Abstract
Background
Colorectal cancer (CRC), the most lethal long-term complication of inflammatory bowel disease (IBD), is the culmination of a complex sequence of molecular and histologic derangements of the colon epithelium that are initiated and at least partially sustained by prolonged chronic inflammation. Dysplasia, the earliest histologic manifestation of this process, plays an important role in cancer prevention by providing the first clinical alert that this sequence is under way and by serving as an endpoint in colonoscopic surveillance of patients at high risk for CRC. Restorative proctocolectomy (RPC) is indicated for patients with IBD, specifically for ulcerative colitis that is refractory to medical treatment, emergency conditions, and/or in case of neoplastic transformation. Even after RPC with mucosectomy, pouch-related carcinomas have recently been reported with increasing frequency since the first report in 1984. We review IBD-associated CRC and pouch-related neoplasia prevalence, adverse events, risk factors, and surveillances.
Methods
Literature of IBD-associated CRC patients and those undergoing RPC surgeries through 2010 were prospectively reviewed.
Results
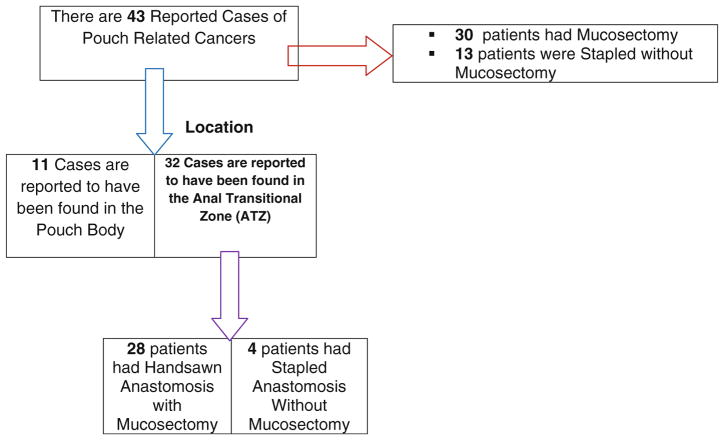
We found 12 studies from retrospective series and 15 case reports. To date, there are 43 reported cases of pouch-related cancers. Thirty-two patients had cancer in the anal transit zone (ATZ); of these, 28 patients had mucosectomy. Eleven patients had cancer found in the pouch body.
Conclusion
RPC with mucosectomy does not necessarily eliminate risks. There is little evidence to support routine surveillance of pouch mucosa and the ATZ except for patients associated with histological type C changes, sclerosing cholangitis, and unremitting pouchitis.
Keywords: Inflammatory bowel disease-associated colorectal cancer, Proctocolectomy, Mucosectomy, Pouch-related cancer
Background
Colorectal cancer (CRC) is such a preventable and treatable condition that has been described as “the disease no one has to die from” [1]. Yet, CRC is one of the most prevalent cancers in the USA, affecting 146,970 in 2008 [2, 3] and 149,810 in 2009 [2, 3], and is the second after lung cancer, as the most common cause of cancer deaths with 49,920 for 2008 [2, 3] and 49,960 death for 2009 [2, 3]. People who suffer from inflammatory bowel disease (IBD), which comprises two major subtypes, Crohn’s colitis (CC) and ulcerative colitis (UC) that are based on clinical, endoscopic, histopathological, and radiological characteristics, are at increased risk for developing CRC [4, 5]. This is true because the tissue of the colon and rectum is inflamed [6–9] for a long period of time [10]. Risks are even greater for patients with pan-colitis [11] and depend on the patient’s age of disease onset, disease duration, and histological severity of inflammation [12, 13]. Prevalence of CRC development risks for patients with UC and CC [14] is identical but higher than normal population [12, 15]. The first IBD-associated CRC report for UC (1925) [16] and CD (1948) [17] recognized inflammation as a leading cause of long-term mortality. Recent studies observed that IBD confers a higher risk of CRC to males compared with females [5, 18], striking most by middle age [18, 19]. The etiology of IBD is to date still unknown. For nearly 30 years, efforts at cancer prevention have been based on an empirical strategy of colonoscopy surveillance with biopsies to identify dysplasia, the earliest recognizable precursor of CRC and the most reliable marker of imminent cancer risk. Ideally, the rationale of surveillance is to allow most patients whose biopsy specimens remain free of dysplasia to avoid colectomy while enabling those with dysplasia to undergo surgery before the development of CRC. Although validation of this strategy has been based largely on indirect evidence, surveillance has been widely embraced and broadly implemented as the standard of care for patients at risk for CRC [19, 20].
