCO3− of 16 ± 3 mM), and rabbits administered NH4Cl for 3 days and then abruptly transitioned to NaHCO3 for 12–18 h (recovery: urine pH of 8.1 ± 0.32 and serum HCO3− of 29 ± 6 mM). As shown in Fig. 2A, acidosis (solid bars) reduced the pendrin cap volume to the extent that 74% of β-ICs exhibited cap volumes of <200 μm3 (acidosis: mean cap volume of 141 ± 16 μm3) compared with 69% of β-ICs in the normal rabbit CCD with cap volumes of >200 μm3 (normal: mean cap volume of 220 ± 24 μm3; Fig. 2B). Recovery from acidosis restored the distribution of pendrin cap volumes to near normal (recovery: mean cap volume of 212 ± 94 μm3), and 63% (190 of 302 cells) of β-ICs had cap volumes of >200 μm3.
To determine whether acidosis altered the localization of pendrin within β-ICs, we examined the distribution of the cap area relative to ZO-1. 3-D reconstructions of individual β-ICs were oriented to view cells from the lateral perspective, as in Fig. 1, E and F, and saved as two-dimensional images from which pendrin cap areas above and below ZO-1 were measured with ImageJ (Fig. 2C). Acidosis reduced the pendrin cap area without shifting the cap distribution with respect to ZO-1; the pendrin cap was equally distributed above and below the zona occludens irrespective of acid-base status (Fig. 2D). Note that pendrin cap areas were larger than normal during recovery (n = 2 rabbits), which may have reflected increased synthesis of pendrin upon transition from an acidotic condition to an alkalotic condition. Thus, rather than a shift in subcellular distribution, changes in pendrin cap size most likely reflected a shift in the rate of pendrin degradation versus synthesis. During acidosis, this would result in a net decrease in the amount of pendrin available for transport at the apical surface of β-ICs.
Acidosis reduces the volume of Rab11a+ apical recycling endosomes but not EEA-1+ early endosomes in β-ICs.
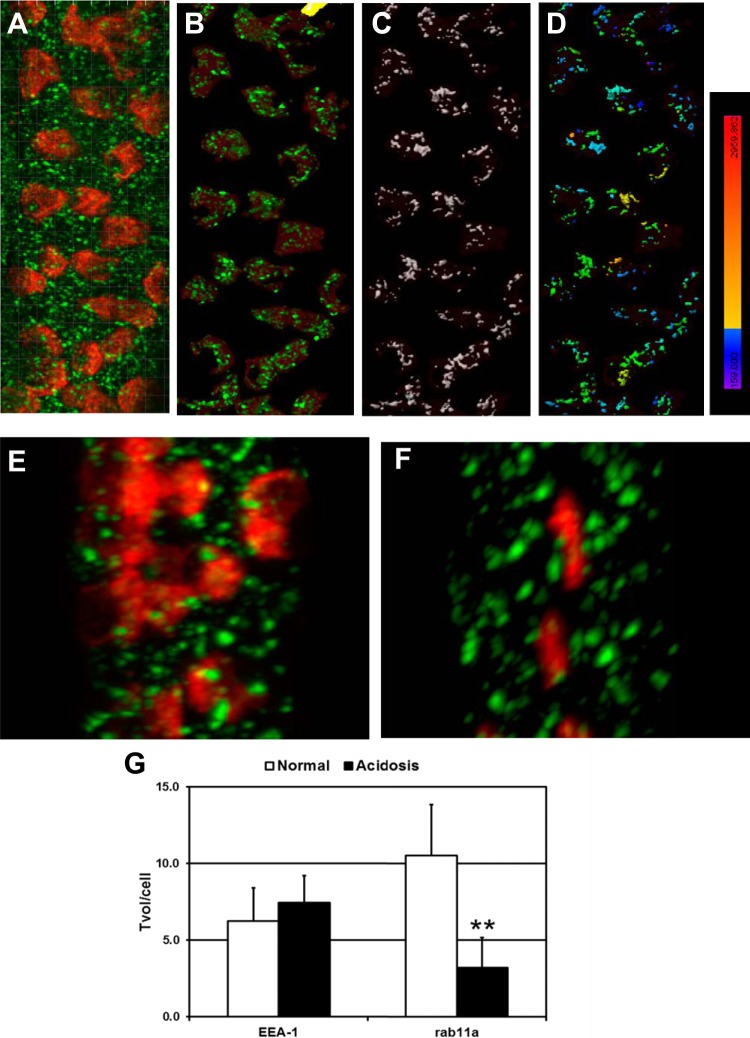
The results presented above suggest that the pendrin cap includes transporters located within vesicular structures. Therefore, we examined pendrin localization within early endosomes and apical recycling endosomal compartments at steady state in the normal condition and during acidosis. Microdissected CCDs were stained for pendrin and the early endosomal marker EEA-1 (7, 18, 27) or Rab11a, which is associated with apical recycling endosomes (15, 19, 32). The volume and pendrin staining intensity of vesicular compartments were quantified via measurements of spacial overlap with Imaris, as shown in Fig. 3. It is important to note that this approach is distinct from determining pixel overlap using colocalization algorithms. Pendrin cap surfaces (red) were identified in 3-D projections of Z-stacks with Imaris (Fig. 3A). EEA-1+ vesicles (green) located outside of pendrin surfaces were excluded from subsequent analyses, since EEA-1-positive vesicles in principle cells and α-ICs were not relevant to the analysis (Fig. 3B). EEA-1+ vesicle surfaces were then identified (white surfaces; Fig. 3C), and pendrin staining intensity in EEA-1+ vesicles was presented as a chromatic scale (Fig. 3D). Nearly all EEA-1+ vesicles contained pendrin signal, with a substantial proportion of vesicles (>90%) exhibiting a staining intensity of >800 (in arbitrary units). Staining of Rab11a+ vesicles in CCDs isolated from normal (Fig. 3E) and acidotic (Fig. 3F) rabbits revealed that, as with EEA-1, 95–99% of Rab11a+ surfaces contained pendrin signal with an intensity of >800 units. The mean fluorescent intensities (±SE) for pendrin caps and the respective vesicular stains in EEA-1+ or Rab11a+ surfaces were comparable (Table 1). Furthermore, acidosis did not alter the pendrin signal intensity of vesicular compartments.
