Abstract
The present study examined whether 20-HETE production is reduced in the renal vasculature and whether this impairs myogenic or tubuloglomerular feedback (TGF) responses of the afferent arteriole (Af-Art). The production of 20-HETE was 73% lower in renal microvessels of Dahl salt-sensitive rats (SS) rats than in SS.5BN rats, in which chromosome 5 from the Brown Norway (BN) rat containing the CYP4A genes was transferred into the SS genetic background. The luminal diameter of the Af-Art decreased by 14.7 ± 1.5% in SS.5BN rats when the perfusion pressure was increased from 60 to 120 mmHg, but it remained unaltered in SS rats. Administration of an adenosine type 1 receptor agonist (CCPA, 1 μM) reduced the diameter of the Af-Art in the SS.5BN rats by 44 ± 2%, whereas the diameter of the Af-Art of SS rats was unaltered. Autoregulation of renal blood flow (RBF) and glomerular capillary pressure (PGC) was significantly impaired in SS rats but was intact in SS.5BN rats. Administration of a 20-HETE synthesis inhibitor, HET0016 (1 μM), completely blocked the myogenic and adenosine responses in the Af-Art and autoregulation of RBF and PGC in SS.5BN rats, but it had no effect in SS rats. These data indicate that a deficiency in the formation of 20-HETE in renal microvessels impairs the reactivity of the Af-Art of SS rats and likely contributes to the development of hypertension induced renal injury.
Keywords: myogenic response, TGF, renal injury, glomerulus
dahl salt-sensitive (ss) rats are salt sensitive (27), insulin resistant (28), hyperlipidemic (24, 26) and rapidly develop proteinuria and glomerulosclerosis when challenged with a high-salt diet (9, 29, 31). Previous studies from our laboratory have revealed that a deficiency in the renal formation of 20-hydroxyeicosatetraenoic acid (20-HETE) in SS rats enhances sodium transport in the thick ascending loop of Henle (15, 17, 18, 30) and contributes to the development of hypertension. Moreover, transfer of chromosome (Chr) 5 containing the CYP4A genes that produce 20-HETE from Brown Norway (BN) rats onto the SS genetic background (SS.5BN strain) increases the renal production of 20-HETE and attenuates the development of hypertension and proteinuria (39).
However, 20-HETE is also a potent vasoconstrictor (2), and we have recently reported that 20-HETE inhibitors and antagonists block both the myogenic and tubuloglomerular feedback (TGF) responses in the isolated, perfused afferent arteriole (Af-Art) of rabbits and mice (12). Thus the purpose of the present study was to determine whether the formation of 20-HETE is also reduced in the renal vasculature of SS rats and whether this contributes to the development of hypertension-induced renal injury by impairing the myogenic and TGF responses of the Af-Art and the transmission of pressure to the glomerulus.
METHODS
General
Experiments were conducted in 6- to 12-wk-old male SS and SS.5BN consomic rats in which Chr 5 was introgressed into the SS background as previously described (6, 7). The animals were bred and housed in the Laboratory Animal Facilities at the University of Mississippi Medical Center and received a Teklab 7034 sterilizable grain-based, low-salt diet containing 0.3% NaCl (Harland Laboratories, Madison, WI). All protocols were approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center and were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Protocol 1: Comparison of Metabolism of Arachidonic Acid in Renal Microvessels Isolated From SS and SS.5BN Rats
These experiments were performed in 6- to 9-wk-old SS, SS.5BN, and Sprague-Dawley (SD) rats. The rats were anesthetized with isoflurane and received an intravenous injection of 6 ml of a 2% solution of Evans Blue in isotonic saline containing 5% albumin to stain the renal microvessels. Five minutes later, the kidneys were collected, and renal microvessels were isolated using a sieving procedure as previously described (8). The microvessels were placed in 1 ml of a cold physiological salt solution containing (in mM) 119 NaCl, 4.7 KCl, 1.17 MgSO4, 1.6 CaCl2, 1.18 NaH2PO4, 24 NaHCO3, 0.03 EDTA, 5.5 dextrose, and 5 HEPES. The vessels were incubated for 60 min at 37°C in the presence of 1 mM NADPH and 40 μM arachidonic acid (AA). The reactions were gently swirled under an atmosphere of 100% O2 to maintain Po2 levels in the incubation media in the range of 100 Torr. To terminate the reaction, 1 M formic acid was added, and the vessels were homogenized in the reaction buffer. An aliquot was collected to determine the protein concentration of the sample, and the remainder of the homogenate was extracted twice with 3 ml of ethyl acetate. Samples were reconstituted in methanol and the metabolites of AA produced were measured using an ABI-Sciex 4000 Q-Trap LC/MS/MS as previously described (10, 15). Values are expressed as picomoles of product formed per minute per milligram of protein.
Protocol 2: Comparison of the Myogenic Response of the Af-Art of SS and SS.5BN Rats
These experiments were performed in 6- to 9-wk-old SS and SS.5BN rats. The kidneys were removed and sliced along the corticomedullary axis. A single superficial Af-Art with attached glomeruli was microdissected and transferred to a temperature-controlled chamber mounted on an inverted microscope and cannulated with concentric glass pipettes. The diameter of the Af-Art was first determined at perfusion pressures of 60 and 120 mmHg (4, 11). Then, HET0016 (1 μM), a selective inhibitor of the synthesis of 20-HETE, was added to the bath, and 15 min later the diameter of the Af-Art was again recorded at 60 and 120 mmHg. Finally, a stable 20-HETE mimetic, 20-hydroxyeicosa-5(Z), 14(Z)-dienoic acid (20-5, 14-HEDE; 10 μM) was added to the perfusate, and after a 15-min equilibration period the pressure-diameter relationships were again assessed.
