Abstract
Ethnopharmacological significance
Nigerian herbalists possess indigenous ethnomedicinal recipes for the management of tuberculosis and related ailments.
Aim of the study
To carry out a collaborative preliminary modern scientific evaluation of the efficacy of some Nigerian ethnomedicines used by traditional medicine practitioners (TMPs) in the management of tuberculosis and related ailments
Materials and methods
Ethnomedicinal recipes (ETMs) were collected from TMPs from locations in various ecological zones of Nigeria under a collaborative understanding. The aqueous methanolic extracts of the ETMs were screened against Mycobacterium bovis, BCG and Mycobacterium tuberculosis (M. tb.) strain H37Rv using the broth microdilution method.
Results
Extracts of ETMs screened against BCG showed 69% activity against the organism. The activities varied from weak, ≤ 2500μg /mL to highly active, 33μg /mL 64% of the extracts were active against M. tb. The activities of the extracts against M.tb. varied from weak, ≤ 2500μg /mL to highly active, 128μg/mL. There was 77% agreement in results obtained using BCG or M. tb. as test organisms
Conclusion
The results show clear evidence for the efficacy of the majority of indigenous Nigerian herbal recipes in the ethnomedicinal management of tuberculosis and related ailments. BCG may be effectively used, to a great extent, as the organism for screening for potential anti-M. tb. agents. A set of prioritization criteria for the selection of plants for initial further studies for the purpose of antituberculsis drug discovery research is proposed.
Keywords: African ethnomedicines, cough, anti-Mycobacterium activity, M.tb., M.bovis (BCG)
1.0 Introduction
1.1 Tuberculosis as a global health problem
Tuberculosis (TB) is a chronic bacterial infection caused by the bacillus, Mycobacterium tuberculosis and easily transmitted from person to person through the air by droplet nuclei (Moulding, 1988). Tuberculosis remains a leading cause of death in the world from a single infectious agent. It is estimated that one-third of the world's population is infected with the tubercule bacillus and about 80% of individuals diagnosed with the disease every year live in the 22 most populous countries (Dye et al., 1999; Dye, 2006). Effective treatment of TB has been hampered by the emergence of drug resistant strains of M. tuberculosis. Particularly, ominous is the emergence of multi-drug resistant TB (MDR-TB) and extensively-drug resistant TB (XDR-TB), which has been accelerated by the rise of Human Immune Virus/Acquire Immune Deficiency Syndrome, HIV/AIDS (CDC, 2006; Smith and Moss, 1994). Despite the introduction of Directly-Observed Therapy Short course (DOTS) by WHO in 1995 (The Economist, 1995), a control strategy to detect and cure TB, millions of TB patients continue to perish (Whalen, 2006).
1.2 Antituberculosis drug discovery efforts and the need for more vigorous drug discovery efforts
Though there are many efforts being made to discover new drugs to treat TB, these efforts are not a major focus of many pharmaceutical companies. There are some notable successes from pharmaceutical companies as exemplified by the recently FDA-approved Bedaquiline The reasons for the lack of more vigorous investments by the industry are mainly economic as the countries most in need of new anti-TB drugs are primarily developing countries whose populations are not able to buy expensive drugs that would arise from the costs of investing huge sums to develop. Current antituberculosis chemotherapy demands the taking of up to four drugs simultaneously over a period of six months which leads to poor adherence by patients and demands close supervision of patients to mitigate the development of drug-resistance. MDR-TB and XDR-TB, which require therapy for up to two full years with multiple poorly-active second-line drugs, have compounded the problem of achieving success and have a high percentage of treatment failure (Gandhi et al., 2010; Mitnick et al., 2003). Many of the drugs used in the treatment of MDR-TB and XDR-TB also have serious toxic effects (Carroll et al., 2012). New drug scaffolds and drugs need to be found and developed which will reduce the current long duration of therapy, reduce the pill burden, successfully treat MDR-TB and XDR-TB, be co-administrable with anti-HIV and anti-diabetes drugs and exhibit less toxic side effects (Barry, 2003).
1.3 TB in Nigeria and the need to investigate the efficacy of ethnomedicines and medicinal plants in Nigeria for the purpose of discovering new TB drugs
Tuberculosis was declared a national emergency in Nigeria in June 2006. The country was ranked one of the most highly TB burdened countries in the world with an estimated incidence of all forms of TB at 311 per 100,000 population (WHO, 2008).
In Nigeria, a large percentage of the population, particularly in the rural areas, depend on traditional medicines for their primary health care. Traditional medicine is a broad term used to describe non-western medicine. Ethnomedicine is a form of traditional medicine that includes the use of plants for healing by humans (Iwu, 2002). Ethnomedicine is a preferred choice for many people as it is readily available and more affordable. Plants have contributed significantly as starting points for the development of modern drugs (Khazir et al., 2013,Newman et al. 2005, Newman and Cragg, 2007) as evidenced by taxol in cancer and artemisinin in malaria. This may be attributed to their chemical diversity, biochemical specificity, possession of a greater number of chiral centres than in synthetic or combinatorial libraries, and evolutionary pressures to create biologically active compounds by interactions with different proteins and biological targets (Queiroz et al., 2009; Wolfender, 2009). Plants therefore, represent potential sources of new drugs acting through novel mechanisms in the search for new and more potent and safe antituberculosis agents. There are a number of natural plant metabolites that have been reported to have inhibitory or bactericidal activities in vitro against Mycobacterium tuberculosis at micromolar concentrations (Copp, 2003; Copp and Pearce, 2007; Okunade et al., 2004). Such reports carry hope of success in fully planned isolation and synthetic strategies to discover new antituberculosis drugs in plants. It is estimated that there are about 250,000 – 500,000 plant species and only about 10 per cent of these has been phytochemically investigated for the purpose of determining biological activity of their components (Hostettmann et al., 1996). A very high percentage of these unstudied plants are endemic to Africa and Asia. Nigeria's bio-resource is massive and diverse and is divided into various climatic zones that include marine mangrove, rainforest, Sudan savannah, derived savannah and the Mediterranean. Nigeria possesses over 5000 plant species and also has a culture and history that is very rich in ethnomedicine.
1.4 The aim of the study
The aim of the work reported here was to initiate a collaborative and preliminary sample study of Nigerian ethnomedicines used by the traditional medicine practitioners (TMPs), living across various ecological zones in the Country, for the management of coughs including bloody cough (tuberculosis), and to evaluate the scientific basis for the use of these traditional remedies.