Fig. 3.
Acidosis reduces the volume of the Rab11a+ apical recycling endosome compartment in β-ICs. A: 3-D projection of a microdissected CCD from normal rabbit kidney stained for pendrin (red) and early endosomal antigen (EEA)-1 (green). Note that colocalization algorithms were not used to identify pixel overlap between red and green stains in this image. B: identification of pendrin cap surfaces with Imaris software enabled the exclusion of EEA-1-labeled vesicles residing outside pendrin caps. C: demarcation of EEA-1 surfaces within pendrin cap boundaries. D: chromatic scale illustrating the intensity of pendrin staining with spatial overlap with EEA-1-labeled surfaces. The numeric values associated with the chromatic scale ranged from 159 (purple) to 2,960 (bright red). E and F: microdissected CCDs from normal (E) or acidotic (F) rabbit kidneys stained for pendrin (red) and Rab11a (green). G: total vesicular volume in β-ICs (Tvol/cell) for EEA-1+ and Rab11a+ compartments. Results are from up to 5 rabbits (n = 3–5), 9–17 CCDs, and 283–659 β-IC per acid-base condition. **The normal → acidosis Rab11a total volume per cell number is different at the 2% confidence interval (by Mann-Whitney U-Test).
Table 1.
Pendrin fluorescent intensities
| EEA-1 |
Rab11a |
|||
|---|---|---|---|---|
| Acid-Base Condition | Cap | Vesicular | Cap | Vesicular |
| Normal | 1,514 ± 90 | 1,541 ± 126 | 1,380 ± 124 | 1,462 ± 121 |
| Acidosis | 1,504 ± 74 | 1,461 ± 57 | 1,444 ± 96 | 1,437 ± 152 |
Values are mean fluorescent intensities ± SE. For early endosomal antigen-1 (EEA-1), n = 4 normal rabbits [10 cortical collecting ducts (CCDs)] and 5 acidotic rabbits (17 CCDs). For Rab11a, n = 3 normal rabbits (9 CCDs) and 3 acidotic rabbits (11 CCDs).
Acidosis reduced the volume of pendrin caps (Fig. 2 and Table 2) and also tended to reduce the volume of Rab11a+ vesicular structures by nearly 50% without changing that of the EEA-1+ early endosome compartment (Table 2). To assess a change in the overall size of the endosome compartment in β-ICs, the total vesicular volume per pendrin cap was calculated from the sum of the respective vesicular volumes divided the number of pendrin-positive cells for each CCD. As shown in Fig. 3G, acidosis did not affect EEA-1+ early endosomes but did induce a 3.3-fold reduction in the size of the Rab11a+ compartment. These results suggest that at steady state, pendrin is localized in early endosomes and apical recycling endosomes and that the pendrin cap volume reduction induced by acidosis is due in part to a decrease in the apical recycling compartment.
Table 2.
Pendrin cap and vesicular volumes
| EEA-1 |
Rab11a |
|||
|---|---|---|---|---|
| Acid-Base Condition | Pendrin cap volume, μm3 | Vesicular volume, μm3 | Pendrin cap volume, μm3 | Vesicular volume, μm3 |
| Normal | 175 ± 18 | 1.1 ± 0.2 | 183 ± 20 | 2.0 ± 0.6 |
| Acidosis | 125 ± 15* | 1.3 ± 0.2 | 117 ± 12* | 1.1 ± 0.2† |
Values are means ± SE.
Normal → acidosis: P < 0.002;
significant at the 10% level (by Mann-Whitney U-test).
AE4 (SLC4A9) is expressed as a “barrel-like structure” in the basolateral membrane of β-ICs.