To ensure that the inhibitory effect of HET0016 on the myogenic response was not due to a nonspecific action, an additional series of experiments was performed before and after the addition of a chemically and mechanistically different inhibitor of the vasoconstrictor actions of 20-HETE, 20 hydroxyeicosa-6(Z), 15(Z)-dienoic acid (20-6, 15-HEDE) was added to the bath.
Protocol 3: Comparison of the Effects of Adenosine on the Diameter of the Af-Art of SS and SS.5BN Rats
Adenosine is a putative mediator of TGF. To access the potential role of 20-HETE in modifying TGF responsiveness, we studied the vasoconstrictor response of Af-Art in SS and SS.5BN rats to an adenosine type 1 receptor (A1AR) agonist, 2-chloro-N-cyclopentyladenosine (CCPA; 10−6 M), before and after blockade of the synthesis of 20-HETE with HET0016. Perfusion pressure was maintained at 60 mmHg during these experiments. We then determined whether addition of a 20-HETE agonist, 20-5, 14-HEDE, to the bath could restore the response to adenosine in the Af-Art of SS and SS.5BN rats. Additional experiments were also performed using the 20-HETE antagonist 20-6, 15-HEDE.
Protocol 4: Comparison of Autoregulation of RBF and PGC in SS and SS.5BN Rats
These experiments were performed in 9- to 12-wk-old SS and SS.5BN rats. The rats were anesthetized with ketamine (30 mg/kg im) and Inactin (50 mg/kg ip), and catheters were placed in the femoral artery for the measurement of mean arterial pressure (MAP) and the femoral vein for an intravenous infusion of 2% BSA in a 0.9% NaCl solution at a rate of 100 μl/min. An ultrasound flow probe (Transonic System, Ithaca, NY) was placed on the left renal artery to measure RBF. After surgery and a 30-min equilibration period, MAP was increased to 140 mmHg by tying the celiac and mesenteric arteries and immediately adjusting back to 100 mmHg using a clamp on the aorta above the renal arteries. After a 20-min equilibration period, RBF was recorded over a 2-min control period. Then, the clamp on the aorta was rapidly released to allow RPP to increase to 150 mmHg, and the transient blood flow response was recorded over the next 5 min. The peak hyperemic and steady-state response were recorded 60 s and 5 min after release of the clamp.
Additional experiments were performed to access autoregulation of PGC by micropuncture in SS and SS.5BN rats. In these experiments, the left kidney was placed in a stainless steel holder and surrounded with 5% agar (Sigma). The surface of the kidney was bathed with a saline solution. RPP was adjusted to 100 mmHg using the clamp on the aorta as described above, and stop-flow pressures were measured in three to five early proximal tubules after blocking of the tubular lumen with high-vacuum grease as previously described (36, 38). Then, the clamp was released and the mesenteric and celiac arteries were tied off to increase RPP to 140 mmHg. PGC was rerecorded in the same tubules that were sampled in the control period.
To further investigate the role of endogenously formed 20-HETE in the autoregulation of RBF and PGC, additional experiments were performed in the second group of SS and SS.5BN rats pretreated with an inhibitor of the formation of 20-HETE. These rats were given a 10 mg/Kg (ip) dose of HET0016 on the evening before the experiment and a second injection just before surgery. We then studied the transient RBF response to elevation in RPP from 100 to 140 mmHg, and measured PGC at RPP of 100 and 140 mmHg.
Statistical Analysis
Mean values ± SE are presented. The significance of differences in control and experimental values within the same animal was determined by a paired t-test. The significance of differences in corresponding mean values between groups was determined by ANOVA followed by a Holm-Sidak test. A value of P < 0.05 was considered to be significant.
RESULTS
Protocol 1: Comparison of Metabolism of Arachidonic Acid in Renal Microvessels Isolated From SS and SS.5BN Rats
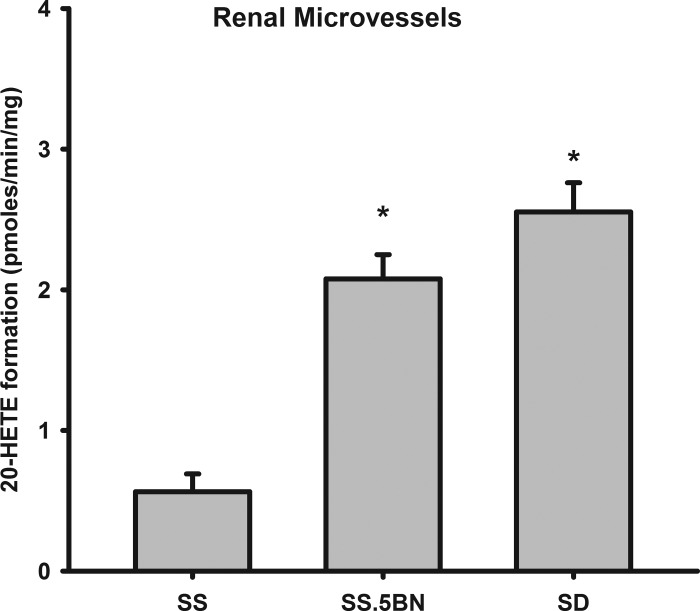
A comparison of the formation of 20-HETE in SS, SS.5BN, and SD rats is presented in Fig. 1. Transfer of Chr 5 from BN rats onto the SS background increased the formation of 20-HETE in renal microvessels in the SS.5BN consomic strain by more than fourfold to about the same level as that seen in SD rats.