2.0 Materials and methods
2.1 Study sites
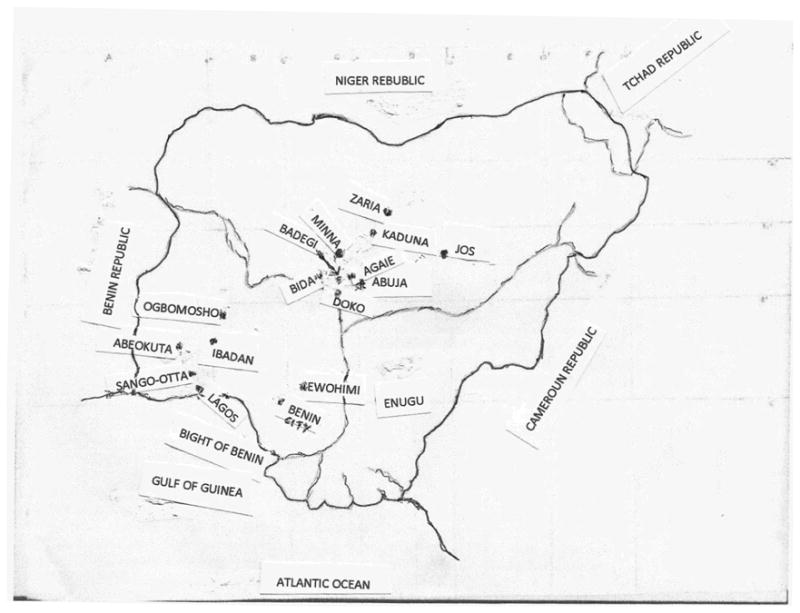
Eight states of the Federation located in various climatic zones (Fig. 1) were visited between August 2005 and February 2006 for the purpose of interviewing individual traditional medicine practitioners (TMPs) about their experience of treating TB and collection of herbal anti-TB recipes and medicines. Ethnobotanical studies were carried out in four geographical regions of Nigeria comprising the South West, South South, South East and North Central. This survey included Lagos, Ogun, Oyo, Edo, Enugu, Niger, Plateau, Kaduna states and the Federal Capital territory, Abuja.
Fig.1. Sketch Map of Nigeria: TB Ethnomedicine Samples Collection Sites.
2.1.1 Interviews
The TMPs were interviewed using copies of the same questionnaire for all the TMPs. The questionnaire was titled “The effectiveness of Nigerian Traditional Medicines for the Treatment of Tuberculosis” and included in it were the following sections: (a) Personal details of the healer, (b) Questions about the healer and his/her practice, (c) Questions about tuberculosis and (d) Herbal remedy. Information was also collected on (i) the nomenclature; botanical, common and native names of the plants used, (ii) part of the plant used (stem, leaves or roots), (iii) special time of collection, (iv) the habitat and mode of growth of plant (wild, cultivated), and (v) mode of collection and drying of plant. The questionnaire requested the TMPs, Table 1 to provide their bio-data, knowledge about TB, their anti-TB recipes, dosage and duration of treatment, plant collection guidelines and procedure for preparing the medicines. In most of the interviews, the TMPs could only communicate in their local language, and a person was at hand to translate and complete the questionnaire in English.
Table 1. Herbalists and their addresses.
| Extract designation ETM numbers | Town | State | Name of traditional healer |
|---|---|---|---|
| 15 | Minna | Niger | Alhassan Alhaji Baba |
| 16 | Badegi | Alhaji Amuda Soma | |
| 17 | Agaie | Ladan Moh'd Ekogi | |
| 18 | Zuba | Abuja | Market purchase: ETM 19 and 20 were purchased from different sellers. |
| 19 | |||
| 20 | Niger | ||
| 21 | Doko | Mohammed Isa | |
| 22 | Badegi | Mohammed Kudu | |
| 23 | Doko | Mohammed Abdullahi | |
| 24 | Agaie | Shehu Ekogi | |
| 25 | Bida | Alhassan Alhaji Baba | |
| 26 | Records not available | ||
| 27 | Records not available | ||
| 28 | Abuja | FCT | Oladele Rahoof |
| 29 | not given | FCT/South East Nigeria | Ndulaka Ngozi Ezeji |
| 30 | Minna | Mohammed Aliyu Alhaji | |
| 31 | Doko | Niger | Jibrilu Abubakar |
| 32 | Zaria | Abubakar Mohammed | |
| 33 | Likoro | Alhassan Umaru | |
| 34 | Zaria | Idris Bala | |
| 35 | Kaduna | Sanusi Abdullahi Maimagani | |
| 36 | Dan Makera Adamu | ||
| 37 | Kaduna | Mama Shehu Mai Magani | |
| 38 | Zaria | Isa N. Mai Magani | |
| 39 | Zaria | Rabiu Sale | |
| 40 | Kaduna | Jibo Haruna | |
| 41 | Jabbi Aliyu Danfulani | ||
| 42 | Zaria | Kaduna | Alhaji Dr. Audu Maimasaki |
| 43 | Gwakura | Mal Tanko Maiyasin | |
| 44 | Tarfa | Idi Abdulahi | |
| 45 | Jos | Plateau | Azijah Oyhu |
| 46 | Dachester Iliya Dung | ||
| 47 | Bazanfara Mohammed Jibril | ||
| 48 | Mailafiaya Audu | ||
| 49 | Maidori Ahmadu | ||
| 50 | Labaran Mohammed | ||
| 51 | Suraj Abdul Azeez | ||
| 52 | Iliya Rahila | ||
| 53 | Yakubu Hassan | ||
| 54 | Ibakwe F.C. | ||
| 55 | Zamani Imil | ||
| 56 | Bomo John | ||
| 57 | Shuaib Abubakar Dlakwa | ||
| 58 | Akaso Adigwu | ||
| 59 | Sankachi Abdullahi | ||
| 60 | Haruna Mary | ||
| 61 | Enugu | Enugu | Nnebe Olisa Isaac |
| 62 | Akajiofor | Anambra | Okeakpu Ifeoma |
| 63 | Enugu | Enugu | Igwe Sosmus Ozonnamalu |
| 64 | Obi D.C.N. Digbo | ||
| 65 | P.C. Uzonze Nkalebe | ||
| 66 | Echieteka Enyi | ||
| 67 | Agu Matthew | ||
| 68 | Obi D.C.N. Digbo | ||
| 69 | Nwabueze Chukwu | ||
| 70 | Abia | Akwakrija U. Alele | |
| 71 | Enugu | Enugu | Ezeakor Chimaeze |
| 72 | Sango-Otta | ||
| 73 | Ogun | Oloyede Akinhanmi Adesola ( | |
| 74 | Abeokuta | Oladehinde Fakemi | |
| 75 | Lagos | A. Immanuel Uwa | |
| 76 | Sango-Otta | Alani Oloyede | |
| 77 | Alani Oloyede | ||
| 78 | Fadipe Rafiu | ||
| 79 | Ogun | Adeyinka Nureni | |
| 80 | Abeokuta | Lawal Sakiru (Sako) | |
| 81 | Salako Ganiyu (Alhaji) | ||
| 82 | Lawal Jimoh | ||
| 83 | Late Bamgboye Emmanuel Akanni | ||
| 84 | Ogun | Oloyede Omoleso | |
| 85 | Sango-Otta | Oloyede Omoleso | |
| 86 | Agbado | Oyewolu Hakeem Abore | |
| 87 | Abeokuta | Global Herbs (Taro' Olu Adeola) | |
| 88 | Bamgboye Morounfoluwa | ||
| 89 | Bamgboye Morounfoluwa | ||
| 90 | Ogbomosho | Oyo | Lawal Suleiman |
| 91 | Oluwofin Alhaja Bintu | ||
| 92 | Awodeji Thomas Agberi | ||
| 93 | Ewohimi | Edo | Innocent Enobakhale |
| 94 | |||
| 95 | |||
| 96 | Francis Agbon | ||
| 97 | |||
| 98, 99, 100 | Bwari | Abuja | Adamu Muhammed |
(c) Many of the TMPs could not be reached for recollection and collection of their respective ETM formulation plant details. Nevertheless, such ETMs have been included and the names and location of the TMPs concerned are in Table 1 to guide further investigation.
