Abstract
Understanding the regulation of death pathways, necrosis and apoptosis, in pancreatitis is important for developing therapies directed to the molecular pathogenesis of the disease. Protein kinase Cε (PKCε) has been previously shown to regulate inflammatory responses and zymogen activation in pancreatitis. Furthermore, we demonstrated that ethanol specifically activated PKCε in pancreatic acinar cells and that PKCε mediated the sensitizing effects of ethanol on inflammatory response in pancreatitis. Here we investigated the role of PKCε in the regulation of death pathways in pancreatitis. We found that genetic deletion of PKCε resulted in decreased necrosis and severity in the in vivo cerulein-induced pancreatitis and that inhibition of PKCε protected the acinar cells from CCK-8 hyperstimulation-induced necrosis and ATP reduction. These findings were associated with upregulation of mitochondrial Bak and Bcl-2/Bcl-xL, proapoptotic and prosurvival members in the Bcl-2 family, respectively, as well as increased mitochondrial cytochrome c release, caspase activation, and apoptosis in pancreatitis in PKCε knockout mice. We further confirmed that cerulein pancreatitis induced a dramatic mitochondrial translocation of PKCε, suggesting that PKCε regulated necrosis in pancreatitis via mechanisms involving mitochondria. Finally, we showed that PKCε deletion downregulated inhibitors of apoptosis proteins, c-IAP2, survivin, and c-FLIPs while promoting cleavage/inactivation of receptor-interacting protein kinase (RIP). Taken together, our findings provide evidence that PKCε activation during pancreatitis promotes necrosis through mechanisms involving mitochondrial proapoptotic and prosurvival Bcl-2 family proteins and upregulation of nonmitochondrial pathways that inhibit caspase activation and RIP cleavage/inactivation. Thus PKCε is a potential target for prevention and/or treatment of acute pancreatitis.
Keywords: apoptosis; necrosis; inhibitors of apoptotic proteins (IAPs), Bcl-2 family proteins; receptor-interacting protein kinase (RIP)
pancreatic acinar cell necrosis is a major pathological response of acute pancreatitis associated with significant morbidity and mortality from the disease (16, 39). Currently, there are no therapies directed to the molecular pathogenesis of necrosis in pancreatitis (39). Thus elucidating the molecular signals that mediate acinar cell necrosis is important for developing new therapeutic strategies.
Apoptosis and necrosis are two major forms of acinar cell death in acute pancreatitis and associated with specific morphological and biochemical features (16, 32). Apoptosis is a tightly regulated process involving the caspase family of cysteine proteases and manifests as chromatin condensation, nuclear shrinkage, and reduction in cell volume but with intact plasma membranes; thus there is no leakage of cellular contents to the extracellular environment. Conversely, necrosis leads to early plasma membrane permeabilization and rupture with release of intracellular constituents to the extracellular space and no sign of nuclear shrinkage (16, 39). Necrosis has been considered a passive, unregulated form of cell death; however, recent studies indicate that necrosis can occur in a tightly regulated fashion, called programmed necrosis (13, 20, 25, 32, 33, 42).
Although the consequences of apoptosis and necrosis are distinct in pancreatitis, the mechanisms underlying these two types of cell death are interrelated and they both involve mitochondria (6, 7, 16, 21, 39, 48). Apoptosis is mediated by the release of mitochondrial cytochrome c into the cytosol through outer membrane permeabilization (OMP) followed by caspase activation, whereas necrosis is associated with injury of inner membrane or opening of the mitochondrial permeability transition pore (mPTP) resulting in mitochondrial depolarization and subsequent ATP depletion (26, 48, 37, 56, 1, 34).
Bcl-2 proteins are known important regulators of mitochondrial permeabilization (1, 26). Proapoptotic Bax and Bak form channels in the outer membrane through which mitochondrial cytochrome c is released into the cytosol (1, 26); BH3-only proteins, such as Bim and Puma, activate Bax/Bak channels. In contrast, prosurvival Bcl-2 proteins such as Bcl-2 and Bcl-xL inhibit apoptosis by sequestering BH3-only proteins and Bax/Bak (1, 26). Notably, the prosurvival Bcl-2 proteins can also stabilize inner membrane and block mPTP opening, thus maintaining energy production and preventing necrosis (52, 53). Our recent studies demonstrated that the predominant effect of Bcl-2/Bcl-xL proteins is to stabilize mitochondrial inner membrane integrity rather than to prevent OMP opening-caused cytochrome c release in pancreatitis (48). Thus the prosurvival Bcl-2 proteins are now recognized to play an important role in protection of acinar cells from necrosis by stabilizing mitochondria against death signals.
Inhibitors of apoptosis proteins (IAPs) are an important family of endogenous proteins that inhibit the caspase system, the essential mediators of apoptotic death pathways (11, 12). The importance of IAPs in regulating the type of death in pancreatitis has been reported by our group (32, 39, 54). NF-κB activation is a key early event in acute pancreatitis (39, 55). A wealth of evidence indicates that NF-κB activation plays an important role in regulation of IAPs such as c-IAP1, c-IAP2, survivin, XIAP, as well as antiapoptotic protein FLICE-inhibitory protein (c-FLIP) (12, 16, 22, 23, 39, 47, 57).
A number of reports indicate that the programmed necrosis requires the receptor-interacting protein kinase (RIP or RIP1) (10, 14, 20, 28, 33, 49). RIP forms a death signaling complex with the Fas-associated death domain and caspases in response to death domain receptor stimulation (10, 28, 49). During apoptosis, RIP is cleaved and inactivated by caspase-3 and -8 (10, 28, 33). The regulation of RIP by caspases has been suggested to be one of mechanisms underlying the protective role of caspases from necrosis in cerulein-induced pancreatitis (20, 32, 54).
Protein kinase Cs (PKCs) are a family of serine/threonine kinases comprising 10 isoforms, namely conventional PKC isoforms (α, βI, βII, and γ), novel PKC isoforms (δ, ε, η, and θ), and atypical PKC isoforms (ζ and λ/ι) (35). Each PKC isoform can be activated independently by specific stimuli and mediates distinct biological functions (36, 4, 19, 8). In pancreatic acinar cell, four PKC isoforms, α, δ, ε, and ζ, have been detected (3), which have been increasingly implicated in the regulation of pathological responses of pancreatitis in pancreatic acinar cells (15). PKCε and δ have been shown to play a role in zymogen activation in pancreatitis (50). Our group demonstrated that PKCε and δ are key regulators of proinflammatory transcription factor NF-κB activation induced by CCK-8 in pancreatitis (43). Furthermore, we demonstrated that ethanol causes specific activation of PKCε in acinar cells and that PKCε mediated the sensitizing effects of ethanol on inflammatory response in alcoholic pancreatitis, suggesting that PKCε activation plays a key role in alcoholic pancreatitis (44).
To better understand the role PKCε in pancreatitis involving necrosis, we designed the present study to explore the molecular mechanisms through which PKCε regulates cell death responses in in vivo and in vitro experimental models of acute pancreatitis. Our findings here show that genetic deletion of PKCε decreases inflammation, necrosis, and the severity of acute pancreatitis. PKCε mediates necrosis in pancreatitis through mechanisms involving mitochondrial proapoptotic and prosurvival Bcl-2 family proteins and upregulation of nonmitochondrial pathways inhibiting caspase activation and RIP cleavage. Thus we suggest that PKCε is a potential target for prevention and/or treatment of necrotizing pancreatitis.
MATERIALS AND METHODS
Reagents.
CCK-8 (CCK) was from American Peptide (Sunnyvale, CA); cerulein was from Peninsula Laboratories (Belmont, CA). Medium 199 was from GIBCO (Grand Island, NY). γ-32P ATP was from Perkin Elmer (Torrance, CA). Nitrocellulose membranes were from Schleicher and Schuell BioScience. Caspase-3 fluorogenic substrates Ac-DEVD-AMC were from Biomol (Plymouth Meeting, PA). Antibodies against PKCε, c-IAP1, c-IAP2, c-FLIP, survivin, or Bcl-2 were from Santa Cruz Biotechnology (Santa Cruz, CA). Cytochrome c and RIP antibodies were from BD Science (San Diego, CA). COX IV antibody was from Molecular Probes (Eugene, OR); antibodies against Bax, Bak, XIAP, Bcl-xL, GAPDH, or ERK1/2 were from Cell Signaling (Beverly, MA). VDAC1 antibody was from Abcam (Cambridge, MA). The specific PKCε translocation inhibitor peptide was synthesized as described previously (43, 44, 55). Protein A-agarose was from Roche Applied Science (Mannheim, Germany) and the specific PKCε peptide substrate was from Calbiochem (La Jolla, CA). Other items were from standard suppliers or as indicated in text.
Experimental pancreatitis.
PKCε-deficient (PKCε−/−) and wild-type (WT, PKCε+/+) littermate mice were both C57BL/6J background and obtained by breeding PKCε−/+ mice generously provided by Dr. Robert O. Messing (University of California, San Francisco, CA). The phenotype of these mice was described (24). PKCε−/− mice were viable, normal in size/body weight, and without display of any gross physical or behavioral abnormalities. There were no gross differences in pancreatic fat. A problem observed by the provider (24) and us was that homozygous females rarely produced litters (0–2 pups). Thus we obtained PKCε−/− by breeding PKCε−/+ mice. Breeding of the PKCε-deficient mice and handling of the animals were approved by the Animal Research Committee of the VA Greater Los Angeles Healthcare System, in accordance with the National Institutes of Health guidelines.
Pancreatitis was induced in WT and PKCε−/− mice (25–30 g) by up to 7 hourly intraperitoneal (IP) injections of 50 μg/kg cerulein, a CCK analog used for experimental pancreatitis models. Control animals received similar injections of physiological saline. The animals were euthanized by CO2-induced asphyxiation at 30 min, 4 h, and 7 h after the first injection, and the blood and pancreas were harvested for measurements. Tissues were immediately removed, frozen in liquid nitrogen, and stored at −80°C.
Preparation of tissue lysates, Western blot analysis, PKCε immunoprecipitation, and in vitro kinase assay.
Pancreatic tissue lysates were prepared and Western blot analyses were performed as described previously (43, 54). PKCε in pancreatic tissue lysates was immunoprecipitated at 4°C overnight with the PKCε antibody (1:100) and protein A-agarose. In vitro kinase assay was performed as described (43).
Preparation and treatments of dispersed pancreatic acini.
Pancreatic acini were isolated from WT and PKCε−/− mice and cultured as described (54, 55). For experimental purposes, the acini were preincubated in medium 199 with or without inhibitors and then treated further with CCK, as described (43, 54, 55).
Enzymatic assays.