Fig. 1.
Comparison of the formation of 20-hydroxyeicosatetraenoic acid (20-HETE) synthesis in preglomerular arteries (afferent arterioles; Af-Art) of Dahl salt-sensitive rats (SS), SS.5BN, and Sprague-Dawley (SD) rats. The vessels were incubated for 30 min with 40 μM arachidonic acid (AA) and 1 mM NADPH for 30 min at 37°C as described in methods. 20-HETE production was determined by liquid chromatography/tandem mass spectrometry (LC/MS/MS). Values are means ± SE expressed as pmol·min−1·mg−1; n = 9 vessel isolations and incubations for each strain. *P < 0.05, significant difference from the corresponding value in SS rats.
Protocol 2: Comparison of the Myogenic Response of Af-Art of SS and SS.5BN Rats
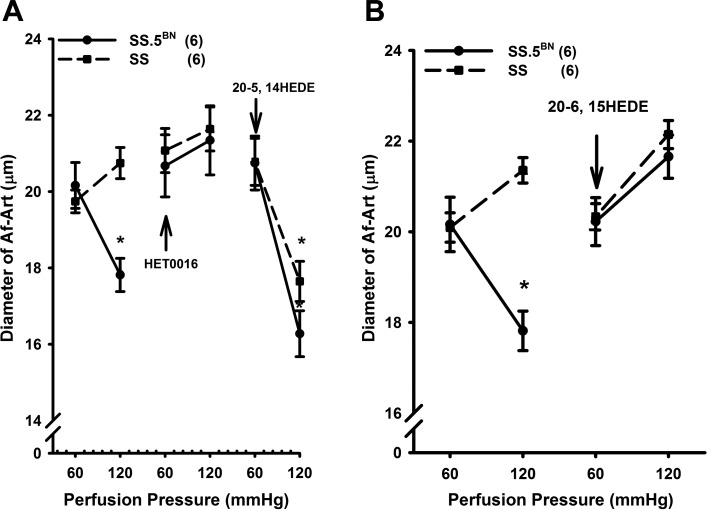
The effects of HET0016 and 20-6, 15-HEDE on the myogenic response of the Af-Art of SS and SS.5BN rats are presented in Fig. 2. The Af-Art constricted in response to an elevation in perfusion pressure from 60 to 120 mmHg in SS.5BN rats. However, the Af-Art of SS rats failed to constrict in response to the same stimulus. HET0016 completely blocked the myogenic response of the Af-Art in SS.5BN rats, but it had no effect in SS rats. Addition of a 20-HETE agonist, 20-5, 14-HEDE (10 μM), restored the myogenic response of the Af-Art in both SS and SS.5BN rats pretreated with HET0016 (Fig. 2A).
Fig. 2.
Comparison of the myogenic response of the Af-Art of SS and SS.5BN rats before and after addition of the HET0016 (1 μM) and 20-5, 14-HEDE (10 μM) to the bath (A). B: effects of 20-6, 15-HEDE (10 μM) on the myogenic response of Af-Art in SS and SS.5BN rats. Numbers in parentheses indicate the number of vessels studied. *P < 0.05, significant difference from the corresponding control value measured at 60 mmHg.
In another series of experiments, we found that the Af-Art of SS.5BN rats constricted in response to an elevation of perfusion pressure, but the Af-Art of SS rats did not. Administration of a selective 20-HETE antagonist, 20-6, 15-HEDE (10 μM, n = 6), completely blocked the myogenic responses in the Af-Art of SS.5BN rats but it had no effect on the Af-Art of SS rats (Fig. 2B).
Protocol 3: Comparison of the Effects of Adenosine on the Diameter of Af-Art of SS and SS.5BN Rats
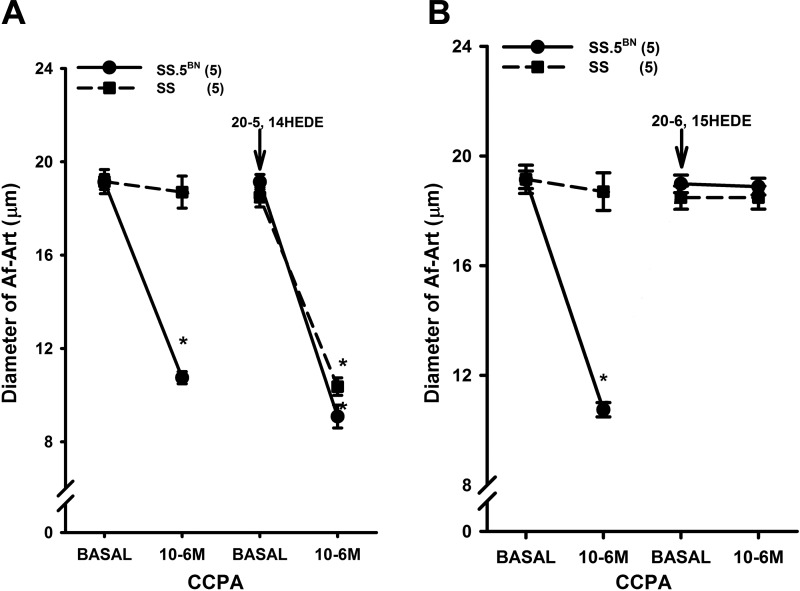
This series of experiments compared the vasoconstrictor response to the A1 receptor agonist CCPA in the Af-Art of SS and SS.5BN rats. Administration of CCPA reduced the diameter of the Af-Art of SS.5BN rats by 50%; however, it had no effect on the Af-Art of SS rats.