2.2 Herbal recipes (ETMs) and material collection
Herbal recipes were fine or coarse powders or liquids. They were collected from the traditional healers as used by the indigenous people to treat TB symptoms such as fever, cough, and blood in the cough sputum etc. Eighty-six preparations were obtained based solely on the recommendations of the traditional healers and no attempt to determine the composition was made at the initial stage unless they volunteered to provide this. At this stage a token sum was paid for each recipe and a one page agreement of collaboration to participate in the research to help in the investigation of a global health problem was signed by the herbalist and a senior member of the research team. The agreement included a clause to the effect that publications arising from work on the unique recipe will include the herbalist's name. Traditional medicine practitioners (TMPs) whose recipes were found active in vitro against BCG/M.tb. were re-visited for the purpose of recollection of their recipes, the composition of the recipes and the provision for taxonomical identification purposes of plant samples used in the preparation of their recipes. The TMPs whose recipes exhibited activities were given letters giving indications about the results of screening the extracts of their recipes against M. tb. together with a slightly higher token sum for services. Fifty-six plant specimens were collected by the individual TMP for each ETM during the time of the team's visit. The plant samples were identified by the taxonomists at the herbarium of the National Institute for Pharmaceutical Research and Development (NIPRD), Abuja where voucher samples were deposited.
2.3 Preparation of Extracts
For recipes that were powders 10 g of raw material was extracted by soaking in 70 % aqueous methanol (100 ml), shaking occasionally for 24 hours. 70% aqueous methanol was used as the solvent of extraction, from experience (Mann et al., 2008), in attempt to extract the whole range of compound polarities in the recipe samples. The extracts were then filtered and concentrated under reduced pressure using rotary evaporator to remove the methanol and some water. The concentrated extract was then lyophilized. For the aqueous recipes, 50 mL of each of the liquid was shaken vigorously for homogeneity and then freeze-dried. The resulting dry powder was transferred to a bottle, weighed and stored.
2.4 Determination of Minimum Inhibitory Concentration (MIC)
The broth microdilution method (Duckworth et al., 2012), which in a survey (Franzblau et al., 2012), was found to be the mostly used protocol in the field, was employed for the preliminary screening. All extracts were screened against both M. tb. strain H37Rv and Mycobacterium bovis BCG after cells were grown to an optical density of 0.2-0.3 at 650 nm in 7H9/ADC/Tween consisting of Middlebrook 7H9 broth supplemented with 0.5% bovine serum albumin fraction V, 0.08% NaCl, 0.2% glucose, 0.2% glycerol and 0.05% Tween 80. The lyophilized plant extract was dissolved in 4 mL DMSO and made up with 7H9/ADC/Tween to a final DMSO concentration of 4%. Each plant extract-medium was vortexed and 100 μL of it dispensed into the empty first column (first row) of a 96-well microtitre plate while each of all the other wells (two to twelve of the column) contained 50 μL of 7H9/ADC/Tween. Two-fold dilutions were performed by sequential transfer of 50 μL from the first column through to column 11 leaving column 12 for the drug-free control. The mycobacterial culture was diluted 1:1000 in 7H9/ADC/Tween, and 50 μL added to all wells giving a final volume of 100 μL. in each well. Isoniazid (INH) was used as positive control. The microtitre plate was incubated for 7-10 days at 37°C, after which the growth or inhibition of growth was read by direct recording of visual growth. All of the MIC determinations and extractions were done in duplicate with the BCG screening at NIPRD, Abuja, Nigeria while the M. tb., H37Rv strain, screening was carried out at TRS, LCID, NIAID/NIH Bethesda, MD USA. The MIC was reported as the highest concentration of extract resulting in complete inhibition of visual growth.
3.0 Results and Discussion
3.1 Ethnobotanical demography and information
Most of the healers interviewed were men with ages ranging from 30 and above. Most of them were also from a family of traditional healers and had been practicing for most of their lives. They also had mostly learnt the practice from an elderly family member confirming the well-known fact that the healing art in most cultures survives through family inheritance and training. Most of the traditional healers also indicated that symptoms such as fever, cough and blood in the sputum together were indications for the treatment of tuberculosis. A number of the recipes contained multiple plant components and decoction was the method used by most of the practitioners in the preparation of these recipes. Most of the plants were obtained from the wild. Some healers recommended special seasons or times for collection of the plant e.g. the dry season or during the day, because they believed that the plants were more potent at the time chosen for collection. The majority of the healers stored the plant parts in aerated sacks. Though the TMPs are not aware of the molecular composition of their herbs, they have learnt over the years that plant metabolites could vary with seasons and environment.
86 ETMs were collected initially when the compositions of the recipes were unknown, 73 were recollected after ensuring compatibility with the first samples and 43 of these had specimens of component plants available for inspection and identification.