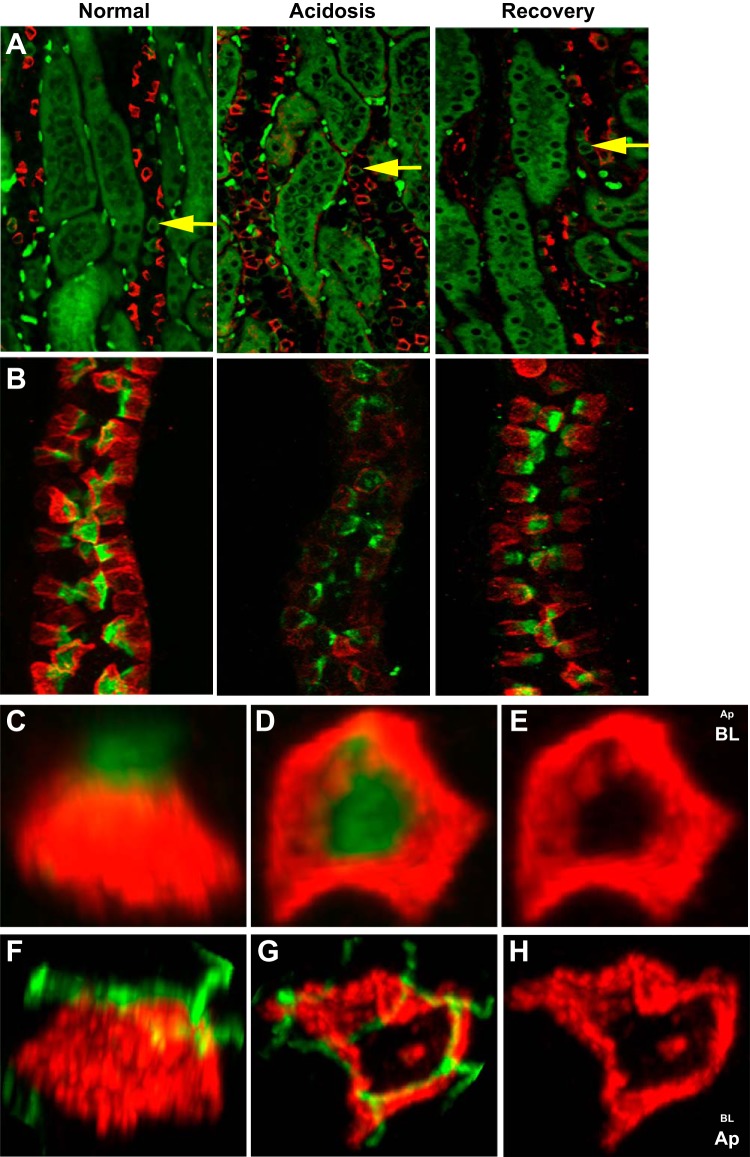
Immunofluorescent staining of rabbit kidney sections for both AE4 (red) and AE1 (green) showed that AE4-expressing cells were limited to the CCD and were not colocalized with AE1 (Fig. 4A) (6). The rank order of AE4 mRNA abundance was the cortex >> outer medulla > inner medulla (data not shown), suggesting that AE4 expression was restricted primarily to β-ICs. The AE4 expression pattern in kidney sections frequently appeared as a ring-like pattern. Although occasionally an apparent apical distribution was observed (see Fig. 4A, right), this most likely reflects the cell orientation (e.g., angle) with respect to the slice through the CCD to produce the thin section (see below). Microdissected CCDs stained with PNA, which specifically labels β-ICs in the rabbit (24, 25), showed strong labeling by AE4, but there was no colabeling (Fig. 4B). AE4 stain was consistently detected below the zona occludens and in a subcellular region distinct from the PNA cap (Fig. 4C and see below). 3-D reconstructions of individual β-ICs labeled with PNA (Fig. 4, C and D) or ZO-1 antibody (Fig. 4, F and G) were performed to rigorously assess the distribution of AE4 in β-ICs. AE4 expression (red) extended well below the apical surface identified by the PNA cap (green; Fig. 4C). Rotation of the cell so that the PNA cap faced the background revealed that AE4 was distributed as a hollow barrel-like structure upon which the apical PNA cap rested, much like ice cream in a cone (Fig. 4, D and E). The lateral view of β-ICs stained for AE4 (red) and ZO-1 (green; Fig. 4F) as well as images of the same cell rotated such that the apical surface and ZO-1 faced the foreground (Fig. 4, G and H) clearly illustrated that AE4 expression was localized below ZO-1. The cellular distribution of AE4 in β-ICs was similar to that observed for AE1 in α-ICs (data not shown), indicating that AE4 functions in transport across the basolateral membrane of β-ICs (6).
Fig. 4.
AE4 (SLC4A9) is expressed as a “barrel-like structure” localized below ZO-1 in β-ICs. A: kidney sections prepared from rabbits with the indicated acid-base conditions were stained for AE4 (red) and AE1 (green) and photographed as described in materials and methods. Yellow arrows point to representative AE1-positive cells located in CCDs. The intensely stained green dots in the interstitium are red blood cells. B: microdissected CCDs isolated from rabbits with the indicated acid-base conditions and stained with peanut agglutinin (PNA; green) and antibody directed against AE4 (red). Z-stack images were obtained by confocal microscopy. C–E: 3-D reconstructions of an individual β-IC from a normal rabbit CCD stained as in B. C: lateral view of a β-IC. D: 90° rotation from the lateral view such that the PNA cap is facing the background and the basolateral surface is in the foreground. E: AE4 distribution sans the PNA cap. F–H: individual β-ICs from a normal rabbit CCD stained for AE4 (red) and ZO-1 (green). F: lateral view of a β-IC. G: 90° rotation bringing ZO-1 and the apical surface to the foreground. H: AE4 distribution sans ZO-1.