CRC in IBD is also the prototype of the inflammation–dysplasia–cancer sequence in the lower gastrointestinal (GI) tract and the culmination of unique histogenetic and molecular pathways, the details of which cannot be simply extrapolated directly from the sporadic adenoma–cancer sequence. Understanding its pathogenesis is the key to future improvements in diagnosis and chemoprevention, as well as basic advances in our understanding of carcinogenesis.
Because the disease is refractory to medical treatment, dysplasia or cancer (found during screening colonoscopy), and retardation of growth in a child or adolescent, one third of patients with UC will eventually require proctocolectomy surgery [21, 22]. In any of the aforementioned circumstances, three operations are recommended, including conventional total proctocolectomy with permanent ileostomy [23], restorative proctocolectomy (RPC) with ileal pouch-anal anastomosis (IPAA), and total abdominal colectomy with ileorectal anastomosis (IRA) [24]. IRA is only suitable for the few patients whose rectum is relatively free of inflammation and where there is no dysplasia or established cancer in the rectum [25]. RPC is now the standard criterion procedure for UC [26] (and familial adenomatous polyposis) while there are still controversies in regard to indeterminate colitis (IC) and CC. The emphasis for each procedure is different with conventional proctocolectomy being indicated, and the surgical options largely lie between colectomy with IRA and RPC [27]. The success of RPC is largely dependent on careful patient selection and of utmost importance is accurate diagnosis (which we are unable to offer in 30% of IBD patients) with meticulous surgical technique [24]. Excision of the entire colon and rectum with mucosectomy of the residual anorectal stump is intended to achieve complete removal of all disease-prone mucosa, while maintaining transanal fecal continence [24, 27]. The procedure, however, inadvertently leaves small mucosal residual islands [24, 27]. Indisputably, ileo-anal pouch mucosa and the anorectal mucosa below the ileo-anal anastomosis is therefore at potential risk of developing subsequent cancer [28, 29] in the pouch and the ileo-anal anastomosis [30, 31]. A substantial number of these patients, after surgical treatment, even with mucosectomy, will not always be prevented from developing cancer in the pouch [32–34] and/or from in the remnant anal transition zone (ATZ) [35].
This review highlights the experience in the clinical literature published on incidence of IBD-associated colorectal and/or ileal pouch cancer following RPC.
Methods
A systematic literature search consisting of retrospective studies and case reports of comparative studies reporting IBD-associated colorectal cancer and post-operative early and late ileal pouch adenomas and adenocarcinoma adverse events were reviewed. Patients diagnosed with IBD-associated CRC between 1925 through June 2010 and those who had undergone RPC surgery since the first pelvic pouch operation in 1978 [26] through August 2010 were prospectively enrolled. Institutional Review Board approval was obtained by each of the participating research Institutions.
The US National Library of Medicine database (MED-LINE), the Excerpta Medica database (EMBASE), the Cochran Library, and Google® search engine were searched for published articles on the “inflammatory bowel disease,” “ulcerative colitis,” Crohn’s colitis, “colectomy,” “restorative proctocolectomy,” “ileo-anal anastomosis,” “ileal pouches,” “villous adenomas,” “adenocarcinoma,” “dysplasia,” “metaplasia,” “pouch dysplasia,” “pelvic pouch”, and “pouch neoplasia.” The initial search covered from January 1975 through December 2007. A second search was performed to include January 2008 through June 2010 to update the initial search. The search excluded foreign language and non-human studies, as well as editorials. Additional articles were identified by cross-referencing papers retrieved in the initial search. Papers were included on the basis of most recent available evidence for each specific point of interest. Final and conclusive agreement was assessed with the k-statistic during the title review and abstract review. If the k value was ≥0.6, the titles were reviewed and divided into two sets; each was reviewed by only one of the two researchers. If the k value was <0.6, reviewers discussed discrepancies, followed by other assessments of agreement. A similar process was used for abstract review, with an increased k value of 0.7 for acceptance.
The same team of authors involved in the original title, abstract, and article review process conducted hands-on searches of bibliography from accepted articles and review articles. These hands-on searches resulted in retrieval of a limited number of additional articles for review (Table 1).
Table 1.