3.2 Biological activities of the ethnomedicines, ETMs
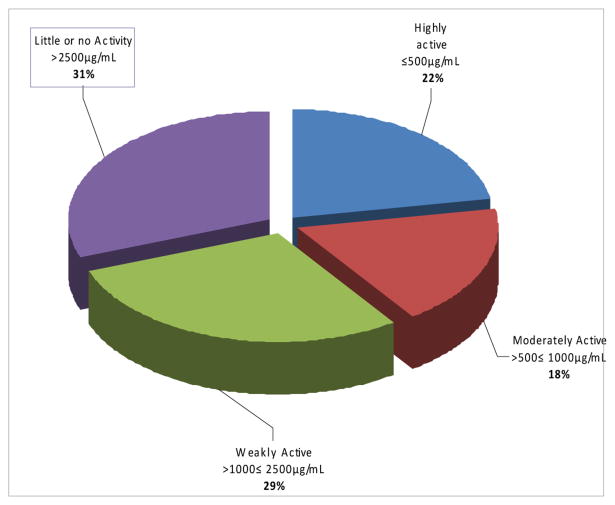
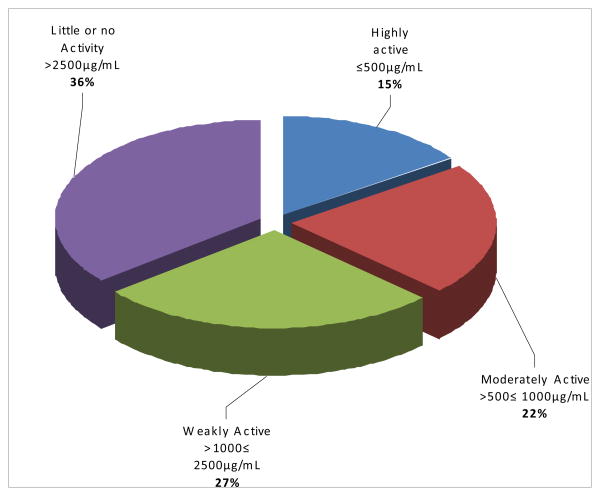
Preliminary screening of the 86 plant recipes showed the various percentage activities of these recipes against BCG and M. tb. in different ranges of concentration (Figs 2 and 3). The activities of the herbal recipes (ETMs) were classified using the MICs into highly active, blue coded in figures (MIC ≤ 500 μg/ml) moderately active, red coded in figures (500μg/ml < MIC ≤ 1 mg/ml) weakly active, green coded in figures (1mg/ml < MIC ≤ 2.5mg/ml), and little or no activity, purple coded in figures ( ≥ 2.5 mg/mL). The individual ETMs and plants making up 43 of these recipes (ETMs) and the MICs of these recipes are shown in Table 2.
Fig 2. Activity percentages of ETMs against BCG.
Fig 3. Activity percentages of ETMs against MTB.
Table 2. Recipes and the MICs.
| ETM* nos. | Plant Composition in Recipe | Physical state of recipe | MIC in μg/mL (MTB) | MIC in μg/mL (BCG) | |
|---|---|---|---|---|---|
| 15 | Abrus precatorius L. (Fabaceae) | Solid | 1838 | 500 | |
| 16 | Erythrina senegalensis DC. (Fabaceae) | Solid | 168 | 1200 | |
| 17 | Ficus exasperata Vahl. (Moraceae) | Solid | 650 | 1000 | |
| 18 | Anogeisus leocarpus (DC.) Guill & Perr (Combretaceae) | Solid | 925 | 266 | |
| 19 | Cassia siberiana DC. (Caesalpiniaceae) | Solid | 150 | 2500 | |
| 20 | Cassia siberiana DC. (Caesalpiniaceae) | Solid | 416 | 1625 | |
| 21 | Tapinanthus sessilifolia Polh. & Wiens.(Loranthaceae) | Solid | 128 | 1000 | |
| 22 | Securidaca longepedunculata Fres. (Polygalaceae) | Solid | >2500 | 2125 | |
| 23 | Guiera senegalensis J. F. Gmel (Combretaceae) | Solid | 919 | 1000 | |
| 24 |
|
Solid | ND | 2250 | |
| 25 | Terminalia avicennioides Guill & Perr (Combretaceae) | Solid | 1200 | 2000 | |
| 26 | Solid | 200 | >2500 | ||
| 27 | Ximenia americana Linn. (Olacaceae) | Solid | >2500 | 875 | |
| 28 | Garcinia kola Heckel (Guttiferae) | Solid | ND | 2000 | |
| 29 | Calliandra portoricensis (Jacq.) Benth (Mimosaceae) | Solid | ND | 563 | |
| 30 | Tapinanthus sessilifolia Polh. & Wiens. (Loranthaceae) | Solid | ND | >2500 | |
| 31 | Combretum molle R. Br ex G. Don (Combretaceae) | Solid | 2000 | 250 | |
| 32 | Solid | >2500 | >2500 | ||
| 33 | Solid | 2100 | 1000 | ||
| 34 |
|
Solid | 850 | 1125 | |
| 35 |
|
Solid | >2500 | >2500 | |
| 36 | Ficus sur Forrsk. (Moraceae) | Solid | 750 | 813 | |
| 37 |
|
Liquid | >2500 | >2500 | |
| 38 |
|
Solid | 220 | 33 | |
| 39 |
|
Solid | 198 | 1475 | |
| 40 | Anogeisus leocarpus (DC.) Guill & Perr (Combretaceae) | Solid | 1013 | 988 | |
| 41 | Solid | 894 | 2200 | ||
| 42 |
|
Solid | 154 | 466 | |
| 43 |
|
Solid | 1488 | 156 | |
| 44 | Solid | 250 | 200 | ||
| 45 |
|
Solid | 1344 | >2500 | |
| 46 | Solid | >2500 | >2500 | ||
| 47 | Liquid | >2500 | ND | ||
| 48 |
|
Solid | 660 | 313 | |
| 49 |
|
Solid | >2500 | 313 | |
| 50 | Crotolaria lachnosema Staph (Fabaceae) | Solid | 1800 | >2500 | |
| 51 |
|
Solid | >2500 | ND | |
| 52 | Solid | 1363 | ND | ||
| 53 | Cassytha filiformis Linn. (Lauraceae) | Solid | 950 | >2500 | |
| 54 |
|
Solid | 875 | 1500 | |
| 55 | Solid | 1813 | ND | ||
| 56 | Solid | >2500 | >2500 | ||
| 57 | Crotolaria lachnosema Staph (Fabaceae) | Solid | 869 | ND | |
| 58 | Solid | 1406 | 1250 | ||
| 59 | Solid | 675 | 300 | ||
| 60 |
|
Solid | 2213 | 488 | |
| 61 | Liquid | >2500 | 1400 | ||
| 62 | Liquid | >2500 | 400 | ||
| 63 | Khaya grandifolia C. Dc. (Meliaceae) | Solid | 663 | 2000 | |
| 64 | Liquid | >2500 | >2500 | ||
| 65 | Liquid | >2500 | >2500 | ||
| 66 | Liquid | >2500 | 1000 | ||
| 67 | Liquid | ND | ND | ||
| 68 | Solid | ND | ND | ||
| 69 | Liquid | 625 | >2500 | ||
| 70 | Liquid | >2500 | ND | ||
| 71 | Ocimum gratissimum Linn. (Lamiaceae) | Liquid | 2400 | >2500 | |
| 72 | Pterocarpus osun Craib (Fabaceae) | Liquid | 1225 | 1100 | |
| 73 | Solid | 388 | ND | ||
| 74 | Solid | >2500 | >2500 | ||
| 75 |
|
Liquid | >2500 | 1125 | |
| 76 | Liquid | >2500 | ND | ||
| 77 | Solid | 506 | 2000 | ||
| 78 | Solid | >2500 | >2500 | ||
| 79 | Liquid | >2500 | >2500 | ||
| 80 | Liquid | ND | 300 | ||
| 81 | Liquid | >2500 | ND | ||
| 82 | Solid | 188 | 350 | ||
| 83 | Liquid | >2500 | >2500 | ||
| 84 | Liquid | >2500 | 2000 | ||
| 85 | Solid | 600 | 1000 | ||
| 86 | Liquid | >2500 | ND | ||
| 87 | Solid | >2500 | ND | ||
| 88 | Solid | 1425 | >2500 | ||
| 89 |
|
Solid | 1513 | 1900 | |
| 90 | Solid | >2500 | >2500 | ||
| 91 | Solid | 1175 | 1125 | ||
| 92 | Solid | 775 | ND | ||
| 93 | Tetrapleura tetraptera (Schum & Thonn.) Taub (Fabaceae) | Solid | 277 | 1125 | |
| 94 | Solid | 1875 | 1000 | ||
| 95 | Pentaclethra macrophylla Benth. (Fabaceae) | Solid | 1625 | 975 | |
| 96 | Calliandria portoricensis (Jacq.) Benth (Mimosaceae) | Solid | 1175 | 228 | |
| 97 | Solid | 1738 | 144 | ||
| 98 | Liquid | ND | >2500 | ||
| 99 |
|
Solid | 638 | 875 | |
| 100 | Solid | >2500 | >2500 | ||
ETM = Recipe. ETMs 1-14 belonged to earlier investigation. Please note that the MIC values are not absolute but are results obtained using our screening procedure that may not have been appropriate for some of the ETMs especially for the ETMs that were not active in our experiments.
