Abstract
Studies suggest that improvements in type 2 diabetes (T2D) post- Roux-en-Y gastric bypass (RYGB) surgery are attributable to decreased intestinal glucose absorption capacity mediated by exclusion of sweet taste-sensing pathways in isolated proximal bowel. We probed these pathways in rat models that had undergone RYGB with catheter placement in the biliopancreatic (BP) limb to permit post-RYGB exposure of isolated bowel to sweet taste stimulants. Lean Sprague Dawley (n = 13) and obese Zucker diabetic fatty rats (n = 15) underwent RYGB with BP catheter placement. On postoperative day 11 (POD 11), rats received catheter infusions of saccharin [sweet taste receptor (T1R2/3) agonist] or saline (control). Jejunum was analyzed for changes in glucose transporter/sensor mRNA expression and functional sodium-glucose transporter 1 (SGLT1)-mediated glucose uptake. Saccharin infusion did not alter glucose uptake in the Roux limb of RYGB rats. Intestinal expression of the glucose sensor T1R2 and transporters (SGLT1, glucose transporter 2) was similar in saccharin- vs. saline-infused rats of both strains. However, the abundance of SGLT3b mRNA, a putative glucose sensor, was higher in the common limb vs. BP/Roux limb in both strains of bypassed rats and was significantly decreased in the Roux limb after saccharin infusion. We concluded that failure of BP limb exposure to saccharin to increase Roux limb glucose uptake suggests that isolation of T1R2/3 is unlikely to be involved in metabolic benefits of RYGB, as restimulation failed to reverse changes in intestinal glucose absorption capacity. The altered expression pattern of SGLT3 after RYGB warrants further investigation of its potential involvement in resolution of T2D after RYGB.
Keywords: Roux-en-Y gastric bypass, obesity, type 2 diabetes mellitus, intestinal sweet taste sensor, sodium-glucose transporter 1
in recent decades, bariatric surgery has evolved into the foremost therapy for obesity. Moreover, one of the weight loss operations, Roux-en-Y Gastric Bypass surgery (RYGB), is associated with a high rate of remission of type 2 diabetes mellitus (T2D), with an early and weight-independent mechanism that improves glucose homeostasis (2, 17). However, the mechanisms mediating the resolution of T2D remain poorly understood.
Intense investigation of contributory factors has implicated several mechanisms, including alterations in incretin levels, the gut microbiome, bile acids, and an area of interest to our lab, namely intestinal nutrient sensing. The surgical isolation of duodenum and proximal jejunum appear to be critical in mediating resolution of T2D, supported by outcomes following endoluminal sleeve device insertion and surgical procedures (e.g., RYGB, biliopancreatic diversion), which exclude a similar region of the intestine from the digestive process (9, 19, 20, 21). Further highlighting the importance of proximal bowel isolation are studies in RYGB models showing that the reintroduction of mixed nutrients into the excluded bowel reverses the beneficial effects of surgery on glucose tolerance (7, 8, 22). Despite recognition of the importance of the proximal small bowel in glucose regulation, a more detailed understanding of the underlying mechanisms remains elusive. RYGB removes a significant part of the proximal small bowel from the physiological enteral tract in the formation of the biliopancreatic (BP) limb and prevents nutrient contact with this region of the bowel. We have hypothesized that prevention of nutrient stimulation of mucosal sensors and luminal nutrient transporters in this bowel segment mediates improvements in glucose homeostasis. We previously confirmed the presence of sweet taste receptors (T1R2/3) along this region of bowel and demonstrated that specific activation of the T1R2/3 receptor by selective agonists resulted in acute upregulation of the critical intestinal sodium-glucose transporter (SGLT1) within the region infused (23). We subsequently showed that RYGB surgery in physiologically healthy Sprague Dawley (SD) rats leads to a decrease in SGLT1-mediated glucose uptake in the Roux limb (also known as the alimentary limb), which could contribute to the metabolic benefits of the surgery (24).
The aim of this study was to extend our previous work by assessing the role of the sweet taste receptor T1R2/3 in the downregulation of intestinal SGLT1-mediated glucose uptake after RYGB in the SD rat and to investigate changes in the intestinal nutrient-sensing pathway following RYGB in the obese Zucker diabetic fatty (ZDF) rat. We hypothesized that decreased SGLT1-mediated intestinal glucose absorption in the Roux limb after RYGB is attributable to exclusion of the sweet taste receptor complex in the isolated BP limb. To test this, we developed a model in which isolated BP mucosa can be reexposed to sweet taste receptor-stimulating agonists via an indwelling catheter. Thus, contrary to most previous studies involving infusion of mixed meals, we restimulated the BP limb with a specific stimulus that has no caloric value to help isolate the contributions of a specific pathway in the antidiabetic effect of RYGB.
MATERIALS AND METHODS
Animals
The Harvard Medical Area Standing Committee on Animals prospectively approved all studies. Thirteen-week-old SD (Harlan) and obese (fa/fa) ZDF rats (Charles River Laboratories) were acclimatized for 5 days at constant temperature and humidity (22°C and 70%, respectively), with a fixed 12-h:12-h light/dark cycle (lights on at 7:00 AM). Standard chow (carbohydrate 58.0%, protein 28.5%, fat 13.5%) and Purina 5008 (carbohydrate 56.5%, protein 26.8%, fat 16.7%) were provided ad libitum to each group, respectively. Animals were caged in pairs preoperatively and individually after surgery.