Acidosis reduces AE4 (SLC4A9) expression in β-ICs.
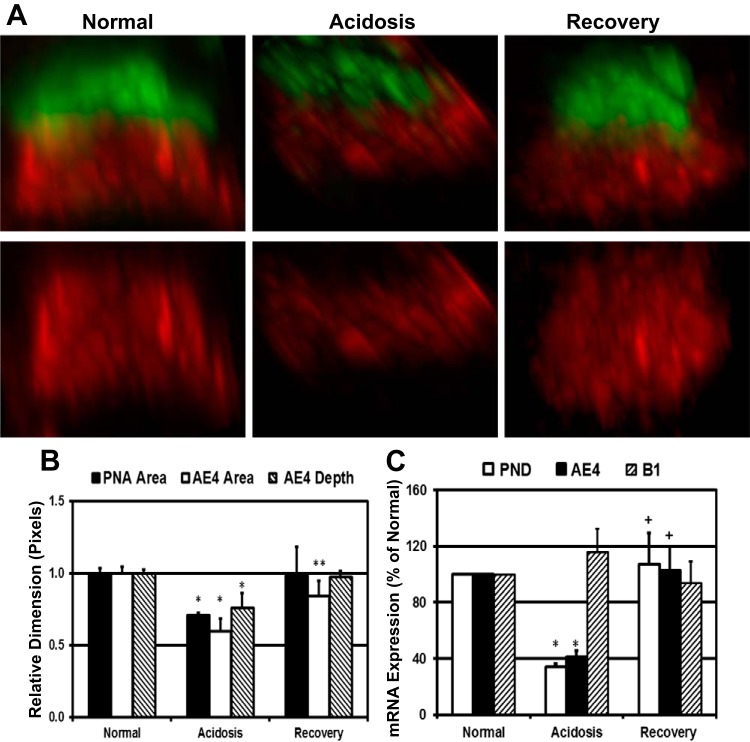
A previous study (13) in rats suggested that AE4 localization was not influenced by acid-base status; however, given the importance of β-ICs in the regulation of HCO3− transport in the CCD, we sought to ascertain the effect of acidosis on AE4 expression in the rabbit CCD. Although stained kidney sections did not reveal dramatic changes in AE4 expression in response to acidosis or upon recovery (Fig. 4A, top, middle, and bottom), AE4 staining in microdissected CCDs isolated from kidneys of acidotic rabbits was consistently attenuated compared with CCDs from normal or recovery animals (Fig. 4, A and B). Staining variations among individual CCDs frequently required the adjustment of acquisition parameters for confocal microscopy and thus precluded a simple comparison of AE4 intensities associated with acid-base conditions. Therefore, morphometric analysis of 3-D reconstructions of individual β-ICs was used to assess the effect of acidosis and its recovery on AE4 expression. Images of β-ICs from a lateral view showed a reduction in the PNA cap and AE4 distribution in response to acidosis and restoration of the PNA cap and AE4 structure upon recovery (Fig. 5A, middle compared with right and left). Using Fluoview, the PNA cap area as well as the AE4 area and depth were measured from images of between 100 and 300 cells from 4 to 6 rabbits for each acid-base condition. Typically, two to three microdissected CCDs were analyzed for each rabbit. To present PNA cap and AE4 staining morphology on the same graph, the value average for normal rabbits was set to 1 (Fig. 5B). Acidosis reduced the PNA cap and AE4 areas by 29% and 40%, respectively. A reduction of AE4 expression was also observed as a 24% reduction in staining depth (normal → acidosis: P < 0.0125). Recovery from acidosis restored PNA cap and AE4 morphology to near normal levels.
Fig. 5.
Acidosis reversibly reduces AE4 (SLC4A9) expression in β-ICs. A: 3-D reconstructions of confocal images from representative β-ICs within microdissected CCDs from rabbit kidneys adapted to the specified acid-base conditions. Top, lateral views of β-ICs stained with AE4 antibody (red) and PNA (green); bottom, AE4 structure sans PNA. B: morphometric analysis of the PNA cap and AE4 staining in the lateral view using Fluoview. For comparison, the average value for normal rabbit CCDs was set to 1. PNA cap area measurements were derived from 2–3 rabbits for each acid-base condition; AE4 morphometrics were generated from 4–6 rabbits/condition. In each case, 2–3 microdissected CCDs from each rabbit were analyzed. Total number of cells analyzed for AE4 morphometrics were as follows: normal, 194; acidosis, 139; and recovery, 265. *Normal → acidosis: P < 0.0125; **acidosis → recovery: P < 0.016. C: relative abundance of pendrin and AE4 mRNA in the rabbit kidney cortex during the specified acid-base conditions. The average pendrin, AE4, and B1-V-ATPase mRNA copy number in the cortex from normal rabbits was set to 100%. Normal: n = 9 rabbits/condition, acidosis: n = 7 rabbits/condition, and recovery: n = 7 rabbits/condition. *Normal → acidosis: P < 0.001; +acidosis → recovery: P < 0.015.