3.2.1 BCG and M. tb. test results
Extracts of 72 ETMs were tested against BCG. Of these 16 (22%) were highly active, 13 (18%) were moderately active, 21 (29%) were weakly active and 22 (31%) had little or no activity. The distribution of activities in MIC ranges against BCG is presented in fig.2.
Extracts of 78 ETMs were tested against M. tb.. Of these 12 (15%) were highly active, 17 (22%) were moderately active, 21 (27%) were weakly active and 28 (36%) had little or no activity. The distribution of activities in MIC ranges against M. tb. is presented in Fig. 3.
Extracts of 66 ETMs were each individually tested against BCG as well as M. tb. Of these, 51 (77%) had highly active to weakly active results for both organisms. 8 (12%) were active against BCG but inactive against M. tb. 7 (11%) were active against M. tb. and inactive against BCG and 13 (20%) were inactive against both organisms.
In this study, there was 77% agreement in the anti-Mycobacterium activities obtained using either BCG or M. tb. strain H37Rv as test organisms.
3.3 Phytochemistry and pharmacology of selected plants listed in the ETMs
The phytochemistry and pharmacology of most of the plants claimed by the TMPs to have been used in formulating their ETMs have been published in various details. The publications have not necessarily been in connection with their anti-Mycobacterium activities. Twelve of the plants stand out owing to the highly active nature of their extracts. Thus the known chemistry and biological activities of all such plants which have been given as the sole components of ETMs and in some cases used in conjunction with other plants are hereby discussed.
Abrus precatorius is the only given component for ETM 18. An active component from the plant extract has been isolated and characterized as the isoflavanoid quinone, abruquinone which was shown (Limmatvapirat et al., 2004) to have an MIC of 12.5μg/mL against the M. tb. strain H37Rv.
Anogeissus leocarpus is listed as a component in 10 of the 43 ETMs whose formulations were divulged by the TMPs and in two of these ETMs, 18 and 40, it is the single component. A. leocarpus is thus the most commonly used plant among the TMPs visited. The chemistry and the antimicrobial activity of the extracts of the Genus Anogeissus have been reviewed (Mann et al 2009a) .The leaves and or stem bark are the parts usually utilized. Our MIC results of 266μg/mL and 988μg/mL respectively for the extracts of ETMs 18 and 40 against BCG are in rough agreement with the MICs, 312μg/mL for the hexane fraction and 1250μg/mL for the methanol fraction (Mann et al., 2008) when their initial 70% aqueous methanol extract was partitioned into hexane and methanol. A. leocarpus extracts contain polyphenols and triterpenoids (Adigun et al, 2000; Chaabi et al, 2008; Mann et al., 2009a). There seems to be clear cases of synergism between the extracts of the plants combined with A. leocarpus in ETMs 38 and 48 while the opposite is the case for ETMs 45, 49 and 60 where A. leocarpus is combined with other plants. These combinations need to be further studied.
Cassia siberiana is the sole component given for ETMs 19 and 20. The leaves and roots extracts of the plant have been shown to possess antibacterial activity (Ndukwe et al, 2004). Anthraquinone and polyphenolic flavonoids 1-epicatechol and leucopelargonidol have been identified in its extracts (Duquenois and Anton, 1968; Ndukwe et al 2004; Paris and Etchepare, 1967) and these compounds could contribute to the antiMycobacterium activity observed in this work.
Combretum molle, the only component in ETM 31 stem bark acetone extract reportedly gave tannin, punicalgin which gave an MIC higher than 600μg/mL against M. tb. strain ATCC 27294 and a clinical isolate (Asres et al., 2001). Extracts of C. mole had MIC of 0.5mg/mL against M. tb. strain H37Rv and inhibited the resistant M. tb. CCK02869V (Lall and Meyer, 1999). A hydroxycycloartenol glycoside, mollic acid has been isolated from the leaf extract of C. molle (Rogers and Thevan, 1996).
Erythrina senegalensis is the lone plant for ETM 16. On a chemotaxonomic basis E. Senegalensis extracts could be expected to contain isoflavonoidsand coumarin derivatives just like E. gibbosa and E. indica respectively. The isoflavonoids pasellidin and erythobissin with MICs respectively lying between 8μg/mL and 25μg/mL against M.tb. have been isolated as active principles from E. gibbosa extracts (Mitscher and Baker, 1998) while a 3-phenyl coumarin derivative indicanine with MIC of 18.5μg/mL against M. smegmatis has been isolated from E. indica extracts ( Waffo et al., 2000).
Garcinia kola is the only component given for ETM 28 and is in ETMs 48 and 75. Its extracts are known to contain anthraquinones, biflavonoids, saponins and xanthones. Four of the bioflavonoids and a xanthone which have antibacterial, antihepatoxic and antidiabetic activities have been identified (Christopher et al., 2007; Iwu et al., 1987, 1990; Oluronke et al, 1999).
Khaya grandifolia, the only component given for ETM 63 is well known to have limonoids (bitter principles) as the main components of its extracts. The antimalarial activity and the effects of its extracts on biochemical and haematological parameters in mice have been reported (Agbedahunsi and Elujoba, 1998a; Bumah, et al., 2005).The limonoids - grandifolilenone, methyl 6-acetoxy angolensate and grandifolin - were isolated from the plant extracts (Agbedahunsi and Elujoba, 1998b; Connolly and McCrindle, 1967). Grandifolin came from the antimalarial active chromatographic fraction of the extract of the stem bark. Adesogan and Taylor (1967) reported the isolation of a steroid hormone from the plant extract. We are aware from direct personal experience of the use of stem bark tannin-containing aqueous decoction in the treatment of dysentery. Tannins are in general antimicrobial and hence could contribute to the observed antimycobacterial activities of the plant extract.
