Abstract
Across three experiments, we explored whether a dog's capacity for inhibitory control is stable or variable across decision-making contexts. In the social task, dogs were first exposed to the reputations of a stingy experimenter that never shared food and a generous experimenter who always shared food. In subsequent test trials, dogs were required to avoid approaching the stingy experimenter when this individual offered (but withheld) a higher-value reward than the generous experimenter did. In the A-not-B task, dogs were required to inhibit searching for food in a previously rewarded location after witnessing the food being moved from this location to a novel hiding place. In the cylinder task, dogs were required to resist approaching visible food directly (because it was behind a transparent barrier), in favor of a detour reaching response. Overall, dogs exhibited inhibitory control in all three tasks. However, individual scores were not correlated between tasks, suggesting that context has a large effect on dogs' behavior. This result mirrors studies of humans, which have highlighted intra-individual variation in inhibitory control as a function of the decision-making context. Lastly, we observed a correlation between a subject's age and performance on the cylinder task, corroborating previous observations of age-related decline in dogs' executive function.
Keywords: Domestic dogs, Inhibitory control, Canine, Cognition
Introduction
The term “inhibitory control” refers to an individual's ability to resist the urge to do something that is immediately tempting, but ultimately harmful or counterproductive. Thus, it is unsurprising that animals have evolved inhibitory control that allows for adaptive responses in a variety of contexts. For example, suppression of immediate pouncing in stalking predators (e.g., cheetahs, African wild dogs, wolves: MacNulty et al. 2007), inhibition of public mating among subordinate males (e.g., Japanese macaques: Soltis et al. 2001), and waiting patiently for a meal of tree sap (e.g., gummivorous common marmosets: Stevens et al. 2005) are all well-documented behaviors that likely recruit inhibitory control. In a study of seven primate species specifically looking at inhibitory control, higher levels of inhibitory control were associated with species living in fission–fusion groups, as opposed to cohesive groups. The authors hypothesized that this result may be explained by these species' superior behavioral flexibility, or ability to respond quickly to changes in their environment and resolve problems using alternative strategies (Amici et al. 2008). Another study found that the performance of male song sparrows in an inhibitory control task was correlated with song repertoire size, a trait predictive of reproductive success in this species (Boogert et al. 2011).
It may be that inhibitory control is subject to interference from other task-specific demands. If so, the performance of different species and individuals should vary across contexts depending on what other cognitive skills are recruited along with inhibitory control to solve a particular problem. Alternatively, given that animals rely on inhibitory control to produce adaptive outcomes across contexts, one might hypothesize that inhibitory control is a highly generalized mechanism that is relatively stable in species and even individuals across decision-making contexts. In the human literature, “context” refers to specific domains, which are often described as categories, such as emotion, exercise, relationships, work ethic, and health-related decisions (e.g., Tsukayama et al. 2011). Obviously, many of these domains are not directly applicable to animals. Another more relevant way to conceptualize these domains is as “functional equivalence classes,” or situations with similar constraints (Mischel 2004). Thus, contexts for animals can be operationalized along these lines, such as tasks that draw on social delay of gratification, overcoming perseverative responses, or making detours.
Much of the human literature supports the notion that inhibitory control in individuals is stable across contexts. In our own species, longitudinal studies have shown that a person's level of inhibitory control is tied to outcomes in numerous domains over the person's lifetime. For example, poor inhibitory control in childhood is correlated with lower grades, poorer social interactions, and lower SAT scores in later adolescence (Shoda et al. 1990). Low levels of inhibitory control in youth are also correlated with a multitude of adverse conditions beyond adolescence, including risky behaviors such as drunk driving, unprotected sex, crime, unemployment, and early mortality (Callender et al. 2010; Moffitt et al. 2011; Wiesner et al. 2010). The fact that childhood inhibitory control is predictive of such a wide variety of outcomes suggests that a single mechanism may influence decision-making in a wide variety of domains. Furthermore, multiple experiments have found that exerting inhibitory control in one context (e.g., by resisting the urge to eat a cookie) leads to impaired performance in a range of subsequent cognitive tasks that also require volition (e.g., persistance toward an unsolvable task; Baumeister et al. 1998, 2002; Gailliot et al. 2007). Interestingly, the converse appears to be true as well; training undertaken in a specific area of self-control, such as adhering to an exercise schedule (Oaten and Cheng 2006b) or a study regimen (Oaten and Cheng 2006a), leads to increased self-regulatory stamina in that area, as well as unrelated domains (e.g., emotional control, leaving dishes in the sink, missing appointments) and a laboratory test of self-control.
In contrast, other human research supports the possibility that inhibitory control is highly variable between contexts. Tsukayama et al. (2011) argued that while willpower itself might still be somewhat generalized, the demands being placed on it vary as a function of the temptation to execute a prepotent response, and this temptation can fluctuate greatly between contexts for different individuals. For example, people who self-identify as being tempted by food are more likely to be impulsive when it comes to dietary decisions, but not in other scenarios, such as alcohol or finance decisions (Tsukayama et al. 2011). While the model suggested by Tsukayama et al. (2011) attempts to reconcile an apparent contradiction by hypothesizing that willpower is generalized while individual temptations are specific to the context, there is still a healthy debate in the human literature. Researchers have presented evidence for both generalized inhibitory control resources (e.g., Baumeister et al. 1998; Duckworth and Seligman 2005) as well as stark intra-individual variances in inhibitory control depending upon context (Tsukayama and Duckworth 2010).
One way to test between a generalized executive control hypothesis and a context-specific hypothesis of inhibitory control is to turn to an animal that has not been influenced by cumulative culture, such as the domestic dog. In the past, a number of tasks have been used to assess inhibitory control in dogs, including a size learning and reversal task (Tapp et al. 2003), social and nonsocial versions of a reversal learning task (Wobber and Hare 2009), and the A-not-B paradigm (Topál et al. 2009). However, no studies have measured intra-individual variation in inhibitory control across different contexts. Such comparisons represent one way that the generality versus context specificity of inhibitory control in dogs can be studied.
To address this question, we exposed dogs to three inhibitory control paradigms, one of which had previously been used in this species. These three contextually different tasks tested the ability of pet dogs to resist a prepotent response in order to make a correct response. In the social task, dogs were required first to learn the reputations of a generous and stingy experimenter, and then to use this social information to inhibit approaching a preferred food reward when the stingy individual possessed it. In the A-not-B task, dogs were required to resist searching for food in a previously rewarded location after they witnessed the reward being moved from that location to another. Lastly, in the detour reaching task, dogs were required to inhibit approaching a desirable food reward directly, in favor of a slight detour response. If a dog's performance on any one task was related to performance on other tasks, this finding would support the executive control hypothesis. Conversely, if a dog's performance on the different tasks was unrelated, the context-specific hypothesis would be supported.
Across all three tasks, we also assessed whether there was a relationship between age and inhibitory control. The prefrontal cortex is thought to play a critical role in inhibitory control (Diamond 1990; Ridderinkhof et al. 2004), and there is evidence in the human literature that mental functions associated with the frontal lobe develop relatively slowly in childhood, but then also decrease with age later in life (Dempster 1992; West 1996). The inhibitory deficit hypothesis posits that distractibility and impaired memory, traits that are consistently found in older rather than younger adults, are in part due to an inability to inhibit irrelevant information (Hasher et al. 1991; Hasher and Zacks 1988; Alain and Woods 1999). Another study specifically linked these age-related cognitive deficits to a decreased functioning of the dopamine system in the prefrontal cortex (Braver et al. 2001). If dogs undergo a similar age-related cognitive decline, we would expect older dogs to make more inhibitory errors during these tasks compared to their younger adult counterparts. This prediction is consistent with previous dog research exploring the relationship between age and executive function (Tapp et al. 2003).
General methods
Recruitment and owner consent
Participating dogs were recruited through the Duke Canine Cognition Center (DCCC) Web site. Owners from the Raleigh-Durham-Chapel Hill, North Carolina area followed a link on the DCCC Web site to fill out a Dog Registration Questionnaire (http://bit.ly/AmWURq), and their information was then added to our database. The database was screened to remove all dogs with an owner-reported history of aggression or debilitating health problems, including any vision-related impairment such as cataracts. Dogs were then selected from the database, and their owners were contacted via email. Owners who were willing to participate brought their dogs for a 1-h-long session at the DCCC. In the majority of cases, the owner was not present in the testing room. However, if the dog was too nervous or distracted with the owner outside of the room, the owner sat in the room behind the dog and out of sight during trials. Some dogs had participated in up to two studies at the DCCC before, but all were naïve to the testing apparatus and procedures in these studies. Owners participated on a voluntary basis and were offered free parking. All dog owners signed informed-consent forms prior to participation. Testing procedures adhered to regulations set forth by the Duke Institutional Animal Care and Use Committee (IACUC # 303-11-12).
Statistical analysis
All data were analyzed using IBM SPSS Statistics 20. Because the data were not normally distributed (Shapiro–Wilk tests), nonparametric tests were used throughout. All tests were two-tailed.
Experiment 1: Social task
To test the role of context on dogs' inhibitory control, we first developed a social inhibitory test that involved experimenters continuously communicating, interacting with, and vocalizing toward the dog. It is clear from past studies that dogs' interactions with humans affect the way that they perform on tasks, as compared to when they are faced with the same task but in the complete absence of humans. For example, Agnetta et al. (2000) found that dogs will use a marker to find food once a human has interacted with it, but will not use a marker that has not been associated with a human. Furthermore, studies have shown that dogs use tone of voice as a cue (Scheider et al. 2011; Pettersson et al. 2011).