Animal Model
Animals were fasted from 7:00 PM the evening before surgery, with ad libitum access to water. Under inhalation anesthesia (isoflurane 2–3% in oxygen), rats were given a dose of 0.1 ml penicillin G. RYGB was undertaken as follows: through a midline laparotomy, the stomach was divided using the Endo GIA stapling device (2–2.5-mm staples) just below the gastroesophageal junction from greater to lesser curve, taking care to preserve the vagus nerve in this region. The jejunum was divided 16 cm distal to the ligament of Treitz and the distal cut end of the jejunum anastomosed to a 1-cm gastrostomy on the anterior surface of the gastric pouch (interrupted, 6/0 PDS). A 1-cm enterotomy was made on the antimesenteric aspect of the jejunum 10 cm distal to the gastrojejunostomy and anastomosed to the proximal cut end of jejunum as an end-to-side anastomosis (interrupted, 6/0 PDS), forming the BP limb.
A soft polyurethane cannula (0.05 inch external diameter) was inserted into the gastric remnant at the time of RYGB and passed into the second part of the duodenum (tip of catheter in proximal BP limb) before being secured with a 4/0 silk suture. The cannula was exteriorized through the left anterolateral abdominal wall and tunneled subcutaneously to emerge at the dorsal neck. It was cut short, capped, and secured in place with 3/0 vicryl. The abdomen was closed in two layers with 3/0 vicryl. Normal saline (10–15 ml, 0.9%) was administered subcutaneously to maintain intraoperative hydration. Postoperatively, animals had free access to tap water, and a liquid diet was provided ad libitum from day 2 postoperatively (CVS Liquid Nutrition 8 oz.: 250 kcal, 40 g carbohydrate, 9 g protein, 6 g fat). After 5 days, animals were returned to preoperative solid diet (standard chow or Purina 5008). Catheters were inspected and flushed daily to ensure that the site of exteriorization was clean and to maintain patency.
Experiment Protocol
Body weights (commencing on the day of surgery) and food intake (preoperatively and once solid diet was resumed postoperatively) of animals were documented on a daily basis. Blood glucose levels were measured before commencing RYGB surgery and immediately before harvest. On the 11th postoperative day (by which time point, the animals' weights had stabilized with no ongoing weight loss and they had been reestablished on their regular preoperative diet), rats were randomized into treatment and control groups. Treatment animals received a 9-ml infusion of 0.3% saccharin via the BP catheter, over a 3-h period commencing at 8:00 AM (SD n = 6, ZDF n = 10). Control animals received 9 ml of 0.9% normal saline following the same infusion regime (SD n = 3, ZDF n = 5). Infusions were delivered via an electronically programmed pump. At harvest, delivery of infuscate into the BP limb was confirmed by ensuring that the tip of the indwelling catheter was correctly positioned in the duodenum.
A further control group of SD rats underwent RYGB with catheter placement but received no infusion at harvest to exclude the effects of mechanical distension in the BP limb (n = 4). At the end of the 3-h infusion period (11:00 AM), animals were harvested and tissues collected for analysis.
Tissue Harvest
Under inhalation anesthesia with isoflurane 2–3% in oxygen, a midline laparotomy was performed and the Roux limb flushed with ice-cold phosphate-buffered saline before the first 5 cm were harvested for everted sleeve studies. This segment was divided into equal thirds, which were everted and mounted onto a glass rod for functional glucose uptake assays. The remainder of the Roux limb as well as the common and BP limbs were harvested for mucosal scrapings, with samples being immediately snap frozen in liquid nitrogen and stored at −80°C for subsequent RNA extraction.
Functional Glucose Transport Assays
Glucose uptake was measured in everted sleeves as previously described (1, 12), using triplicate sleeves from the Roux limb. Uptake experiments were performed with Mammalian Ringer's (128 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 20 mM NaHCO3) containing 50 mM D-glucose (saturating concentration) and trace quantities of [14C]D-glucose (Perkin Elmer) and [3H]L-glucose (Moravek Biochemicals) (1 min, 37°C). Another set of sleeves was similarly incubated in uptake solution containing 500 μM phloridzin, an SGLT1-specific inhibitor (Sigma). The glucose uptake rate was calculated from the difference between the uptake of [14C]D-glucose and that of the nontransported [3H]L-glucose used as a passive control.
RNA Analysis
RNA was extracted from frozen tissue samples with a mirVana mRNA Isolation Kit (Ambion) and quantified with a microplate reader (Spectramax M5; Molecular Devices). Reverse transcription was performed simultaneously on 0.5–1.0 μg of RNA from each rat with Superscript III and oligo-dT (Invitrogen). To facilitate inter- and intragroup comparisons of gene expression, quantitative PCR of all cDNA samples were run on a single 384-well plate. The cDNA product was diluted and added to forward and reverse primers [Sglt1, T1r2, Glut2, Sglt3b, Cyclophylin A (housekeeping gene), Invitrogen; Table 1], together with SYBR Green Master Mix (Applied Biosystems). Quantitative PCR was performed in duplicate or triplicate with diluted cDNA primers and SYBR green master mix using an Applied Biosystems ABI 7900HT Thermal Cycler (2 min, 50°C; 10 min, 95°C; 40 cycles of 15 s, 95°C and 1 min, 60°C).