Pentaclethra macrophylla (ETM 95) seed and root bark extracts gave a phenolic and steroidal glycosides but with no reports of antiMycobacterium screening (Folefoc et al., 2005).
Pterocarpus osun (ETM 72) extracts have been shown to have antimicrobial activities and to contain glycosides, saponins, steroids and tannins (Ebi and Ofoefule, 2001) which could be responsible for the observed anti Mycobacterium activity.
Securidaca longepedunculata methanol and hexane extracts yielded hydroxybenzoic acids and xanthones which had MICs of 312μg/mL and 1250μg/mL respectively against BCG (Green et al., 2010; Lannang et al., 2006; Mann et al., 2009b). The ethanolic extract of the plant was shown to be active at a concentration of 0.050g/mL against M. tb. strain H37Rv and a clinical isolate (Adeleye et al., 2008)
Tapinanthus sessifolia extract is highly active as the sole component in ETM 21 but inactive also as the sole component given for ETM 30. This inconsistency could arise from incorrect information but more likely from the taxonomic problems associated with the genus in Nigeria which has now been resolved (Ibrahim and Ayodele, 2011). The plant, mistletoe, being a parasite might also exhibit metabolite differences depending on its hosts.
Terminalia avicennioides is the only component given for ETM 25. Its extracts have been reported (Mann et al., 2008, 2011) to yield arjunolic acid and friedelin which respectively had MICs against BCG of 156 g/mL and 4.9 g/mL. Our observations and the claims of the TMPs are in accord with these reports.
Tetrapleura tetraptera is the only component given for ETM 93. Oleanane type saponins and sulphates have been reported in the plant extracts and shown to be highly toxic to Mollusca (Aladesanmi, 2007). The same compounds may be the principles responsible for the antiMycobacterium activities observed and reported in this work.
4.0 Conclusion
4.1 Efficacy of Nigerian herbal ethnomedices
Our study clearly showed that Nigerian herbalists have recipes that have likely been effective to some extent for the management of tuberculosis among the rural population of the Country. The recipes need to be fully analyzed for the purpose of potentially identifying new antituberculosis drug scaffolds and in the process, assist in the standardization of the local antituberculosis herbal recipes. The case has been made for applying ‘omics’ technologies to phytomedicines and traditional recipes which have historically been used over decades or centuries for the treatment of tuberculosis symptoms as a starting point for the discovery of new drugs and drug scaffolds (Shyur and Yang, 2008; Wells, 2011; Ngo et al, 2013). We anticipate that using ‘omics’ technologies in systems biology approaches combined with chemical informatics of various scaffolds characterized in active at least partially purified extracts, could make studies initiated around plants and indigenous herbal recipes relatively efficient in the rapid identification of new drug leads for tuberculosis (Boshoff and Lun, 2010; Cho et al., 2006; Sundramurthia et al., 2012; Tang and Marshall, 2011).
4.2 BCG as test organism for potential anti-M. tb. principles
On the basis of the fact that there is 77% qualitative agreement between the MIC results observed using BCG in place of M. tb. we conclude that usable information can be obtained using BCG to assess potential sources of anti-M. tb. principles where no facilities suitable for handling the more dangerous pathogenic M. tb. exist.
4.3 Prioritization criteria for selection of plants for detailed analysis
The following criteria are recommended for the prioritization of the plants for further studies: (i) potency of the extract based on the MIC values, (ii) published work on the biology and chemistry of the plants, (iii) novelty of information of the plant's use as anti-TB remedy and (iv) the frequency of occurrence of the plants in the collected recipes. Using these criteria, the following plants are recommended for the initial further studies: Ficus sur, Pavetta crassipes, Combretum molle, Waltheria indica and Crotolaria lachnosema, Anogiessus leocarpus, Calliandra portoricensis, Cassia sieberiana, Abrus precatorius and Cussonia arborea.
Acknowledgments
We thank the NIAID-NIH for support and for the award of postgraduate fellowships to N. Ibekwe and J. Nvau. Engineer B. Dogonyaro and M. Tsado and members of the NIAID-NIH IT team gave instrument and information technology supports. Bitrus Pam drove the vehicle that took the team safely to various parts of Nigeria to visit the TMPs. We appreciate his contributions to the success of the project. We thank Dr. Jemilat Ibrahim and Grace Ugbabe of the Herbarium, National Institute For Pharmaceutical Research And Development, Abuja, Nigeria who assisted in the identification of plant specimen samples. We acknowledge the administrative and logistics support enthusiastically given to the project by the then Director General of the National Institute for Pharmaceutical Research and Development, Dr. Uford S. Inyang. We thank the management and staff of NIPRD for their support. We are grateful to Katherine M. Perry who was the project manager and a source of strength and encouragement to the team.