Administration of the 20-HETE agonist 20-5, 14-HEDE restored the vascular responsiveness to CCPA in Af-Art of SS rats, while it only slightly increased the response in SS.5BN rats (Fig. 3A). In other experiments, administration of the 20-HETE antagonist 20-6, 15-HETE blocked the vasoconstrictor response to CCPA in SS.5BN rats, but it had no effect on the Af-Art of SS rats that are deficient in the formation of 20-HETE (Fig. 3B).
Fig. 3.
Comparison of the response of the Af-Art of SS and SS.5BN rats to the adenosine type 1 agonist 2-chloro-N-cyclopentyladenosine (CCPA) before and after administration of 20-5, 14-HEDE (10 μM; A). B: effects of 20-6, 15-HEDE on the vasoconstriction response of Af-Art to CCPA in SS and SS.5BN rats. Numbers in parentheses indicate the number of vessels studied. *P < 0.05, significant difference from the corresponding control value.
Protocol 4: Comparison of Autoregulation of RBF and PGC in SS and SS.5BN Rats
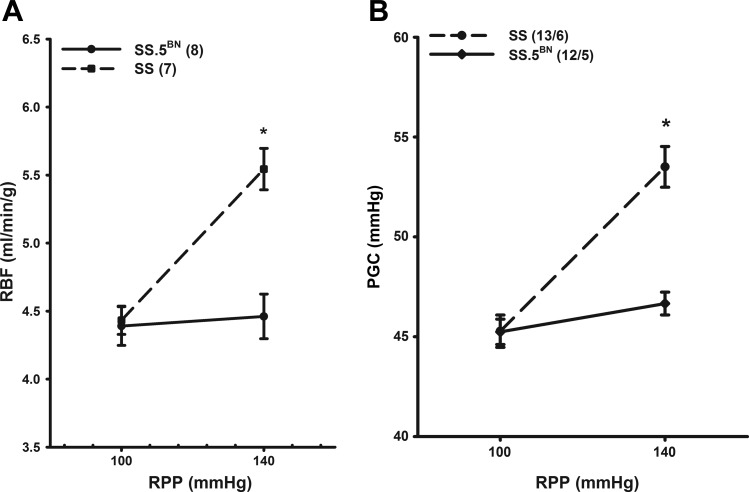
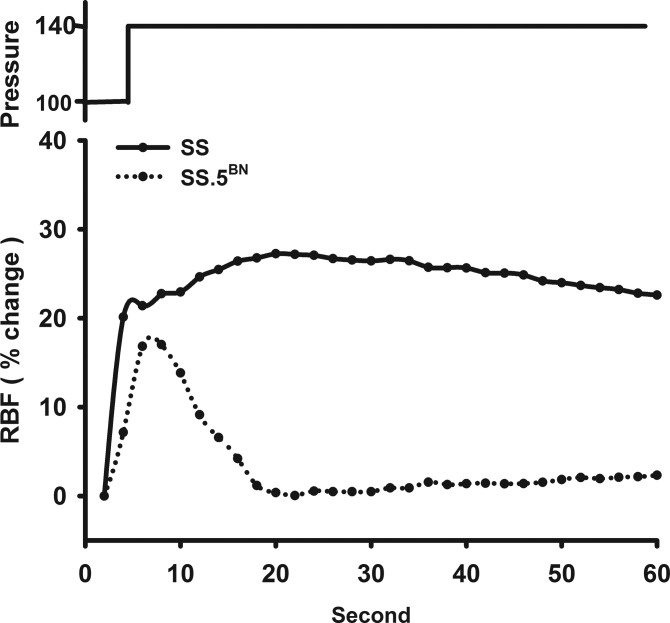
Autoregulation was markedly impaired in SS rats, and RBF increased by 25.2 ± 2.9% in response to an elevation in RPP from 100 to 140 mmHg. In contrast, autoregulation of RBF was intact in the SS.5BN rats, and RBF changed very little when RPP was increased over the same range (Fig. 4A). PGC increased significantly by 17.9 ± 1.7% in SS rats in response to an elevation in RPP from 100 to 140 mmHg. In contrast, PGC only increased by 3.2 ± 0.6% in the SS.5BN rats (Fig. 4B). Figure 5 presents a representative tracing of the transient RBF response to an elevation in RPP in SS and SS.5BN rats. RBF increased in response to step elevation in RPP and then quickly returned to control over the next 30 s in SS.5BN rats. The RBF hyperemic response was greater in SS rats than in SS.5BN rats, and RBF remained elevated for a prolonged time (>5 min) after RPP was elevated.
Fig. 4.
Comparison of the changes in renal blood flow (RBF; A) and glomerular capillary pressure (PGC; B) to the elevations in renal perfusion pressure (RPP) from 100 to 140 mmHg in SS and SS.5BN rats. Values are means ± SE. Numbers in parentheses indicate the number of tubules and rats studied per group. *P < 0.05, significant difference from the corresponding control value.
Fig. 5.
Representative tracings of the RBF response to a transient increase in RPP in SS and SS.5BN rats.