Table 1.
RT-PCR primers for rat genes
| Gene of Interest | Primer Sequence- Forward | Primer Sequence- Reverse |
|---|---|---|
| Sglt1 | CCAAGCCCATCCCAGACGTACACC | CTTCCTTAGTCATCTTCGGTCCTT |
| Glut2 | CTCGGGCCTTACGTGTTCTTCCTT | TGGTTCCCTTCTGGTCTGTTCCTG |
| T1r2 | TTCTTTCACGAGGTGCTCCG | AGGACTCAGAGGCGATCCACAC |
| Sglt3 | GAACATGTCCCACGTGAAGGC | TGCAGAAGATGGCAAGCAAGAAC |
| Cyclophylin A | TATCTGCACTGCCAAGACTGAGTG | CTTCTTGCTGGTCTTGCCATTCC |
Thermal Cycler conditions used were 2 min, 50°C; 10 min, 95°C; 40 cycles of 15 s, 95°C, and 1 min, 60°C. Dissociation curves were obtained to ensure a single amplicon. Sglt1, sodium glucose cotransporter; Glut2, glucose transporter; T1r2, sweet taste receptor component; Sglt3b, sodium glucose cotransporter; Cyclophylin A, housekeeping gene.
Statistical Analyses
All data are presented as means ± SE. Data were analyzed using Student's t-test or ANOVA with post hoc correction. Differences were considered significant at P ≤ 0.05. Analyses were undertaken using SPSS 16 software or Excel.
RESULTS
Body Weight, Food Intake, and Blood Glucose
SD rats.
Mean body weight of rats decreased from 364.8 ± 3.3 g at baseline (day of surgery) to 315.1 ± 7.2 g at harvest on POD 11 (13.5 ± 2.1% decrease in baseline body weight) (Fig. 1). There was no difference in weight loss between those randomized to saline vs. saccharin infusion (P = 0.7). Food intake was decreased in the postoperative period (24 ± 0.5 vs. 19.1 ± 1.5 g/rat per 24 h standard chow pre- and postoperatively, respectively, P < 0.05) although this decrease was not evident when corrected for body weight (0.064 ± 0.001 vs. 0.060 ± 0.004 g/g body wt per 24 h pre- and postoperatively, respectively, P = 0.4). Blood glucose did not change significantly in physiologically healthy SD rats after RYGB.
Fig. 1.
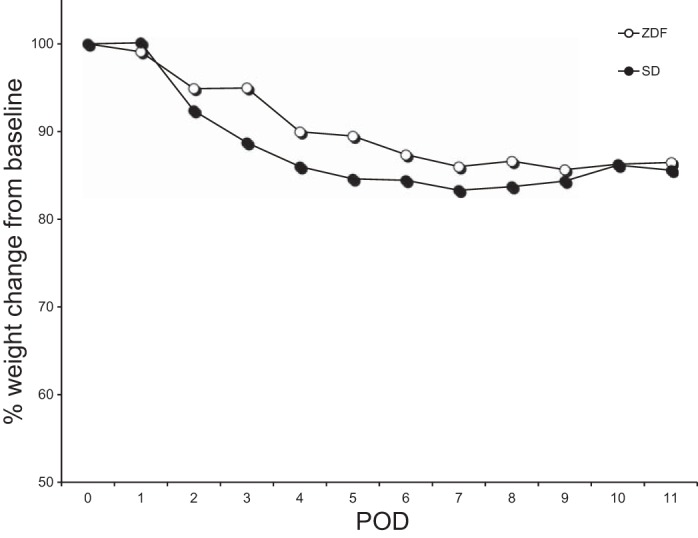
Weight change in Sprague Dawley (SD) and Zucker diabetic fatty (ZDF) rats after Roux-en-Y gastric bypass (RYGB) with indwelling catheter placement in the biliopancreatic (BP) limb. POD, postoperative day.
ZDF rats.
ZDF body weights were lowest at POD 9 and remained at this level until harvest on POD 11 (mean body wt 390.4 ± 11.4 g at baseline vs. 337.6 ± 8.2 g at harvest; 13.1 ± 1.8% decrease in baseline body weight) (Fig. 1). There was no difference in weight loss between the two infusion arms (P = 0.9). Food intake was decreased in the postoperative period (34.5 ± 0.9 preoperative vs. 22.2 ± 2.3 postoperative g/rat per 24 h, Purina 5008, P < 0.01). This decrease remained significant when corrected for body weight (0.085 ± 0.004 preoperative vs. 0.064 ± 0.007 postoperative g/g body wt per 24 h, P < 0.05). Rats in the two infusion arms consumed similar amounts of chow in the postoperative period (P = 0.2). Mean blood glucose decreased by more than 30% in all ZDF groups after surgery (331 ± 37 preoperative vs. 200 ± 29 mg/dl postoperative, P = 0.02).
Functional Glucose Uptake
SD rats.