To confirm the regulation of AE4 expression by acid-base status, we compared steady-state levels of AE4 and pendrin mRNA in the rabbit kidney cortex by quantitative RT-PCR. The abundance of B1-V-ATPase mRNA was unaffected by acidosis or recovery from acidosis (4, 21), and thus relative B1-V-ATPase abundance provided an assurance of comparable IC sampling in kidney cortex preparations normalized for total RNA input for which ubiquitous housekeeping gene (e.g., GAPDH) expression is consistently equivalent. To show changes in pendrin and AE4 mRNA abundance on the same graph, the relative copy number for pendrin, AE4, and B-1-V-ATPase abundance mRNA in normal rabbits was set to 100% (Fig. 5C). Acidosis reduced pendrin and AE4 mRNA abundance by 66 ± 3.4% and 59 ± 5.6%, respectively (normal → acidosis: P < 0.001), and recovery from acidosis restored pendrin and AE4 mRNA to essentially normal levels (normal → recovery for pendrin: P > 0.1 and normal → recovery for AE4: P > 0.85). The coordinated regulation of pendrin and AE4 expression by acid-base status suggests that these transporters collaborate in the regulation of ion transport across β-ICs.
DISCUSSION
In our previous report, we characterized changes in pendrin cap expression in kidney sections. We found that acidosis reversibly reduced pendrin staining intensity and the apparent pendrin cap size, which reflects, in part, a reduction in steady-state protein and mRNA expression in β-ICs (21). It was also plausible that the change in cap morphology was caused by the redistribution of pendrin from the apical region of β-ICs concomitant with a reduction in apical Cl−/HCO3− exchange at the surface (23). However, imaging kidney sections does not allow a detailed investigation of epithelial cell morphology, since a 3-D tubular structure is reduced to a two-dimensional cross-section of between 4 and 10 μm. In addition, the orientation of the cut with respect to cell axes is variable due to differential positioning of ICs within the cylindrical structure of the collecting duct. Therefore, morphological analysis of cellular structure in sections can produce substantial artifacts. For example, studies in our laboratory (see Fig. 4A) and others have suggested that AE4 expression in kidney sections is either apical or basolateral or perhaps both (6, 13, 28). Figure 4A in the present study shows AE4 staining in kidney sections that is consistent with a lateral distribution; however, AE4 staining in sections frequently appears apical (arrows). This observation prompted an investigation of whether the distribution of AE4 is regulated by acid-base status. We performed extensive morphometric analysis of kidney sections from normal, acidosis, and recovery from acidosis. The analysis of thousands of cells suggested that there was a small shift toward an apparent apical AE4 distribution upon recovery from acidosis (unpublished results). It was only after careful 3-D morphometric analysis of AE4 with respect to other structural proteins defining cell polarity (e.g., ZO-1) that we discovered that AE4 expression occurs below ZO-1 regardless of acid-base status. Thus, we concluded that the apical distribution of AE4 observed in kidney sections was an artifact of the tissue preparation.
The present study focused on the morphology of pendrin and AE4 expression in β-ICs that manifests during well-defined steady-state acid-base conditions. Our present approach is to isolate CCDs, fix and stain for acid-base transporters, and prepare 0.4- to 1-μm optical sections via confocal microscopy. To preserve the cylindrical shape, we mounted CCDs on slides separated from the coverslip by 120-μm-thick spacers; the ZO-1 label was used to orient each cell and distinguish apical from basolateral domains. These experiments confirmed some aspects of pendrin regulation by acid-base status, and described below are novel findings regarding the structure of the pendrin cap and AE4 distribution as well as how these parameters are modulated by acid/base status. First, the pendrin cap includes transporter expression beyond apical surface expression (Figs. 1 and 2) and includes transporter trafficking via the EEA-1+ early endosome [associated with Rab5 (15) and Rab11a+ apical recycling endosomes (Fig. 3)]. Second, acidosis reduced the volume of the pendrin cap expressed in β-ICs without changing the distribution of the structure with respect to ZO-1 (Fig. 2, C and D), and the reduction in pendrin cap volume was due in part to a reduction of the Rab11a+ apical recycling compartment (Fig. 3). Third, AE4 exhibited a lateral distribution in β-ICs, below the tight junction, and expression, but not distribution, was altered by acid-base status. Fourth, steady-state levels of AE4 mRNA were coordinately regulated with pendrin in response to a perturbation of the acid-base status. Finally, and perhaps most importantly, the present study provides a proof of concept for the utilization of 3-D morphometric analysis as an approach for the study of IC structure, function, and adaptation.
