Abstract
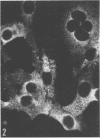
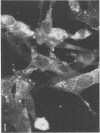
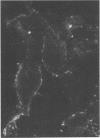
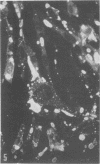
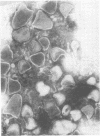
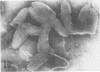
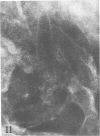
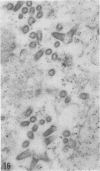
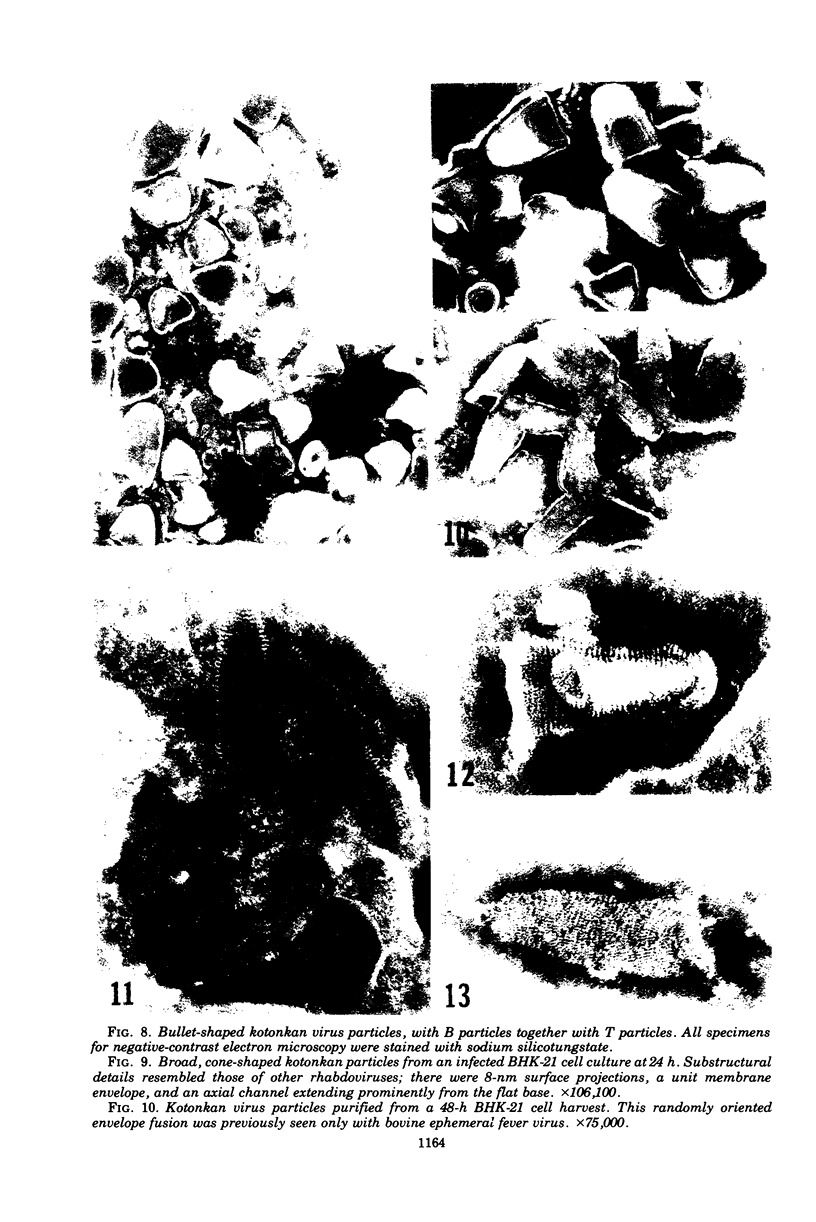
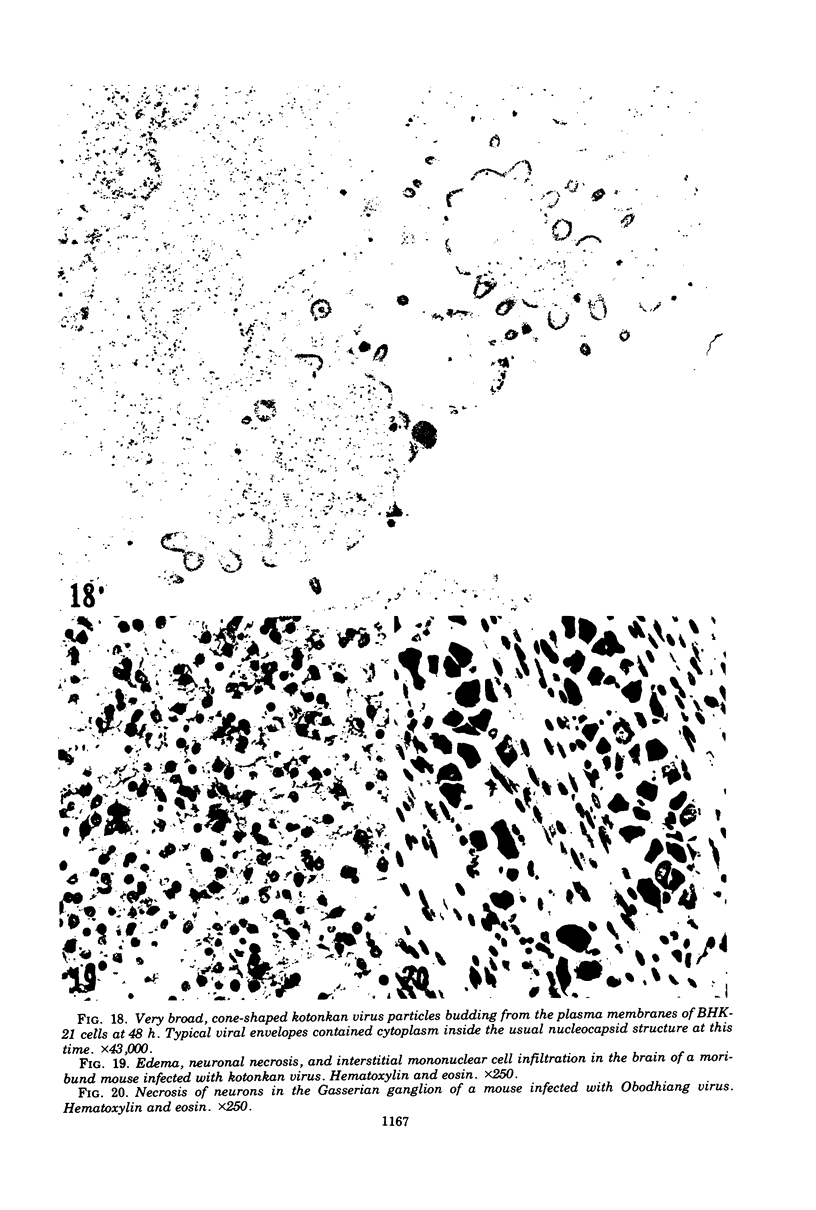
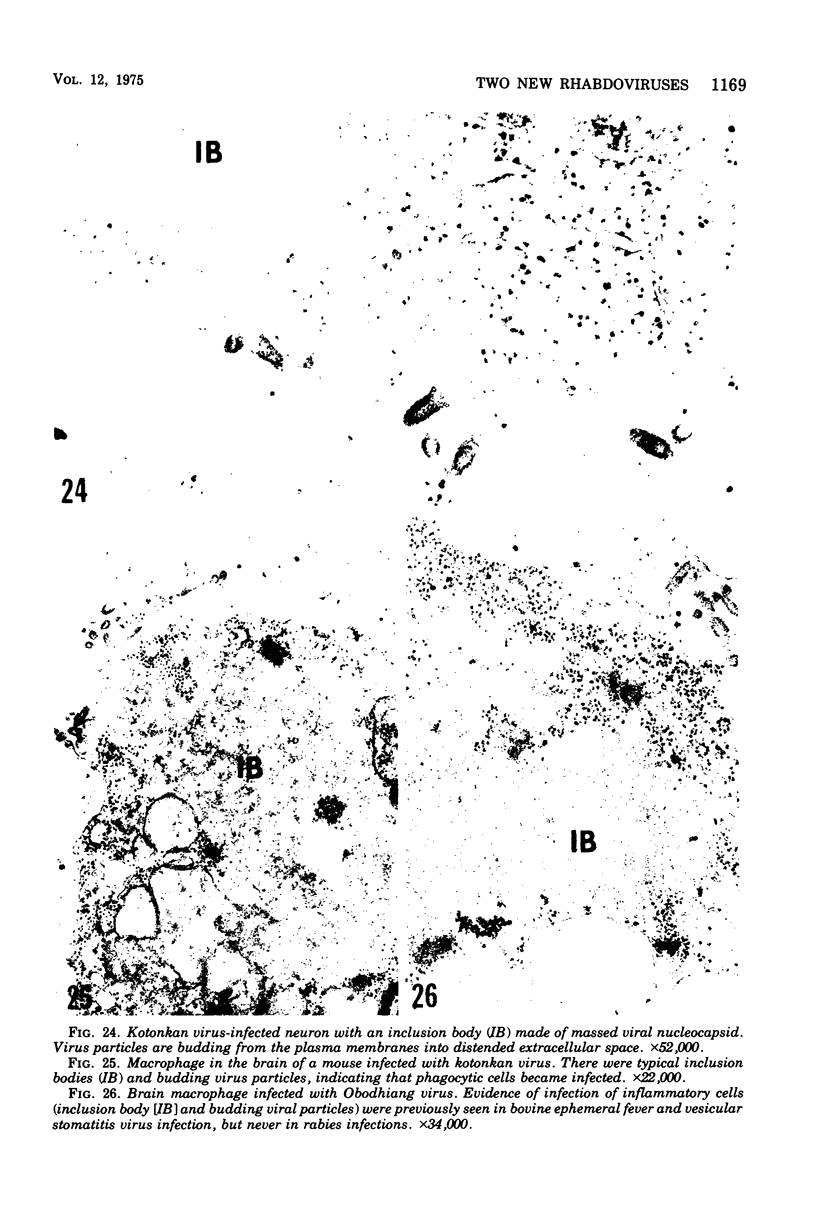
Indirect immunofluorescence confirmed the antigenic relationship between kotankan and Obodhiang viruses and Mokola virus that had originally been shown by complement fixation test. This relationship suggests inclusion of these two arthropod isolates in the rabies subgroup of the Rhabdoviridae family. Cross-reactivity