Mean functional glucose uptake rates in the Roux limb of the rats were 6.65 ± 0.77, 7.10 ± 0.10, and 5.76 ± 1.53 nmol/mg per min in the saccharin-infused, saline-infused, and noninfused groups, respectively. Differences were not significant. Approximately 90% reduction of glucose uptake in the presence of phloridzin confirmed that SGLT1 accounted for the majority of glucose absorption (Fig. 2).
Fig. 2.
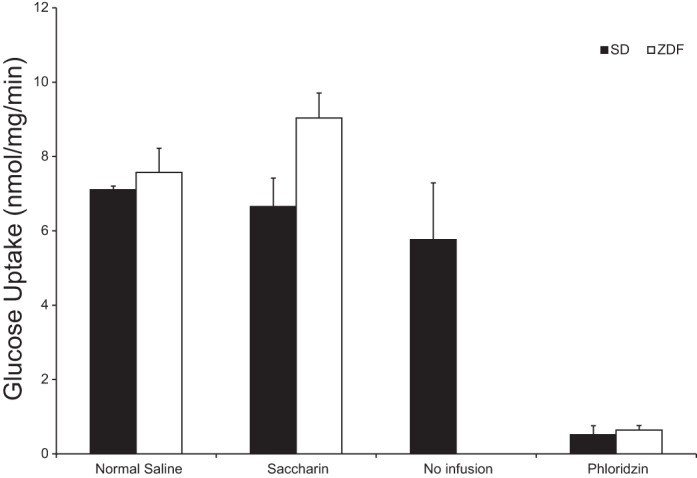
Mean functional glucose uptake in Roux limb of RYGB SD and ZDF rats following BP limb infusion of 0.3% saccharin vs. 0.9% normal saline vs. no infusion (SDs only), as measured by the everted sleeve technique. Phloridzin inhibition of glucose uptake demonstrates sodium-glucose transporter (SGLT1)-mediated uptake.
ZDF rats.
There was no difference in SGLT1-mediated glucose uptake in the Roux limb of RYGB rats receiving BP infusions of saccharin (n = 10) vs. normal saline (n = 5) (9.03 ± 0.68 vs. 7.57 ± 0.65 nmol/mg per min, P = 0.15) (Fig. 2). Phloridzin inhibition was >90%.
Intestinal Gene Expression
Glucose transporters, Sglt1 and Glut2.
At POD 11 after RYGB, there was no difference in mRNA expression of the glucose transporters Sglt1 and Glut2 between rats receiving a BP infusion of saccharin and the groups receiving control saline infusions in either SD rats or the ZDF model (assessed regionally per bypass limb or average expression levels throughout bowel). Topographic variation of mRNA expression of these transporters was not evident in SD or ZDF rats after RYGB (Fig. 3, A and B).
Fig. 3.
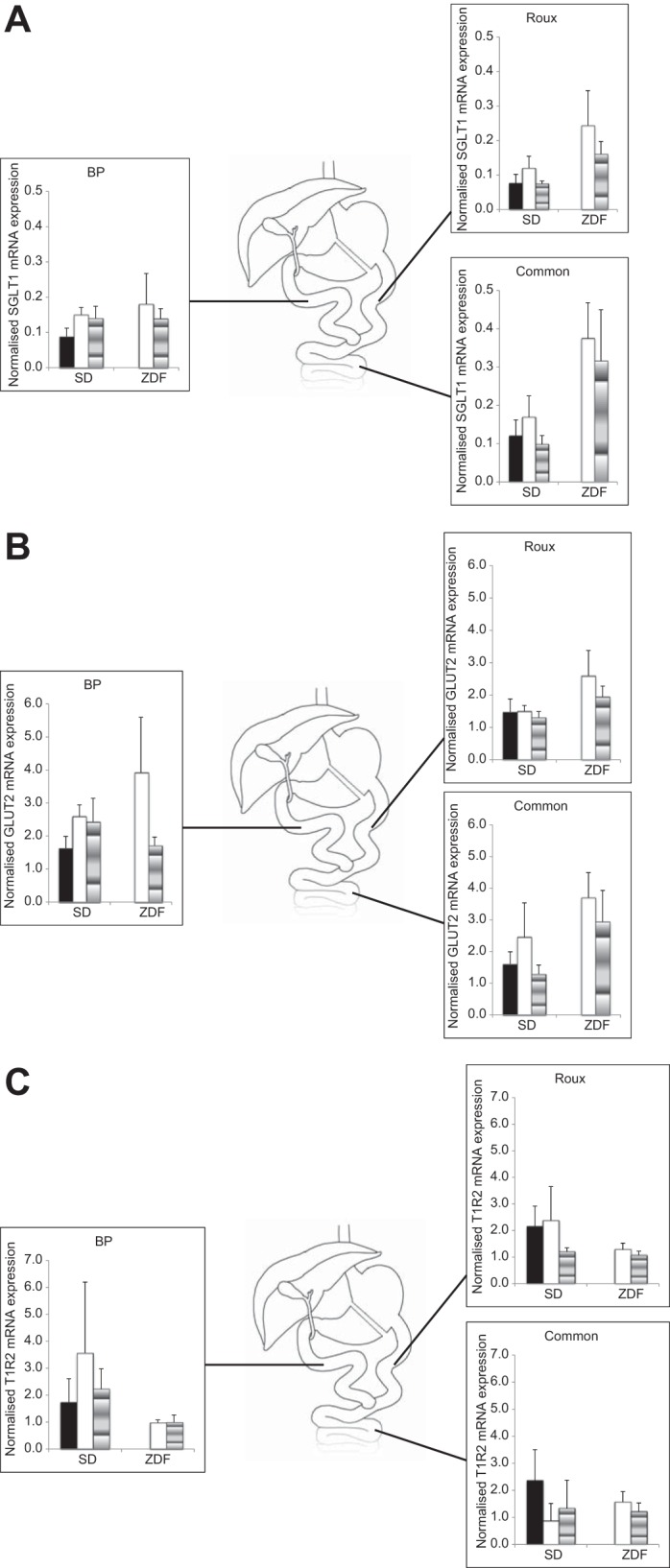
A: SGLT1 mRNA expression in BP, common, and Roux limbs of RYGB SD and ZDF rats 11 days after surgery. Solid bar, no infusion; open bar, normal saline infusion; shaded bar; saccharin infusion. B: glucose transporter 2 (GLUT2) mRNA expression in BP, common, and Roux limbs of RYGB SD and ZDF rats 11 days after surgery. Solid bar, no infusion; open bar; normal saline infusion; shaded bar, saccharin infusion. C: sweet taste receptor (T1R2) mRNA expression in BP, common, and Roux limbs of RYGB SD and ZDF rats 11 days after surgery. Solid bar, no infusion; open bar, normal saline infusion; shaded bar, saccharin infusion.