Using a 3-D morphology-based approach, we found that the pendrin cap on β-ICs extended beyond the apical region. Figure 1 shows pendrin expression in the cytoplasm well inferior to the ZO-1 marker. This area between the nucleus and apical cap may serve as a reservoir of pendrin transporters, for a rapid response to sudden HCO3− loads, or to help reclaim additional NaCl in the setting of acute volume contraction. Acidosis reduced the volume of the pendrin cap in β-ICs without changing the distribution of the structure with respect to ZO-1 (Fig. 2, C and D). It is known that acidosis and low pH decrease the rate of HCO3− secretion and apical Cl−/HCO3− exchange (17, 20, 26, 30, 37). Our experiments in individual cells showed that acidosis reduced the pendrin cap size and cap volume without changing the ratio of pendrin staining between apical and subapical domains (∼50%:50%). Recovery from acidosis, accomplished by 12–18 h of HCO3− loading, fully reversed the changes in pendrin cap size and volume without affecting the ratio of apical to subapical distribution. We cannot formally exclude retraction of pendrin from the apical surface or perhaps retraction of apical microvilli as the cause for the reduction in pendrin area above ZO-1, but pendrin distribution below ZO-1 is more likely reduced by a decrease in the pendrin trafficking pool, which reflects the balance between steady-state synthesis and degradation (Figs. 2, C and D, and 5C).
The overall volume of the pendrin cap also reflects fluctuations in the amount of pendrin in vesicular structures that mediate pendrin trafficking. The pendrin cap is composed of transporters localized in vesicular structures including EEA-1+ early endosomes and apical recycling endosomes associated with Rab11a. As shown in Fig. 3, pendrin staining intensity spatially overlapped with both EEA-1+ and Rab11a+ vesicular compartments in the normal condition in which net HCO3− secretion occurs in the rabbit CCD (23, 26). These results represent the current understanding of the “normal” steady state in which pendrin is endocytosed through EEA-1+ vesicles and a portion of this pool is returned to the apical surface via apical recycling endosomes (15, 19, 32, 36). The acidotic condition establishes a new steady state in which the size of the early endosomal pendrin pool is unchanged, whereas the Rab11a+ apical recycling endosome volume is decreased (Fig. 3G). Thus, the amount of endocytosed pendrin potentially available for return to the apical surface is substantially reduced. It is plausible that in response to acidosis, pendrin localized in early endosomes is redirected toward vesicular components associated with degradative pathways (2). Reversal of acidosis is likely to result in a new steady state in which the early endocytic pool of pendrin is unchanged but the Rab11a+ apical recycling endosomal volume is increased. Pendrin synthesis is increased to accommodate this adaptation.
We extended our observations to AE4 (SLC4A9) because of its role in distal nephron salt and proton transport (6). AE4 is primarily expressed by PNA-positive β-ICs in the form of a barrel-like structure, as shown in Fig. 4. There was little basal staining when the cell was looked down on (Fig. 4E), and so the expression was mainly lateral and below the ZO-1 demarcation; this differs from AE1 distribution in α-ICs, where there is typically basal and lateral localization (data not shown). The significance of strictly lateral localization of AE4 is not obvious; however, we can speculate that this lateral location might be the result of an interaction with scaffolding, the cytoskeleton, or chaperone proteins in the cell, such as cadherins, which are also laterally located (9). Alternatively, the lateral location might move the transporter closer to the substrates entering via apical transporters. AE4 expression was restricted to β-ICs, as there was no colabeling with AE1. Acidosis caused remodeling of this transporter as well, such that there was less AE4-labeled staining by measurement of either area or depth (Fig. 5B). However, this remodeling was not accompanied by a change in AE4 distribution with respect to ZO-1, demonstrating that polarization of AE4 expression is not regulated by acid-base status, as has been suggested by a study of AE4 expression in kidney sections (28). AE4 expression returned essentially to normal after 12–18 h of HCO3− loading. The larger changes in microdissected CCDs compared with those in sections suggests the possibility that acidosis destabilized AE4 during the microdissection of CCDs. However, it is more likely that examination of microdissected CCDs provides more reliable information than that obtainable from sections. There are several reasons for this expectation. First, we maintained 3-D morphology by using spacers to alleviate compression of the tissue. Second, the cross-sectioning was performed digitally rather than physically, so that cellular and tubular morphology could be viewed in its entirety and was not subjected to mechanical disruption. Third, the 3-D volumetric software enabled viewing ICs from multiple perspectives. Finally, the 3-D software allowed the quantitation of multiple parameters for thousands of identified cells.
We have found that steady-state levels of AE4 mRNA are coordinately regulated with pendrin in response to a perturbation of the acid-base status. When mRNA was isolated from the kidney cortex, it was apparent (Fig. 5C) that AE4 mRNA was regulated similarly to pendrin, decreasing by ∼60% during acidosis and recovering quickly to normal after HCO3− loading. We suspect that in the mouse, AE4 is coordinately regulated with pendrin in β-ICs, where it mediates electroneutral NaCl reabsorption coupled with apical transport activity of pendrin and Slc4A8 (6, 10). The function of Slc4A8 has not yet been investigated in rabbit CCDs. In mice lacking the forkhead transcription factor Foxi1, both AE4 and pendrin are not expressed (5), suggesting that Foxi1 is a regulator of β-IC anion transporter expression. Therefore, it was not surprising to observe coordinated regulation of AE4 and pendrin in response to acidosis and alkalosis. Indeed, Foxi1 directly activates the AE4 promoter in vitro (14); in addition, low pH decreases and high pH increases human pendrin gene expression in transfected kidney cells (1). Acid loading for 24–48 h causes a reduction in pendrin protein expression in the mouse kidney along with a shift of expression from the apical membrane to the cytosol (33), and these changes were reversed with alkali loading (33). Thus, there appears to be coordinated regulation of pendrin and AE4 via both transcriptional and nontranscriptional mechanisms.