Gene targets associated with colorectal neoplasia in inflammatory bowel disease
| Gene (chromosomal locus studies) | Function | Prevalence in IBD-related CRC | Timing in the dysplasia–cancer sequence | Pathogenesis or mechanisms of mutation | References |
|---|---|---|---|---|---|
| APC (5q21) | Tumor suppressor gene (cell adhesion) | Rare (<15%) | Late | HGD, CRC | Greewald et al. [191], Kern et al. [192], Tarmin et al. [193], Redston et al. [194], Willenbucher et al. [195], Tomlinson et al. [200], Willenbucher et al. [234], Umetani et al. [196], Aust et al. [197], Aust et al. [198], Aust et al. [199], Maia et al. [235] |
| DCC/DPC (18Q21) | Tumor suppressor gene (cell adhesion) | Common (>50%0 | Early | Dysplasia | Lei et al. [201], Willenbucher et al. [195], Hoque et al. [202], Tomlinson et al. [200], Willenbucher et al. [234], Aust et al. [197] |
| TP53 (17p13) | Tumor suppressor gene (cell cycle, apoptosis) | Common (>50%) | Early | May precede dysplasia | Burmer et al. [203], Greewald et al. [191], Yin et al. [204], Harpaz N et al. [205], Brentnall et al. [206], Chaubert et al. [207], Klump et al. [208], Holzman et al. [209], Rabinovitch et al. [210], Aust et al. [197], Wong et al. [211], Hussain et al. [212], Maia et al. [235], Nathanson et al. [213] |
| CDKN2A (9P21) | Tumor suppressor gene (cell cycle inhibitor) | Frequent | Early | Hypermethylation | Hsieh et al. [214], Issa et al. [215] |
| CDKN2A (9P21) | Tumor suppressor gene (indirect p53 regulator) | Frequent | Early | Hypermethylation | Sato et al. [216], Moriyama et al. [217] |
| CDKN2A (12p13) | Tumor suppressor gene (cell cycle regulation) | Under-expressed in CRC | Walsh et al. [218] | ||
| E-cadherin | Tumor suppressor gene (cell adhesion) | Frequent | Early | Hypermethylation | Ilyas et al. [219], Tomlinson et al. [200], Wheeler et al. [220], Azarschab et al. [221], Van Dekken et al. [222] |
| K-ras (12p12) | Oncogene (cell cycle regulation) | Uncommon (<25%) | Late | HGD, CRC | Burmer et al. [223], Meltzer et al. [224], Bell et al. [225], Chaubert et al. [207], Redston et al. [194], Umetani et al. [196], Lyda et al. [226], Holzmann et al. [227], Aust et al. [228] |
| C-src TGFβR2 RII |
Oncogene TGF-β1 receptor gene |
Intermediate MSI-related CRC (less common than sporadic MSI CRC) | Unknown Unknown |
LGD to HGD MSI |
Cartwright et al. [229], Souza et al. [230] |
| MSH2 (2p22) | DNA mismatch repair gene | MSI-related CRC | Unknown | MSI | Brentnall et al. [231], Noffsinger et al. [232] |
| MLH1 | DNA mismatch repair gene | Intermediate MSI-related CRC (less common than sporadic MSI CRC) | Unknown | MSI, hypermethylation | Fleisher et al. [233] |
Abbreviations: APC adenomatous polyposis coli, CRC colorectal carcinoma, DCC deleted in colon cancer, HGD high-grade dysplasia, LGD low-grade dysplasia, MSI microsatellite instability, TGF transforming growth factor
Results
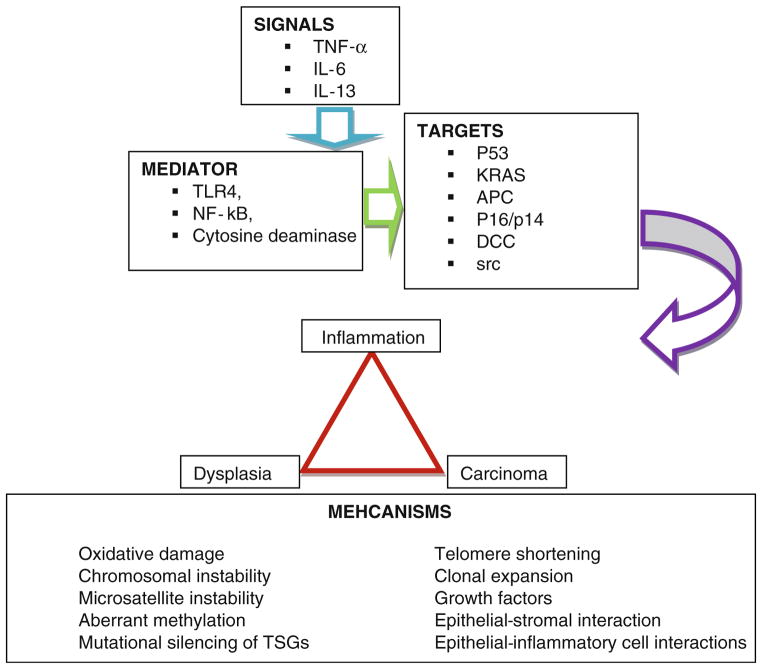
Summary of molecular signals, mediators, targets, and mechanism implicated in progression from inflammation to cancer are depicted in Fig. 1. Approximately two thirds of the IBD-associated CRC patients are males [36]. This happens because of protective effect of female sex on inflammation-associated cancer, attributed to estrogens [36–38] on the propagation of inflammation and inflammation-associated cancer. All IBD complicating CRC are reported to have occurred in stages of colitis segment [39]. Proctocolectomy, even with mucosectomy, does not always prevent subsequent development of cancer of the pouch and/or in the remnant ATZ [34]. The summary of all reported ileal pouch or the ileo-anal outflow cancer results following RPC for UC, to date, are depicted in Tables 2 and 3, respectively. To date, there are 43 reported cases of IBD-pouch-related cancer of which 11 cases had cancer from the pouch body and 32 cases had it originating from the ATZ [40–65]. Interestingly, out of the 32 patients with cancer at ATZ, 28 patients were mucosectomized and three patients had stapled anostomosis without mucosectomy (Table 2).