Glucose sensors, T1r2 and Sglt3b.
Topographic variation of mRNA expression of the sweet taste receptor T1r2 was not evident in SD or ZDF rats after RYGB. There were no differences in expression levels overall or regionally (within BP/Roux/common limb) between saccharin- and saline-infused rats of either strain (Fig. 3C).
Topographic variation of Sglt3b was apparent in both SD and ZDF rats but was more pronounced in the ZDF rats with seven- to tenfold higher levels of Sglt3b in the common limb than in the Roux and BP limbs (P ≤ 0.01 for both infusion groups) (Fig. 4A). Levels of Sglt3b were approximately three- to fivefold higher in the common limb of bypassed SD rats compared with Roux and BP limb levels (P < 0.05 for all SD groups combined) (Fig. 4B). There was no difference in SGLT3b expression between infusion groups of the SD rats. However, in ZDF rats, there was a 2.4-fold downregulation on infusion of saccharin vs. normal saline (P < 0.05).
Fig. 4.
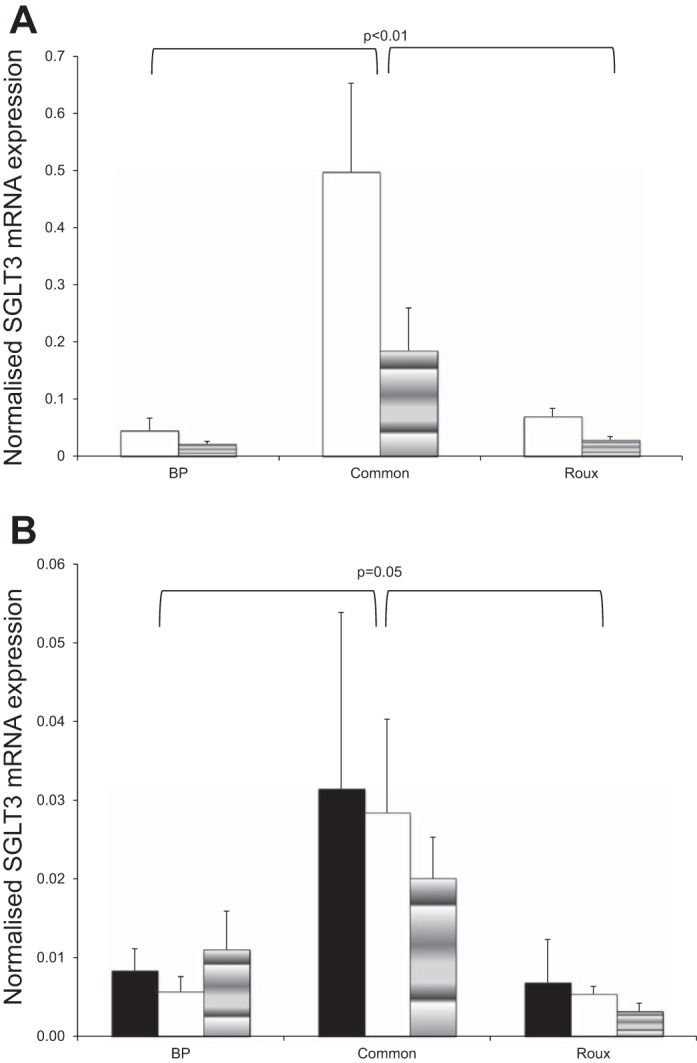
A: SGLT3b mRNA expression in BP, common, and Roux limbs of RYGB ZDF rats 11 days after surgery. Open bar, normal saline infusion; shaded bar, saccharin infusion. B: SGLT3b mRNA expression in BP, common, and Roux limbs of RYGB SD rats 11 days after surgery. Solid bar, no infusion; open bar, normal saline infusion; shaded bar, saccharin infusion.