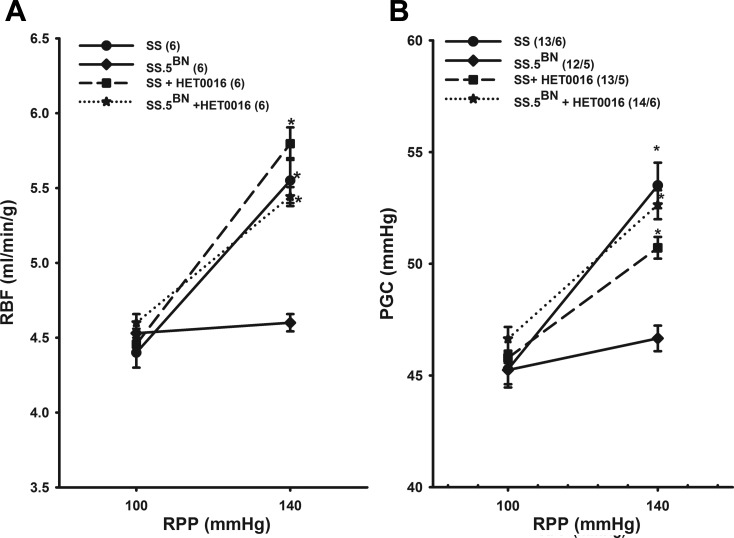
Administration of an inhibitor of the synthesis of 20-HETE, HET0016, completely blocked autoregulation of RBF in the SS.5BN rats, but it had no effect in SS rats, in which we found that the vascular synthesis of 20-HETE was reduced (Fig. 6A). The effect of HET0016 on autoregulation of PGC is presented in Fig. 6B. Administration of HET0016 completely blocked autoregulation of PGC in SS.5BN rats, but it had little effect in SS rats.
Fig. 6.
Effect of HET0016 on autoregulation of RBF (A) and PGC (B) in SS and SS.5BN rats. Values are means ± SE. Numbers in parentheses indicate the number of rats studied per group. *P < 0.05, significant difference from the corresponding control value measured at a RPP of 100 mmHg.
DISCUSSION
Previous studies from our laboratory have indicated that the expression of CYP4A protein and the production of 20-HETE are reduced, especially in the renal outer medulla of SS rats relative to a variety of other inbred strains (40), and this contributes to the development of hypertension in SS rats by enhancing sodium transport in the thick ascending loop of Henle (15, 41). More recently, we have reported that transfer of Chr 5 containing the CYP4A genes that produce 20-HETE from BN rats onto the SS genetic background restores the renal production of 20-HETE and attenuates the development of hypertension (39). The present study now extends these findings to the renal microcirculation and indicates that the production of 20-HETE is also reduced in the renal microcirculation of SS rats relative to the levels seen in a SS.5BN consomic strain and normotensive SD rats.
The functional significance of the deficiency in the formation of 20-HETE in the renal microcirculation was studied by examining myogenic and TGF responses in isolated, perfused Af-Art with attached glomerulus from SS and SS.5BN rats. We also compared autoregulation of RBF and PGC in these strains in vivo. The results of our experiments indicate that the myogenic response is absent in the Af-Art of SS rats with reduced vascular 20-HETE production, and it was intact in SS.5BN rats in which vascular 20-HETE production is restored. Blockade of the synthesis of 20-HETE with HET0016 eliminated the myogenic response of isolated, perfused Af-Art of SS.5BN rats, but it had little effect in SS rats. Similar results were obtained using a chemically dissimilar 20-HETE antagonist that has been reported to block the vasoconstrictor actions of 20-HETE (1, 23, 32). The effects of HET0016 were fully reversed by exogenous administration of a 20-HETE mimetic, 20-5, 14-HEDE, to the Af-Art, suggesting that endogenously formed 20-HETE plays an essential permissive role in the myogenic response. These findings are consistent with our previous results indicating that blockade of the synthesis of 20-HETE and EETs with 17-ODYA attenuated pressure-induced constriction of the Af-Art of rat using the isolated, perfused juxtamedullary preparation (14) and our recent finding in the isolated, perfused Af-Art of rabbits and mice (12) that 20-HETE plays an essential role in the myogenic and TGF response.
While the present results are the first to indicate that deficiency in the production of 20-HETE contributes to an impaired myogenic response in SS rats, it is important to note that the present findings are not the first to recognize that the myogenic response of the Af-Art is impaired in SS rats and that it likely contributes to renal injury. Indeed, Azar (3) reported that Af-Art resistance and PGC were elevated in SS rats challenged with a high-salt diet. Takenaka et al. (35) reported the myogenic response of the Af-Art was markedly reduced in SS rats using the hydronephrotic kidney microvascular preparation (35). The results of the present study are also consistent with a previous report that dynamic autoregulation of RBF in the high-frequency range (0.1–0.3 Hz) is impaired in hypertensive SS rats, which is indicative of an impaired myogenic response (16).