Animal serum amylase and lipase activities were determined by Antech Diagnostics (Irvine, CA) Custom Service. Active trypsin in pancreatic tissue or acini homogenates was measured by using Boc-Gln-Ala-Arg-AMC as a substrate by a fluoroenic assay as described previously (29). Caspase-3 activities in pancreatic tissue homogenates were measured by using a fluorogenic assay with substrates specific for caspase-3 (Ac-DEVDAMC) as we previously described (32, 48). ATP level in pancreatic acini samples was measured as described (48) with a luciferin/luciferase-based ATP determination kit (Molecular Probes).
Quantification of necrosis.
Necrosis in pancreatic acini was determined by the release of lactate dehydrogenase (LDH) into the incubation medium, as described previously (16, 17, 48, 54). LDH activity was measured by use of a cytotoxicity detection kit (Roche Diagnostics, Indianapolis, IN).
Quantification of necrosis in in vivo pancreatitis was performed on pancreatic tissue (collected after 7 hourly cerulein injections) sections stained with hematoxylin and eosin (H&E). Cells with swollen cytoplasm, loss of plasma membrane integrity, and leakage of organelles into interstitium were considered necrotic. A total of at least 2,000 acinar cells were counted on tissue sections from each animal and three to five animals per condition were counted.
Quantification of apoptosis.
Apoptosis was quantified in pancreatic tissue sections (7 hourly cerulein injections) stained with TdT-mediated dUTP nick end-labeling (TUNEL) to measure DNA breaks, as we described previously (16, 32) by use of the DeadEnd Fluorometric TUNEL System kit (Promega, Madison, WI). For these and other quantifications of histological measurements, a total of at least 3,000 acinar cells were counted on tissue sections from each animal and three to five animals per condition were counted.
Histological analysis for pancreas inflammatory cell infiltration and vacuolization and measurement of edema.
Quantification of inflammatory cell infiltration and vacuolization was performed on H&E-stained pancreatic tissue (7 hourly cerulein injections) sections from at least four mice per group and expressed as the number of inflammatory cells or vacuoles per 100 acinar cells. Neutrophil infiltration in pancreas was further quantified by measuring myeloperoxidase (MPO) activity by using a MPO Activity Assay kit (Sigma, no. MAK068) according to the manufacturer's instructions. Pancreatic edema was evaluated by measuring the wet-to-dry weight ratio as described (18). The results were calculated and expressed as a water index (wet wt/dry wt).
Tissue and cell fractioning and measurement of cytochrome c release.
Cytosolic and mitochondria-enriched membrane fractions from pancreatic tissue or isolated pancreatic acini were prepared as described in detail (32, 48) and used as samples for Western blot analysis.
Preparation of nuclear extracts and NF-κB DNA binding activity measurement.
Pancreatic tissue nuclear protein extracts were prepared by using ActiveMotif nuclear extract kit (Carlsbad, CA) following the manufacturer's instructions. NF-κB DNA binding activities in pancreas tissue collected 30 min after 1 injection of cerulein were measured with EMSA as described previously (43, 44, 55).
Immunofluorescence.
For immunolabeling, pancreas was dissected and fixed for 6 h in formalin. Sections were stained with primary antibodies against PKCε and mitochondrial marker protein voltage-dependent anion channel (VDAC1), followed by incubation with secondary antibodies conjugated with Alexa 488 or 594 (Life Technologies, Grand Island, NY). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Images were acquired with a Zeiss LSM 710 confocal microscope using a ×63 objective.
Measurements of mRNA expression by RT-PCR.
RNA isolation and conventional quantitative RT-PCR using gene specific, intron-spanning primers (Table 1) were performed as we described previously (48). Mouse 18S ribosomal RNA (m18S) was used as a reference (housekeeping) control. The m18S and our target sequences were amplified at the annealing temperature 57°C during 29 or 32 cycles, respectively, to yield visible products within linear amplification range. PCR products were visualized and quantified with a FluorChem-HD2 imager (Alpha Innotech).
Table 1.
Primer Sequences for Quantitative RT-PCR
| Transcript | Sense Primer | Antisense Primer | Amplicon, bp | GeneBank Accession No. |
|---|---|---|---|---|
| Mouse Bcl-2 | 5′-GGTGGTGGAGGAACTCTTCAG | 5′-TAGTTCCACAAAGGCATCCCAG | 208 | NM_009741.4 |
| Mouse Bcl-xL | 5′-TGAATGACCACCTAGAGCCTTG | 5′-CAGAACCACACCAGCCACAG | 155 | NM_009743.4 |
| Mouse Bak | 5′-CCCAACAGCATCTTGGGTCA | 5′-ATTGGCCCAACAGAACCACA | 409 | NM_007523.2 |
| Mouse Survivin | 5′-CTTCATCCACTGCCCTACCG | 5′-GCTCCTCTATCGGGTTGTCA | 104 | NM_006989.2 |
| Mouse Flips | 5′-TACACCCAGTCCAGCCAAGGA | 5′-CTGGTACTCCATACACTGGCTC | 118 | NM_009805.4 |
| Mouse 18S | 5′-AGTCCCTGCCCTTTGTACACA | 5′-CGATCCGAGGGCCTCACTA | 75 | NR_003278.3 |
18S, 18S ribosomal RNA.
Statistical analysis of data.
Results are means ± SE and represent data from at least three independent experiments. The experimental data were evaluated by the analysis of variance (ANOVA) followed by Bonferroni multiple comparison post hoc tests with the GraphPad Prism software (GraphPad Software, La Jolla, CA), and t-tests were used to analyze differences between two groups. P < 0.05 was considered statistically significant.
RESULTS
PKCε is activated in cerulein-induced experimental pancreatitis.
We first examined whether PKCε was activated in vivo in experimental pancreatitis. WT mice received an IP injection of pancreatitis-causing doses of the CCK analog (cerulein, 50 μg/kg) or vehicle (saline). The pancreas was collected 15, 30, and 45 min after the cerulein injection. We then performed in vitro kinase assays using PKCε isoform-specific immunoprecipitates from the pancreatic tissue lysate and a specific PKCε peptide substrate. PKCε was activated as early as 15 min after the initiation of pancreatitis and reached maximal activation at 30 min and then declined at 45 min after cerulein injection (Fig. 1). These results indicated that PKCε activation was an early event in acute pancreatitis, consistent with the data we reported previously in an in vitro model of pancreatitis using isolated pancreatic acini stimulated with a supramaximal dose of CCK (43).
Fig. 1.
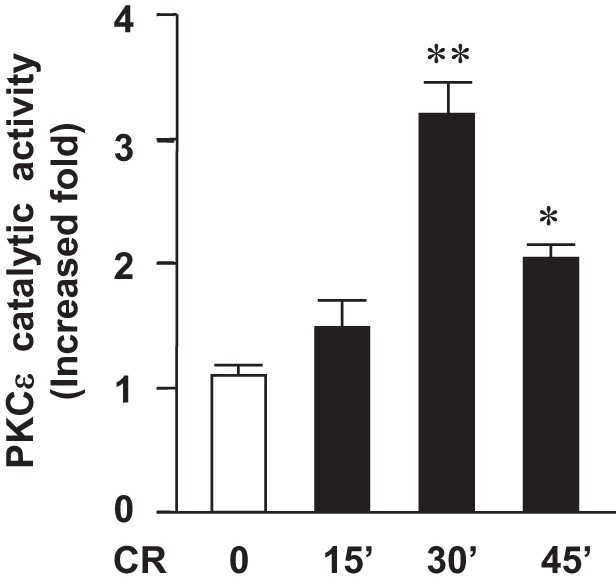
PKCε is activated in the early stage of cerulein-induced pancreatitis. Wild-type (WT) mice received intraperitoneal (IP) injections of cerulein (CR) or saline. The pancreata were collected in 15, 30, and 45 min after the cerulein injection. The catalytic activity of PKCε immunoprecipitated from pancreatic tissue lysates was determined by in vitro kinase assay. Values (means ± SE, n = 3 for each condition) were compared with their basal activity in the animals injected with saline. **P < 0.01 or *P < 0.05 vs. saline control.
Characterization of PKCε−/− mice.
To investigate the role of PKCε in pancreatitis, we used mice with genetic deletion of PKCε (PKCε−/−) and their littermate WT controls (PKCε+/+). Genetic deletion of PKCε was confirmed by PCR (Fig. 2A) and Western blot analysis of pancreatic acinar cell lysate with PKCε antibody (Fig. 2B). Importantly, PKCε deletion did not alter protein expression levels of the other three major PKC isotypes (α, δ, ζ) in pancreatic acinar cells during pancreatitis (Fig. 2C).
Fig. 2.
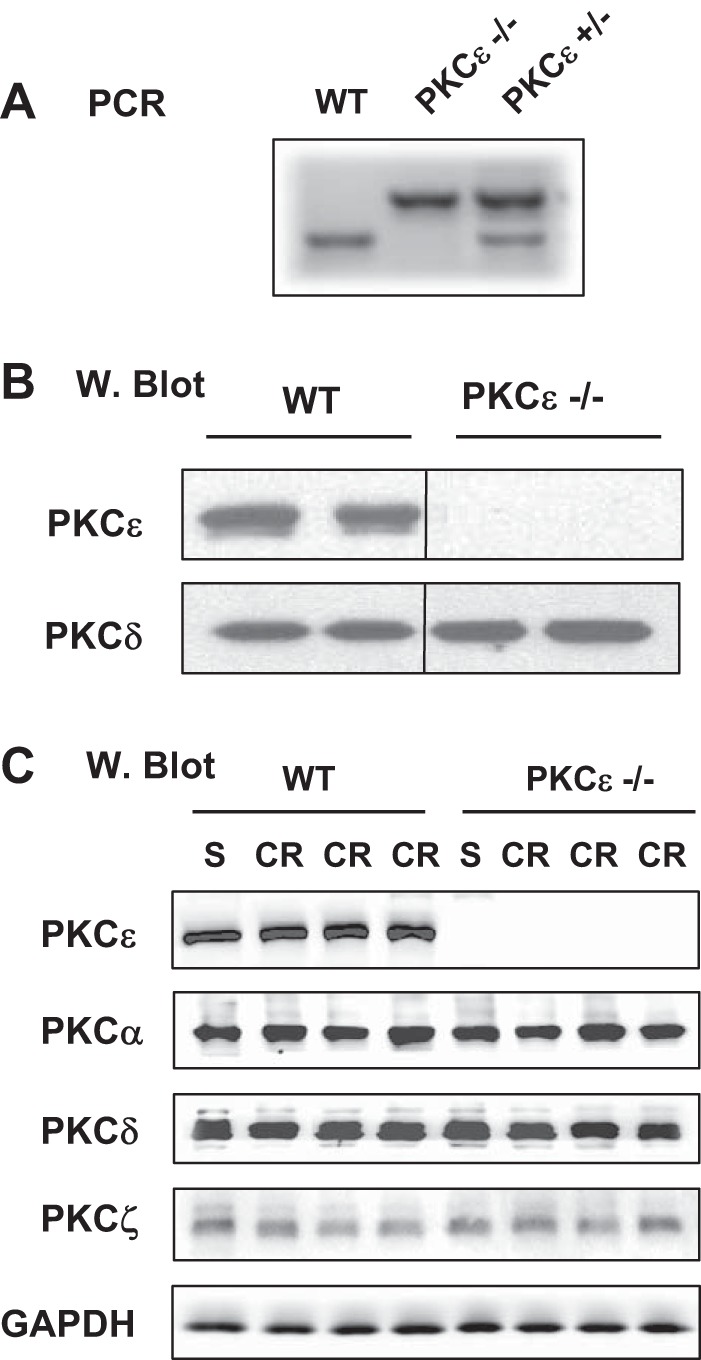
Genetic deletion of PKCε does not affect expression of the other 3 PKC isoforms presenting in pancreatic acinar cells. A: genotyping of PKCε mouse colonies with PCR. B: Western blot (W. Blot) analysis of PKCε and PKCδ in lysates of pancreatic acini isolated from WT mice and PKCε−/− mice. Samples were run in a single gel but were not continuous, as indicated by a line between lanes. C: Western blot analysis of PKC isoforms, ε, α, δ, and ζ in the lysates of pancreatic tissue collected from WT mice and PKCε−/− mice IP injected with saline (S) or cerulein (CR). GAPDH: for loading controls.