DISCUSSION
Despite widespread acceptance of RYGB as a treatment for obesity and T2D, the mechanisms through which improvements in glycemic control are achieved are not well understood. Numerous studies have examined feeding patterns, weight, and hormonal changes after RYGB in either obese or diabetic rodent models (4, 13, 25), but few have analyzed changes in the obese and diabetic rat or attempted to delineate the mechanisms mediating change. To this end, we elected to study changes in healthy SD as well as obese ZDF rats. The obese ZDF rat carries an autosomal recessive mutation in the leptin receptor gene, leading to hyperphagia, obesity, and progressive insulin resistance, closely paralleling the metabolic syndrome in humans.
We had previously shown that RYGB leads to downregulation of intestinal glucose absorption capacity through changes in SGLT1 function (24). We hypothesized that this post-RYGB reduction in intestinal SGLT1-mediated glucose uptake is attributable to exclusion of the sweet taste receptor T1R2/3 otherwise positioned to detect the presence of glucose in the anatomically normal bowel. We confirmed the presence of the sensor in both models and observed no changes in mRNA expression of the T1R2 component or of the glucose transporter genes SGLT1 and GLUT2 in response to a sweet taste receptor agonist infused into the surgically isolated bowel in either rat strain. However, we did find changes in the putative glucose sensor SGLT3b.
Studies have shown that the T1R2/3 complex is necessary for SGLT1 upregulation in response to an increased carbohydrate load (18) and that nonnutritive sweeteners acting at the sweet taste receptor as an alternative to glucose can reproduce this upregulation as well as elicit an incretin hormone response (15). In our study of the post-RYGB model, however, the sweet taste receptor stimulant saccharin did not alter Roux limb glucose uptake when infused into the isolated BP limb. The lack of response to saccharin infusion could be due to one of several reasons. First, the T1R2/3-SGLT1 signaling pathway may be irrecoverably disrupted by the surgical intervention, e.g., due to disruption of vagal nerve branches or division of enteric nerve plexus within the bowel wall. These factors may also explain why others have found no effect of sweet taste receptor agonists on intestinal glucose absorption in isolated segments of bowel that had been ligated proximally and distally (5). Alternatively, T1R2/3 may exert a more local effect on SGLT1 (autocrine/paracrine) but not one that reaches as far distally as the jejunum of the Roux limb. Given reports of diminished desire for sweet-tasting food post-RYGB and descriptions of altered perception of sweet foods on the palate, it is even plausible that either sensitivity or ability of intestinal T1R2/3 to perceive sweet substrates as it did preoperatively is altered post-RYGB (3, 27). To preempt reduced sensitivity post-RYGB, we infused a dose of saccharin that is 10 times greater than that in saccharin-sweetened sodas.
Another possibility for our negative result is that the relevant sweet-sensing pathway is one that is independent of the T1R2/3 complex and one for which saccharin is not an agonist. This possibility made us explore SGLT3, another putative glucose sensor. SGLT3 is a neuronally located member of the solute carrier family of proteins that appears to function as a sensor rather than a transporter of glucose (6, 10). Binding of glucose (but not saccharin) to SGLT3 leads to membrane depolarization. Thus, if SGLT3 is an important nutrient sensor after bariatric surgery, our infusion of saccharin to the BP limb would not reverse the antiglucose-absorptive effects of surgery, consistent with what we have seen. We found higher levels of the putative glucose sensor SGLT3 in the common limb of bowel vs. the BP and Roux limbs in both SD and ZDF rats. The decrease in SGLT3 mRNA level in the Roux limb of RYGB ZDF rats after saccharin infusion is an interesting finding and should prompt further investigation of its role in glucose sensing, the pathophysiology of metabolic disease, and resolution of T2D after RYGB, including measurement of both its known isomeric forms in rodents (SGLT3a and b). Other groups have also studied changes in glucose transporters and sensors after RYGB in other rodent models. In a study to assess taste preference after RYGB, Bueter et al. (3) found decreased T1R2 levels in the BP limb of bypassed Wistar rats but increased levels in the Roux limb. In their study on SD rats, Taqi et al. (26) found an increase in GLUT2 mRNA levels in both the Roux and common limbs, with decreased SGLT1 in the Roux limb. These studies differ from ours in several ways, however, including the fact that they compared RYGB rats to sham animals rather than expression levels of transporters in RYGB limbs after BP limb infusion with different agents.
To clarify the role of the foregut in diabetes amelioration, limited studies with catheter placement into the gastric remnant or BP limb have been done. In separate studies, one group conducted glucose tolerance tests post-RYGB in obese SD and ZDF rats and compared results from orally administered glucose to administration via gastrostomy tube in the gastric remnant (8, 22). RYGB led to a significant decrease in glucose area under the curve following orally administered glucose. This post-RYGB improvement was lost when glucose was administered per gastrostomy. In these studies, administration of glucose rather than a specific sweet taste receptor agonist as in our study may exert a different effect on glucose sensors in the isolated BP limb, e.g., also activate SGLT3. In humans, single case studies report improved glucose, incretin, and insulin responses with liquid mixed meals administered orally vs. via gastrostomy tube in the remnant (7, 16). Most recently, Lindqvist et al. (14) showed an increased insulin and GLP-1 response to a mixed meal test delivered orally vs. via the gastric remnant in four female patients post-RYGB. Although Hansen et al. (11) found no difference in glucose homeostasis parameters in human subjects post-RYGB following mixed meals administered orally or into the isolated gut, most studies suggest that restimulation of the proximal bowel with glucose or mixed meal leads to deterioration of glucose tolerance. Our studies take these observations further, attempting to identify the mechanisms involved in these observations by testing specific nutrient-sensing pathways.