Fig. 1.
Molecular pathogenesis of colorectal dysplasia and cancer associated with inflammatory bowel disease. Summary of molecular signals, mediators, targets, and mechanisms implicated in progression from inflammation to cancer.
Abbreviations: IL interleukin, TLR Toll-like receptor, TNF tumor necrosis factor, TSG tumor suppressor gene between microscopic and clinical indices of disease severity activity [83–85].
Table 2.
Shows the number of reported pouch-related cancer, the nature of the anastomosis weather was hand-sewn or stapled with or without mucosectomy and the location of the cancer
|
This table underscores that mucosectomy does not always prevent the development of ATZ pouch-related cancer
Table 3.
Reported ileal pouch neoplasia following restorative proctocolectomy for ulcerative colitis
| Author, published year, nature of study | Age at UC diagnosis, years | Interval, UC diagnosis to cancer, years | Operation technique | Interval, surgery to cancer, years | Age at cancer diagnosis | Number of patients | Location | Histology | |
|---|---|---|---|---|---|---|---|---|---|
| Ravitch, 1984 | Case report | Not reported | Not reported | RPC | Not reported | Not reported | 1 | Not reported | T4N0M0 |
| Stern, 1990 | Case report | 21 | 35 | RPC | 4 | 56 | 1 | Not reported | T2N2M0 |
| Puthu, 1992 | Case report | 34 | 11 | RPC | 6 | 45 | 1 | Pouch | T4N0M1 |
| R-Sanjuan, 1995 | Case report | 30 | 22 | RPC | 1 | 52 | 1 | Pouch | T1N0M0 |
| Sequens, 1997 | Case report | 45 | 9 | RPC | 1.3 | 54 | 1 | ATZ | T3N0M0 |
| Vieth, 1998 | Case report | 18 | 16 | RPC | 2 | 34 | 1 | Pouch | T2N0M0 |
| Iwama, 2000 | Case report | 27.5 | 21 | RPC | 1.5 | 50 | 1 | IAA | T4N2M1 |
| Rotholtz, 2001 | Case report | 59 | 6 | RPC | 7 | 65 | 1 | ATZ | T3N0M0 |
| Heuschen, 2001 | Case report | 11.8 | 23 | RPC | 3.2 | 38 | 1 | Pouch | T3N0M0 |
| Hyman, 2002 | Case report | 23 | 18 | RPC | 5 | 41 | 1 | Rectal stump | T2N2M0 |
| Laureti, 2002 | Case report | 26 | 28 | RPC | 2 | 48 | 1 | IAA | T3N1M0 |
| Baratsis, 2002 | Case report | 23 | 26 | RPC | 2 | 49 | 1 | ATZ | T4N0M0 |
| Hassan, 2003 | Case report | 28 | 10 | RPC | 2 | 40 | 1 | Pouch | Dysplasia |
| Herline, 2003 | Routine surveillance | Not reported | Not reported | RPC | 10 | Not reported | 1 | Not reported | T4N0M0 |
| Bell, 2003 | Case report | 24 | 27 | RPC | 12 | 51 | 1 | IAA | T3N1M0 |
| Negi, 2003 | Case report | Not reported | Not reported | RPC | 5 | 28 | 1 | Rectal stump | T4N0M0 |
| Bentrem, 2003 | Case report | 19 | 30 | RPC | 14 | 63 | 1 | Pouch | T3N0M0 |
| Lee, 2005 | Case report | 18 | RPC and MUC | 15 | 1 | IAA | T4N2M0 | ||
| Knupper, 2006 | Case report | 12 | 20 | RPC | 2 | 34 | 1 | Pouch | T4N2M0 |
| Das, 2007 | Case report | 27 | RPC | 25 | 1 | Rectal stump | Adenocarcinoma | ||
| Walker, 2006 | Case report | 26 | 20 | RPC | 12 | 52 | 1 | Pouch mucosa | High-grade dysplasia |
| Schaus, 2007 | Perspective study | Not reported | RPC | Not reported | |||||
| Chia, 2007 | Case report | Not reported | 11 | RPC | Not reported | 1 | ATZ | T4N0M0 | |
| Das, 2007 | Case report | 120 and 528 (median 246) | RPC | 16 and 216 (median 6) | 2 | Not reported | Adenocarcinoma | ||
| Koh, 2008 | Case report | 54 | RPC | 14 | 1 | Pouch inlet | Adenocarcinoma | ||
| Branco, 2008 | Case report | 8 | 29 | RPC | 6 | 37 | 1 | Pouch | Adenocarcinoma |
| Pedersen, 2008 | Case report | 45 | 11 RPC MUC | 9 | 56 | 1 | ATZ | Adenocarcinoma T2N0 (4 pts) T3N0 (3 pts) |