Deletion of PKCε decreases NF-κB activation in cerulein-induced pancreatitis.
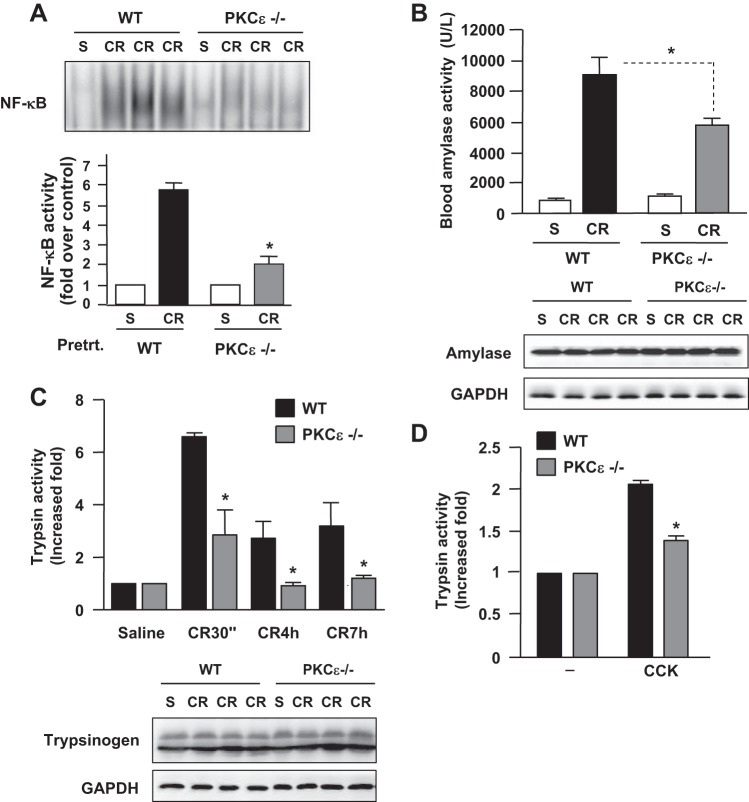
Our previous studies using specific PKCε pharmacological inhibitor in an in vitro pancreatitis model in isolated pancreatic acini demonstrated that PKCε was required for CCK-induced NF-κB activation (43, 44). Here, using the in vivo pancreatitis model, we showed that genetic deletion of PKCε largely prevented NF-κB activation in cerulein-induced pancreatitis (Fig. 3A), supporting the critical role of PKCε in NF-κB activation in acute pancreatitis.
Fig. 3.
Deletion of PKCε decreases NF-κB activation, blood amylase activity, and intrapancreatic trypsin activation in pancreatitis. A: NF-κB binding activities in 30 min after 1 injection of cerulein (CR) were measured in nuclear extracts by EMSA. Bottom: EMSA band intensities were quantified and normalized on the saline control. Values are means ± SE (n = 3), *P < 0.05 vs. WT mice with cerulein. B: serum amylase activity at 7 hourly cerulein injection. Values are means ± SE (n = 3), *P < 0.05 vs. WT mice with cerulein; Western blot analysis (bottom) shows similar pancreatic amylase expression levels in WT and PKCε−/−. C: intrapancreatic trypsin activation in pancreatitis. Values are means ± SE (n = 3), *P < 0.05 vs. WT mice treated with cerulein at the same indicated time points. Western blot analysis of pancreatic trypsinogen (bottom) shows that PKCε deletion does not alter pancreatic expression of this enzyme. Blots of GAPDH in B and C for loading controls. D: intracellular trypsin activation induced by stimulation with CCK (100 nM) for 30 min in isolated pancreatic acini. Values are means ± SE (n = 3), *P < 0.05 vs. WT mouse acini stimulated with CCK.
Deletion of PKCε ameliorates necrosis and other pancreatitis parameters in cerulein-induced pancreatitis.
Next, we evaluated the effect of PKCε−/− on the parameters of cerulein-induced pancreatitis. Cerulein caused a marked increase in blood amylase activity after 7 hourly IP injections of cerulein in WT mice (Fig. 3B). This increase was significantly attenuated in PKCε−/− mice. Cerulein-induced intracellular trypsinogen conversion to trypsin (as measured by trypsin activity) was markedly reduced in PKCε−/− mice (Fig. 3C). Reduction of trypsin conversion in PKCε−/− mice was also observed in an in vitro experimental pancreatitis model (Fig. 3D) using isolated pancreatic acini stimulated with a supramaximal dose (100 nM) of CCK, which was known to cause cell pathologies of pancreatitis in vitro (17, 43, 48). Western blot analyses of pancreatic tissue lysates showed similar protein expression levels of amylase and trypsinogen between PKCε−/− mice and WT mice (Fig. 3B and 3C, bottom), which excluded the possibility that the decreased activities of blood amylase and intracellular trypsin were caused by the alteration of their expression levels in the pancreas of PKCε−/− mice.
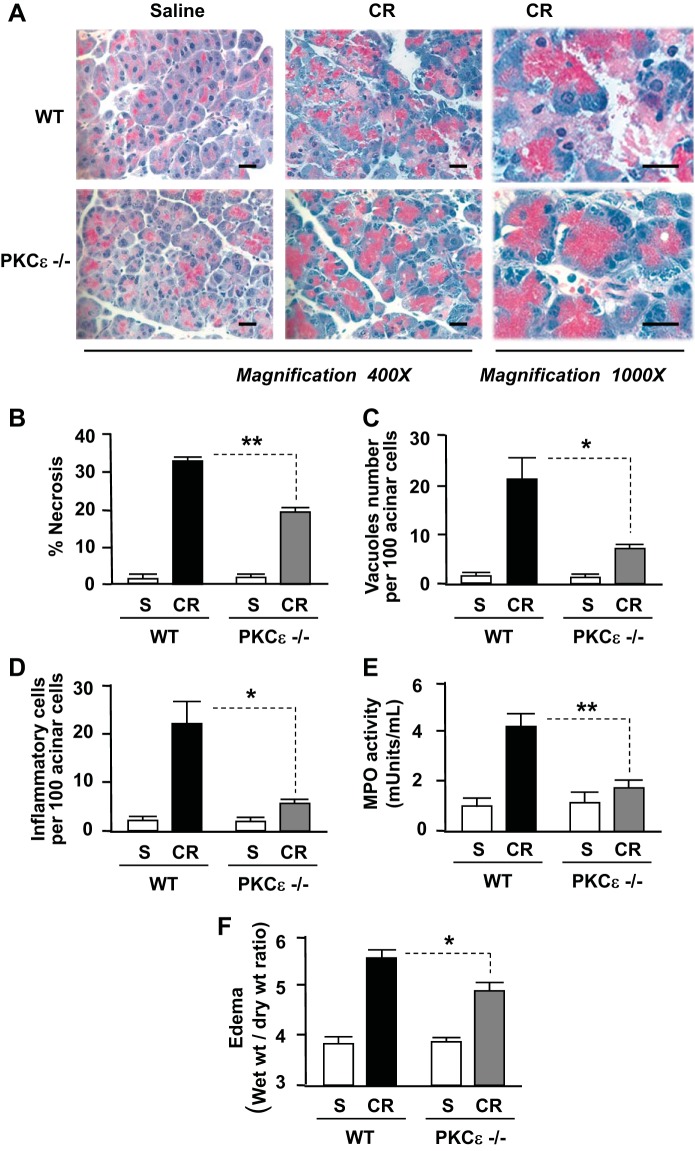
In addition to the above findings, PKCε deletion dramatically ameliorated the histological damage in cerulein-induced pancreatitis (Fig. 4A). The first salient feature of the pancreatic histological changes in PKCε−/− mice compared with WT mice was that cerulein-induced acinar cell necrosis was significantly attenuated (Fig. 4B). Other histopathological features of pancreatitis including accumulation of cytoplasmic vacuoles and inflammatory cell infiltration were also greatly attenuated in PKCε−/− mice (Fig. 4, C and D). Measurements of MPO activities further confirmed the attenuated inflammatory cell infiltration in pancreatitis in PKCε−/− mice (Fig. 4E). Measurements of the wet-to-dry weight ratio of pancreatic tissue (Fig. 4E) indicated that the edema was significantly reduced in pancreatitis in PKCε−/− mice. All these results demonstrate that PKCε deletion ameliorates necrosis, inflammation, and the severity of acute pancreatitis.
Fig. 4.
Deletion of PKCε attenuates necrosis and severity in cerulein pancreatitis. A: hematoxylin and eosin (H&E) staining of pancreatic tissue sections from mice with 7 hourly cerulein (or saline) injections. Each image was representative of at least 3 mice with similar results at each condition. Bars represent 20 μm. B: necrosis was measured on H&E-stained pancreatic tissue sections, as described in materials and methods. C and D: number of vacuole and inflammatory cells were counted on H&E-stained pancreatic tissue sections and expressed as percentage of total acinar cells. E: neutrophil infiltration within pancreas was assessed by measuring myeloperoxidase (MPO) activity in tissue homogenates and expressed as milliunits of MPO activity per milliliter homogenate (with same protein concentration). F: pancreatic edema (water content) was evaluated by measuring the wet-to-dry weight ratio of the pancreas tissue samples. Results in graphs (B–F) represent means ± SE (n = 3–6 mice) for each condition. **P < 0.01 or *P < 0.05 vs. WT with cerulein injections as indicated.