In this task, we gave dogs a series of choices to determine their baseline preference for larger or smaller amounts of food, therein determining which option they found most tempting. In the next phase, they interacted with a “stingy” experimenter who never shared her food and a “generous” experimenter who always shared her food. In a past social eavesdropping study looking at whether dogs could learn the reputations of a selfish and generous donor, the results indicated that 65–75 % of the dogs did approach the generous donor first, therefore seeming to base their choices on the reputation, while the remainder of the dogs either made an ambiguous approach or one to the selfish donor (Marshall-Pescini et al. 2011). In the final test phase of our experiment, we examined the dogs' ability to avoid the stingy experimenter, who was closer with the most tempting option, in favor of the generous experimenter, who was further away with a less valuable reward. To choose correctly, dogs had to inhibit approaching the proximately located food of greater value possessed by the stingy experimenter and choose the distantly located food of lesser value possessed by the generous experimenter.
Subjects
Forty-eight dogs came to the center to be tested, but 15 of these dogs were unable to finish the task. According to our predetermined abort criteria, a dog was excluded if it did not make a choice on four consecutive trials, did not make a choice on eight trials total at any point during the experiment, or if the dog did not eat food within 30 s when the food was placed directly in front of it. If any of these conditions were met, the session immediately ended. Dogs were unable to complete the task for a variety of reasons (see Online Resource 1). Three successful dogs were later excluded due to abort criteria in Experiment 2. Thus, thirty dogs, 15 male and 15 female dogs (mean age = 5.33 years ± 0.57; range 1–11 years), were included for analysis in this study. This study was conducted from July to November 2011. See Table 1 for a list of subjects' breeds, sexes, and ages.
Table 1. Dogs participating in Experiments 1, 2, and 3 (N = 30).
| Dog Name | Breed | Sex | Age (years) |
|---|---|---|---|
| Friday | Platt Hound | M | 7 |
| Dax | Labradoodle | M | 1 |
| Gus | Mixed: Tolling Retriever | M | 2 |
| Zen | Border Collie | M | 5 |
| Ellie | Mixed: Corgi/Spitz | F | 2 |
| Harbor | Golden Retriever | M | 4 |
| Morgan | Mixed: Greyhound/Lab | M | 11 |
| Cricket | Cairn Terrier | F | 3 |
| Diva | German Shepherd | F | 9 |
| Griffin | Golden Retriever | M | 5 |
| Ludo | Mixed: Shepherd/Lab | M | 2 |
| Poppy | Standard Poodle | M | 6 |
| Mazie | Labrador Retriever | F | 7 |
| Zephyr | Mixed: Border Collie | M | 3 |
| Ela | Mixed: Lab/Sheltie | F | 10 |
| Clara | Mixed: Basenji/Hound | F | 8 |
| Cassie | Mixed: Shepherd | F | 7 |
| Zoey | Mixed: Beagle/Lab | F | 1 |
| Lucky | Labrador Retriever | M | 7 |
| Bowie | Mixed: Basenji/Lab | M | 5 |
| Vespa | Springer Spaniel | F | 1 |
| Kali | German Shepherd | F | 9 |
| Rex | Mixed: Staffordshire | M | 8 |
| Murphy | Wheaton Terrier | M | 2 |
| Ginger | Mixed: Terrier | F | 3 |
| Tiger | Mixed: Pit/Lab/Shepherd | F | 9 |
| Buffy | Mixed: Samoyed/Shepherd | F | 6 |
| Bender | Border Collie | M | 1 |
| Bonnie | Australian Shepherd | F | 9 |
| Gertie | Mixed: Hound | F | 5 |
Procedure and design
Experimenters played one of three roles during this task: the dog-handler, the stingy experimenter, or the generous experimenter. The dog-handler positioned the dog appropriately throughout the trials, centering the leashed dog behind the start line at the beginning of every trial. The stingy experimenter remained aloof to the dog as soon as it entered the testing room, never feeding it, speaking to it, or giving it praise or attention. In contrast, the generous experimenter enthusiastically greeted the dog and praised it. The generous experimenter also conducted the pre-test value discrimination trials. The first author, who was present during every session, played the role of the generous experimenter for half of the dogs and the stingy experimenter for the other half. With the exception of one testing session in which the stingy experimenter role was filled by a male, all experimenters were female.
During every trial, dogs were presented with two paper plates mounted on wooden bases at a 45° angle so that each plate's contents were easily visible to the dog from its position at the start line. One plate was always located 1.4 m in front of the dog and 60 cm off center (the “proximal position”), and the other was placed approximately 2 m in front of the dog and 60 cm off center to the opposite side (“distal position”). Whether the proximal plate was placed on the dog's right or left was counterbalanced across trials within each session, and its location on the first trial was counter-balanced across subjects. Throughout the experiment, each plate contained either a “low-value reward” consisting of a lesser amount of food (1/2 Zuke's© dog treat OR 1 slice of Vienna Sausage1), a “high-value reward” consisting of a larger amount of food (1/2 Zuke's© dog treat, 2 pieces of cheese, 2 pieces of Vienna Sausage OR 2 pieces of cheese, 3 pieces of Vienna Sausage1), or no food (control trials). We used a 1:5 ratio (one piece of food as the low-value and five pieces of food as the high-value) because previous research has shown that dogs are most successful at discriminating between quantities when the ratio between the two amounts is small, but the numerical distance between the amounts is large (Ward and Smuts 2007). No attempts were made to control for odor cues, as there was no hiding of food during any of the trials. Rather, dogs were allowed full visual and olfactory access to all rewards in making their decisions. All sessions were video-recorded with a Sony DCR-SX65/S 4 GB Flash Memory Camcorder on a tripod.
Pre-test: value discrimination
Before the test began, dogs received an initial exposure trial in which the generous experimenter gave the dog a sample of all the foods to be used throughout the experiment (cheese, Vienna Sausage, and/or Zuke's© Mini Naturals dog treats) to ensure that the dog willingly ate all of these items. Following this trial, we evaluated dogs' preferences for the high-value versus the low-value rewards across 10 trials. At the start of the trial, the dog-handler centered the dog behind the start line. The generous experimenter approached the dog in a rolling chair, allowed the dog to inspect two plates (one contained the high-value reward and the other contained the low-value reward), and then moved the plates to their respective locations. The plate with the high-value reward was placed in the proximal location, and the plate containing the low-value reward was placed in the distal location. Whether the proximal plate was placed on the dog's right or left was counterbalanced across trials within each session, and its location on the first trial was counterbalanced across subjects (Fig. 1a). The experimenter then moved to the back of the room, faced the wall, and issued a release command (either “go get it” or “OK”) while looking straight ahead. At this point, the dog-handler released the leash and remained behind the dog. In a single familiarization trial, the dog was allowed to make two choices, approaching and eating from both plates, in order to acquaint the dog with the task and show that both plates contained food. In the subsequent 10 food discrimination trials, the dog was only allowed to approach and eat from one plate. As soon as the dog made a choice, defined as consuming one of the rewards, the experimenter removed the other plate and the dog-handler returned the dog to the start line.
Fig. 1.
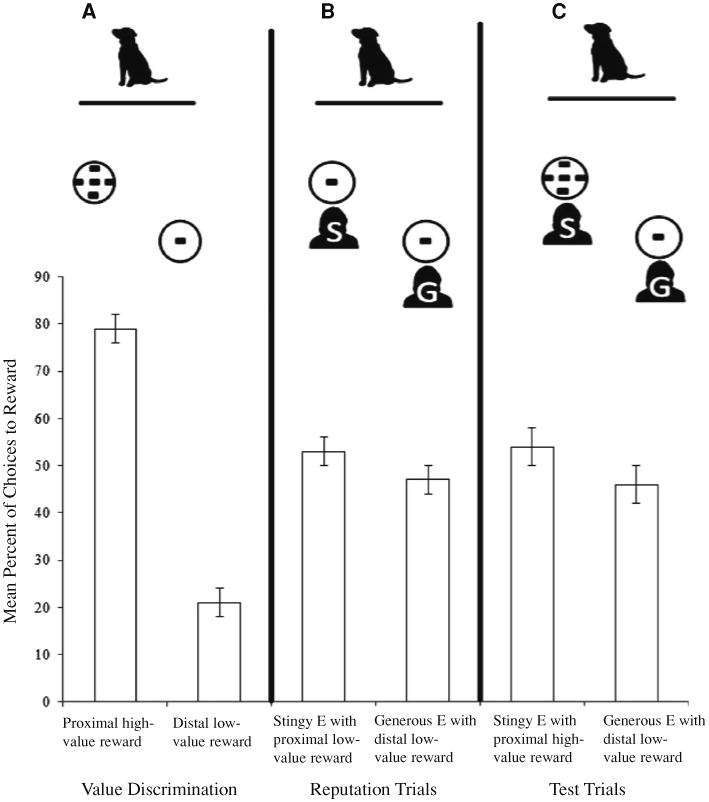
The setup and mean percentage of choices to either option for the three phases of Experiment 1. a Overall value discrimination. b Reputation trials—Stingy experimenter (indicated by ‘S’) was always closest to the dog, while generous experiment (indicated by ‘G’) was always farthest. c Inhibitory control test trials. Error bars represent the standard error of the means
Reputation formation
The next 20 trials served to expose the dog to the generous and stingy experimenters' reputations. Both experimenters sat on rolling chairs, each holding a plate containing the low-value reward. At the start of each trial, both experimenters sat equidistant from the dog (2.5 m) looking at the subject's face and spoke to the dog according to the following script. The generous experimenter said, “You can have my food,” in a high-pitched, inviting tone, whereas the stingy experimenter said, “You can't have my food,” in a stern, low-pitched tone. The experimenters vocalized in this way because, across mammals, high-pitched tones tend to be associated with affiliative behaviors, whereas low-pitched tones are associated with threatening behaviors (Morton 1977). Further, in at least two studies, dogs primarily relied on tone of voice to discern an experimenter's cooperative intent, correctly interpreting her communicative cues when the experimenter spoke in a high-pitched voice and ignoring them when the experimenter spoke in a low, forbidding tone (Pettersson et al. 2011; Scheider et al. 2011). The stingy experimenter then broke eye contact with the dog for the duration of the trial and instead looked at her feet, while the generous experimenter maintained friendly eye-contact throughout. The experimenter to the dog's right always vocalized first, followed immediately by the experimenter to the dog's left. Because the left/right locations of the stingy and generous experimenter were counterbalanced across trials, half of the time the generous experimenter spoke first and the other half of the time the stingy experimenter spoke first. The location of the stingy and generous experimenters was also counterbalanced on the first reputation trial across subjects.