In humans, resolution of T2D occurs early post-RYGB, before substantial weight loss. For this reason, we conducted catheter infusion studies in rats early (POD 11) to investigate changes in the sweet taste-sensing pathway in this early postoperative period. We chose to selectively interrogate one pathway at a time, using saccharin, which is specific for T1R2/3 activation, rather than challenging the isolated limb of bowel with a mixed meal, which may activate multiple pathways and introduce confounding. We have previously demonstrated an acute response (within 3 h) of intestinal glucose uptake to saccharin stimulation of T1R2/3 and designed the current study based on these data and did not pursue models of longer infusions or repeated infusions over a longer period.
The possibility of a type 2 statistical error should be considered because of use of ZDF rats with less predictable disease phenotype expression. To account for this, however, we used an n = 10 in the ZDF saccharin-infused group and n = 5 in the ZDF saline-infused group. We would expect this to provide adequate power to detect any meaningful and significant differences.
Transcription and posttranslational modifications mean that mRNA results cannot be extrapolated/interpreted to make assumptions about protein levels. However, technical difficulties with antibodies against SGLT1 and T1R2 precluded protein assays in this study. We believe that measurement of functional SGLT1-mediated glucose uptake is an acceptable and likely better surrogate for SGLT1 protein levels, as it measures the final step in the process and the extent of functional protein.
Changes in intestinal glucose sensing in the pathophysiology of obesity and T2D and following RYGB require further research to enhance understanding of the gut as an endocrine organ, the effects of RYGB, and its scope as a metabolic as well as bariatric procedure. Analysis of changes in intestinal sensors and transporters at the protein level and challenging the isolated gut after RYGB with different agonists in a selective manner will add valuable insight into the mechanisms effecting improvements in T2D after surgery.
GRANTS
This study was funded by National Institute of Health Grant 1 R01 DK084064 (A. Tavakkoli).
DISCLOSURES
A. Tavakkoli has an equity interest in Avaxia Biologics, a company that is developing oral antibodies for treatment of intestinal disorders, with potential applications for treatment of diabetes and obesity. His interests were reviewed and are managed by the Brigham and Women's Hospital and Partners HealthCare in accordance with their conflict of interest policies. No other conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: H.Y.B., C.W.L.R., S.W.A., D.B.R., and A.T. conception and design of research; H.Y.B. and T.E.D. performed experiments; H.Y.B., D.B.R., and A.T. analyzed data; H.Y.B., C.W.L.R., S.W.A., D.B.R., and A.T. interpreted results of experiments; H.Y.B. prepared figures; H.Y.B., D.B.R., and A.T. drafted manuscript; H.Y.B., T.E.D., C.W.L.R., S.W.A., D.B.R., and A.T. edited and revised manuscript; H.Y.B., T.E.D., C.W.L.R., S.W.A., D.B.R., and A.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jan Rounds and Amy Blass for invaluable managerial support. Aspects of this manuscript have been presented as poster presentations at the American College of Surgeons Annual Congress (Chicago, Oct 2012) and at Digestive Disease Week (Florida, May 2013).
REFERENCES
- 1.Bhutta HY, Deelman TE, Ashley SW, Rhoads DB, Tavakkoli A. Disrupted circadian rhythmicity of the intestinal glucose transporter SGLT1 in Zucker Diabetic Fatty rats. Dig Dis Sci 58: 1537–1545, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 122: 248–256; e5, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Bueter M, Miras AD, Chichger H, Fenske W, Ghatei MA, Bloom SR, Unwin RJ, Lutz TA, Spector AC, le Roux CW. Alterations of sucrose preference after Roux-en-Y gastric bypass. Physiol Behav 104: 709–721, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Chambers AP, Stefater MA, Wilson-Perez HE, Jessen L, Sisley S, Ryan KK, Gaitonde Sorrell S, JE, Toure M, Berger J, D'Alessio DA, Sandoval DA, Seeley RJ, Woods SC. Similar effects of roux-en-Y gastric bypass and vertical sleeve gastrectomy on glucose regulation in rats. Physiol Behav 105: 120–123, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudhry RM, Garg A, Abdelfatah MM, Duenes JA, Sarr MG. Lack of functionally active sweet taste receptors in the jejunum in vivo in the rat. J Surg Res 183: 606–11, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diez-Sampedro A, Hirayama BA, Osswald C, Gorboulev V, Baumgarten K, Volk C, Wright EM, Koepsell H. A glucose sensor hiding in a family of transporters. Proc Natl Acad Sci USA 100: 11753–11758, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dirksen C, Hansen DL, Madsbad S, Hvolris LE, Naver LS, Holst JJ, Worm D. Postprandial diabetic glucose tolerance is normalized by gastric bypass feeding as opposed to gastric feeding and is associated with exaggerated GLP-1 secretion: a case report. Diabetes Care 33: 375–377, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eldar