|
| Zmora, 2009 | Prospective study | 18 | 28 Proctectomt & IPAA | 10 | 46 | 1 | Rectal cuff One in the pouch inlet |
Cancer cells Lympoma |
|
| IRA Stapled | 28 | Three SCC of the AZT | |||||||
| Kariv, 2010 | Routine surveillance | 34.4±14.4 | 23.3±12.5 | IPAA MUC | 9.7±6.4 | 57.6±13.6 | 15 | Eleven in the pouch or AZT One in the pouch inlet |
3 |
UC ulcerative colitis, RPC restorative proctocolectomy, IAA ileal anal anastomosis, ATZ anal transitional zone, MUC mucosectomy, SCC squamous cell cancer
Colorectal neoplasia in IBD
IBD, often characterized by UC and CD (or IC, when non-definitive evaluations have been made), increases the risk of developing CRC [12, 15]. In general, the longer a person has had IBD, the greater the chance of developing CRC [12, 66].
Risk factors
Despite the fact that the magnitude of risk for CRC conferred by IBD has been difficult to quantify, there is unanimous agreement in the literature [67, 68] that it exceeds that of the age-matched general population three-to fivefold and that it depends critically on duration and the anatomic extent of colonic disease inflammation. A widely referenced comprehensive meta-analysis of 19 studies with age-stratified data [69] reported that the cumulative incidence of CRC in UC is 2% after 10 years, 8% after 20 years, and 18% after 30 years of disease. Other recent studies [68, 70] observed lower incident rates attributed to, among other factors, the benefit of endoscopic surveillance and anti-inflammatory chemoprophylaxis [71].
Evaluations of the risk of CRC in patients with CD show compelling evidence that the magnitude of risk associated with CD is identical to that for UC of comparable duration and extent [71–73]. Direct comparable hospital-based studies of CRC in UC and CC [72–74] have reported close similarities with respect to multiple clinical and pathologic parameters. One study [33], which compared the incidence of CRC in two identically selected cohorts of patients with extensive UC and CC, reported a cumulative incidence of CRC of 185 at 22 years from onset of symptoms in the CC patients and 7% at 20 years from onset of symptoms in the UC group. Intriguingly, a recent meta-analysis [75] has estimated the relative risk for CRC in patients with CC to be 4.5, similar to that in UC, even without factoring in the effects of early colectomy.
CRC complicating pan-colitis is diagnosed at an average age of 45 years, or 15 to 20 years younger than patients with cancer in the general population [5, 76, 77] corresponding to a mean colitis–cancer interval of approximately 20 years. The onset of IBD in childhood or adolescence poses a substantial cumulative risk for development of CRC [76], as high as 40% and accounts for nearly one third of all IBD-related cancer cases. However, most authorities do not consider early disease onset to be an independent risk factor per se but a reflection of prolonged risk exposure and high prevalence of pan-colitis in children [78].
Approximately 80% of cancer in UC occurs in the setting of pancolitis or extensive colitis, colitis extending as far as the hepatic flexure [68]. Left-sided UC confers an intermediate degree of risk for neoplasia, whereas proctitis and proctosigmoiditis confer little or none [76, 79]. The onset of left-sided colitis and of associated CRC each occurs 5 to 10 years later, on average, than for pan-colitis, but the mean 20-year colitis–cancer interval remains the same [80].