Both experimenters then approached the dog and simultaneously presented the plates as before, though now each of the plates contained the low-value reward. The experimenters then moved to the designated locations, holding the plates on the floor in front of them. The stingy experimenter was always at the proximal location, while the generous experimenter was always at the distal location. The dog-handler then let the leash go slack, while the dog was allowed up to 30 s to choose between the plates held by the two experimenters.
For the first 10 trials, the dog was allowed to make two choices in order to facilitate reputation learning. A choice was defined as the dog's snout crossing a marked 30.5 × 30.5 cm perimeter around the plate. If the dog approached the generous experimenter's plate, she allowed the dog to eat the food and briefly praised and petted it. If the dog approached the stingy experimenter's plate, this experimenter pulled the plate of food away, preventing the dog from feeding, and turned to face the back of the room. Following their initial choice, dogs were given 15 s to make an additional choice. If the dog first approached the stingy experimenter, the generous experimenter waited a few seconds to see whether the dog would approach on its own, and then called the dog over to retrieve food off of the plate, at this point praising and petting the dog. If the dog first approached the generous experimenter, the stingy experimenter remained stationed behind her plate, either for the next 15 s or until the dog approached her, but did not actively call the dog. If the dog did not make an initial choice after 15 s, the trial was repeated.
In the following 10 trials, the dog was only allowed to make one choice in order to impose a higher cost for choosing the stingy experimenter. After the dog made its choice, the dog-handler immediately walked the dog back to the start line before it had the opportunity to make a second choice. The contingencies for choosing the generous and stingy experimenters were the same as in the preceding trials (i.e., the stingy experimenter withheld food, while the generous experimenter provided food).
Inhibitory control test
The next 20 trials consisted of 10 trials that tested the dog's inhibitory control and 10 control trials to validate other aspects of the procedure (see below). The order of these trial types was counterbalanced across the session, with no more than two trials of each type presented consecutively. The procedure was identical to the one-choice reputation trials, except that the amount and quality of food on the stingy and generous experimenters' plates differed by condition. As before, the side of the room on which the experimenters started each trial was counterbalanced within the session and across dogs, and in all trials, the generous experimenter again positioned herself at the distal location, while the stingy experimenter was always closest to the dog.
Test trials
The generous experimenter presented the low-value reward on her plate, while the stingy experimenter presented the high-value reward. Therefore, the food discrimination was identical to that from the pre-test value discrimination trials, but the high-value food was rendered unobtainable because it was possessed by the stingy experimenter (Fig. 1c).
Control trials
These trials were identical to the test trials except that the stingy experimenter offered no food on her plate. Thus, the inhibitory demand of bypassing the stingy experimenter's plate was minimized in these trials compared to the test trials.
Scoring and analysis
In the pre-test, a choice was defined as a dog approaching a plate and consuming the food. In the reputation and test trials, we used a different criterion because dogs were only allowed to eat one of the two options, so a choice was defined as the dog's snout crossing a marked 30.5 × 30.5 cm perimeter around the plate. A choice to the generous experimenter's plate led to the dog consuming the reward, whereas a choice to the stingy experimenter's plate led to the experimenter immediately removing the plate before the dog could consume the reward. Twenty percent of trials were coded by a second individual, and agreement was perfect on the value discrimination and reputation trials. For test trials, interrater agreement was excellent, with a kappa of 0.982. In cases of disagreement, the original live coding was used.
To test the hypothesis that dogs would exhibit some level of inhibitory control in a socially mediated scenario, we compared dogs' choices to the distal plate containing the low-value reward in the pre-test trials, when there was no cost associated with the closer high-value reward, to their choices to that same plate in test trials, when the high-value reward became unattainable. Additionally, in order to test the hypothesis that our manipulation presented at least an initial inhibitory challenge to dogs, we compared dogs' choices to the generous experimenter on the last five reputation trials to their choices to the generous experimenter on the first five test trials.
Results
Pre-test: value discrimination
In the food preference trials, 27 out of 30 dogs chose the proximal plate containing the high-value reward on the first trial. Throughout the pre-test, dogs chose this plate on the vast majority of trials (mean = 79 ± 3 %, Fig. 1a). Twenty-seven of the 30 dogs chose the high-value reward more frequently than the low-value reward (when all analyses were rerun excluding the three dogs that showed no preference, all results were the same; See Online Resource 2), and no dogs chose the low-value reward more frequently than the high-value reward (Wilcoxon signed-ranks test: Z = −0.767, N = 30, P < 0.001). Thus, dogs exhibited a robust preference for the high-value reward during this phase of the experiment. These results also served as a baseline for each dog in terms of which choice they found most desirable when both choices were obtainable.
Reputation formation
In the reputation trials, 15 out of 30 dogs chose the generous experimenter on the first trial. To determine whether dogs approached the generous experimenter (associated with the distal plate) with increasing frequency across the reputation trials, we assessed the percent of dogs choosing the generous experimenter as a function of trial number. A Spearman's Rank-Order Correlation revealed a positive but non-significant correlation between trial number and the percent of dogs choosing the generous experimenter (rs(18) = 0.365, P = 0.114).
We also evaluated whether dogs chose the generous experimenter at different frequencies in the first versus the second half of reputation trials. Although dogs approached the generous experimenter more often in the second half of trials, this difference was not significant (mean 1st half: 44 ± 4 %; mean 2nd half: 50 ± 4 %; Wilcoxon signed-ranks test: Z = −1.767, N = 30, P = 0.077).
Inhibitory control test
Because dogs are sensitive to distance when making choices, we did not compare their performance to chance. Dogs tend to naturally approach food that is closer to them than farther (see discussion in Hare et al. 1998). In the current test, this bias is strengthened since the larger amount of food is always closest. Therefore, we used subjects' choices in the value discrimination as a baseline instead of using 50 % as chance. Specifically, we compared performance between the two conditions in which the most desirable choice was near to the dog and A) was obtainable (value discrimination) or B) was not obtainable (test). If dogs were able to inhibit the desire to approach the stingy experimenter even when she presented the high-value reward, we expected that they would approach the low-value reward significantly more often in test trials than during the baseline value discrimination trials. To test this hypothesis, we evaluated whether dogs chose the distal plate, which always contained the low-value reward, more frequently in the 10 test trials (when the more desirable closer plate was unobtainable) than the 10 value discrimination trials (when the more desirable closer plate was obtainable). This analysis revealed a significant difference between conditions (Wilcoxon signed-ranks test: Z = −3.800, N = 30, P < 0.001, Fig. 2) with dogs choosing the low-value reward more frequently in the test trials (46 ± 4 %, Fig. 1c) than in the initial value discrimination (21 ± 3 %, Fig. 1a). These results confirmed a deviation from baseline performance, with the majority of dogs showing a change of behavior in the predicted direction (mean difference between conditions = 25 %).
Fig. 2.
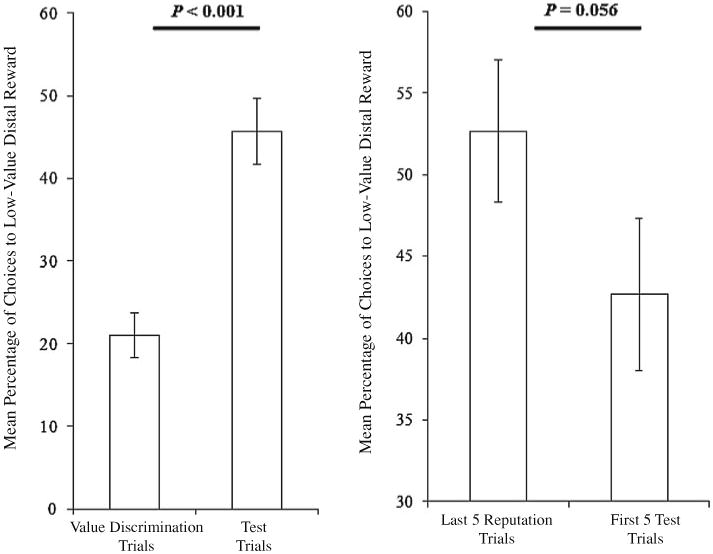
Left The mean percent of trials that dogs choose the low-value distal reward on value discrimination trials compared to inhibitory control test trials of Experiment 1. Right The percentage of choices to the low-value distal reward always associated with the “generous” experimenter in the last five reputation formation trials and the first five test trials of Experiment 1. Please note that the scales on the y-axes differ across panels. Error bars represent the standard error of the means
To evaluate whether the experimental manipulation of endowing the stingy experimenter with a more desirable reward affected dogs' behavior, we compared the last 5 trials from the reputation phase (both experimenters had equal amounts of food) to the first 5 test trials (stingy experimenter possessed the high-value reward). We predicted that if this manipulation made the task more difficult for dogs, subjects would exhibit a decreased tendency to approach the generous experimenter (correct choice) once the stingy experimenter offered the high-value reward. The results of this analysis were not significant, but did indicate a trend in the predicted direction (Wilcoxon signed-ranks test: Z = −1.909, N = 30, P = 0.056) in which dogs tended to approach the generous experimenter less frequently in the first 5 test trials (43 ± 5 %) than in the last 5 reputation trials (53 ± 4 %) (Fig. 2). On the first test trial, 14 out of 30 dogs chose the generous experimenter with the low-value reward.