Previous studies that investigated the influence of acid-base status of IC morphology have interpreted structural adaptation in terms of the changes in apical surface area and degree of apical endocytosis versus exocytosis. Chronic acidosis in rats induced by acetazolamide induced more developed apical microvilli and increased α-IC size, bulk, and protrusion into the tubule lumen while possibly decreasing the number of intracellular vesicles (3). High-density plating of clone C ICs in culture changed the shape of ICs from low and flat, appearing to tall and columnar with exuberant apical surface infolding and microvilli (31) reminiscent of the ultrastructure of cells actively involved in luminal H+ secretion. In an earlier study of rat ICs (presumably acid-secreting ICs) using ultrastructural analysis, Dørup (8) found that acidosis resulted in a higher surface density of the luminal membranes and that alkalosis reduced the surface density. He concluded that the luminal membrane was internalized during alkalosis and that the membrane that bounds apical vesicles is transferred to the luminal membrane during acidosis. Similar endocytosis of apical membrane (and functional Cl−/HCO3− exchangers) was seen in rabbit β-ICs subjected to acid incubation (23). The present study is the first to define alteration in the steady-state size of the vesicular compartment, specifically Rab11a+ recycling endosomes, within β-ICs during acidosis and thereby provides a proof of concept for utilization of these 3-D morphometric analyses as a tool for the study of vesicular structure and function.
GRANTS
G. J. Schwartz was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-050603. E. V. Heintz was a Strong Children's Summer Scholar and was funded in part by the Department of Pediatrics, University of Rochester Medical Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.M.P. and G.J.S. conception and design of research; J.M.P., E.V.H., A.N., and G.J.S. performed experiments; J.M.P., E.V.H., A.N., and G.J.S. analyzed data; J.M.P. and G.J.S. interpreted results of experiments; J.M.P. prepared figures; J.M.P. and G.J.S. drafted manuscript; J.M.P. and G.J.S. edited and revised manuscript; J.M.P., A.N., and G.J.S. approved final version of manuscript.
REFERENCES
- 1.Adler L, Efrati E, Zelikovic I. Molecular mechanisms of epithelial cell-specific expression and regulation of the human anion exchanger (pendrin) gene. Am J Physiol Cell Physiol 294: C1261–C1276, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Apodaca G, Gallo LI, Bryant DM. Role of membrane traffic in the generation of epithelial cell asymmetry. Nat Cell Biol 14: 1235–1243, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagnis C, Marshansky V, Breton S, Brown D. Remodeling the cellular profile of collecting ducts by chronic carbonic anhydrase inhibition. Am J Physiol Renal Physiol 280: F437–F448, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Bastani B, Purcell H, Hemken P, Trigg D, Gluck S. Expression and distribution of renal vacuolar proton-translocating adenosine triphosphatase in response to chronic acid and alkali loads in the rat. J Clin Invest 88: 126–136, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blomqvist SR, Vidarsson H, Fitzgerald S, Johansson BR, Ollerstam A, Brown R, Persson AE, Bergstrom G, Enerback S. Distal renal tubular acidosis in mice that lack the forkhead transcription factor Foxi1. J Clin Invest 113: 1560–1570, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambrey R, Kurth I, Peti-Peterdi J, Houillier P, Purkerson JM, Leviel F, Hentschke M, Zdebik AA, Schwartz GJ, Hubner CA, Eladari D. Renal intercalated cells are rather energized by a proton than a sodium pump. Proc Natl Acad Sci USA 110: 7928–7933, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christoforidis S, McBride HM, Burgoyne RD, Zerial M. The Rab5 effector EEA1 is a core component of endosome docking. Nature 397: 621–625, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Dørup J. Structural adaptation of intercalated cells in rat renal cortex to acute metabolic acidosis and alkalosis. J Ultrastruct Res 92: 119–131, 1985 [DOI] [PubMed] [Google Scholar]
- 9.Eaton S, Simons K. Apical, basal, and lateral cues for epithelial polarization. Cell 82: 5–8, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Eladari D, Chambrey R, Peti-Peterdi J. A new look at electrolyte transport in the distal tubule. Annu Rev Physiol 74: 325–349, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hafner P, Grimaldi R, Capuano P, Capasso G, Wagner CA. Pendrin in the mouse kidney is primarily regulated by Cl− excretion but also by systemic metabolic acidosis. Am J Physiol Cell Physiol 295: C1658–C1667, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Jennings ML, Anderson MP, Monaghan R. Monoclonal antibodies against human erythrocyte band 3 protein. Localization of proteolytic cleavage sites and stilbenedisulfonate-binding lysine residues. J Biol Chem 261: 9002–9010, 1986 [PubMed] [Google Scholar]
- 13.Ko SB, Luo X, Hager H, Rojek A, Choi JY, Licht C, Suzuki M, Muallem S, Nielsen S, Ishibashi K. AE4 is a DIDS-sensitive Cl−/HCO3− exchanger in the basolateral membrane of the renal CCD and the SMG duct. Am J Physiol Cell Physiol 283: C1206–C1218, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Kurth I, Hentschke M, Hentschke S, Borgmeyer U, Gal A, Hubner CA. The forkhead transcription factor Foxi1 directly activates the AE4 promoter. Biochem J 393: 277–283, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol 5: 121–132, 2004 [DOI] [PubMed] [Google Scholar]