The intensity of microscopic inflammation in colonic biopsy specimens has been implicated as an independent risk factor for development of neoplasia [15, 81]. After using similar scales to grade inflammation in colonic biopsy specimens [79–81], the actuarial risk of neoplasia increased three- to fivefold for every unit increase in the grade of inflammation. In most cases, CRC is often diagnosed during subsidence of manifestations of a disease [72–82]. The seemingly contradictory fact that CRC is often diagnosed in patients with clinically quiescent disease may be explained by (1) the greater likelihood that they will retain their colon indefinitely, (2) their lower likelihood of seeking regular medical care [82], and (3) poor correlation
The development of neoplasia in IBD may reflect underlying genetic predispositions in some cases. Case–control [86] and cohort studies [87] have reported a greater than twofold increased risk of CRC in patients with IBD who have a first-degree relative with CRC and a ninefold increased risk if the relative had CRC before the age of 50 years [87]. Early epidemiologic studies [88, 89] suggested that the incidence and prevalence of IBD in the USA, particularly CD, is significantly lower in African Americans than in Caucasians. It is difficult and unrealistic to draw conclusions about disease incidence rates among black Americans based on disease rates among South African blacks [90]. One study found that the incidence of CD among African American was 0.04 cases/100,000 persons compared with an incidence of 1.35/100,000 among Caucasians in the USA while the incidence of UC was 0.45/100,000 for African American vs. 3.5/100,000 for Caucasians. In an epidemiologic study conducted at the University of Chicago [91], 58/1557 (3.7%) patients with IBD were black [88, 91]. Following epidemiologic studies, however, suggested that the prevalence of IBD among African American may be higher than initially recognized. A mail-based survey study conducted in a large health maintenance organization in California revealed prevalence rates of 43.6/100,000 for Caucasians and 29.8/100,000 for African Americans [92]. However, one has to bear in mind that such studies often suffer from low rates of minority enrollment, which may provide poor indicators for disease incidence among minorities.
Synchronous primary sclerosing cholangitis (PSC) in UC confers a substantially increased risk for development of CRC [93]. One American [94] and three European [95, 96] population-based studies agreeably reported the incidence of CRC 10, 20, and 30 years after the diagnosis of UC to be 10%, 33%, and 40%, respectively. Nevertheless, since PSC is prevalent in patients with extensive but mild or asymptomatic colitis, it may serve merely as a surrogate marker for long-standing extensive UC, rather than as an independent risk factor for CRC [97].
Inflammation
The triggering role of inflammation in the pathogenesis of neoplasia in IBD is implied by disease duration, anatomic extent, and intensity of inflammation [98, 99]. The interrelationships between chronic inflammation and cancer predisposition have, to date, merged as a common theme in medicine, particularly in the GI tract, but the specific molecular signals that mediate them in IBD are only now beginning to be clarified [100–104].
Research based mostly on murine colitis models or human colon cancer cell lines suggests that various inflammatory mediators are active participants in tumor promotion and progression. The proinflammatory cytokines TNF-α (tumor necrosis factor α), IL-6, and IL-23 have each been elucidated in the interplay between inflammatory and intestinal epithelia cells during IBD-associated carcinogenesis [98–105]. For instance, transforming growth factor β-dependent IL-6 transsignaling, derived from tumor-infiltrating T cells, has been implicated in tumor progression in the azoxymethane/dextran sulfate sodium (AOM/DSS) mouse colitis model [106]. Intraluminal bacterial endotoxins, TNF-α, and other proinflammatory cytokines act through extracellular receptors such as Toll-like receptors to initiate phosphorylation cascades that transmit signals to key transcription factors such as nuclear factor (NF)–kB [107, 108]. Toll-like receptor 4, which responds specifically to bacterial lipopolysaccharide ligand and is expressed at low levels in normal intestinal mucosa, was shown to be upregulated in the mucosa of patients with IBD, in UC-associated CRC, and in colon tumors that develop in the AOM/DSS mouse models [109]. Conversely, Toll-like receptor 4-deficient mice developed fewer and smaller tumors and produced reduced levels of cyclooxygenase-2 expression and prostaglandin E2 production, both of which are mediators of colorectal tumorigenesis. Mice deficient in cytoplasmic NOD1, another innate immune receptor that signals through NF–Kb, were reported to develop more severe colitis and a greater tumor burden when exposed to AOM/DSS than controls [110]. Interestingly, prior to depletion of the gut, microbial flora abrogated these effects, echoing an earlier study in which intestinal microflora were essential for the induction of inflammation-dependent carcinogenesis in transforming growth factor β1-deficient mice [111]. The link between colitis and tumor progression was reinforced by a study [112] reporting that deletion of IKKβ, the major positive regulator of NF–kB, in murine intestinal epithelial cells, resulted in decreased tumor incidence, but not tumor size, in AOM/DDS-treated mice. This decrease was attributable to increased apoptosis during tumor promotion despite no decrease in inflammation. However, the same deletion in myeloid cells resulted in decreased tumor size through diminished expression of proinflammatory cytokines, implicating that later as tumor growth factors [112]. Conversely, deletion of CYLD, a deubiquitinating enzyme that functions to down-regulate NF–Kb, resulted in more severe colonic inflammation and increased incidence of colonic adenocarcinoma [113].