with Mokola virus was also demonstrated by direct immunofluorescence but was easily eliminated when conjugates were diluted. No crossreactivities were found by neutralization tests or by surface immunofluorescence. Other than these immunological ties to the rabies serogroup, other biological characteristics of kotonkan and Obodhiang viruses were distinct. Maximum yield of infectivity of kotonkan and Obodhiang in cell culture was at 30 C, antigen usually filled the cytoplasm of infected cells diffusely, and syncytia were formed before severe cytonecrosis. By electron microscopy, virus particles and their nucleocapsids appeared cone shaped (mean lengths: kotonkan, 182 nm; Obodhiang, 170 nm). Viral morphogenesis took place on plasma membranes of cells in culture, mouse brain neurons, and inflammatory cells (macrophages) in brain lesions. All of these characteristics of the two viruses, and the known association of kotonkan virus with an acute, febrile illness of cattle in Nigeria, suggest a biological relationship with bovine ephemeral fever virus. The latter is known to exist in the same geographic area but exhibits no serological cross-reaction with either kotonkan or Obodhiang virus. The question of whether these two viruses deserve placement in an expanded rabies subgroup (at the cost of a less precise definition of the subgroup) or in a separate subgroup (which would include bovine ephemeral fever virus) of the Rhabdoviridae family will only be answered by further physicochemical characterization and comparison.