Inhibition of PKCε attenuates acinar cell necrosis and ATP reduction in the in vitro model of pancreatitis.
To corroborate the findings that PKCε promoted necrosis in the in vivo model of pancreatitis, we performed experiments on isolated pancreatic acinar cells. The acinar cells were stimulated with supramaximal (100 nM) CCK, which was known to induce pancreatitis responses in acinar cells, such as activation of trypsinogen and NF-κB, necrosis, and apoptosis (17, 43, 48, 54, 55). Therefore, this system was considered in vitro model of acute pancreatitis.
We applied two approaches to inactivate PKCε in acinar cells in this study: using the pancreatic acini isolated from PKCε−/− mice and using a pharmacological isoform-specific peptide PKCε inhibitor to treat WT acinar cells. WT pancreatic acini were preincubated with a cell-permeable specific PKCε translocation inhibitor peptide or a scrambled peptide (10 μM, 1 h). Then PKCε−/− acini or the pretreated WT acini were hyperstimulated with CCK (100 nM). Changes in cellular ATP levels and LDH release into the extracellular medium were measured after 3-h incubation with CCK. We observed that CCK hyperstimulation caused increased LDH release, an indicator of cell necrosis (Fig. 5A), which was associated with a dramatic decrease in cellular ATP (Fig. 5B), but either genetic or pharmacological inhibition of PKCε protected the acinar cells from CCK-induced necrosis and ATP reduction.
Fig. 5.
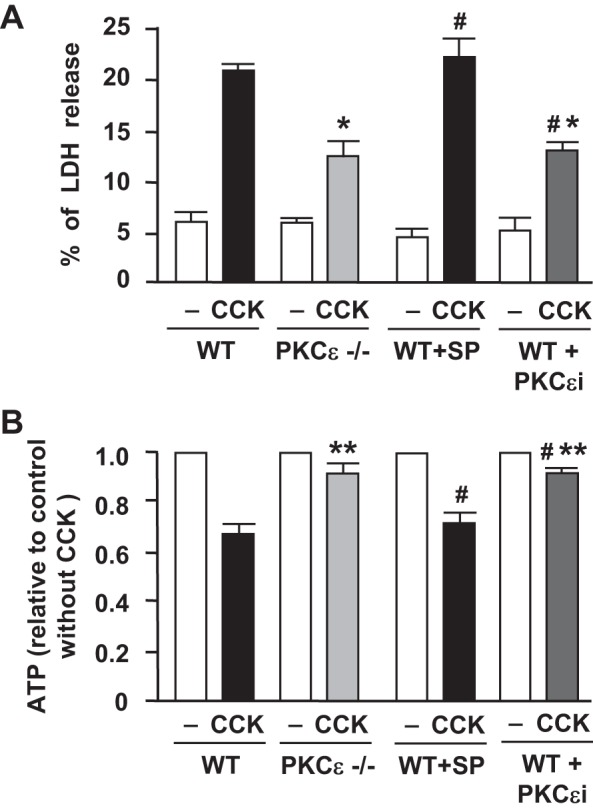
Inhibition of PKCε prevents ATP depletion and necrosis in CCK-hyperstimulated pancreatic acinar cells. Pancreatic acini isolated from PKCε−/− mice or WT mice were incubated for 3 h without or with 10 μM PKCε translocation inhibitor (PKCεi) or a scrambled peptide (SP) followed by stimulation with 100 nM CCK-8. A: percentage of lactate dehydrogenase (LDH) released into the extracellular medium. B: cellular ATP was determined via ATP determination kit. ATP values in PKCε−/− or WT acinar cells were compared with their own control cells incubated without CCK. Values are means ± SE (n ≥ 3). **P < 0.01 and *P < 0.05 vs. WT acinar cells pretreated without or with a scrambled peptide (WT+SP, #) and then incubated with CCK (black bar) as indicated.
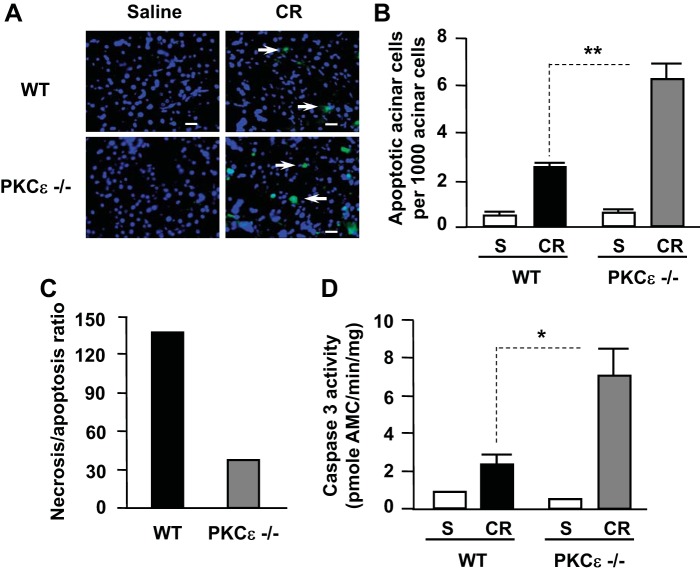
Deletion of PKCε promotes apoptosis and caspase activation in cerulein-induced pancreatitis.
We investigated the effects of PKCε deletion on apoptosis in the tissue slides with in situ TUNEL assays. Our results showed that, in contrast to the inhibitory effect of PKCε deletion on necrosis in pancreatitis, cerulein-induced apoptosis increased twofold in PKCε−/− mice compared with WT controls (Fig. 6, A and B). These results demonstrate the antiapoptotic effect of PKCε on acinar cell death in pancreatitis. Analysis of the effect of PKCε on the two death pathways (necrosis vs. apoptosis) showed that the ratio of necrosis to apoptosis in cerulein-induced pancreatitis was dramatically decreased (∼5 times less) in the PKCε−/− mice compared with WT (Fig. 6C).
Fig. 6.
Deletion of PKCε enhances apoptosis and caspase activation in pancreas during cerulein-induced pancreatitis. A: TUNEL staining of pancreatic tissue sections. Each image was representative of at least 3 mice with similar results at each condition. B: TUNEL-positive cells were quantified on pancreatic tissue sections from mice receiving 7 hourly injections of cerulein (CR) or saline (S) as described in materials and methods. C: necrosis/apoptosis ratios were compared in WT mice and PKCε−/− mice. The ratio calculation was based on the percentage of necrotic cells vs. percentage of apoptotic cells using the data from B and Fig. 4B. D: caspase-3 activity was measured in pancreatic tissue homogenates. Graphs (B and D) show means ± SE (n = 3). **P < 0.01 or *P < 0.05 vs. WT mice with 7 hourly injections of cerulein as indicated.
Apoptosis in pancreatic acinar cells is mediated mainly by activation of caspases. Supramaximal doses (100 nM) of CCK and its analog cerulein have been shown to activate caspases leading to apoptosis of pancreatic acinar cells in pancreatitis (5, 7, 17, 32, 48). We here found that caspase-3 activation was significantly increased in PKCε−/− mice in cerulein-induced pancreatitis compared with WT mice (Fig. 6D). These results indicate that PKCε regulates death pathways in pancreatitis by promoting necrosis and inhibiting caspase activation/apoptosis. Deletion of PKCε removes the blockade of PKCε on caspase activation, resulting in increased apoptosis in pancreatitis.
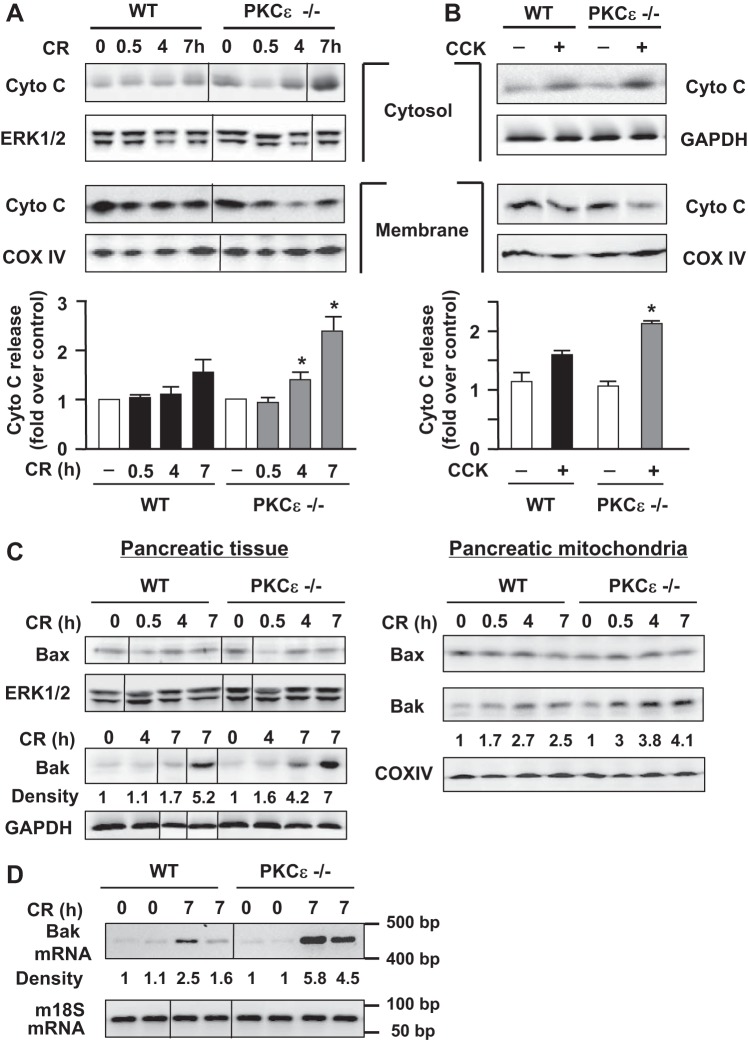
Deletion of PKCε promotes mitochondrial cytochrome c release in cerulein-induced pancreatitis.
Apoptosis is mediated by the release of cytochrome c into the cytosol followed by caspase activation. We evaluated the effect of PKCε−/− on cytochrome c release in cerulein-induced pancreatitis.