As a measure of inhibitory control for individual subjects, we assigned each dog a difference score, calculated as the percent of trials that the dog chose the generous experimenter (distal location, low-value reward) in the test trials minus the percent of trials that the dog chose the low-value reward (distal location) in the value discrimination trials. Accordingly, this difference score measured how an individual dog's performance in the test deviated from its performance in the food preference trials, with each dog serving as its own baseline. A positive score indicated that a dog demonstrated inhibitory control by choosing the high-value reward less often during test trials, when it was unobtainable, than during the initial value discrimination. A negative score indicated that a dog demonstrated lower levels of inhibitory control by choosing the preferred food more often during test trials, despite it being unobtainable, than during food preference trials. These scores ranged from −30 to 70 (mean = 25 ± 5), indicating a range of individual differences in inhibitory control.
Finally, to examine whether dogs were able to bypass the stingy experimenter's plate on control trials when she presented no food, we examined whether dogs showed greater preference for the generous experimenter and the low-value reward during (a) the 10 control trials, when the stingy experimenter possessed no food, or (b) the 10 test trials, when the stingy experimenter possessed the high-value reward. This analysis indicated a significant difference (Wilcoxon signed-ranks test: Z = −2.747, N = 30, P < 0.01), showing that dogs approached the generous experimenter more often when the stingy experimenter possessed no reward (mean = 55 ± 4 %) than when the stingy experimenter possessed the high-value reward (mean = 46 ± 4 %).
There was no effect of age or sex on the overall number of test trials that dogs went to the generous experimenter (rs(28) = −0.07, P = 0.724; Mann–Whitney Test: U = 98.5, P = 0.56, r2 = 0.01) or on the social difference score (rs(28) = −0.18, P = 0.354; Mann–Whitney Test: U = 93.0, P = 0.414, r2 = 0.02).
Discussion
Despite some individual variation, we found that dogs as a group discriminated between food quantities, preferring the high-value reward during pre-test value discrimination trials. This result echoes past studies in which dogs have shown some numerical competence by discriminating between larger and smaller quantities of food and the group sizes of conspecifics (Ward and Smuts 2007; Bonanni et al. 2011). We then evaluated whether dogs could bypass the preferred food reward when it was unobtainable (because it was possessed by the stingy experimenter) in favor of a lower-value reward that was obtainable (because it was possessed by the generous experimenter). Overall, dogs approached the lower-value reward offered by the generous experimenter significantly more during test trials than during the initial value discrimination trials when they were allowed to consume either the low- or high-value reward. These results illustrate that dogs learned to distinguish between the generous and stingy experimenters and also exerted some degree of inhibitory control during the test. However, dogs found it difficult to bypass the stingy experimenter when she possessed the high-value reward, indicating that subjects were sensitive to the experimental manipulation of reward values between the different trial types.
It is interesting and surprising that during control trials when the stingy experimenter's plate contained no reward, dogs still approached it at relatively high rates (albeit significantly less than during test trials, when it contained the high-value reward). This high rate of choosing the empty plate in control trials may have occurred for a number of reasons. First, dogs may have made a perceptual error, and simply not realized until approaching this plate that it was empty. Second, the error could be due to a perseverative search strategy, and thus, evidence for an inhibitory control problem in that some dogs may have been unable to inhibit a habit of searching at the nearest location. This possibility is supported by other studies showing that dogs are highly susceptible to inflexible search patterns once a habitual response has been established (Kaminski et al. 2008; Osthaus et al. 2010).
In this study, we found some evidence for inhibitory control within a social context—that is, a context in which dogs were receiving communicative signals throughout the entire experiment, including during the choice phase of each reputation and test trial. To determine whether a dog's level of inhibitory control differs depending on context or remains constant despite being tested under different circumstances, we conducted a nonsocial task that examined inhibitory control in the same dogs. The task was still conducted by a human experimenter, but social interaction was no longer a main component of the task.
Experiment 2: A-not-B task
While Experiment 1 explicitly placed dogs in a social context, the second experiment minimized social interaction and instead used a variant of the A-not-B task (Piaget 1954) to test whether subjects could inhibit searching in a previously rewarded location after witnessing the reward moved from this location to a novel hiding place. Topál et al. (2009) used a comparable paradigm in a previous study and found that dogs reached high levels of success when tested in a non-communicative or nonsocial context, when an experimenter either had her back to the dog or was out of view of the dog. In contrast, their social-communicative condition, which involved the experimenter shifting her gaze back and forth between the target and the dog at each bucket, led to significantly more search errors. A follow-up study by Kis et al. (2012) re-emphasized the role of dogs' sensitivity to human communication, finding that dogs still made the error when they were not given the opportunity to actively search and be rewarded at location A during the familiarization trials. In this experiment, we used a procedure similar to Topál's nonsocial and non-communicative conditions, in which the dog always made choices while the experimenter's back was turned. This methodology ensured that the dog solved the task in the absence of any social cues relevant to the problem.
Methods
Subjects
The 33 dogs that successfully completed Experiment 1 were contacted by email and invited back to participate in a follow-up test session. These studies were conducted from July to December 2011. Three dogs that successfully completed Experiment 1 did not complete this experiment because their owners did not respond to the invitation to return (two dogs) or the dog was fearful of the apparatus (one dog).
Apparatus
In this task, three buckets were placed in an array, each 2.1 m from the dog and 1.2 m apart from each other. Which of these buckets (either left or right) was baited in test trials was counterbalanced between dogs. The food rewards used were either Zuke's© Mini Naturals or Real Meat® Jerky. As in Experiment 1, all sessions were video-recorded.
Procedure and design
Prior to beginning the task, all dogs participated in a short warm-up that served to familiarize them with finding food in the buckets. In these trials, the experimenter held a dog treat between her thumb and forefinger, approached the dog, called the dog's name, and showed the dog the treat. The experimenter then walked to one of the buckets at the end of the array, placed the treat in the bucket, walked to the back of the room, and faced the wall. The experimenter then issued the release command (either “OK” or “Go get it”) to indicate the dog-handler should drop the dog's leash. This process was repeated at each of the three buckets until the dog correctly retrieved the treat from each of them on its first choice.
Next, the experimenter began the familiarization trials in which dogs repeatedly experienced locating the food in one of the buckets at the end of the array. The procedure was identical to the warm-up trials, except that the experimenter placed the treat in the same bucket (either at the left or right end of the array) for three consecutive trials. If the dog successfully retrieved the treat from that bucket on all of these trials, it continued on to a test trial. If it failed to approach the baited bucket first in one or more of the three trials, the experimenter finished the set of trials and began a new set of familiarization trials immediately afterward. Once subjects had correctly selected the baited bucket in all three familiarization trials, a test trial was conducted.
In test trials, the experimenter stood in front of the dog holding the treat, called the dog's name, and then walked to the same bucket that had been baited during the familiarization trials and visibly placed the treat inside this bucket. The experimenter then stood behind this bucket for approximately 1 s before reaching back into the bucket and visibly removing the treat. The experimenter then walked behind the array of buckets with the treat in plain view of the dog and placed the treat in the bucket on the other end of the array. The experimenter then walked to the back of the room, faced the wall, and gave the release command, indicating for the handler to release the dog's leash. The first location that the dog searched was recorded as the dependent measure. Each dog participated in two complete sets of familiarization trials which were each followed by a test trial, resulting in two test trials per dog. The location of which bucket was baited was consistent within dogs. When dogs were making their choices and the treat was concealed within a bucket, no attempts were made to control for odor cues. However, many past studies have repeatedly ruled out the possibility that dogs rely on olfactory cues in these testing scenarios—rather, dogs have been shown time and again to perform at chance levels in control trials, where food is surreptitiously hidden and no cues are given (e.g., Miklósi et al. 1998; Hare and Tomasello 1999; Ittyerah and Gaunet 2009; Riedel et al. 2008; see Hare and Woods 2013 for review).
Scoring and analysis
A choice was defined as the dog's snout crossing the edge of the bucket. Twenty percent of trials were coded by a second individual, and agreement was perfect. In order to test whether dogs were exhibiting inhibitory control, we compared how often they went to the correct bucket to chance expectation (0.33).