References
- Adeleye IA, Onubogu CC, Ayolabi CI, Isawumi AO, Nshiogu ME. Screening of crude extracts of twelve medicinal plants and “wonder-cure” concoction used in Nigeria unorthodox medicine for activity against Mycobacterium tuberculosis isolated from tuberculosis patients sputum. African Journal of Biotechnology. 2008;7:3182–3187. [Google Scholar]
- Adesogan EK, Taylor DHR. Isolation of a steroid hormone from Khaya gradifoliola. Chemical Communications. 1967:1365. [PubMed] [Google Scholar]
- Adigun JO, Amupitan JO, Kelly DR. Isolation and investigation of antimicrobial effect of 3,4,31- tri-O-methyl flagellic acid and its glucoside from Anogeissus leocarpus. Bulletin of the Chemical Society of Ethiopia. 2000;14:169–174. [Google Scholar]
- Agbedahunsi JM, Elujoba AA. Anti-malarial activity of Khaya grandifoliola stembark. Pharmaceutical Biology. 1998a;36:8–12. [Google Scholar]
- Agbedahunsi JM, Elujoba AA. Grandifolin from Khaya grandifoliola stem bark. Nigerian Journal of Natural Products and Medicine. 1998b;2:34. [Google Scholar]
- Aladesanmi JA. Tetrapleura tetraptera mollusicidal activity and chemical constituents. African J of Traditional, Complimentary and Alternative Medicine. 2007;4:23–36. [PMC free article] [PubMed] [Google Scholar]
- Asres K, Bucar F, Edelsbrunner S, Kartnig T, Höger G, Thiel W. Investigations on antimycobacterial activity of some Ethiopian medicinal plants. Phytotherapy Research. 2001;15:323–326. doi: 10.1002/ptr.724. [DOI] [PubMed] [Google Scholar]
- Barry CE., III . Modern drug development. In: Kaufmann SHE, Hahn H, editors. Mycobacterial and TB. Issues in Infectious Diseases 2. Karger; Basel: 2003. pp. 137–150. [Google Scholar]
- Boshoff HI, Lun DS. Systems biology approaches to understanding mycobacterial survival mechanisms. Drug Discovery Today, Disease Mechanisms. 2010;7:75–82. doi: 10.1016/j.ddmec.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumah VV, Essien EU, Agbedahunsi JM, Eka OU. Effect of Khaya grandifoliola on red blood cells and bone mineral content in rats. Journal of Ethnopharmacology. 2005;102:446–449. doi: 10.1002/ptr.1750. [DOI] [PubMed] [Google Scholar]
- Carroll MW, Lee M, Cai Y, Hallahan CW, Shaw PA, Min JH, Goldfeder LC, Alekseyev V, Grinkrug S, Kang HS, Hwang S, Park HM, Kang E, Lee SY, Jin B, Park HE, Min S, Park SK, Jeon DS, Via LE, Barry CE., 3rd Frequency of adverse reactions to first- and second-line anti-tuberculosis chemotherapy in a Korean cohort. International Journal of tuberculosis Lung Disorder. 2012;16:961–969. doi: 10.5588/ijtld.11.0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control. Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs worldwide, 2000-2004. Weekly. 2006;55(11):301–305. [PubMed] [Google Scholar]
- Chaabi M, Benayache S, Benayache F, N'Gom S, Kone M, Anton R, Weniger B, Lobstein A. Triterpenes and polyphenols from Anogeissus leocarpus(Combretaceae) Systematics Ecology. 2008;36:59–62. [Google Scholar]
- Cho CR, Labow M, Reinhardt M, van Oostrum J, Peitsch MC. The application of systems biology to drug discovery. Current Opinion in Chemical Biology. 2006;10:294–302. doi: 10.1016/j.cbpa.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Christopher O, Slarko K, Geogie F, Alexander P, David MR, Petre IA, Yoichiro I, Ilya R. Preparative isolation and identification of tryrosinase inhibitors from the seed of Garcinia kola by high-speed counter-current chromatography. Journal of Chromatography A. 2007;1151:45–50. doi: 10.1016/j.chroma.2007.02.085. [DOI] [PubMed] [Google Scholar]
- Connolly JD, McCrindle R. Constitution of grandifolilenone, a novel triterpenoid from Khaya gradifoliola. Chemical Communications. 1967:1193–1194. [Google Scholar]
- Copp BR. Antimycobacterial natural products. Natural Product Reports. 2003;20:535–557. doi: 10.1039/b212154a. [DOI] [PubMed] [Google Scholar]
- Copp BR, Pearce NA. Natural product growth inhibitors of Mycobacterium tuberculosis. Natural Product Reports. 2007;24:278–297. doi: 10.1039/b513520f. [DOI] [PubMed] [Google Scholar]
- Duckworth BP, Wilson DJ, Nelson KM, Boshoff HI, Barry CE., 3rd Development of a selective activity-based probe for adenylating enzymes: profiling MbtA Involved in siderophore biosynthesis from Mycobacterium tuberculosis. ACS Chemical Biology. 2012;7(10):1653–1658. doi: 10.1021/cb300112x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duquenois P, Anton R. Chemical study of Cassia sieberiana leaves. Planta. 1968;16:184–90. [PubMed] [Google Scholar]
- Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. Journal of the American Medical Association. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- Dye C. Global epidemiology of tuberculosis. Lancet. 2006;367:938–940. doi: 10.1016/S0140-6736(06)68384-0. [DOI] [PubMed] [Google Scholar]
- Ebi GC, Ofoefule SI. Antimicrobial activity of Pterocarpus Osun stems. Fitoterapia. 2001;71:433–435. doi: 10.1016/s0367-326x(00)00130-1. [DOI] [PubMed] [Google Scholar]
- Folefoc GN, Bisseck JP, Fomum ZT, Bodo B. Constituents from the roots of Pentaclethra macrophylla. Biochemical Systematics and Ecology. 2005;33:1280–1282. [Google Scholar]
- Franzblau SG, DeGroote MA, Cho SH, Andries K, Nuermberger E, Orme IM, Mdluli K, Angulo-Barturen I, Dick T, Dartois V, Lenaerts AJ. Comprehensive analysis of methods used for the evaluation of compounds against Mycobacterium tuberculosis. Tuberculosis. 2012;92:453–488. doi: 10.1016/j.tube.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Gandhi NR, Nunn P, Dheda K, Schaaf HS, Zignol M, Soolingel D, Jensen P, Bayona J. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet. 2010;375:1830–1843. doi: 10.1016/S0140-6736(10)60410-2. [DOI] [PubMed] [Google Scholar]
- Green E, Samie A, Obi CL, Bessong PO, Ndip RN. Inhibitory properties of selected South African medicinal plants against Mycobacterium tuberculosis. Journal of Ethonopharmacology. 2010;130:151–157. doi: 10.1016/j.jep.2010.04.033. [DOI] [PubMed] [Google Scholar]
- Hiserodt RD, Franzblau SG, Rosen RT. Isolation of 6-, 8-, and 10-gingerol from ginger rhizome by HPLC and preliminary evaluation of inhibition of Mycobacterium avium and Mycobacterium tuberculosis. Journal of Agricultural and Food Chemistry. 1998;46:2504–2508. [Google Scholar]
- Hostettmann K, Wolfender JL, Rodriguez S, Marston A. Strategy in the search for bioactive plant constituents. In: Hostettmann K, Chinyanganya F, Maillard M, Wolfender JL, editors. Chemistry, Biological and Pharmacological Properties of African Medicinal Plants. University of Zimbabwe; Harare: 1996. pp. 21–42. [Google Scholar]
- Ibrahim JA, Ayodele AE. Taxonomic revision of Loranthaceae in Nigeria. Nigerian Journal of Botany. 2011;24:153–188. [Google Scholar]