Cytochrome c protein levels in both cytosolic and membrane fractions were analyzed with Western blot (Fig. 7). Interestingly, we found that genetic inhibition of PKCε promoted mitochondrial cytochrome c release (Fig. 7A), exhibited by a faster and greater increase in cytochrome c levels in cytosol and a decrease in membrane-bound cytochrome c in PKCε−/− mice during pancreatitis. The blots were reprobed with an antibody against complex IV cytochrome c oxidase (COX IV, a mitochondrial integral membrane protein). The COX IV antibody did not detect any bands in cytosol (data not shown), indicating no contamination of cytosol with mitochondria. To substantiate the findings obtained with our in vivo model of experimental pancreatitis, we also measured cytochrome c release in isolated pancreatic acinar cells. The enhancement of pancreatic mitochondrial cytochrome c release-associated PKCε deletion was also observed in isolated pancreatic acini stimulated with a supramaximal dose of CCK (100 nM) (Fig. 7B), as evidenced by a more prominent decrease in the membrane and increase in the cytosolic 14-kDa cytochrome c in the PKCε−/− mice acini (Fig. 7B).
Fig. 7.
Cytochrome c release and upregulation of mitochondrial proapoptotic Bcl-2 protein Bak are higher in PKCε−/− mice during cerulein pancreatitis. A and B: cytochrome c (Cyto C) was measured by Western blot analyses in mitochondrial-enriched membrane and cytosolic fractions from pancreatic tissue of WT or PKCε−/− mice at indicated time points after induction of pancreatitis (A) or from pancreatic acini incubated with or without 100 nM CCK-8 for 3 h (B). Blots of membrane fractions were reprobed with complex IV cytochrome c oxidase (COX IV) antibody to confirm equal membrane protein loading. Blots from cytosolic fractions were further reprobed with an antibody against ERK1/2 or GAPDH for loading controls. Bar graphs: the band densities of the cytochrome c release from mitochondria into cytosol were quantified and normalized to the controls. Values are means ± SE (n = 2–3). *P < 0.05 vs. WT mice (black bars) at the same time points, respectively (A), or vs. WT acini stimulated with CCK (black bar) as indicated (B). C: showing greater upregulation of Bak but not Bax protein in PKCε−/− mice during cerulein pancreatitis, measured by Western blot analyses of the pancreatic tissue homogenates (left) and mitochondrial fractions (right). Left: the Bak blot was reblotted with GAPDH to confirm equal loading. For Bax blot and its loading control, the membrane was cut across a 36-kDa molecular weight marker, the bottom half membrane was blotted with Bax antibody, and the top part of the membrane was blotted with ERK1/2 antibody for loading control. Right: reblots of COX IV were to confirm equal protein loading. D: RT-PCR shows higher pancreatic Bak mRNA expression in PKCε−/− mice with cerulein (CR) pancreatitis. Mouse 18S ribosomal RNA was used as a reference (“housekeeping”) gene. Numbers to the right of the gel are DNA size markers in base pairs. The densities of Bak protein bands (C) or mRNA bands (D) were quantified by densitometry and normalized to the controls. A, C, D: samples were run in a single gel but were not continuous, as indicated by a line between lanes.
Thus both in vivo and in vitro results indicate that genetic deletion of PKCε stimulates cytochrome c release from the mitochondria into the cytoplasm during pancreatitis, accounting for the increased caspase activation and apoptosis in PKCε−/− mice during pancreatitis.
Deletion of PKCε increases pancreatic mitochondrial levels of proapoptotic protein Bak in cerulein-induced pancreatitis.
The proapoptotic Bax and Bak proteins form channels in the outer mitochondrial membrane through which cytochrome c is released into the cytosol (1, 26). Therefore, we analyzed the levels of these two proapoptotic Bcl-2 proteins in pancreatitis.
Western blot analysis showed that Bax levels did not change in either pancreatic tissue or pancreatic mitochondria in response to cerulein-induced pancreatitis in the WT or PKCε−/− mice (Fig. 7C). However, the other key proapoptotic Bcl-2 protein, Bak, was markedly upregulated at levels of both protein and mRNA during cerulein-induced pancreatitis in both mouse genotypes (Fig. 7, C and D). More importantly, the increases of both Bak protein and its mRNA were greater (∼2-fold) in PKCε−/− mice than in WT mice in pancreatitis, which was consistent with the greater increases in cytochrome c release seen in PKCε−/− mice (Fig. 7A).
Deletion of PKCε causes a more prominent upregulation of pancreatic mitochondrial prosurvival Bcl-2 proteins in cerulein-induced pancreatitis.
To provide insights into the mechanism of protection against cell necrosis in PKCε−/− mice, we next analyzed Bcl-2 and Bcl-xL, two mitochondrial prosurvival Bcl-2 proteins. Recent studies from our group indicated that these two Bcl-2 proteins stabilized pancreatic mitochondria and protected against necrosis in pancreatitis and that upregulation of Bcl-2 and Bcl-xL was a key protective mechanism against necrosis in pancreatitis (38, 48).
We found that Bcl-2 and Bcl-xL protein levels increased both in the whole pancreatic homogenates (Fig. 8A, left) and pancreatic mitochondria in cerulein-induced pancreatitis in WT mice (Fig. 8B), in accord with previous results from our group (48). More importantly, the upregulation of Bcl-2 and Bcl-xL proteins in pancreatitis was more pronounced in PKCε−/− mice than in WT mice (Fig. 8). Consistent with their greater protein levels, the mRNA expression of Bcl-2 and Bcl-xL was also more increased in PKCε−/− mice compared with WT mice (Fig. 8A, right). The greater extent of upregulation of the prosurvival Bcl-2 and Bcl-xL proteins in PKCε−/− mice may account, in part, for the attenuation of necrosis in pancreatitis in PKCε−/− mice and suggests that the negative regulation of Bcl-2 and Bcl-xL proteins by PKCε might be a mechanism whereby PKCε promotes necrosis in pancreatitis.
Fig. 8.
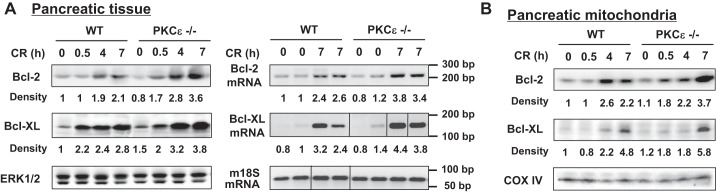
Deletion of PKCε leads to a higher upregulation of pancreatic prosurvival Bcl-2 proteins in cerulein pancreatitis. Protein levels of Bcl-2 and Bcl-xL were measured by Western blot analysis for pancreatic tissue homogenates (A, left) and mitochondrial membrane fractions (B) at indicated times after the induction of pancreatitis. The densities of Bcl-2 and Bcl-xL bands were quantified and normalized to the control. Blots of ERK1/2 or COX IV were for loading controls. RT-PCR (A, right) shows higher Bcl-2 and Bcl-xL mRNA expression in pancreatic tissue of PKCε−/− mice with cerulein (CR) pancreatitis. Mouse 18S ribosomal RNA was used as a reference (housekeeping) gene. For either Bcl-xL or m18S PCR, samples were run in a single gel but were not continuous, as indicated by a line between lanes. The densities of Bcl-2 and Bcl-xL mRNA bands were quantified and normalized to the controls. Numbers to the right of the gel are DNA size markers in base pairs.
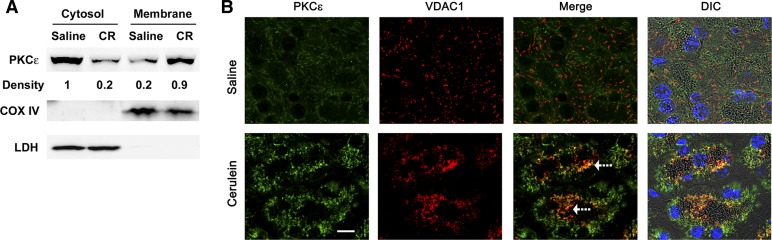
PKCε translocates to mitochondria in cerulein-induced pancreatitis.
Translocation from cytosol to membranes is a characteristic of activated PKCs. Activated PKCε has been reported to translocate to intracellular membranes in previous studies (44, 50). The effect of PKCε on mitochondrial proteins prompted us to address the possibility of mitochondrial translocation of PKCε in cerulein pancreatitis. Again we used two approaches: subcellular fractionation, and immunostaining of the pancreatic tissue collected in cerulein pancreatitis (60 min after one IP injection) in WT mice. Comparing PKCε level in cytosolic fractions and mitochondria-enriched membrane fractions (Fig. 9A), we found that PKCε localized predominantly in the cytosolic fraction of pancreas from control mice injected with saline and that cerulein treatment dramatically decreased the presence of PKCε in the cytosolic fraction and increased PKCε in the membrane fraction, indicating mitochondrial membrane translocation of this kinase (Fig. 9A). The same blots were also probed with antibody against COX IV or LDH for loading controls and to assess the quality of our separation of membrane from cytosolic fractions.
Fig. 9.
PKCε translocates to mitochondria in cerulein-induced pancreatitis. A: subcellular distribution of PKCε in early stage (30 min) of pancreatitis was determined in cytosolic and mitochondrial-enriched membrane fractions by Western blot analysis. The densities of PKCε bands were quantified and normalized to the saline controls. The blots were reprobed with an antibody against COX IV or LDH for loading controls and to assess the quality of separating membrane fractions from cytosolic fractions. Shown are representative Western blots from 3 independent experiments. B: colocalization of PKCε with VDAC1, a mitochondria marker in CR pancreatitis. Pancreatic tissue sections were double immunostained for PKCε (green) and VDAC1 (red), and secondary antibodies were conjugated with Alexa 488 or 594. Nuclei were counterstained with DAPI. Images were visualized under confocal microscope. The original magnification is ×63. Bar represents 10 μm. DIC, differential interference contrast.
The mitochondrial translocation of PKCε was further confirmed by immunostaining of the pancreatic tissue sections. Confocal immunofluorescence (Fig. 9B) showed that in saline control mice, the staining of PKCε (shown in green) distributed throughout the cytosol. Mitochondrial marker (VDAC1) was seen as sharp punctate structures (shown in red) and distributed around the nuclei, granules, and cell periphery [see DIC (differential interference contrast) image in Fig. 9B], as described before (41). Little colocalization (shown in yellow) of PKCε and VDAC1 was observed in the pancreatic tissue sections from saline control mice. Upon cerulein administration, we observed a condensed, ringlike pattern of staining for both PKCε and VDAC1, indicating the redistribution of PKCε and VDAC1-positive compartments. More importantly, we observed a dramatically increased colocalization of PKCε and VDAC1-positive compartments (around granules, Fig. 9B), supporting that PKCε might translocate to mitochondria to effect death regulating proteins directly during pancreatitis. Similar results were also obtained when we used another mitochondrial marker protein, Tom20, instead of VDAC1 in immunostaining (data not shown).