Results
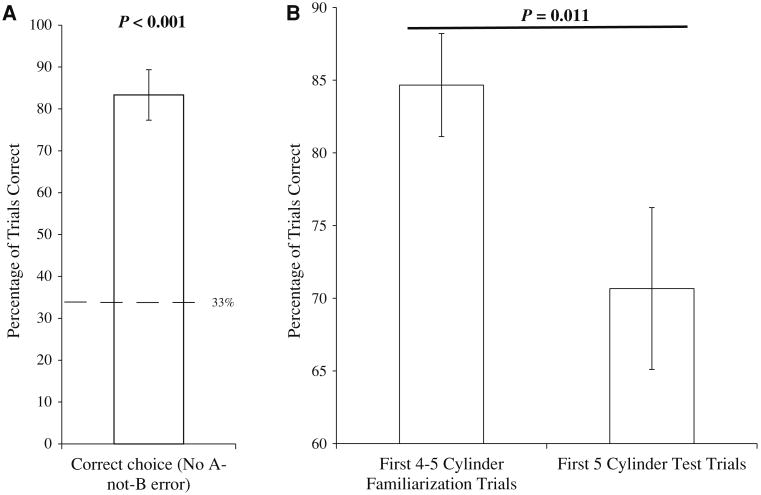
Twenty-four of 30 dogs did not commit the A-not-B error on the first trial and instead chose the correct bucket containing the treat. Furthermore, dogs chose successfully on the vast majority of test trials (mean = 83 ± 6 %), and their performance was significantly better than chance (Wilcoxon signed-ranks test: Z = −4.671, N = 30, P < 0.001, Fig. 3a). Thus, while some dogs did commit the A-not-B error, most did not.
Fig. 3.
a Mean percent correct choices for the A-not-B task in Experiment 2. The dashed line represents chance performance. b The mean percent of trials that dogs made the correct detour on the first five familiarization trials compared to the first five test trials in Experiment 3. In both graphs, error bars represent the standard error of the means
There was no effect of age (rs(28) = 0.151, P = 0.426) or sex (Mann–Whitney Test: U = 94.0, P = 0.299, r2 = 0.04) on how often dogs chose correctly during test trials.
Discussion
The results indicate that this variant of the A-not-B task was relatively easy for most dogs, and very few exhibited the classic perseverance error. While the mean of correct choices was 83 %, the median was 100 %, indicating that the majority of dogs performed perfectly. Our results differ from a past study that, using different methods, reported 29 of 30 dogs made perseveration errors in a detour task (Osthaus et al. 2010). Despite the fact that Topál et al. (2009) more explicitly removed the human cues in their nonsocial condition (the experimenter was invisible to the dog in their version but not in ours), our results were the same: In both cases, dogs made the error in only 17 % of all trials. Dogs performed substantially better on both Topál et al's. (2009) and our version of the task. The contrasting results might have arisen from one of the main differences between the two studies: The Osthaus et al. (2009) study did not familiarize the dogs with each of the response options before the test, whereas our A-not-B paradigm and Topál's both included an opportunity for the animals to practice each of the possible responses. Furthermore, the food was always visible during the Osthaus task, as compared to our task where the food was temporarily out of sight. Perhaps the continued presence of food was over-arousing for the dogs, causing them to experience initial dips in inhibitory control.
Experiment 2 provided evidence that dogs exhibit inhibitory control in at least one nonsocial context. In the third and final experiment, the same dogs were tested in a different nonsocial context with the goal of comparing the same dogs' performance across all three tasks. Determining whether or not dogs' scores were correlated across all three tasks allows us to test between the executive control hypothesis and the context-specific hypothesis.
Experiment 3: Cylinder task
The third task was a detour reaching task modeled after other studies of inhibitory control (Boogert et al. 2011; Santos et al. 1999; Vlamings et al. 2010; Diamond 1990). In some of the past studies, researchers placed a reward inside of a transparent Plexiglas box and then varied which side of the box was open. Both children (Diamond 1981) and capuchin monkeys (Santos et al. 1999) experienced difficulty performing a detour reaching response when the opening was on the side, as they could not inhibit their desire to reach straight for the reward. A similar task in song sparrows revealed that they also were prone to incorrectly peck at the front of a transparent barrier when a mealworm was visible, rather than obtaining the food through a side opening (Boogert et al. 2011).
Methods
Subjects
The same 30 dogs from Experiments 1 and 2 participated, and this task was conducted immediately following the conclusion of the dog's participation in Experiment 2.
Apparatus
The apparatus consisted of a cylinder (22 cm in length × 20 cm in diameter) that was open on both sides and attached to a wooden base for support. In familiarization trials, this cylinder was opaque, while in test trials, it was transparent. The side from which the experimenter baited the cylinder was counterbalanced across dogs. The treat was always placed in the middle of the tube, making it accessible to the dog via either side. All sessions were video-recorded.
Procedure and design
The experiment began with a series of familiarization trials to acquaint dogs with the solution to the task (Santos et al. 1999). The handler positioned the dog 1 m from the apparatus, facing the opaque cylinder. The experimenter sat behind the cylinder apparatus, showed the dog a treat, said “look,” placed the treat into the tube, said “look” again, removed her hand, and then said “OK,” indicating that the dog-handler should release the dog. After dropping the leash, the handler remained behind the start line. On each trial, the experimenter recorded whether the dog first made a correct or incorrect choice. In order to move on to the test trials, the dog was required to make a correct first choice in four of five consecutive familiarization trials. No attempts were made to control for odor cues throughout the task. The familiarization trials were designed to introduce dogs to the solution to the task without the critical manipulation of making the food visible at the time of the dog's choice.
The 10 test trials were identical to the familiarization trials except that the opaque cylinder was replaced with the transparent cylinder. Thus, in test trials, the dogs could see the food through the cylinder, introducing a competition between the correct motor response (established during the familiarization trials) and visual input (which could lead the dog to approach the food directly, bumping into the front of the cylinder). Even if the barrier was not visible, it was immediately perceivable through tactile feedback, and the challenge was to adjust behavior flexibly in response. As in familiarization trials, the experimenter recorded whether the dog made a correct or incorrect choice on each trial, using the same criteria. Trials in which the response was not clear were subsequently coded by video by the first author.
Scoring and analysis
A choice was coded as “correct” if the dog's snout entered the open end of the cylinder without the dog first touching the exterior of the cylinder with any part of his or her head or paw. Conversely, a choice was coded as “incorrect” if the dog touched the front or back of the cylinder with its snout or paw prior to finding the treat. Twenty percent of trials were coded by a second individual, and interrater reliability was excellent (Pearson's R = 0.941, n = 6, P = 0.005). To test the hypothesis that test trials would present more of an inhibitory challenge for dogs than the familiarization trials, we compared the percentage of correct responses on the first 5 familiarization trials to the percentage of correct responses on the first 5 test trials. In the cases where dogs met the familiarization criterion in only 4 trials, their familiarization score of 100 % was based on only those 4 trials.
Finally, to test between the two competing hypotheses about the nature of dogs' inhibitory control, we performed correlations between the success measures of the social, A-not-B, and cylinder tasks to assess whether scores on these tasks were related.
Results
Fourteen of the 30 dogs required more than the four mandatory familiarization trials, and of those dogs who did, there was a large range of total familiarization trials (from 5 to 11). There was no effect of age (rs(28) = 0.043, P = 0.821) or sex (Mann–Whitney Test: U = 100, P = 0.585, r2 = 0.0099) on number of familiarization trials completed. There was an effect of number of familiarization trials on dogs' test performance. A Spearman's Rank-Order Correlation revealed a significant negative correlation between number of familiarization trials and the number of correct test trials (rs(28) = −0.398, P = 0.029). Thus, the lower the number of familiarization trials required, the more test trials that the dogs subsequently succeeded on.
On average, dogs made correct choices on 70.3 ± 5 % of test trials. As we would predict if the inhibitory control manipulation affected dogs' response strategies, the percentage of correct responses during the first 5 familiarization trials was significantly greater than the percentage of correct responses during the first 5 test trials (mean familiarization trials: 85 ± 4 %; mean first 5 test trials: 71 ± 6 %; Wilcoxon signed-ranks test: Z = −2.552, N = 30, P = 0.011, Fig. 3b). In order to analyze whether learning occurred over the course of the 10 test trials, we compared dogs' choices on the first 5 trials to their choices on the last 5 trials. There was no significant difference between the first and second half of the session (Wilcoxon signed-ranks test: Z = −1.090, N = 30, P = 0.276).
There was an effect of age on dogs' performance. A Spearman's Rank-Order Correlation revealed a significant negative correlation between age and the percent of correct responses on test trials (rs(28) = −0.453, P = 0.012). Thus, older dogs were more likely to exhibit a lack of inhibitory control, attempting to retrieve the food through the front of the apparatus more frequently than younger dogs. There was no effect of sex on the number of correct responses that dogs made during test trials (Mann–Whitney Test: U = 102.0, P = 0.654, r2 = 0.010).
To evaluate whether inhibitory control was stable within individuals across tasks, we explored the correlations between the primary dependent measures from Experiments 1–3. A Spearman's Rank-Order Correlation revealed no correlation between any of the tasks (Table 2). Thus, there were no stable individual differences in performance across the three inhibitory control tasks.