Among the implications of these studies is the realization that links exist between commensal bacterial components and elements of the innate immune response and inflammation-induced tumorigenesis [114] and that corresponding mechanisms, important for the initiation and maintenance of both chronic inflammation and tumor progression, involve NF–kB-regulated factors such as cytokines and chemokines, proangiogenic and antiapoptotic factors, and matrix proteases.
The mechanisms by which inflammatory cytokines promote the epithelial DNA mutations necessary for the initiation of neoplasia remain largely undefined. A potential clue has recently emerged from a study of activation-induced cytidine deaminase (AID), an enzyme that, under physiologic conditions, regulates class switch recombination and somatic hypermutation in immunoglobulin genes of activated B cells. The study demonstrated that AID induction by proinflammatory cytokines in human colon cancer cell lines could lead to the accumulation of TP53 mutation [115]. The deaminase was overexpressed in both dysplastic and nondysplastic colonic epithelium from patients with UC and CC, as well as in some sporadic colon cancer specimens. Other mechanisms of this type may account for the production of potentially carcinogenic mutations in colonic epithelial cells under the control of proinflammatory cytokines.
The innate immune system may also provide a micro-environment that permits or even promotes tumor progression in states of chronic inflammation. A good example is IL-23, a proinflammatory cytokine that promotes the Th17 T cell differentiation pathway and plays an important role in the pathogenesis of IBD [116]. IL-23 is upregulated in some human tumors, whereas its deletion leads to increased infiltration of cytotoxic T cells and growth restriction of transformed or transplanted tumors [117]. It is likely that other tumor cell products are capable of recruiting and activating inflammatory cells capable of enhancing or limiting tumor growth. Unraveling the complex relationships between tumor cells, their microenvironment and the roles of cytokines within it may afford future targets for chemoprophylaxis.
Molecular pathogenesis
The typical pathogenetic characteristics of cancer development in IBD have attracted many studies [102, 118] seeking to identify the molecular differentiation between the colitides, corresponding etiologic factors, molecular pathways and mechanisms, as well as markers that might be exploited for clinical purposes (Fig. 1). Most of these studies have dealt with UC, and it remains to be seen how closely the shared features of colorectal neoplasia in UC and CC are, to date, mirrored at the molecular level. Clearly, while emerging technological advances in endoscopy promise to make surveillance more effective, the greatest promise for cancer prevention in IBD lies in expanding our, thus far, limited understanding of the molecular pathogenetic relationships between neoplasia and chronic inflammation. Currently, there are multiple ongoing studies on molecule signature differences between the inflammatory colitides [79, 84, 100–104]. Delineation of the differential molecular features may aid not only in the understanding of disease biopathophysiology but accurately early diagnosis, easy, cheap, noninvasive and fast screening, and developing appropriate personalized therapies.
Genetic predisposition
In spite of the fact that the importance of genetic susceptibility in the cause of IBD is irrefutable, its role as a risk factor for CRC is not well understood. Research studies have reported that first-degree relatives of patients with IBD complicated by CRC are more than twice as likely as the general population to have CRC themselves [86, 119]; however, a similar risk increment is observed in the families of patients with sporadic CRC. The relatives of patients with uncomplicated IBD face no significantly increased risk of developing CRC [119, 120], but conversely, a family history of sporadic CRC confers a doubling of the already increased rates of CRC among patients with UC [86, 119]. Analogous findings have been reported in the cotton-top Tamarin model of UC [121, 122]. Concisely, the risk for development of colitis-associated neoplasia may be influenced by heritable factors; however, these are not necessarily the same ones that predispose to sporadic CRC. For example, a mutation in the APC gene, which has been linked to development of sporadic CRC in Ashkenazi Jewish patients, is not increased in frequency among patients with IBD [122, 123].