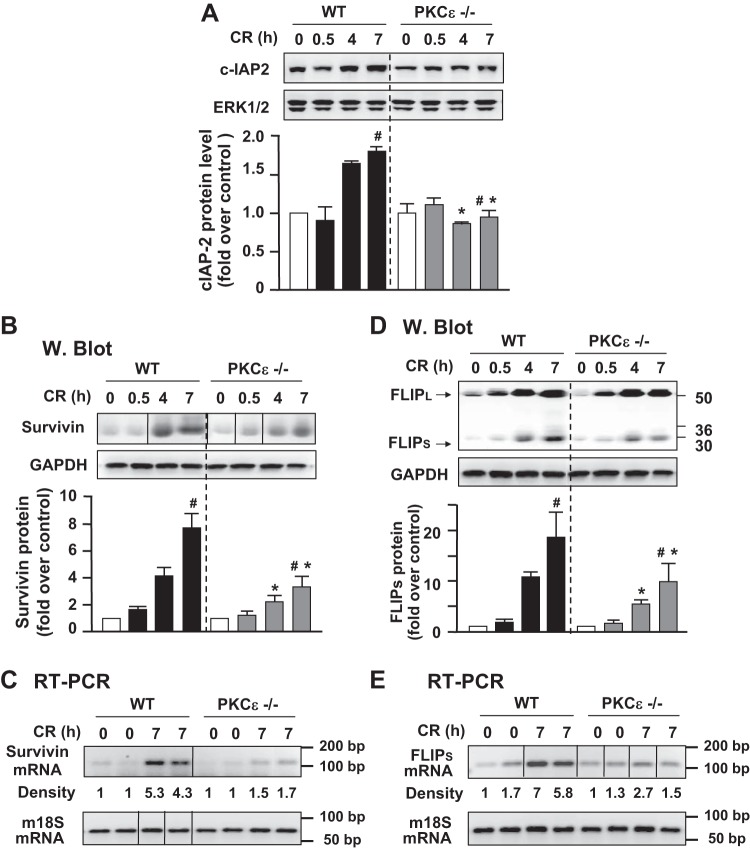
Deletion of PKCε decreases pancreatic levels of antiapoptotic proteins c-IAP2, survivin, and c-FLIPs in cerulein-induced pancreatitis.
Next, we determined whether PKCε regulated cell death also through nonmitochondrial pathways. IAPs are potent endogenous suppressors of caspases and play a pivotal role in regulation of cell death (11, 12, 16, 22, 23, 32, 39, 47, 55).
We first determined whether PKCε regulates c-IAP1 and c-IAP2, two key members of the eight mammalian IAPs and endogenous inhibitors of caspase-3, 7, and 9 (12, 57), in pancreatitis. We did not detect any alteration of c-IAP1 in pancreatitis in WT and PKCε−/− mice (data not shown). However, we found that c-IAP2 was upregulated markedly and time dependently in pancreatitis in WT mice (Fig. 10A), and importantly, this upregulation of c-IAP2 was significantly suppressed in PKCε−/− mice. We next examined the level of survivin, an endogenous inhibitor of caspase-3 (22). Similar to c-IAP2, the time-dependent upregulation of survivin during cerulein-induced pancreatitis was also dramatically inhibited in PKCε−/− mice (Fig. 10B).
Fig. 10.
Deletion of PKCε decreases antiapoptotic proteins levels in cerulein pancreatitis. Western blot analyses of pancreas tissue lysate using antibodies against c-IAP2 (A), survivin (B), c-FLIP (C) to measure the pancreatic levels of these proteins in cerulein (CR)-induced pancreatitis at indicated times (ERK1/2 or GAPDH: loading controls). Bar figures are the quantification of these protein band densities that were compared with the saline control. Graphs show means ± SE (n = 2–3) for each condition. *P < 0.05 vs. WT mice with cerulein at the same time point as indicated. C and E: RT-PCR shows less mRNA expression of survivin and FLIPs in pancreatic tissue of PKCε−/− mice with cerulein pancreatitis. Mouse 18S ribosomal RNA was used as a reference (housekeeping) gene. The densities of survivin and FLIPs mRNA bands were quantified and normalized to the controls. B, C, E: samples were run in a single gel but were not continuous, as indicated by a line between lanes.
c-FLIP, an important endogenous protein inhibitor of caspase-8, has two splice isoforms: c-FLIPS (short) and c-FLIPL (long). c-FLIPS completely inhibits caspase-8 processing, whereas c-FLIPL allows partial processing of caspase-8 (27, 40). We found that both c-FLIPS and c-FLIPL were markedly upregulated in pancreatitis in WT mice (Fig. 10D). There was no significant difference in the magnitude of the upregulation of c-FLIPL between WT and PKCε−/− mice. However, the upregulation of c-FLIPS occurred to a greater extent in WT mice than in PKCε−/− mice (Fig. 10D), indicating that the upregulation of c-FLIPS in pancreatitis was inhibited in PKCε−/− mice.
Furthermore, using survivin and c-FLIPS as the representatives of antiapoptotic proteins, we examined whether the observed inhibitory effect on upregulation of the antiapoptotic proteins in pancreatitis in PKCε−/− mice was associated with decreased mRNA levels. We found that the increased mRNA expression of either survivin or c-FLIPS during cerulein pancreatitis was dramatically suppressed in PKCε−/− mice (Fig. 10, C and E), consistent with their protein levels observed in Western blotting (Fig. 10, B and D).
We also examined whether deletion of PKCε had any effect(s) on XIAP, which was shown to significantly decrease in rat but not mouse pancreatitis models (32, 54). We found that XIAP remained unchanged in the course of cerulein-induced pancreatitis in WT mice, and no difference was observed between WT mice and PKCε−/− mice (data not shown).
Thus our results indicate that genetic inhibition of PKCε has effects on several endogenous inhibitors of caspases, c-IAP2, survivin, and c-FLIPs, accounting for, at least partially, the effects observed on caspase activation and apoptosis in vivo, and indicating that PKCε probably inhibits apoptosis and caspase activation in mouse pancreatitis by enhancing the levels of these IAPs.
Deletion of PKCε promotes degradation of RIP1 in cerulein-induced pancreatitis.
RIP kinase has recently emerged as a key mediator of programmed necrosis in acute pancreatitis (20, 32, 54) and other diseases (10, 14, 28, 33, 49). As we discussed earlier, RIP is cleaved and inactivated by caspase-3 and -8 during apoptosis (10, 28, 33), which has been suggested to be one of mechanisms underlying the protective role of caspases from necrosis in cerulein-induced pancreatitis (20, 32, 54).
Here we determined the effect of the genetic deletion of PKCε on RIP degradation (Fig. 11). In agreement with our previous findings (32), RIP remained at a stable level during cerulein-induced pancreatitis in WT mice. Of note, the expression level of RIP in control PKCε−/− mice injected with saline was similar to that in WT mice. However, RIP underwent a dramatic cleavage (∼60–70%) to a ∼30-kDa product in PKCε−/− mice after seven hourly cerulein injections (Fig. 11). The results correlated with the change in caspase activities (Fig. 6D) and IAPs levels (Fig. 10) in the two mouse genotypes, suggesting that PKCε-regulated IAPs/caspases pathways played a role in RIP cleavage/inactivation in cerulein-induced pancreatitis.
Fig. 11.
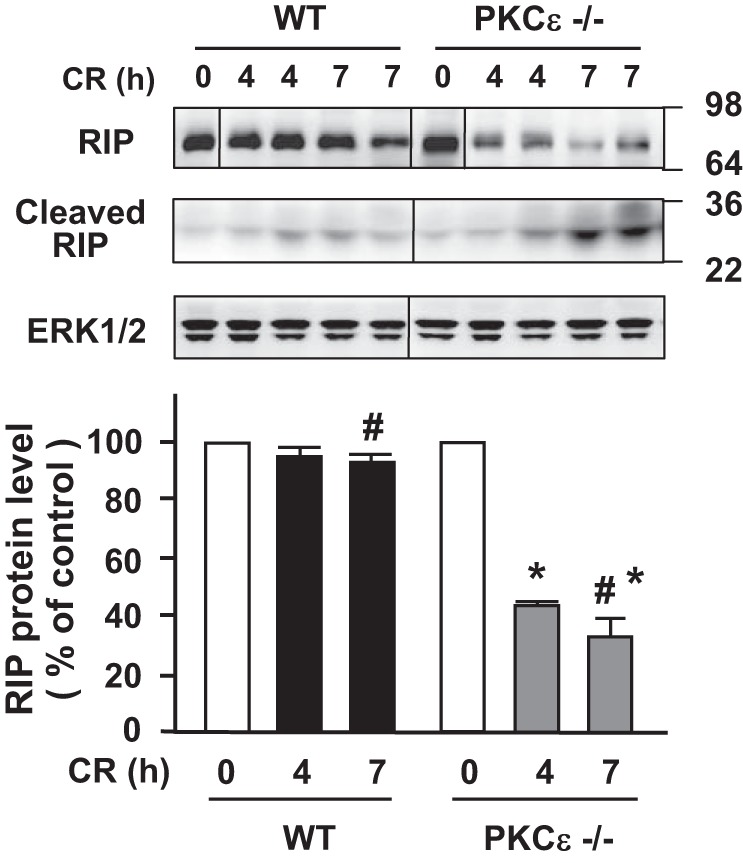
Deletion of PKCε promotes receptor-interacting protein kinase (RIP) degradation/inactivation in cerulein-induced pancreatitis. Western blot analysis of pancreas tissue lysate using antibodies against RIP to measure the protein levels of the protein in cerulein (CR) pancreatitis at indicated times. The same samples were used to rerun gel and blotted with ERK1/2 antibody to confirm equal protein loading. Samples were run in a single gel but were not continuous, as indicated by a line between lanes. Bar figures are the quantification of these protein band densities that were compared with saline (S) control. Values are means ± SE (n = 2–3) for each condition. *P < 0.05 vs. WT mice with CR at the same time point as indicated; #, the 2 bars are compared statistically. Numbers to the right of the gels are protein size (kDa).
DISCUSSION
Previous work demonstrated a role for PKCε in the inflammatory response (43, 44) and zymogen activation (50) in pancreatitis. We designed the present study to investigate the molecular mechanisms through which PKCε regulates cell death responses in in vivo and in vitro experimental models of acute pancreatitis.
We first demonstrated that PKCε activation was an early event in in vivo experimental model of pancreatitis. PKCε activation promoted necrosis whereas genetic inhibition of PKCε significantly ameliorated necrosis, inflammation, and the severity of pancreatitis. We then showed that the decreased necrosis in PKCε−/− mice was associated with marked increases in caspase activation and apoptosis pathways associated with pancreatitis. Thus the study evolved to determining both mitochondrial proapoptotic and prosurvival proteins and nonmitochondrial mechanisms that could account for necrosis regulated by PKCε.