We also examined whether the deficiency in 20-HETE impairs TGF responsiveness in SS rats since previous studies have indicated that blockade of the formation of 20-HETE also attenuates this response (12, 43). Recent studies have indicated that adenosine released from the macula densa acts on the adenosine type 1 receptor (A1AR) to initiate the TGF response by constricting the Af-Art (33). 20-HETE production is known to be increased by vasoconstrictor agonists such as ANG II through activation of PLA2, and it augments vascular responsiveness by inhibiting large-conductance, calcium-sensitive potassium channel activity (5, 25, 42). In addition, Kunduri et al. (19) have recently reported that the vasoconstrictor response to adenosine mediated by activation of the A1AR involves activation of PLA2, release of arachidonic acid, and the formation of 20-HETE followed by activation of protein kinase C-α and ERK1/2. We found that 1 μM CCPA reduced the diameter of the Af-Art by ∼50% in SS.5BN rats, but it had no effect on the Af-Art of SS rats. Administration of 20-HETE agonist 20-5, 14-HEDE restored the vasoconstriction response to CCPA in SS rats. Administration of a 20-HETE antagonist, 20-6, 15-HETE, completely blocked the vasoconstriction response in SS.5BN rats. These results are consistent with our recent findings that inhibitors of the formation of 20-HETE block the TGF response in the isolated, perfused juxtaglomerular apparatus of mice and rabbits and our previous findings in vivo (12) that addition of CYP4A inhibitors to the tubular perfusate blocks the TGF response in the rat (43). Together, these results confirm that endogenously formed 20-HETE augments the vasoconstrictor responsiveness of the Af-Art and play an essential role in modulating the TGF response. They also indicate that the deficiency in the formation of 20-HETE in the vasculature also likely contributes to increased transmission of pressure to the glomerulus and renal injury during the development of hypertension in SS rats. These findings are consistent with the results of a previous micropuncture study by Wilcox and Welch (37), who found that the TGF response to elevation in flow rate in the distal nephron was reduced in SS rats.
A question that remains to be answered is why the myogenic and TGF response is completely blocked in SS rats but the formation of 20-HETE is not. One possibility is that there is a threshold concentration needed to initiate the myogenic response. In this regard, previous studies have indicated that elevations in perfusion pressure rapidly increase the formation and release of 20-HETE in less than a minute in isolated arterioles (13), but the threshold concentration needed to constrict renal arterioles under basal condition is ∼100 nM (21). Thus it may be that the fivefold reduction in the formation of 20-HETE seen in SS rats is not sufficient to increase 20-HETE levels to the threshold concentration needed to elicit a myogenic response. Another possibility is that 20-HETE is produced by many CYP4A and 4F isoforms that are likely differentially expressed in vessels. We know that some of these isoforms are more catalytically active and might be involved in the rapid formation of free 20-HETE following stretch of vessels and release of AA from membrane phospholipid pools, whereas other isoforms may be more involved in the w-hydroxylation of fatty acids for β-oxidation and constitutive generation of bound 20-HETE that may not be inhibited in SS rats. Indeed, we often observed that inhibitors of the formation of 20-HETE completely block the myogenic response in isolated vessels in minutes, but the levels of 20-HETE in the vessels do not immediately fall as 20-HETE is avidly bound to proteins inside the cell and stored in membrane phospholipid pools.
Since autoregulation of RBF and PGC is mediated via the myogenic and TGF responses acting in concert and both of these responses were impaired in SS rats, we examined whether this affected autoregulation of RBF and PGC in response to step changes in renal perfusion pressure (RPP) in SS rats. We found that autoregulation was fully intact, and RBF remains relatively constant in SS.5BN rats in response to a step change in RPP from 100 to 140 mmHg. Moreover, PGC did not increase in SS.5BN rats when RPP was elevated. In contrast, autoregulation of RBF was impaired in SS rats and it rose by 26% in response to a 40% increase in RPP from 100 to 140 mmHg. More importantly, the rise in systemic pressure was transmitted to the glomerulus, and PGC rose by 8 mmHg when RPP was increased from 100 to 140 mmHg in SS rats. We also confirmed that the impaired autoregulation of RBF and PGC was due to a deficiency in the formation of 20-HETE by showing that blockade of the synthesis of 20-HETE with HET0016 eliminated autoregulation of both RBF and PGC in SS.5BN rats, but it had no effect on SS rats in which the formation of 20-HETE is already low.
The present study, indicating that the production of 20-HETE is reduced in renal microvessels of Dahl S rats relative to SS.5BN and SD rats, is consistent with previous findings that the production of 20-HETE is markedly reduced in renal cortical and medullary microsomes (22, 34, 40) and isolated glomeruli of SS rats relative to other strains (39). However, our findings differ from a recent report that the expression of CYP4A protein is higher in cerebral arteries from SS rats than SS.5BN rats and that elevated levels of 20-HETE may contribute to endothelial dysfunction (20). We do not think that this is due to a difference in the expression of CYP4A enzymes in cerebral vs. renal vessels because in our hands we find that the production of 20-HETE is markedly reduced in the middle cerebral artery of SS rats relative to SS.5BN and SD rats (RJ Roman and Fan F, unpublished observations). The reason for the differences remains to be determined. It may have something to do with diet, antibodies, and animal strains. For instance, the rats in the previous study were fed a purified AIN76 diet while the rats in the present study were fed a Teklab 7034 grain-based, low-salt diet. In addition, the previous study did not directly measure the formation of 20-HETE in cerebral vessels but inferred that there may be differences based on the expression of CYP4A protein and the differential effect of HET0016 on the vasodilator response to acetylcholine.
Perspective
The results of the present study provide the first direct evidence that the production of 20-HETE is reduced in the renal vasculature of SS rats and that this contributes to impaired myogenic and TGF responses of the Af-Art. These findings further support the view that the endogenous formation of 20-HETE in the renal microcirculation plays a key role in modulating both the myogenic and TGF responses of the Af-Art. These findings support our hypothesis that a deficiency in the renal production of 20-HETE impairs renal autoregulatory responses and contributes to the susceptibility of SS rats to develop hypertension-induced renal injury vs. other hypertensive strains like spontaneously hypertensive rats, in which the renal vascular production of 20-HETE is elevated and autoregulation of RBF is intact.