- 16.McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem 22: 1077–1083, 1974 [DOI] [PubMed] [Google Scholar]
- 17.Merot J, Giebisch G, Geibel J. Intracellular acidification induces Cl/HCO3 exchange activity in the basolateral membrane of β-intercalated cells of the rabbit cortical collecting duct. J Membr Biol 159: 253–262, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Mu FT, Callaghan JM, Steele-Mortimer O, Stenmark H, Parton RG, Campbell PL, McCluskey J, Yeo JP, Tock EP, Toh BH. EEA1, an early endosome-associated protein. EEA1 is a conserved α-helical peripheral membrane protein flanked by cysteine “fingers” and contains a calmodulin-binding IQ motif. J Biol Chem 270: 13503–13511, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Parent A, Hamelin E, Germain P, Parent JL. Rab11 regulates the recycling of the β2-adrenergic receptor through a direct interaction. Biochem J 418: 163–172, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Petrovic S, Wang Z, Ma L, Soleimani M. Regulation of the apical Cl−/HCO3− exchanger pendrin in rat cortical collecting duct in metabolic acidosis. Am J Physiol Renal Physiol 284: F103–F112, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Purkerson JM, Tsuruoka S, Suter DZ, Nakamori A, Schwartz GJ. Adaptation to metabolic acidosis and its recovery are associated with changes in anion exchanger distribution and expression in the cortical collecting duct. Kidney Int 78: 993–1005, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED. Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci USA 98: 4221–4226, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Satlin LM, Schwartz GJ. Cellular remodeling of HCO3−-secreting cells in rabbit renal collecting duct in response to an acidic environment. J Cell Biol 109: 1279–1288, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuster VL, Bonsib SM, Jennings ML. Two types of collecting duct mitochondria-rich (intercalated) cells: lectin and band 3 cytochemistry. Am J Physiol Cell Physiol 251: C347–C355, 1986 [DOI] [PubMed] [Google Scholar]
- 25.Schwartz GJ, Barasch J, Al-Awqati Q. Plasticity of functional epithelial polarity. Nature 318: 368–371, 1985 [DOI] [PubMed] [Google Scholar]
- 26.Schwartz GJ, Tsuruoka S, Vijayakumar S, Petrovic S, Mian A, Al-Awqati Q. Acid incubation reverses the polarity of intercalated cell transporters, an effect mediated by hensin. J Clin Invest 109: 89–99, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simonsen A, Lippe R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, Stenmark H. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 394: 494–498, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Tsuganezawa H, Kobayashi K, Iyori M, Araki T, Koizumi A, Watanabe SI, Kaneko A, Fukao T, Monkawa T, Yoshida T, Kim DK, Kanai Y, Endou H, Hayashi M, Saruta T. A new member of the HCO3− transporter superfamily is an apical anion exchanger of β-intercalated cells in the kidney. J Biol Chem 276: 8180–8189, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Tsuruoka S, Schwartz GJ. Adaptation of rabbit cortical collecting duct HCO3− transport to metabolic acidosis in vitro. J Clin Invest 97: 1076–1084, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsuruoka S, Schwartz GJ. Metabolic acidosis stimulates H+ secretion in the rabbit outer medullary collecting duct (inner stripe) of the kidney. J Clin Invest 99: 1420–1431, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vijayakumar S, Takito J, Hikita C, Al-Awqati Q. Hensin remodels the apical cytoskeleton and induces columnarization of intercalated epithelial cells: processes that resemble terminal differentiation. J Cell Biol 144: 1057–1067, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vossenkamper A, Nedvetsky PI, Wiesner B, Furkert J, Rosenthal W, Klussmann E. Microtubules are needed for the perinuclear positioning of aquaporin-2 after its endocytic retrieval in renal principal cells. Am J Physiol Cell Physiol 293: C1129–C1138, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Wagner CA, Finberg KE, Stehberger PA, Lifton RP, Giebisch GH, Aronson PS, Geibel JP. Regulation of the expression of the Cl−/anion exchanger pendrin in mouse kidney by acid-base status. Kidney Int 62: 2109–2117, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Wall SM, Kim YH, Stanley L, Glapion DM, Everett LA, Green ED, Verlander JW. NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: role in Cl− conservation. Hypertension 44: 982–987, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Wallenstein S, Zucker CL, Fleiss JL. Some statistical methods useful in circulation research. Circ Res 47: 1–9, 1980 [DOI] [PubMed] [Google Scholar]
- 36.Woodman PG. Biogenesis of the sorting endosome: the role of Rab5. Traffic 1: 695–701, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Yasoshima K, Satlin LM, Schwartz GJ. Adaptation of rabbit cortical collecting duct to in vitro acid incubation. Am J Physiol Renal Fluid Electrolyte Physiol 263: F749–F756, 1992 [DOI] [PubMed] [Google Scholar]