Full text
PDF


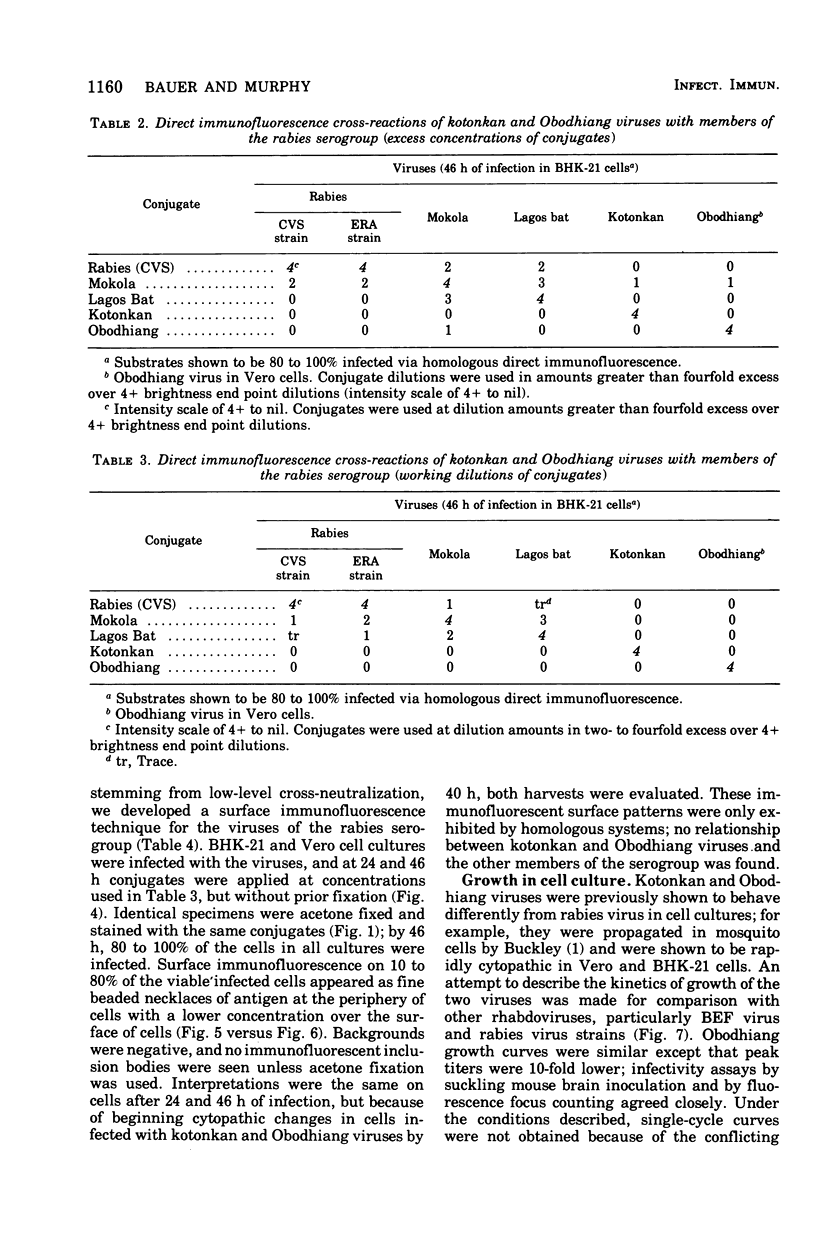
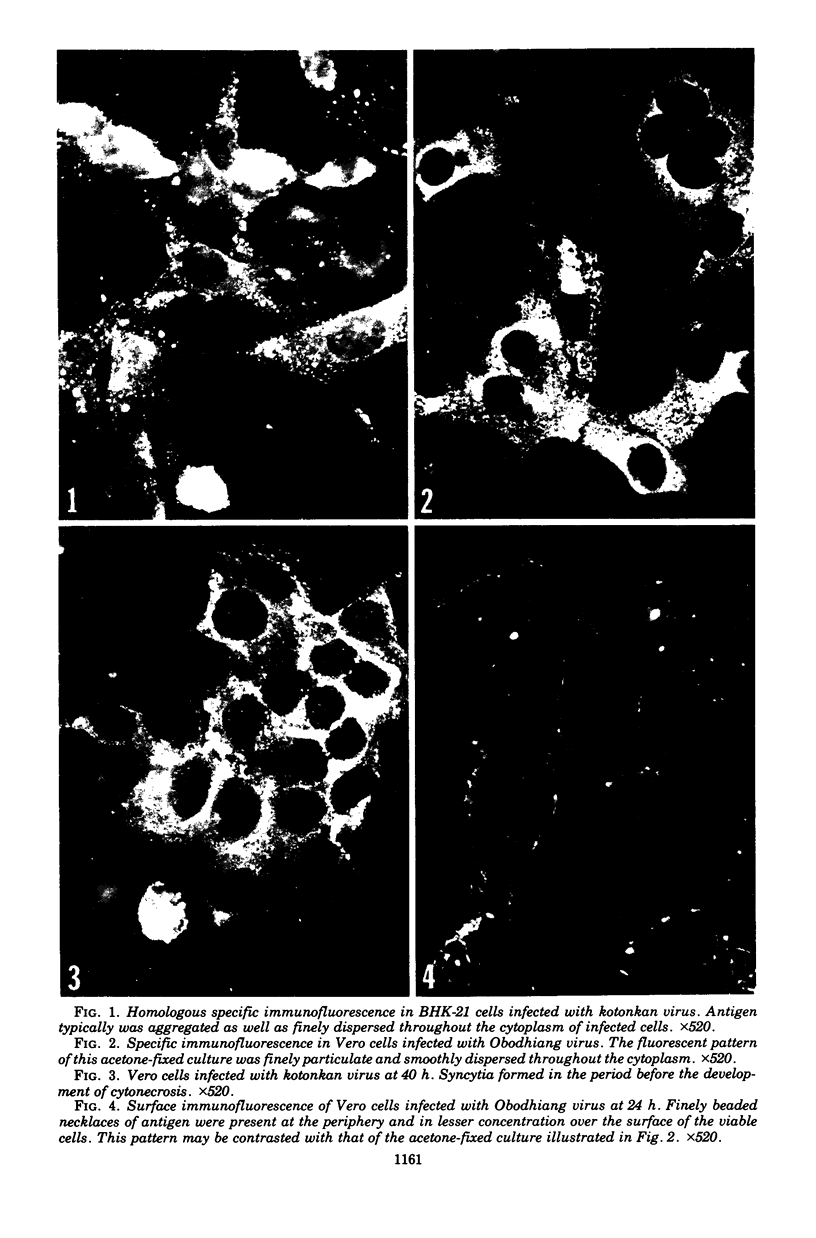
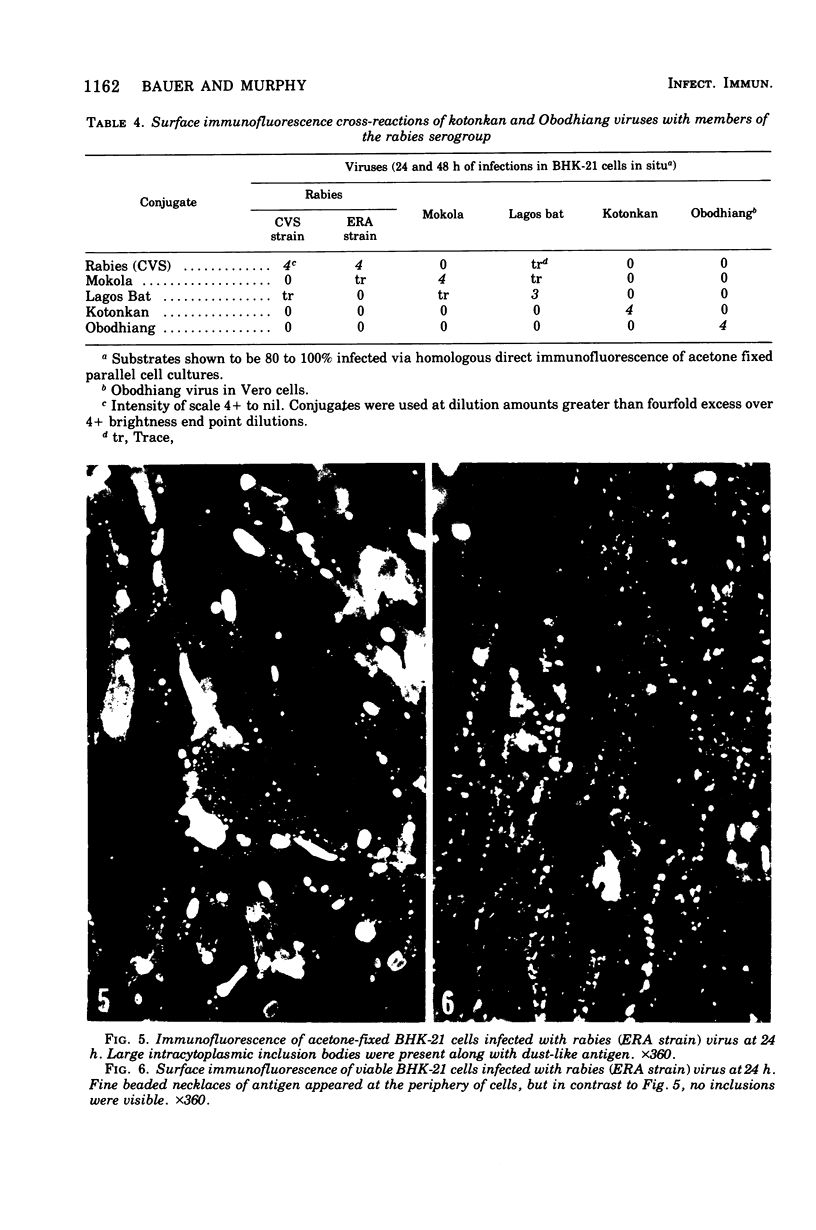
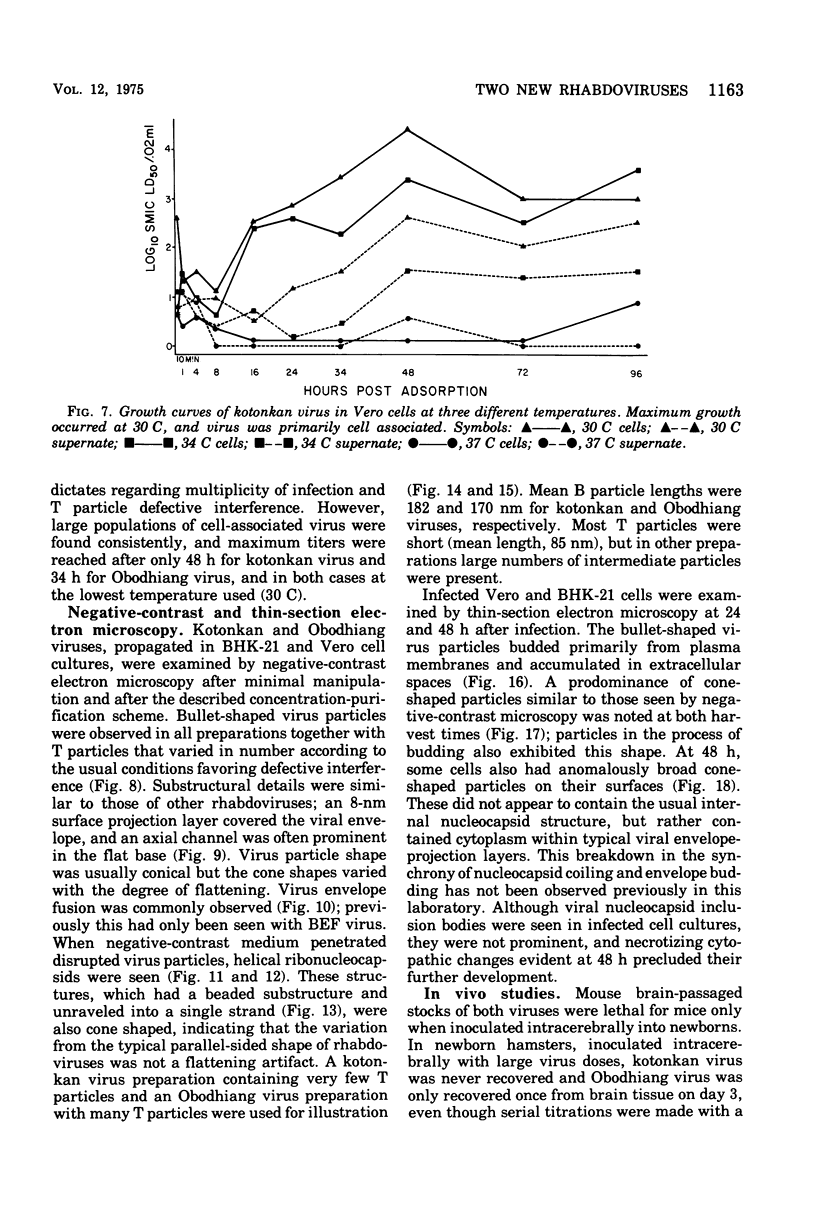

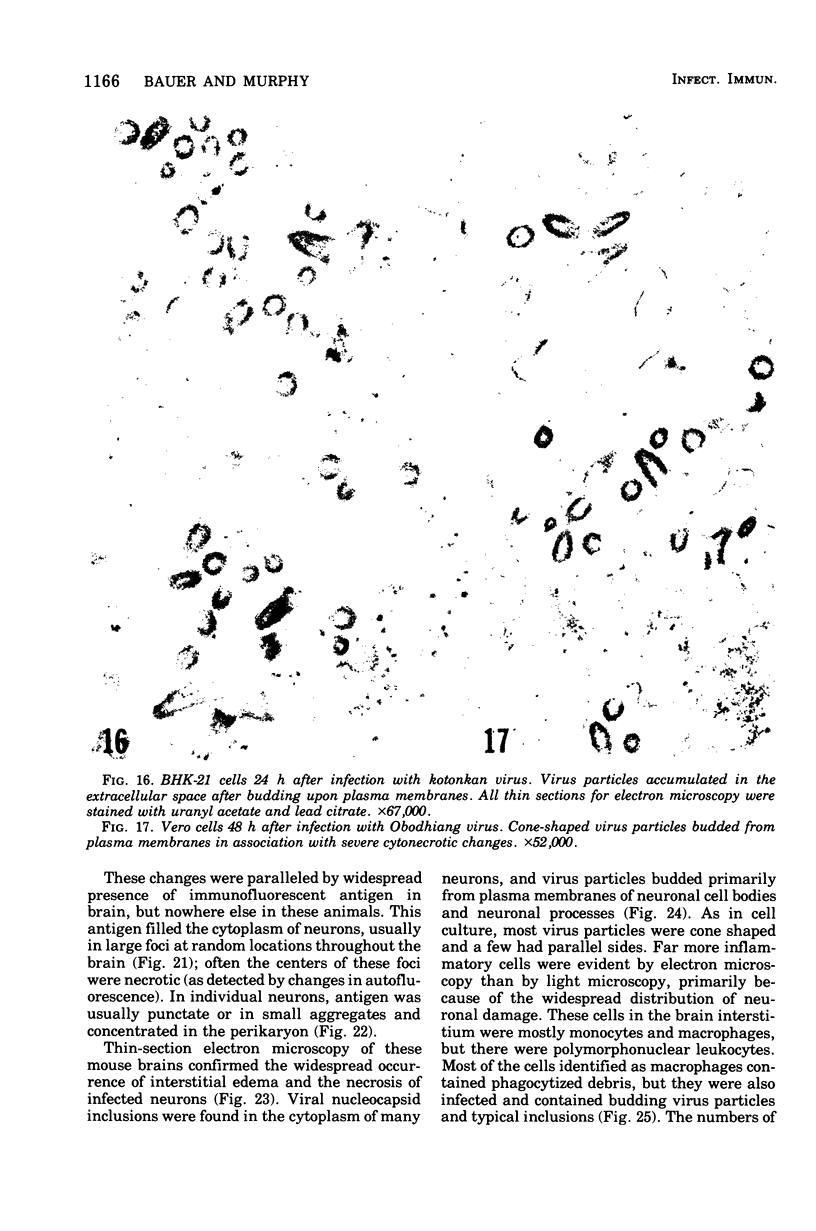




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckley S. M. Singh's Aedes albopictus cell cultures as helper cells for the adaptation of Obodhiang and kotonkan viruses of the rabies serogroup to some vertebrat cell cultures. Appl Microbiol. 1973 Apr;25(4):695–696. doi: 10.1128/am.25.4.695-696.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley S. M., Tignor G. H. Plaque assay for rabies serogroup viruses in vero cells. J Clin Microbiol. 1975 Feb;1(2):241–242. doi: 10.1128/jcm.1.2.241-242.