Mitochondrial membrane permeability occupies a central position in regulation of cell death in pancreatitis. The outer mitochondrial membrane permeabilization (OMP) causes release of cytochrome c into the cytosol, which leads to activation of caspases. In contrast, opening the mitochondrial permeability transition pore causes the loss of mitochondrial membrane potential, results in subsequent decrease in ATP synthesis, loss of plasma membrane integrity, and necrosis (1, 26, 37, 48, 56). Mitochondrial Bcl-2 proteins play a critical role in the regulation of mitochondrial permeabilization. PKCε has been known to primarily exert antiapoptotic signaling by influencing the levels/activation status of Bcl-2 family proteins that regulate mitochondrial integrity (4, 46).
We evaluated the effect of PKCε deletion on cytochrome c release through OMP in pancreatitis. Both in vivo and in vitro results suggested that genetic inhibition of PKCε stimulated cytochrome c release from the mitochondria to the cytoplasm during pancreatitis. More interestingly, the increased cytochrome c release in PKCε−/− mice was closely associated with marked increases in Bak levels in both whole pancreatic tissue homogenate and mitochondria in PKCε−/− mice. These results suggest that inhibition of PKCε promotes OMP opening, leading to cytochrome c release and caspase activation, at least partially via upregulation of mitochondrial Bak.
Next, we assessed whether PKCε inhibition had an effect on cellular ATP and cell necrosis induced by supramaximal CCK stimulation in an in vitro pancreatitis. Our results showed that inhibition of PKCε with either genetic deletion or PKCε translocation inhibitor prevented supramaximal CCK-induced ATP decrease and cell necrosis in pancreatitis.
Of note, it has been controversial whether ATP depletion plays a causal or primary role in the pathophysiology of acute pancreatitis in earlier studies (30, 31). For example, an equivalent ATP decrease was observed in response to physiological doses of cerulein (30) in mice and in rats with supraphysiological cerulein (31), which did not develop necrosis. Recent evidence (1, 26, 48, 56) indicated, however, that necrosis in pancreatitis was associated with ATP depletion. The present study showing that inhibition of PKCε prevented ATP reduction and necrosis suggested a key role for PKCε in regulating ATP production and necrosis. The decrease in ATP itself is important but is not the only effect mediating the actions of PKCε in pancreatitis as shown in this report.
We further investigated other factors mediating the effect of PKCε on necrosis.
We analyzed expression of Bcl-2 and Bcl-xL, which were mitochondrial prosurvival Bcl-2 proteins in pancreatitis. The recent studies in our group (38, 48) indicated that these two Bcl-2 proteins could stabilize pancreatic mitochondria and protect against necrosis in pancreatitis and that upregulation of Bcl-2 and Bcl-xL was a key protective mechanism against necrosis in pancreatitis. We found more prominent upregulation of the pancreatic mitochondrial prosurvival Bcl-2 proteins in cerulein-induced pancreatitis in PKCε−/− mice, which may have provided the protection against cell necrosis.
Notably, previous studies have demonstrated a difference between cancer cells and pancreatic acinar cells regarding the predominant role of mitochondrial prosurvival/antiapoptotic Bcl-2 proteins (2, 9, 48). For example, the major effect of Bcl-xL/Bcl-2 inhibitors in cancer cells is increased apoptosis (2, 9) resulting from stimulation of cytochrome c release (9). In contrast, the predominant effect of the Bcl-xL/Bcl-2 inhibitors in pancreatitis is ATP depletion and necrosis but not apoptosis, as shown in our recent studies (48, 38).
The mechanism by which PKCε upregulates mitochondrial Bcl-2 proteins is unknown. We found that the pancreatitis treatment caused translocation of PKCε to the mitochondria. Our Western blot analyses of tissue fractions showed that PKCε translocated from the cytosol to mitochondrial membranes upon cerulein stimulation. Using immunofluorescence, we observed that PKCε distribution after stimulation was strongly colocalized with the mitochondrial marker protein VDAC1, confirming the presence of PKCε at cellular sites occupied by mitochondria. These studies suggest that PKCε may directly target mitochondria and modulate mitochondrial functions related to necrosis. These results could also account in part for the decreased ATP reduction and attenuated necrosis we found in pancreatitic acini, either treated with PKCε translocation inhibitor or isolated from PKCε−/− mice.
In addition to mitochondrial death pathways, the present study shows that PKCε has effects on nonmitochondrial mechanisms of caspase activation downstream of cytochrome c release. IAPs and antiapoptotic protein c-FLIP play a pivotal braking role on apoptotic signaling by inhibiting caspases (12, 16, 22, 23, 39, 47). Some of these molecules have been shown to be key determinants of the type of cell death during pancreatitis in our studies (32, 54). Here we found that deletion of PKCε resulted in significant decreases in pancreatic levels of antiapoptotic proteins c-IAP2, survivin, and c-FLIPs in cerulein-induced pancreatitis. These results indicate that PKCε regulates pathways that inhibit apoptosis through both mitochondrial and nonmitochondrial mechanisms.
The mechanisms of the downregulation of IAPs and FLIPs in pancreatitis caused by genetic inhibition of PKCε remain to be studied. Our results suggested that this downregulation is mediated at least in part through transcriptional mechanism, possibly through NF-κB inhibition. A wealth of evidence indicates that transcriptional activation of survivin and c-FLIP through NF-κB pathways is crucial in regulating cell death (12, 22, 23, 39, 47, 57). PKCε was shown to be required for NF-κB activation in isolated pancreatic acini (43, 44). Here we demonstrated that NF-κB activation in cerulein-induced pancreatitis model was potently inhibited in PKCε−/− mice (Fig. 3A). These findings support the potential role of PKCε activation in regulation of IAPs through NF-κB activation. Nevertheless, the complete mechanism of reduction in IAPs with PKCε inhibition needs further study.
Of additional importance, our study showed that deletion of PKCε promoted degradation/inactivation of RIP in cerulein-induced pancreatitis. RIP, a key mediator of programmed necrosis in many diseases including acute pancreatitis (10, 14, 20, 28, 33, 49), was previously found to be cleaved/inactivated by caspase-3 and -8. Our findings suggest that increased RIP cleavage/inactivation may be a mechanism through which deletion of PKCε ameliorates necrosis in cerulein-induced pancreatitis.
In summary, our studies present the first in vivo evidence that PKCε is a critical mediator of necrosis in experimental pancreatitis through its effects on mitochondrial and nonmitochondrial death pathways. Genetic inhibition of PKCε decreases necrosis and the severity of pancreatitis. As we summarized in Fig. 12, activation of PKCε in pancreatitis promotes necrosis through regulating mitochondrial death pathways involving Bcl-2 family proteins, OMP and cytochrome c release, and ATP depletion; and nonmitochondrial pathways involving IAPs, c-FLIPs, caspase activation/apoptosis, and RIP.
Fig. 12.
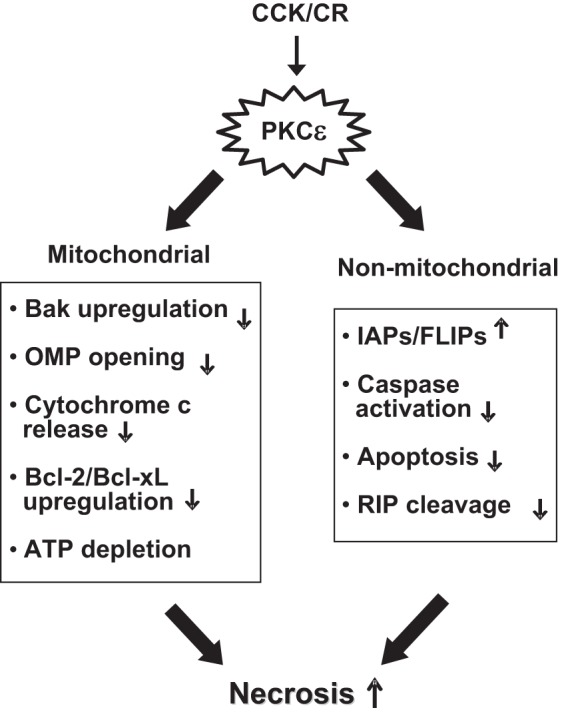
Scheme illustrating the regulation of necrosis in pancreatitis by PKCε through mitochondrial and nonmitochondrial pathways. OMP, outer membrane permeabilization.
Although our findings on the role of PKCε in pancreatitis were based on cerulein pancreatitis models, evidence supports a role for PKCε in other models of experimental pancreatitis and human pancreatitis. PKCε activation mediates key pathobiological processes in alcohol-induced pancreatitis (44). Furthermore, the cholinergic system is known to mediate exocrine pancreas functions and pancreatitis responses in humans, which we have recapitulated in a model of alcoholic pancreatitis (29). Hyperstimulation with cholinergic agonist carbachol also induces pancreatitis responses such as NF-κB activation and zymogen activation in rat pancreatic acinar cells (51, 55), and carbachol-induced stimulation of PKCδ and PKCε activities are required for these pathological responses (51, 55). In addition, PKCε activation is observed in an early stage of arginine model of pancreatitis (our unpublished data). PKC inhibitors significantly reduce biliary acute pancreatitis-induced tissue injury (45). These findings indicate a potential role for PKCε across animal models and human pancreatitis. Thus we suggest that targeting PKCε may be a novel approach for prevention or treatment of necrosis in acute pancreatitis.
GRANTS
This study was supported by the Southern California Research Center for Alcoholic Liver and Pancreatic Diseases (NIH Grant P50-A11999); UCLA Center of Excellence in Pancreatic Diseases (NIH Grant 1P01AT0003960-01); the Department of Veterans Affairs Merits Grant; and Lee Summer Student Research Award from Southern California Research Center for Liver and Pancreatic Diseases (to T. Tan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Y.L., J.Y., T.T., W.J., O.A.M., and R.T.W. performed experiments; Y.L., J.Y., T.T., W.J., A.L., O.A.M., and S.J.P. analyzed data; Y.L., J.Y., T.T., A.L., and S.J.P. interpreted results of experiments; Y.L., J.Y., T.T., W.J., A.L., O.A.M., R.T.W., and S.J.P. approved final version of manuscript; J.Y. and S.J.P. conception and design of research; J.Y. and O.A.M. prepared figures; J.Y. drafted manuscript; J.Y., R.T.W., and S.J.P. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Dr. Robert O. Messing (University of California, San Francisco) for providing the initial breeding pairs of PKCε deficiency mice. We also thank Dr. Anna Gukovskaya for discussion in this study.