GRANTS
This study was supported in part by National Institutes of Health Grants HL-36279 and -29587 (R. J. Roman), -DK098582 (R. Liu), and DK-38226, the Robert A. Welch Foundation GL625910 (J. R. Falck), and 13POST14220024 (Y. Ge) from the American Heart Association.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Y.G., S.R.M., and F.F. performed experiments; Y.G. analyzed data; Y.G. prepared figures; Y.G. drafted manuscript; Y.G., J.M.W., J.R.F., R.L., and R.J.R. edited and revised manuscript; S.R.M., F.F., J.M.W., and R.J.R. interpreted results of experiments; J.M.W. and R.J.R. provided conception and design of research; R.J.R. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Chris Purser for technical assistance with LC/MS analysis of the metabolites of AA.
REFERENCES
- 1.Alonso-Galicia M, Falck JR, Reddy KM, Roman RJ. 20-HETE agonists and antagonists in the renal circulation. Am J Physiol Renal Physiol 277: F790–F796, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Arima S, Omata K, Ito S, Tsunoda K, Abe K. 20-HETE requires increased vascular tone to constrict rabbit afferent arterioles. Hypertension 27: 781–785, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Azar S, Limas C, Iwai J, Weller D. Single nephron dynamics during high sodium intake and early hypertension in Dahl rats. Jpn Heart J 20: 138–140, 1979 [Google Scholar]
- 4.Burke M, Pabbidi M, Fan F, Ge Y, Liu R, Williams JM, Sarkis A, Lazar J, Jacob HJ, Roman RJ. Genetic basis of the impaired renal myogenic response in FHH rats. Am J Physiol Renal Physiol 304: F565–F577, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carroll MA, Balazy M, Huang DD, Rybalova S, Falck JR, McGiff JC. Cytochrome P450-derived renal HETEs: storage and release. Kidney Int 51: 1696–1702, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Cowley AW, Jr, Liang M, Roman RJ, Greene AS, Jacob HJ. Consomic rat model systems for physiological genomics. Acta Physiol Scand 181: 585–592, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Cowley AW, Jr, Roman RJ, Jacob HJ. Application of chromosomal substitution techniques in gene-function discovery. J Physiol 554: 46–55, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahly-Vernon AJ, Sharma M, McCarthy ET, Savin VJ, Ledbetter SR, Roman RJ. Transforming growth factor-beta, 20-HETE interaction, and glomerular injury in Dahl salt-sensitive rats. Hypertension 45: 643–648, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Donnelly TH, Shergold JH, Southgate PN. Anomalous geochemical signals from phosphatic middle Cambrian rocks in the southern Georgina basin, Australia. Sedimentology 35: 549, 1988 [Google Scholar]
- 10.Fan F, Sun CW, Maier KG, Williams JM, Pabbidi MR, Didion SP, Falck JR, Zhuo J, Roman RJ. 20-Hydroxyeicosatetraenoic acid contributes to the inhibition of K+ channel activity and vasoconstrictor response to angiotensin II in rat renal microvessels. PLoS One 8: e82482, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ge Y, Gannon K, Gousset M, Liu R, Murphey B, Drummond HA. Impaired myogenic constriction of the renal afferent arteriole in a mouse model of reduced betaENaC expression. Am J Physiol Renal Physiol 302: F1486–F1493, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ge Y, Murphy SR, Lu Y, Falck J, Liu R, Roman RJ. Endogenously produced 20-HETE modulates myogenic and TGF response in microperfused afferent arterioles. Prostaglandins Other Lipid Mediat 102: 42–48, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gebremedhin D, Lange AR, Lowry TF, Taheri MR, Birks EK, Hudetz AG, Narayanan J, Falck JR, Okamoto H, Roman RJ, Nithipatikom K, Campbell WB, Harder DR. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ Res 87: 60–65, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Gebremedhin D, Ma YH, Imig JD, Harder DR, Roman RJ. Role of cytochrome P-450 in elevating renal vascular tone in spontaneously hypertensive rats. J Vasc Res 30: 53–60, 1993 [DOI] [PubMed] [Google Scholar]
- 15.Ito O, Roman RJ. Role of 20-HETE in elevating chloride transport in the thick ascending limb of Dahl SS/Jr rats. Hypertension 33: 419–423, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Karlsen FM, Andersen CB, Leyssac PP, Holstein-Rathlou NH. Dynamic autoregulation and renal injury in Dahl rats. Hypertension 30: 975–983, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Kirchner KA. Greater loop chloride uptake contributes to blunted pressure natriuresis in Dahl salt sensitive rats. J Am Soc Nephrol 1: 180–186, 1990 [DOI] [PubMed] [Google Scholar]
- 18.Kirchner KA. Increased loop chloride uptake precedes hypertension in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 262: R263–R268, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Kunduri SS, Mustafa SJ, Ponnoth DS, Dick GM, Nayeem MA. Adenosine A1 receptors link to smooth muscle contraction via CYP4a, protein kinase C-alpha, and ERK1/2. J Cardiovasc Pharmacol 62: 78–83, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lukaszewicz KM, Falck JR, Manthati VL, Lombard JH. Introgression of Brown Norway CYP4A genes on to the Dahl salt-sensitive background restores vascular function in SS-5(BN) consomic rats. Clin Sci (Lond) 124: 333–342, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma YH, Gebremedhin D, Schwartzman ML, Falck JR, Clark JE, Masters BS, Harder DR, Roman RJ. 20-Hydroxyeicosatetraenoic acid is an endogenous vasoconstrictor of canine renal arcuate arteries. Circ Res 72: 126–136, 1993 [DOI] [PubMed] [Google Scholar]