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzschold B., Schneider L. G., Cox J. H. Serological characterization of the three major proteins of vesicular stomatitis virus. J Virol. 1974 Jul;14(1):1–7. doi: 10.1128/jvi.14.1.1-7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes I. H., Doherty R. L. Morphology and development of bovine ephemeral fever virus. J Virol. 1970 Jan;5(1):91–96. doi: 10.1128/jvi.5.1.91-96.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Tanaka Y., Inaba Y., Omori T. Electron microscopic observations of bovine epizootic fever virus. Natl Inst Anim Health Q (Tokyo) 1969 Spring;9(1):35–44. [PubMed] [Google Scholar]
- Kemp G. E., Mann E. D., Tomori O., Fabiyi A., O'Connor E. Isolation of bovine ephemeral fever virus in Nigeria. Vet Rec. 1973 Jul 28;93(4):107–108. doi: 10.1136/vr.93.4.107. [DOI] [PubMed] [Google Scholar]
- Lecatsas G., Theodoridis A., Erasmus B. J. Electron microscopic studies on bovine ephemeral fever virus. Arch Gesamte Virusforsch. 1969;28(3):390–398. doi: 10.1007/BF01240952. [DOI] [PubMed] [Google Scholar]
- MOLLENHAUER H. H. PLASTIC EMBEDDING MIXTURES FOR USE IN ELECTRON MICROSCOPY. Stain Technol. 1964 Mar;39:111–114. [PubMed] [Google Scholar]
- Matumoto M., Inaba Y., Tanaka Y., Ito H., Omori T. Behavior of bovine ephemeral fever virus in laboratory animals and cell cultures. Jpn J Microbiol. 