REFERENCES
- 1.Armstrong JS. Mitochondrial membrane permeabilization: the sine qua non for cell death. BioEssays 28: 253–260, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Azmi AS, Mohammad RM. Non-peptidic small molecule inhibitors against Bcl-2 for cancer therapy. J Cell Physiol 218: 13–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastani B, Yang L, Baldassare JJ, Pollo DA, Gardner JD. Cellular distribution of isoforms of protein kinase C (PKC) in pancreatic acini. Biochim Biophys Acta 1269: 307–315, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Basu A, Sivaprasad U. Protein kinase Cepsilon makes the life and death decision. Cell Signal 19: 1633–1642, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beil M, Leser J, Lutz MP, Gukovskaya A, Seufferlein T, Lynch G, Pandol SJ, Adler G. Caspase 8-mediated cleavage of plectin precedes F-actin breakdown in acinar cells during pancreatitis. Am J Physiol Gastrointest Liver Physiol 282: G450–G460, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Bhatia M, Wallig MA, Hofbauer B, Lee HS, Frossard JL, Steer ML, Saluja AK. Induction of apoptosis in pancreatic acinar cells reduces the severity of acute pancreatitis. Biochem Biophys Res Commun 246: 476–483, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Bhatia M. Apoptosis vs. necrosis in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 286: G189–G196, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Cacace AM, Guadagno SN, Krauss RS, Fabbro D, Weinstein IB. The epsilon isoform of protein kinase C is an oncogene when overexpressed in rat fibroblasts. Oncogene 8: 2095–2104, 1993 [PubMed] [Google Scholar]
- 9.Campàs C, Cosialls AM, Barragán M, Iglesias-Serret D, Santidrián AF, Coll-Mulet L, de Frias M, Domingo A, Pons G, Gil J. Bcl-2 inhibitors induce apoptosis in chronic lymphocytic leukemia cells. Exp Hematol 34: 1663–1669, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Chan FK, Shisler J, Bixby JG, Felices M, Zheng L, Appel M, Orenstein J, Moss B, Lenardo MJ. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem 278: 51613–51621, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Chu ZL, McKinsey TA, Liu L, Gentry JJ, Malim MH, Ballard DW. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc Natl Acad Sci USA 94: 10057–10062, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deveraux QL, Reed JC. IAP family proteins-suppressors of apoptosis. Genes Dev 13: 239–252, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol 16: 663–669, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Galluzzi L, Kepp O, Kroemer GI. RIP kinases initiate programmed necrosis. J Mol Cell Biol 1: 8–10, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Gorelick F, Pandol S, Thrower E. Protein kinase C in the pancreatic acinar cell. J Gastroenterol Hepatol Suppl 1: S37–S41, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Gukovskaya AS, Pandol SJ. Cell death pathways in pancreatitis and pancreatic cancer. Pancreatology 4: 567–586, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Gukovskaya AS, Gukovsky I, Jung Y, Mouria M, Pandol SJ. Cholecystokinin induces caspase activation and mitochondrial dysfunction in pancreatic acinar cells. Roles in cell injury processes of pancreatitis. J Biol Chem 277: 22595–22604, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Hackert T, Sperber R, Hartwig W, Fritz S, Schneider L, Gebhard M, Werner J. P-selectin inhibition reduces severity of acute experimental pancreatitis. Pancreatology 9: 369–374, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Hafeez BB, Zhong W, Weichert J, Dreckschmidt NE, Jamal MS, Verma AK. Genetic ablation of PKC epsilon inhibits prostate cancer development and metastasis in transgenic mouse model of prostate adenocarcinoma. Cancer Res 71: 2318–2327, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He S, Wang L, Miao L, Wang T, Du F, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 137: 1100–1111, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Kaiser AM, Saluja AK, Sengupta A, Saluja M, Steer ML. Relationship between severity, necrosis, and apoptosis in five models of experimental acute pancreatitis. Am J Physiol Cell Physiol 269: C1295–C1304, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Kawakami H, Tomita M, Matsuda T, Ohta T, Tanaka Y, Fujii M, Hatano M, Tokuhisa T, Mori N. Transcriptional activation of survivin through the NF-kappaB pathway by human T-cell leukemia virus type I tax. Int J Cancer 115: 967–974, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Kerbauy DM, Lesnikov V, Abbasi N, Seal S, Scott B, Deeg HJ. NF-kappaB and FLIP in arsenic trioxide (ATO)-induced apoptosis in myelodysplastic syndromes (MDSs). Blood 106: 3917–3925, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, Green PG, Hodge CW, Levine JD, Messing RO. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron 24: 253–260, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krantic S, Mechawar N, Reix S, Quirion R. Apoptosis-inducing factor: a matter of neuron life and death. Prog Neurobiol 81: 179–196, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev 87: 99–163, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Krueger A, Schmitz I, Baumann S, Krammer PH, Kirchhoff S. Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex. J Cell Biochem 276: 20633–20640, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev 13: 2514–2526, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lugea A, Gong J, Nguyen J, Nieto J, French SW, Pandol SJ. Cholinergic mediation of alcohol-induced experimental pancreatitis. Alcohol Clin Exp Res 34: 1768–1781, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lüthen RE, Niederau C, Ferrell LD, Grendell JH. Energy metabolism in mouse pancreas in response to different dosages of a CCK analogue. Pancreas 11: 141–146, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Lüthen RE, Niederau C, Grendell JH. Intrapancreatic zymogen activation and levels of ATP and glutathione during caerulein pancreatitis in rats. Am J Physiol Gastrointest Liver Physiol 268: G592–G604, 1995 [DOI] [PubMed] [Google Scholar]
- 32.Mareninova OA, Sung KF, Hong P, Lugea A, Pandol SJ, Gukovsky I, Gukovskaya AS. Cell death in pancreatitis: caspases protect from necrotizing pancreatitis. J Biol Chem 281: 3370–3381, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Moquin D, Chan FK. The molecular regulation of programmed necrotic cell injury. Trends Biochem Sci 35: 434–441, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434: 652–658, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Newton AC. Protein kinase C: structure, function, and regulation. J Biol Chem 270: 28495–28498, 1995 [DOI] [PubMed] [Google Scholar]
- 36.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 258: 607–614, 1992 [DOI] [PubMed] [Google Scholar]
- 37.Odinokova IV, Sung KF, Mareninova OA, Hermann K, Evtodienko Y, Andreyev A, Gukovsky I, Gukovskaya AS. Mechanisms regulating cytochrome c release pancreatic mitochondria. Gut 58: 431–442, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Odinokova IV, Sung KF, Mareninova OA, Hermann K, Evtodienko Y, Andreyev A, Gukovsky I, Gukovskaya AS. Bcl-xL and Bcl-2 prosurvival proteins protect against necrosis in pancreatitis. Pancreas 35: 420, 2007 [Google Scholar]
- 39.Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology 132: 1127–11251, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Peter ME. The flip side of FLIP. Biochem J 382: e1–e3, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petersen OH. Specific mitochondrial functions in separate sub-cellular domains of pancreatic acinar cells. Pflügers Arch 464: 77–87, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Proskuryakov SY, Konoplyannikov AG, Gabai VL. Necrosis: a specific form of programmed cell death? Exp Cell Res 283: 1–16, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Satoh A, Gukovskaya AS, Nieto JM, Cheng JH, Gukovsky I, Reeve JR, Jr, Shimosegawa T, Pandol SJ. PKC-δ and -ε regulate NF-κB activation induced by cholecystokinin and TNF-α in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 287: G582–G591, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Satoh A, Gukovskaya AS, Reeve JR, Jr, Shimosegawa T, Pandol SJ. Ethanol sensitizes NF-κB activation in pancreatic acinar cells through effects on protein kinase C-ε. Am J Physiol Gastrointest Liver Physiol 291: G432–G438, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Shi C, Zhao X, Wang X, Zhao L, Andersson R. Potential effects of PKC or protease inhibitors on acute pancreatitis-induced tissue injury in rats. Vascul Pharmacol 46: 406–411, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Soh JW, Lee YS, Weinstein IB. Effects of regulatory domains of specific isoforms of protein kinase C on growth control and apoptosis in MCF-7 breast cancer cells. J Exp Ther Oncol 3: 115–126, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Stehlik C, Martin R, Kumabashiri I, Schmid JA, Binder BR, Lipp J. NF-κB regulated X-chromosome-linked IAP gene expression protects endothelial cells from TNF-α induced apoptosis. J Exp Med 188: 211–216, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sung KF, Odinokova IV, Mareninova OA, Rakonczay Z, Jr, Hegyie P, Pandol SJ, Gukovsky I, Gukovskaya AS. Prosurvival Bcl-2 proteins stabilize pancreatic mitochondria and protect against necrosis in experimental pancreatitis. Exp Cell Res 315: 1975–1989, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trichonas G, Murakami Y, Thanos A, Morizane Y, Kayamaa M, Debouckc CM, Hisatomi T, Miller JW, Vavvas DG. Receptor interacting protein kinases mediate retinal detachment-induced photoreceptor necrosis and compensate for inhibition of apoptosis. Proc Natl Acad Sci USA 107: 21695–21700, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thrower EC, Osgood S, Shugrue CA, Kolodecik TR, Chaudhuri AM, Reeve JR, Jr, Pandol SJ, Gorelick FS. The novel protein kinase C isoforms -δ and -ε modulate caerulein-induced zymogen activation in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 294: G1344–G1353, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thrower EC, Yuan J, Usmani A, Liu Y, Jones C, Minervini SN, Alexandre M, Pandol SJ, Guha S. A novel protein kinase D inhibitor attenuates early events of experimental pancreatitis in isolated rat acini. Am J Physiol Gastrointest Liver Physiol 300: G120–G129, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsujimoto Y, Nakagawa T, Shimizu S. Mitochondrial membrane permeability transition and cell death. Biochim Biophys Acta 1757: 1297–1300, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Tsujimoto Y, Shimizu S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis 12: 835–840, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Yuan J, Liu Y, Tan T, Guha S, Gukovsky I, Gukovskaya A, Pandol SJ. Protein kinase D regulates cell death pathways in experimental pancreatitis. Front Physiol 3: 60, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuan J, Lugea A, Zheng L, Gukovsky I, Edderkaoui M, Rozengurt E, Pandol SJ. Protein kinase D1 mediates NF-κB activation induced by cholecystokinin and cholinergic signaling in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 295: G1190–G1201, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zong WX, Thompson CB. Necrotic death as a cell fate. Genes Dev 20: 1–15, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Zou T, Rao JN, Guo X, Liu L, Zhang HM, Strauch ED, Bass BL, Wang JY. NF-κB-mediated IAP expression induces resistance of intestinal epithelial cells to apoptosis after polyamine depletion. Am J Physiol Cell Physiol 286: C1009–C1018, 2004 [DOI] [PubMed] [Google Scholar]