- 22.Ma YH, Schwartzman ML, Roman RJ. Altered renal P-450 metabolism of arachidonic acid in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 267: R579–R589, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Miyata N, Taniguchi K, Seki T, Ishimoto T, Sato-Watanabe M, Yasuda Y, Doi M, Kametani S, Tomishima Y, Ueki T, Sato M, Kameo K. HET0016, a potent and selective inhibitor of 20-HETE synthesizing enzyme. Br J Pharmacol 133: 325–329, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Donnell MP, Kasiske BL, Katz SA, Schmitz PG, Keane WF. Lovastatin but not enalapril reduces glomerular injury in Dahl salt-sensitive rats. Hypertension 20: 651–658, 1992 [DOI] [PubMed] [Google Scholar]
- 25.Pabbidi MR, Mazur O, Fan F, Farley JM, Gebremedhin D, Harder DR, Roman RJ. Enhanced large conductance K+ channel activity contributes to the impaired myogenic response in the cerebral vasculature of Fawn Hooded Hypertensive rats. Am J Physiol Heart Circ Physiol 306: H989–H1000, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raij L, Azar S, Keane W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int 26: 137–143, 1984 [DOI] [PubMed] [Google Scholar]
- 27.Rapp JP. Dahl salt-susceptible and salt-resistant rats. A review. Hypertension 4: 753–763, 1982 [DOI] [PubMed] [Google Scholar]
- 28.Reaven GM, Twersky J, Chang H. Abnormalities of carbohydrate and lipid metabolism in Dahl rats. Hypertension 18: 630–635, 1991 [DOI] [PubMed] [Google Scholar]
- 29.Roman RJ, Alonso-Galicia M, Wilson TW. Renal P450 metabolites of arachidonic acid and the development of hypertension in Dahl salt-sensitive rats. Am J Hypertens 10: 63S–67S, 1997 [PubMed] [Google Scholar]
- 30.Roman RJ, Kaldunski ML. Enhanced chloride reabsorption in the loop of Henle in Dahl salt-sensitive rats. Hypertension 17: 1018–1024, 1991 [DOI] [PubMed] [Google Scholar]
- 31.Roman RJ, Ma YH, Frohlich B, Markham B. Clofibrate prevents the development of hypertension in Dahl salt-sensitive rats. Hypertension 21: 985–988, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Sato M, Ishii T, Kobayashi-Matsunaga Y, Amada H, Taniguchi K, Miyata N, Kameo K. Discovery of a N′-hydroxyphenylformamidine derivative HET0016 as a potent and selective 20-HETE synthase inhibitor. Bioorg Med Chem Lett 11: 2993–2995, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Schnermann JB, Traynor T, Yang T, Huang YG, Oliverio MI, Coffman T, Briggs JP. Absence of tubuloglomerular feedback responses in AT1A receptor-deficient mice. Am J Physiol Renal Physiol 273: F315–F320, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Stec DE, Deng AY, Rapp JP, Roman RJ. Cytochrome P4504A genotype cosegregates with hypertension in Dahl S rats. Hypertension 27: 564–568, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Takenaka T, Forster H, De Micheli A, Epstein M. Impaired myogenic responsiveness of renal microvessels in Dahl salt-sensitive rats. Circ Res 71: 471–480, 1992 [DOI] [PubMed] [Google Scholar]
- 36.van Dokkum RP, Sun CW, Provoost AP, Jacob HJ, Roman RJ. Altered renal hemodynamics and impaired myogenic responses in the fawn-hooded rat. Am J Physiol Regul Integr Comp Physiol 276: R855–R863, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Wilcox CS, Welch WJ. TGF and nitric oxide: effects of salt intake and salt-sensitive hypertension. Kidney Int Suppl 55: S9–S13, 1996 [PubMed] [Google Scholar]
- 38.Williams JM, Burke M, Lazar J, Jacob HJ, Roman RJ. Temporal characterization of the development of renal injury in FHH rats and FHH. 1BN congenic strains. Am J Physiol Renal Physiol 300: F330–F338, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams JM, Fan F, Murphy S, Schreck C, Lazar J, Jacob HJ, Roman RJ. Role of 20-HETE in the antihypertensive effect of transfer of chromosome 5 from Brown Norway to Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 302: R1209–R1218, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams JM, Sarkis A, Hoagland KM, Fredrich K, Ryan RP, Moreno C, Lopez B, Lazar J, Fenoy FJ, Sharma M, Garrett MR, Jacob HJ, Roman RJ. Transfer of the CYP4A region of chromosome 5 from Lewis to Dahl S rats attenuates renal injury. Am J Physiol Renal Physiol 295: F1764–F1777, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zou AP, Drummond HA, Roman RJ. Role of 20-HETE in elevating loop chloride reabsorption in Dahl SS/Jr rats. Hypertension 27: 631–635, 1996 [DOI] [PubMed] [Google Scholar]
- 42.Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman RJ. Stereospecific effects of epoxyeicosatrienoic acids on renal vascular tone and K+-channel activity. Am J Physiol Renal Fluid Electrolyte Physiol 270: F822–F832, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Zou AP, Imig JD, Ortiz de Montellano PR, Sui Z, Falck JR, Roman RJ. Effect of P-450 ω-hydroxylase metabolites of arachidonic acid on tubuloglomerular feedback. Am J Physiol Renal Fluid Electrolyte Physiol 266: F934–F941, 1994 [DOI] [PubMed] [Google Scholar]