1970 Sep;14(5):413–421. doi: 10.1111/j.1348-0421.1970.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Bauer S. P., Harrison A. K., Winn W. C., Jr Comparative pathogenesis of rabies and rabies-like viruses. Viral infection and transit from inoculation site to the central nervous system. Lab Invest. 1973 Mar;28(3):361–376. [PubMed] [Google Scholar]
- Obijeski J. F., Marchenko A. T., Bishop D. H., Cann B. W., Murphy F. A. Comparative electrophoretic analysis of the virus proteins of four rhabdoviruses. J Gen Virol. 1974 Jan;22(1):21–33. doi: 10.1099/0022-1317-22-1-21. [DOI] [PubMed] [Google Scholar]
- Schneider L. G., Dietzschold B., Dierks R. E., Matthaeus W., Enzmann P. J., Strohmaier K. Rabies group-specific ribonucleoprotein antigen and a test system for grouping and typing of rhabdoviruses. J Virol. 1973 May;11(5):748–755. doi: 10.1128/jvi.11.5.748-755.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shope R. E., Murphy F. A., Harrison A. K., Causey O. R., Kemp G. E., Simpson D. I., Moore D. L. Two African viruses serologically and morphologically related to rabies virus. J Virol. 1970 Nov;6(5):690–692. doi: 10.1128/jvi.6.5.690-692.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigley N. G. The lattice spacing of crystalline catalase as an internal standard of length in electron microscopy. J Ultrastruct Res. 1968 Sep;24(5):454–464. doi: 10.1016/s0022-5320(68)80048-6. [DOI] [PubMed] [Google Scholar]