Table 2. Scores on the A-not-B, cylinder, and social tasks were not correlated.
| Tasks | 1 | 2 | 3 |
|---|---|---|---|
| 1. Social task | – | ||
| 2. A-not-B task | 0.035 P = 0.855 |
– | |
| 3. Cylinder task | −0.056 P = 0.767 |
0.111 P = 0.559 |
– |
Discussion
Overall, dogs were able to inhibit their desire to approach the visible food directly on the majority of trials in the cylinder task. However, in some cases, dogs found the visual stimulus to be overpowering, despite tactile feedback from the barrier. A similar effect has been documented in an object retrieval task in human infants (Diamond 1981). This study confirmed perseverative reaching errors to be a failure of inhibitory control. The same infants who could successfully reach up and into an opaque topless box to retrieve a centrally located toy experienced difficulty when the box was transparent (Diamond 1981). Their tendency became to reach straight ahead, in a direct line to the toy, despite tactile resistance from the side of the Plexiglas box. Our task similarly illustrates that animals that make errors do not lack the mechanical knowledge required to solve the task, because they successfully execute this response in warm-up trials with an opaque cylinder. Rather, the errors during test trials appear to be driven by a failure of inhibitory control.
Dogs who took more familiarization trials initially tended to perform worse on test trials, while those who required minimal familiarization trials tended to perform better on test trials. There are a few possible interpretations of these results. It could be that dogs with less inhibitory control rush into the task and are more likely to make contact with the front of the cylinder even during familiarization trials. Also, it could be that poor inhibitory control is associated with taking longer to learn the solution to the problem, which was the primary purpose of the familiarization trials.
Dogs, who typically begin to reach “old age” around 8 years old (e.g., Adams et al. 2000), have been proposed as an appropriate model of human cognitive aging (Studzinski et al. 2005). Both species begin to show decline of executive functioning at advanced ages, although it is variable among individuals (Studzinski et al. 2005). In our task, we found that age was negatively correlated with performance. This finding parallels the results of Tapp et al. (2003), who showed that older dogs performed worse on reversal learning tasks, as well as studies of humans demonstrating age-related decline in executive function (Hasher and Zacks 1988). One possible explanation for these results is that older dogs showed less capacity for inhibitory control due to age-related decline in the function of the prefrontal cortex (West 1996), a region of the brain that is critical for inhibitory control (Diamond and Goldman-Rakic 1989). In the future, it would be informative to test puppies in this paradigm, given that we did not test any dogs younger than 1 year of age in the current study. If the human pattern of age-related development and decline of executive function is paralleled in dogs, we would predict a parabolic function, with the youngest and oldest dogs exhibiting the lowest levels of inhibitory control (Dempster 1992).
General discussion
Across three studies, we explored dogs' abilities to solve a range of problems requiring inhibitory control. Our results support the hypothesis that inhibitory control is context dependent, as dogs' scores were not correlated between the social, A-not-B, and cylinder tasks. On the one hand, this finding is somewhat surprising. Because inhibitory control has been linked with such a wide array of correlated outcomes in our own species, one might expect it to be a highly generalized skill. However, there is also evidence from humans indicating that cognitive skills for self-control can vary greatly between contexts (Tsukayama et al. 2011).
It is worth noting that there was a possible ceiling effect in Experiment 2, with only six dogs committing the A-not-B error on the first trial. The outstanding performance of almost all of the dogs may have limited our ability to detect a correlation between this task and the other two tasks. Future research might be able to address this problem by using a social version of the task, as Topál et al. (2009) found that version led to more variation in behavior. However, scores were far more variable in Experiments 1 and 3 and were not correlated across subjects.
Taken together, the lack of correlation between the three different experiments can be interpreted in two possible ways. First, it may be that the inhibitory control mechanism itself differs between contexts. For example, avoiding a tempting but unobtainable reward in a social context may draw on different cognitive resources than those required to solve a physical problem necessitating a detour reach. Second, the inhibitory control mechanism may be stable within an individual, but other task demands (e.g., quantity discrimination, reputation-like inferences, learning, physical problem solving) may also greatly influence performance differently across individuals and tasks. Consequently, individual differences in skills relevant to other task demands may interact with skills for inhibitory control, yielding large intra-individual differences between contexts. Further studies should be conducted to determine whether the negative results were specific to the tasks we used in order to rigorously test the hypothesis of context-specific inhibitory control.
Another complementary way to test the hypothesis would be to ask whether dogs can become better at inhibitory control with practice, and if such training would be task-specific or translate across multiple contexts. Studies in humans have shown that subjects are capable of improved inhibitory control following a practice period—not by exerting a higher baseline level of inhibitory control, but rather by becoming more impervious to inhibitory control fatigue (Oaten and Cheng 2006a, b). In humans, training in just one area led to improvement that could be observed across multiple domains. Future research should strive to develop an inhibitory control training program for dogs and identify which tasks and contexts are affected.
Our design controlled for redundancy in contexts, instead using tasks that drew on dogs' social and physical cognition. As a result of comparing different contexts, each of the inhibitory control tasks necessarily had diverse requirements that may have contributed to the individual variance in performance. In the social task, dogs' performance was contingent on their ability to make reputation-like judgments, an ability that has been demonstrated by some dogs, but not all (Marshall-Pescini et al. 2011). Furthermore, our main manipulation in the social task was intended to tempt the dog by providing a high-value food reward. However, just as Tsukayama et al. (2011) found certain domains to be more tempting for some humans, it could be that some of our dog subjects were more motivated by food, while others were more motivated by toys or praise. In this case, primarily food-motivated dogs would have faced difficulty bypassing the high-value food reward, while socially motivated dogs may have done so easily, instead focusing on engaging in a positive social interaction with the generous experimenter. The A-not-B task required object permanence, as the dogs had to realize that once food disappeared into the bucket, it had not disappeared from the room. The cylinder task necessitated an understanding of physical properties, such as the solidity principle (Kundey et al. 2010).
These diverse requirements might have been part of the reason why we found a correlation between performance and age in the cylinder task, but not the other two tasks. Assuming that old age is correlated with worse performance due to a deficit in inhibitory control, it could be that the cylinder task was the most valid measure of inhibitory control. In other words, when performing the cylinder task dogs faced the least amount of interference from other cognitive demands that remain constant with age and appear to play a larger role in the other tasks (i.e., food motivation or object permanence). An alternative though less likely hypothesis is also possible: Perhaps the age-related decline in performance was not related to inhibitory control, but a deterioration of the other cognitive demands that were unique to the cylinder task (i.e., solidity principle).
There are many factors affecting inhibitory control in dogs that remain to be explored. For example, one purposeful way that humans overcome constraints of inhibitory control is through the use of certain mental devices, such as pre-commitment strategies (Ariely and Wertenbroch 2002) and psychological distance (Mischel and Rodriguez 1993). There is evidence that even chimpanzees might be capable of using self-distraction and future planning in order to overcome impulsive tendencies (Osvath and Osvath 2008; Evans and Beran 2007). An empirical question is whether dogs are similarly capable of employing spatial strategies (purposefully placing distance between themselves and the reward), temporal strategies (waiting before completing a task), or self-distraction strategies (using diverting behaviors, such as sniffing around, walking in circles, or playing with a provided toy) to overcome impulsive tendencies. One promising approach for future research will be to explore whether certain dogs use such strategies to compensate for inhibitory weaknesses, utilizing past experiences to productively alter their actions in the future.
Recent research points to potential parallels in dogs and humans by illustrating that the same biological mechanism may regulate inhibitory control in both species. In one study, dogs who exerted self-control by remaining in a sit position did not persevere as long at a subsequent unsolvable task compared to dogs who had not previously exerted self-control (Miller et al. 2010). This difference between the two groups disappeared when the first group of dogs was given a drink containing glucose before being presented with the unsolvable task (Miller et al. 2010). The proposed explanation was that dogs that performed an obedience task had depleted inhibitory control, similar to when humans are forced to make an extended series of choices and subsequently suffer from decision fatigue (Baumeister 2002; Baumeister et al. 2007). A similar phenomenon was demonstrated in humans (Gailliot et al. 2007). Gailliot et al. (2007) proposed that exerting willpower is mentally exhausting because it diminishes blood glucose levels, the primary energy source for the brain. However, a more recent study has revealed that the glucose as energy hypothesis does not hold: Even using a glucose mouthwash (that is never digested) produces the same self-control enhancing effects, which potentially implicates special glucose reward receptors in the brain that increase motivation when activated (Sanders et al. 2012).
Even though the specific mechanisms remain unknown, the same mechanism appears to be at work in both species. Moreover, a different study reported that high impulsivity is linked to lower levels of serotonin and dopamine in dogs, a phenomenon which has previously been reported in humans (Wright et al. 2012). In light of our findings of behavioral differences across contexts, it would be instructive to see whether the neurobiological data also reflect differences in depletion and chemical levels depending upon context.
Furthermore, it would also be informative to look at breed differences, as the sample size of different breeds in the current study was not large enough to permit such an analysis. For example, one might predict that dogs that have been bred for working roles would have better inhibitory control than dogs that have been bred solely to be house pets and companions. Specifically, herding dogs might possess particularly impressive inhibitory control skills because their job involves nipping at the heels of sheep, but stopping themselves before actually attacking as a wolf might. Consistent with this theory, Border collies have innate eye, stalk, and chase patterns, but weaker bite and dissect responses than other catch dogs (Coppinger and Schneider 1995). Retrieving dogs, too, face inhibitory challenges by carrying downed fowl back to their handlers while simultaneously resisting the urge to eat the animal. This “soft mouth” characteristic has been artificially selected for in retrievers (Schmutz and Schmutz 1998). Thus, selection for these traits may have yielded corresponding breed differences in inhibitory control. In conducting these studies, researchers should also take into account the purpose for which individual dogs have been bred, even within a specific breed. After all, one would expect that Labrador retrievers that have been bred for many generations to work as service dogs would potentially differ in inhibitory control from Labrador retrievers that have been bred as pets or hunting dogs.