S, Heneghan HM, Dan O, Kirwan JP, Schauer PR, Brethauer SA. Gastrostomy tube placement in gastric remnant at gastric bypass: a rat model for selective gut stimulation. Surg Obes Relat Dis 9: 442–446, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Escalona A, Pimentel F, Sharp A, Becerra P, Slako M, Turiel D, Munoz R, Bambs C, Guzman S, Ibanez L, Gersin K. Weight loss and metabolic improvement in morbidly obese subjects implanted for 1 year with an endoscopic duodenal-jejunal bypass liner. Ann Surg 255: 1080–1085, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Freeman SL, Bohan D, Darcel N, Raybould HE. Luminal glucose sensing in the rat intestine has characteristics of a sodium-glucose cotransporter. Am J Physiol Gastrointest Liver Physiol 291: G439–G445, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Hansen EN, Tamboli RA, Isbell JM, Saliba J, Dunn JP, Marks-Shulman PA, Abumrad NN. Role of the foregut in the early improvement in glucose tolerance and insulin sensitivity following Roux-en-Y gastric bypass surgery. Am J Physiol Gastrointest Liver Physiol 300: G795–G802, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karasov WH, Pond RS, 3rd, Solberg DH, Diamond JM. Regulation of proline and glucose transport in mouse intestine by dietary substrate levels. Proc Natl Acad Sci USA 80: 7674–7677, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kindel TL, Martins PJ, Yoder SM, Jandacek RJ, Seeley RJ, D'Alessio DA, Obici S, Tso P. Bypassing the duodenum does not improve insulin resistance associated with diet-induced obesity in rodents. Obesity (Silver Spring) 19: 380–387, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindqvist A, Spegel P, Ekelund M, Mulder H, Groop L, Hedenbro J, Wierup N. Effects of ingestion routes on hormonal and metabolic profiles in gastric-bypassed humans. J Clin Endocrinol Metab 98: E856–E861, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA 104: 15075–15080, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLaughlin T, Peck M, Holst J, Deacon C. Reversible hyperinsulinemic hypoglycemia after gastric bypass: a consequence of altered nutrient delivery. J Clin Endocrinol Metab 95: 1851–1855, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Miras AD, Risstad H, Baqai N, Law S, Sovik TT, Mala T, Olbers T, Kristinsson JA, le Roux CW. Application of the International Diabetes Federation and American Diabetes Association criteria in the assessment of metabolic control after bariatric surgery. Diabetes Obes Metab 16: 85–89, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Moran AW, Al-Rammahi MA, Arora DK, Batchelor DJ, Coulter EA, Ionescu C, Bravo D, Shirazi-Beechey SP. Expression of Na+/glucose co-transporter 1 (SGLT1) in the intestine of piglets weaned to different concentrations of dietary carbohydrate. Br J Nutr 104: 647–655, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Munoz R, Carmody JS, Stylopoulos N, Davis P, Kaplan LM. Isolated duodenal exclusion increases energy expenditure and improves glucose homeostasis in diet-induced obese rats. Am J Physiol Regul Integr Comp Physiol 303: R985–R993, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pata G, Crea N, Di Betta E, Bruni O, Vassallo C, Mittempergher F. Biliopancreatic diversion with transient gastroplasty and duodenal switch: long-term results of a multicentric study. Surgery 153: 413–422, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Sandler BJ, Rumbaut R, Swain CP, Torres G, Morales L, Gonzales L, Schultz S, Talamini M, Horgan S. Human experience with an endoluminal, endoscopic, gastrojejunal bypass sleeve. Surg Endosc 25: 3028–3033, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Shimizu H, Eldar S, Heneghan HM, Schauer PR, Kirwan JP, Brethauer SA. The effect of selective gut stimulation on glucose metabolism after gastric bypass in the Zucker diabetic fatty rat model. Surg Obes Relat Dis 10: 29–35, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Stearns AT, Balakrishnan A, Rhoads DB, Tavakkolizadeh A. Rapid upregulation of sodium-glucose transporter SGLT1 in response to intestinal sweet taste stimulation. Ann Surg 251: 865–871, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stearns AT, Balakrishnan A, Tavakkolizadeh A. Impact of Roux-en-Y gastric bypass surgery on rat intestinal glucose transport. Am J Physiol Gastrointest Liver Physiol 297: G950–G957, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki S, Ramos EJ, Goncalves CG, Chen C, Meguid MM. Changes in GI hormones and their effect on gastric emptying and transit times after Roux-en-Y gastric bypass in rat model. Surgery 138: 283–290, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Taqi E, Wallace LE, de Heuvel E, Chelikani PK, Zheng H, Berthoud HR, Holst JJ, Sigalet DL. The influence of nutrients, biliary-pancreatic secretions, and systemic trophic hormones on intestinal adaptation in a Roux-en-Y bypass model. J Pediatr Surg 45: 987–995, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Tichansky DS, Glatt AR, Madan AK, Harper J, Tokita K, Boughter JD. Decrease in sweet taste in rats after gastric bypass surgery. Surg Endosc 25: 1176–1181, 2011 [DOI] [PubMed] [Google Scholar]