- Iwu MM, Igboko OA, Onwuchekwa UA, Okunji CO. Evaluation of the antihepatoxic activity of the bioflavanoids of Garcinia kola seeds. Journal of Ethnopharmacology. 1987;21:127–138. doi: 10.1016/0378-8741(87)90123-1. [DOI] [PubMed] [Google Scholar]
- Iwu MM, Igboko OA, Okunji CO, Tempesta MS. Antidiabetic and aldose reductase activities of biflavanones of Garcinia kola. Journal of Pharmacy and Pharmacology. 1990;42:290–292. doi: 10.1111/j.2042-7158.1990.tb05412.x. [DOI] [PubMed] [Google Scholar]
- Iwu MM. Introduction: therapeutic agents from ethnomedicine. In: Iwu MM, Wootton JC, editors. Ethnomedicine and Drug Discovery. Elsevier Science B.V.; Amsterdam, The Netherlands: 2002. pp. 1–22. [Google Scholar]
- Khazir J, Mir BA, Mir SA, Cowan D. Natural products as lead compounds in drug discovery. Journal of Asian Natural Products Research. 2013;15:764–788. [Google Scholar]
- Lall L, Meyer JJM. In vitro inhibition of drug-resistant and drug-sensitive Strain of Mycobacterium tuberculosis by ethnobotanically selected South Africa plants. Journal of Ethnopharmacology. 1999;66:347–354. doi: 10.1016/s0378-8741(98)00185-8. [DOI] [PubMed] [Google Scholar]
- Lannang AM, Lontsi D, Ngounou FN, Sondengan BL, Nkengfack AE, Van-Heeerden FR, Assob JCN. Securidacaxanthone A, a heptaoxygenated xanthone from Securidaca longepedunculata. Fitoterapia. 2006;77:199–202. doi: 10.1016/j.fitote.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Limmatvapirat C, Sirisopanaporn S, Kittakoop P. Antitubercular and antiplasmodial constituents of Abrus precatorius. Planta Medica. 2004;70:276–278. doi: 10.1055/s-2004-818924. [DOI] [PubMed] [Google Scholar]
- Mann A, Amupitan JO, Oyewale AO, Okogun JI, Ibrahim K, Oladosu P, Lawson L, Olajide I, Nnamdi A. Evaluation of in vitro antimycobacterial activity of Nigerian plants used for treatment of respiratory diseases. African Journal of Biotechnology. 2008;7:1630–1636. [Google Scholar]
- Mann A, Amupitan JO, Oyewale AO, Okogun JI, Ibrahim K. Chemistry of secondary metabolites and their antimicrobial activity in the drug development process: a review of the Genus Anogeissus. Medicinal Plants. 2009a;1:1–23. [Google Scholar]
- Mann A, Ibrahim K, Oyewole AO, Amupitan JO, Okogun JI. Antimycobacterial activity of some medicinal plants in Niger State, Nigeria. African J Infectious diseases. 2009b;3:44–48. [Google Scholar]
- Mann A, Ibrahim K, Oyewale AO, Amupitan JO, Fatope MO, Okogun JI. Antimycobacterial Friedelane-terpenoid from the Root Bark of Terminalia avicennioides. American Journal of Chemistry. 2011;1(2):52–55. [Google Scholar]
- Mitnick C, Bayona J, Palacious E, Shin S, Furin J, Alcantara F, Sanchez E, Sarria M, Becerra M, Fawzi MCS, Kapiga S, Neuberg D, Maguire JH, Kim JY, Farmer P. Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. New England Journal of Medicine. 2003;348:119–128. doi: 10.1056/NEJMoa022928. [DOI] [PubMed] [Google Scholar]
- Mitscher LA, Baker WR. Tuberculosis: a search for novel therapy starting with natural products. Medicinal Research Reviews. 1998;18:363–374. doi: 10.1002/(sici)1098-1128(199811)18:6<363::aid-med1>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Moulding T. Pathogenesis, Pathophysiology and Immunology. In: Schlossberg D, editor. Tuberculosis. 2nd. Springer-Verlag; New York: 1988. pp. 13–22. [Google Scholar]
- Ndukwe KC, Lamikanra A, Okeke IN. Antibacterial in plants used as chewing sticks in Africa. Drugs of the future. 2004;29:1221. [Google Scholar]
- Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981-2002. Journal of Natural Products. 2005;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. Journal of Natural Products. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- Ngo LT, Okogun JI, Folk WR. 21st Century natural products research and drug development and traditional medicine. Natural Products Reports. 2013;30:584–592. doi: 10.1039/c3np20120a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okunade AL, Elvin-Lewis MPF, Lewis WH. Natural antimycobacterial metabolites: current status. Phytochemistry. 2004;65:1017–1032. doi: 10.1016/j.phytochem.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Oluronke T, Hoxg-Xi X, Song FL. Antibacterial activities of extracts from Nigeria chewing stick. Phytotherapy Research. 1999;13:675–679. doi: 10.1002/(sici)1099-1573(199912)13:8<675::aid-ptr513>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Paris RR, Etchepare S. Cassia sieberiana polyphenols: Isolation of 1-epicatechol and leucopelargonidol. Annales pharmaceutiques francaides. 1967;25:343–346. [PubMed] [Google Scholar]
- Queiroz EF, Wolfender JL, Hostettmann K. Modern approaches in the search for new lead antiparasitic compounds from higher plants. Current Drug Targets. 2009;10:202–211. doi: 10.2174/138945009787581113. [DOI] [PubMed] [Google Scholar]
- Rogers CB, Thevan I. Identification of mollic acid a-l-arabinose, hydroxycycloartenoid from C. molle leaves. Phytochemistry. 1996;25:1759–1761. [Google Scholar]
- Shyur LF, Yang NS. Metabolomics for phytomedicine research and drug development. Current Opinion in Chemical Biology. 2008;12:66–71. doi: 10.1016/j.cbpa.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Smith PG, Moss AR. Epidemiology of Tuberculosis. In: Bloom BR, editor. Pathogenesis, Protection and Control. American Society for Microbiology Press; 1994. pp. 47–59. [Google Scholar]
- Sundarmurthi JC, Brindha S, Reddy TBK, Hanna LE. Informatics resources for tuberculosis – Towards drug discovery. Tuberculosis. 2012;92:133–138. doi: 10.1016/j.tube.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Tang YT, Marshall GR. Virtual screening for lead discovery. Methods in Molecular Biology. 2011;716:1–22. doi: 10.1007/978-1-61779-012-6_1. [DOI] [PubMed] [Google Scholar]
- TB: Join the DOTS. The Economist. 1995 May 20;:89. [Google Scholar]
- Waffo K, Azebaze GA, Nkengfac AE, Fomum ZT, Meyer M, Bodo B, van Heerden FR. Indicannines B and C, two isoflavonoid derivatives from the root bark of Erythrina indica. Phytochemistry. 2000;53:981–985. doi: 10.1016/s0031-9422(99)00615-9. [DOI] [PubMed] [Google Scholar]
- Wells TNC. Natural products as starting points for future anti-malarial therapies: going back to our roots? Malaria Journal. 2011;10(Suppl. 1):1–12. doi: 10.1186/1475-2875-10-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen CC. Failure of Directly observed treatment for tuberculosis in Africa: a call for new approaches. Clinical Infectious Diseases. 2006;42:1048–1050. doi: 10.1086/501022. [DOI] [PubMed] [Google Scholar]
- Wolfender JL. HPLC in natural product analysis: The detection issue. Planta Medica. 2009;75:719–734. doi: 10.1055/s-0028-1088393. [DOI] [PubMed] [Google Scholar]
- World Health Organisation. Global tuberculosis - control surveillance, planning and financing. WHO Report WHO/HTM/Tb/2008.393 2008 [Google Scholar]