From an applied perspective, the results of the current studies are informative because dogs are so widely used in our society. Humans rely on dogs for everything from companionship to guidance to military services to search and rescue. Dogs can also use scent to accurately detect multiple types of cancer, including melanoma, colorectal, lung, and breast cancer (McCulloch et al. 2006; Sonoda et al. 2011), and can even identify cancer when traditional methods initially fail to do so (Pickel et al. 2004). All of these tasks rely on a range of problem solving skills that must work in concert to produce the desired outcome (e.g., the dog must sustain attention to the task, discriminate stimuli accurately, ignore environmental distractors, and execute the correct behavioral responses). Interestingly, two studies of working dog performance implicate the absence of distractibility as a predictor of success in both drug detection dogs (Maejima et al. 2007) and guide dogs (Batt et al. 2008; Goddard and Beilharz 1983). Thus, the ability to inhibit responses to task-irrelevant stimuli may be especially important for working dog populations. By understanding both the factors that influence inhibitory control and the nature of individual differences in these skills, we may be better able to identify and effectively train dogs likely to succeed in these important societal roles.
The studies reported here provide evidence for inhibitory control in dogs across a range of contexts. These cognitive skills are critical to effective problem solving (Diamond 1990) and have been shown to vary both between and within other animal species. Our data reveal that skills for inhibitory control may also differ between contexts within the same individual, mirroring the results of studies of humans.
Supplementary Material
Acknowledgments
We thank S. Mitroff and A. Joh for helpful discussion, as well as J. Serpell, D. Cheney, P. Costanzo, M. Golonka, M. Ritchey, and two anonymous reviewers for their valuable feedback on drafts. We also thank T. Jones, A. Madison, Z. Best, and J. Clift for their help with coding, in addition to all members of the Duke Canine Cognition Center for help with data collection and testing. This work was supported in part by the Vertical Integration Program, the Duke Undergraduate Research Support Office, Office of Naval Research Grant No. N00014-12-1-0095, and National Institute of Health Grant 5 R03 HD070649-02.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10071-013-0633-z) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have no conflict of interest.
Ethical standards The experiments comply with the current laws of the United States.
The high- and low-value rewards were consistent within individual subjects but varied between subjects due to dietary restrictions or refusal to eat particular foods.
Contributor Information
Emily E. Bray, Email: ebray90@gmail.com, ebray@sas.upenn.edu, Department of Psychology, University of Pennsylvania, Philadelphia, PA, USA.
Evan L. MacLean, Department of Evolutionary Anthropology, Duke University, Durham, NC, USA
Brian A. Hare, Department of Evolutionary Anthropology, Duke University, Durham, NC, USA; Center for Cognitive Neuroscience, Duke University, Durham, NC, USA
References
- Adams B, Chan A, Callahan H, Siwak C, Tapp D, Ikeda-Douglas C, Atkinson P, Head E, Cotman C, Milgram N. Use of a delayed non-matching to position task to model age-dependent cognitive decline in the dog. Behav Brain Res. 2000;108(1):47–56. doi: 10.1016/s0166-4328(99)00132-1. [DOI] [PubMed] [Google Scholar]
- Agnetta B, Hare B, Tomasello M. Cues to food location that domestic dogs (Canis familiaris) of different ages do and do not use. Anim Cogn. 2000;3(2):107–112. [Google Scholar]
- Alain C, Woods DL. Age-related changes in processing auditory stimuli during visual attention: evidence for deficits in inhibitory control and sensory memory. Psychol Aging. 1999;14(3):507–519. doi: 10.1037//0882-7974.14.3.507. [DOI] [PubMed] [Google Scholar]
- Amici F, Aureli F, Call J. Fission-fusion dynamics, behavioral flexibility, and inhibitory control in primates. Curr Biol. 2008;18(18):1415–1419. doi: 10.1016/j.cub.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Ariely D, Wertenbroch K. Procrastination, deadlines, and performance: self-control by precommitment. Psych Sci. 2002;13(3):219–224. doi: 10.1111/1467-9280.00441. [DOI] [PubMed] [Google Scholar]
- Batt LS, Batt MS, Baguley JA, McGreevy PD. Factors associated with success in guide dog training. J Vet Behav. 2008;3(4):143–151. [Google Scholar]
- Baumeister RF. Ego depletion and self-control failure: an energy model of the self's executive function. Self Identity. 2002;1(2):129–136. [Google Scholar]
- Baumeister RF, Bratslavsky E, Muraven M, Tice DM. Ego depletion: is the active self a limited resource? J Pers Soc Psychol. 1998;74(5):1252–1265. doi: 10.1037//0022-3514.74.5.1252. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Vohs KD, DeWall CN, Zhang L. How emotion shapes behavior: feedback, anticipation, and reflection, rather than direct causation. Pers Soc Psychol Rev. 2007;11(2):167–203. doi: 10.1177/1088868307301033. [DOI] [PubMed] [Google Scholar]
- Bonanni R, Natoli E, Cafazzo S, Valsecchi P. Free-ranging dogs assess the quantity of opponents in intergroup conflicts. Anim Cogn. 2011;14(1):103–115. doi: 10.1007/s10071-010-0348-3. [DOI] [PubMed] [Google Scholar]
- Boogert NJ, Anderson RC, Peters S, Searcy WA, Nowicki S. Song repertoire size in male song sparrows correlates with detour reaching, but not with other cognitive measures. Anim Behav. 2011;81(6):1209–1216. [Google Scholar]
- Braver TS, Barch DM, Keys BA, Carter CS, Cohen JD, Kaye JA, Janowsky JS, Taylor SF, Yesavage JA, Mumenthaler MS. Context processing in older adults: evidence for a theory relating cognitive control to neurobiology in healthy aging. J Exp Psychol [Gen] 2001;130(4):746–763. [PubMed] [Google Scholar]
- Callender KA, Olson SL, Kerr DCR, Sameroff AJ. Assessment of cheating behavior in young school-age children: distinguishing normative behaviors from risk markers of externalizing psychopathology. J Clin Child Adolesc. 2010;39(6):776–788. doi: 10.1080/15374416.2010.517165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppinger R, Schneider R. Evolution of working dogs. In: Serpell J, editor. The domestic dog: its evolution, behaviour and interactions with people. Cambridge University Press; Cambridge: 1995. pp. 21–47. [Google Scholar]
- Dempster FN. The rise and fall of the inhibitory mechanism: toward a unified theory of cognitive development and aging. Dev Rev. 1992;12(1):45–75. [Google Scholar]
- Diamond A. Retrieval of an object from an open box: the development of visual-tactile control of reaching in the first year of life. Society for Research in Child Abstracts. 1981:78. [Google Scholar]
- Diamond A. Developmental time course in human infants and infant monkeys, and the neural bases of, inhibitory control in reaching. Ann NY Acad Sci. 1990;608(1):637–676. doi: 10.1111/j.1749-6632.1990.tb48913.x. [DOI] [PubMed] [Google Scholar]
- Diamond A, Goldman-Rakic PS. Comparison of human infants and rhesus monkeys on Piaget's AB task: evidence for dependence on dorsolateral prefrontal cortex. Exp Brain Res. 1989;74(1):24–40. doi: 10.1007/BF00248277. [DOI] [PubMed] [Google Scholar]
- Duckworth AL, Seligman ME. Self-discipline outdoes IQ in predicting academic performance of adolescents. Psychol Sci. 2005;16(12):939–944. doi: 10.1111/j.1467-9280.2005.01641.x. [DOI] [PubMed] [Google Scholar]
- Evans TA, Beran MJ. Chimpanzees use self-distraction to cope with impulsivity. Biol Lett. 2007;3(6):599–602. doi: 10.1098/rsbl.2007.0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailliot MT, Baumeister RF, DeWall CN, Maner JK, Plant EA, Tice DM, Brewer LE, Schmeichel BJ. Self-control relies on glucose as a limited energy source: willpower is more than a metaphor. J Pers Soc Psychol. 2007;92(2):325–336. doi: 10.1037/0022-3514.92.2.325. [DOI] [PubMed] [Google Scholar]
- Goddard ME, Beilharz RG. Genetics of traits which determine the suitability of dogs as guide-dogs for the blind. Appl Anim Ethol. 1983;9(3–4):299–315. doi: 10.1016/0304-3762(83)90010-x. [DOI] [Google Scholar]
- Hare B, Tomasello M. Domestic dogs (Canis familiaris) use human and conspecific social cues to locate hidden food. J Comp Psychol. 1999;113(2):173–177. [Google Scholar]
- Hare B, Woods V. The genius of dogs: how dogs are smarter than you think. Penguin Group; New York: 2013. [Google Scholar]
- Hare B, Call J, Tomasello M. Communication of food location between human and dog (Canis familiaris) Evol Commun. 1998;2(1):137–159. [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: a review and a new view. Psychol Learn Motiv. 1988;22:193–225. [Google Scholar]
- Hasher L, Stoltzfus ER, Zacks RT, Rypma B. Age and inhibition. J Exp Psychol Learn. 1991;17(1):163–169. doi: 10.1037//0278-7393.17.1.163. [DOI] [PubMed] [Google Scholar]
- Ittyerah M, Gaunet F. The response of guide dogs and pet dogs (Canis familiaris) to cues of human referential communication (pointing and gaze) Anim Cogn. 2009;12(2):257–265. doi: 10.1007/s10071-008-0188-6. [DOI] [PubMed] [Google Scholar]
- Kaminski J, Fischer J, Call J. Prospective object search in dogs: mixed evidence for knowledge of what and where. Anim Cogn. 2008;11(2):367–371. doi: 10.1007/s10071-007-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kis A, Topál J, Gácsi M, Range F, Huber L, Miklósi Á, Virányi Z. Does the A-not-B error in adult pet dogs indicate sensitivity to human communication? Anim Cogn. 2012;15(4):737–743. doi: 10.1007/s10071-012-0481-2. [DOI] [PubMed] [Google Scholar]
- Kundey SMA, De Los Reyes A, Taglang C, Baruch A, German R. Domesticated dogs' (Canis familiaris) use of the solidity principle. Anim Cogn. 2010;13(3):497–505. doi: 10.1007/s10071-009-0300-6. [DOI] [PubMed] [Google Scholar]
- MacNulty DR, Mech LD, Smith DW. A proposed ethogram of large-carnivore predatory behavior, exemplified by the wolf. J Mammal. 2007;88(3):595–605. [Google Scholar]
- Maejima M, Inoue-Murayama M, Tonosaki K, Matsuura N, Kato S, Saito Y, Weiss A, Murayama Y, Ito S. Traits and genotypes may predict the successful training of drug detection dogs. Appl Anim Behav Sci. 2007;107(3–4):287–298. [Google Scholar]
- Marshall-Pescini S, Passalacqua C, Ferrario A, Valsecchi P, Prato-Previde E. Social eavesdropping in the domestic dog. Anim Behav. 2011;81(6):1177–1183. [Google Scholar]
- McCulloch M, Jezierski T, Broffman M, Hubbard A, Turner K, Janecki T. Diagnostic accuracy of canine scent detection in early-and late-stage lung and breast cancers. Integr Cancer Ther. 2006;5(1):30–39. doi: 10.1177/1534735405285096. [DOI] [PubMed] [Google Scholar]
- Miklósi Á, Polgárdi R, Topál J, Csányi V. Use of experimenter-given cues in dogs. Anim Cogn. 1998;1(2):113–121. doi: 10.1007/s100710050016. [DOI] [PubMed] [Google Scholar]
- Miller HC, Pattison KF, DeWall CN, Rayburn-Reeves R, Zentall TR. Self-control without a “self”?: common self-control processes in humans and dogs. Psychol Sci. 2010;21(4):534–538. doi: 10.1177/0956797610364968. [DOI] [PubMed] [Google Scholar]
- Mischel W. Toward an integrative science of the person. Annu Rev Psychol. 2004;55:1–22. doi: 10.1146/annurev.psych.55.042902.130709. [DOI] [PubMed] [Google Scholar]
- Mischel W, Rodriguez ML. Psychological distance in self-imposed delay of gratification. In: Cocking R, editor. The development and meaning of psychological distance. Lawrence Erlbaum Associates, Inc.; England: 1993. pp. 109–121. [Google Scholar]
- Moffitt TE, Arseneault L, Belsky D, Dickson N, Hancox RJ, Harrington HL, Houts R, Poulton R, Roberts BW, Ross S. A gradient of childhood self-control predicts health, wealth, and public safety. Proc Natl Acad Sci USA. 2011;108(7):1–6. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton ES. On the occurrence and significance of motivation-structural rules in some bird and mammal sounds. Am Nat. 1977;111(981):855–869. [Google Scholar]
- Oaten M, Cheng K. Improved self-control: the benefits of a regular program of academic study. Basic Appl Soc Psych. 2006a;28(1):1–16. [Google Scholar]
- Oaten M, Cheng K. Longitudinal gains in self-regulation from regular physical exercise. Br J Health Psych. 2006b;11(4):717–733. doi: 10.1348/135910706X96481. [DOI] [PubMed] [Google Scholar]
- Osthaus B, Marlow D, Ducat P. Minding the gap: spatial perseveration error in dogs. Anim Cogn. 2010;13(6):881–885. doi: 10.1007/s10071-010-0331-z. [DOI] [PubMed] [Google Scholar]
- Osvath M, Osvath H. Chimpanzee (Pan troglodytes) and orangutan (Pongo abelii) forethought: self-control and pre-experience in the face of future tool use. Anim Cogn. 2008;11(4):661–674. doi: 10.1007/s10071-008-0157-0. [DOI] [PubMed] [Google Scholar]
- Pettersson H, Kaminski J, Herrmann E, Tomasello M. Understanding of human communicative motives in domestic dogs. Appl Anim Behav Sci. 2011;133(3–4):235–245. [Google Scholar]
- Piaget J. The construction of reality in the child. Basic Books; New York: 1954. [Google Scholar]
- Pickel D, Manucy GP, Walker DB, Hall SB, Walker JC. Evidence for canine olfactory detection of melanoma. Appl Anim Behav Sci. 2004;89(1):107–116. [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WPM, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56(2):129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Riedel J, Schumann K, Kaminski J, Call J, Tomasello M. The early ontogeny of human–dog communication. Anim Behav. 2008;75(3):1003–1014. [Google Scholar]
- Sanders MA, Shirk SD, Burgin CJ, Martin LL. The gargle effect rinsing the mouth with glucose enhances self-control. Psychol Sci. 2012;23(12):1470–1472. doi: 10.1177/0956797612450034. [DOI] [PubMed] [Google Scholar]
- Santos LR, Ericson BN, Hauser MD. Constraints on problem solving and inhibition: object retrieval in cotton-top tamarins (Saguinus oedipus oedipus) J Comp Psychol. 1999;113(2):186–193. [Google Scholar]
- Scheider L, Grassmann S, Kaminski J, Tomasello M. Domestic dogs use contextual information and tone of voice when following a human pointing gesture. PLoS One. 2011;6(7):e21676. doi: 10.1371/journal.pone.0021676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutz S, Schmutz J. Heritability estimates of behaviors associated with hunting in dogs. J Hered. 1998;89(3):233–237. doi: 10.1093/jhered/89.3.233. [DOI] [PubMed] [Google Scholar]
- Shoda Y, Mischel W, Peake PK. Predicting adolescent cognitive and self-regulatory competencies from preschool delay of gratification: identifying diagnostic conditions. Dev Psychol. 1990;26(6):978–986. doi: 10.1037/0012-1649.26.6.978. [DOI] [Google Scholar]
- Soltis J, Thomsen R, Takenaka O. The interaction of male and female reproductive strategies and paternity in wild Japanese macaques, Macaca fuscata. Anim Behav. 2001;62(3):485–494. [Google Scholar]
- Sonoda H, Kohnoe S, Yamazato T, Satoh Y, Morizono G, Shikata K, Morita M, Watanabe A, Kakeji Y. Colorectal cancer screening with odour material by canine scent detection. Gut. 2011;60(6):814–819. doi: 10.1136/gut.2010.218305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JR, Hallinan EV, Hauser MD. The ecology and evolution of patience in two New World monkeys. Biol Lett. 2005;1(2):223–226. doi: 10.1098/rsbl.2004.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studzinski CM, Araujo JA, Milgram NW. The canine model of human cognitive aging and dementia: pharmacological validity of the model for assessment of human cognitive-enhancing drugs. Prog Neuro-Psychopharmacol Biol Psychiatry. 2005;29(3):489–498. doi: 10.1016/j.pnpbp.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Tapp PD, Siwak CT, Estrada J, Head E, Muggenburg BA, Cotman CW, Milgram NW. Size and reversal learning in the beagle dog as a measure of executive function and inhibitory control in aging. Learn Memory. 2003;10(1):64–73. doi: 10.1101/lm.54403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topál J, Gergely G, Erdohegyi Á, Csibra G, Miklósi Á. Differential sensitivity to human communication in dogs, wolves, and human infants. Science. 2009;325(5945):1269–1272. doi: 10.1126/science.1176960. [DOI] [PubMed] [Google Scholar]
- Tsukayama E, Duckworth AL. Domain-specific temporal discounting and temptation. Judgment Decision Mak. 2010;5(2):72–82. [Google Scholar]
- Tsukayama E, Duckworth AL, Kim B. Resisting everything except temptation: evidence and an explanation for domain-specific impulsivity. Eur J Pers. 2011;26(3):318–334. [Google Scholar]
- Vlamings PHJM, Hare B, Call J. Reaching around barriers: the performance of the great apes and 3–5-year-old children. Anim Cogn. 2010;13(2):273–285. doi: 10.1007/s10071-009-0265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward C, Smuts BB. Quantity-based judgments in the domestic dog (Canis lupus familiaris) Anim Cogn. 2007;10(1):71–80. doi: 10.1007/s10071-006-0042-7. [DOI] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120(2):272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Wiesner M, Kim HK, Capaldi DM. History of juvenile arrests and vocational career outcomes for at-risk young men. J Res Crime Delinq. 2010;47(1):91–117. doi: 10.1177/0022427809348906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobber V, Hare B. Testing the social dog hypothesis: are dogs also more skilled than chimpanzees in non-communicative social tasks? Behav Process. 2009;81(3):423–428. doi: 10.1016/j.beproc.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Wright HF, Mills DS, Pollux PMJ. Behavioural and physiological correlates of impulsivity in the domestic dog (Canis familiaris) Physiol Behav. 2012;105(3):676–682. doi: 10.1016/j.physbeh.2011.09.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.