Pathology of CRC in IBD
IBD-associated CRC occurs in a background of chronically inflamed mucosa, although direct endoscopic visualization, compared with microscopic assessment, often underestimates the intensity and extent of inflammation [124]. Most observations [68, 125, 126] have reported UC-associated CRC predominated in the descending colon, especially the sigmoid colon and rectum, but others [5, 72, 79, 127] have reported a roughly even distribution of lesions on either side of the splenic flexure in both UC and CC.
Compared with sporadic CRC, cancer in IBD is macroscopically heterogeneous and tends to be poorly delimited, irregular, and multifocal. Some cases mimic inflammatory strictures, ulcers, and inflammatory polyps, while others are deceptively flat and inconspicuous [68, 128]. The frequency of multiple synchronous cancers in IBD is 10% to 30%, especially in younger patients, and the simultaneous occurrence of three or more lesions is not unusual; by comparison, the frequency of two synchronous cancers in the general population is 3% to 5% and that of 3% or more is virtually nil [72, 79, 125, 127–129].
Most cases of CRC in IBD are histologically conventional adenocarcinomas. However, mucinous adenocarcinomas account for 15% to 30% of these cancers, compared with 10% to 15% in the general population [72, 74], and signet ring cell adenocarcinomas account for up to 7%, compared with approximately 1% in the general population [72]. In addition, extremely well-differentiated adenocarcinomas that are rarely encountered outside the setting of IBD account for 11% of IBD-associated intestinal cancers that are resected [130].
Pathologists were the first to recognize dysplasia as a precursor to CRC in IBD [17, 131] and proposed periodic monitoring for dysplasia using rectal biopsies as a means of controlling cancer mortality [131]. These observations led to the first endoscopic surveillance program for patients with UC [132], which became emulated widely and has now become the standard of care for those patients with UC and CC who are at high risk for CRC [19, 20, 133, 134]. As a result of such surveillance, evidence has been found supporting the role of dysplasia in the pathogenesis of CRC in IBD and can be summarized as follows: first, studies of resected colons have shown that dysplasia occurs in proximity to cancer in approximately 90% of resected colons, including nearly all cases with multiple cancers [72, 125, 129, 135, 136] and elsewhere in the colon in approximately 75% of cases with UC [76, 125, 127, 137] and 27% to 100% of cases of CC [136, 138]. Second, patients with IBD who undergo colectomy with a prior biopsy diagnosis of dysplasia are diagnosed with CRC in 20% to 50% of cases [139, 140]. Third, nearly all published results of endoscopic surveillance examinations have demonstrated neoplastic progression to CRC or high-grade dysplasia by way of lower-grade dysplasia. Fourth, IBD-associated dysplasia and CRC share common gene mutations (e.g., K-ras, TP53, and APC) and altered gene expression (e.g., Ki-67, bcl-2, surviving, YB-1, topoisomerase IIα) profiles [141, 142]. Lastly, although ethical constraints preclude randomized prospective studies to prove that endoscopic surveillance can reduce the incidence of CRC or prolong survival in the population with IBD, direct evidence from case–control studies [143] indicates that surveillance leads to the detection of cancers at relatively early stages, and circumstantial evidence [143] suggests that it is an effective strategy to reduce cancer-related mortality [143].
CRC in minority population
African American IBD patients are more likely than whites to develop colorectal cancer/disease (OR=1.9; 95% Cl 1.1–3.4) [144]. African American CD patients, but not UC patients, show lower prevalence of family history of IBD than white counterparts [144]. While the reduction in mortality attributable to CRC in the USA is evidence of the effectiveness of early screening interventions, overall survival rate for CRC among minority and disadvantaged population remains poor partly because most cancers are diagnosed at an advanced stage [145, 146]. Indeed, CRC racial disparities have widened during the last 30 years, and racial differences in prevention practice patterns among health providers have partly been blamed for excessive morbidity among minority population [147]. Study participants with higher level of CRC risk factors, like IBD, were twice as likely to report during studies that physician had ever recommended CRC screening (OR= 1.9; Cl=1.3–2.8; p<0.001) [148]. Studies detected a sizeable gap between recommended, scheduled, and completed CRC testing. This strongly suggests that increasing physician communication of CRC risk and barriers to compliance will optimize actual CRC screening rates. However, it is reasonable—and to a larger extent practical—to design a non-physician-based navigator system that reinforces physicians’ recommendation by providing information and motivation to remove personal perceived barrier of patients who are at risk for non-compliance to complete the screening.
Blacks have the highest incidence and death rates for CRC among all major races or ethnicities in the USA (Table 4) and have a higher proportion of CRC under the age of 50 years compared with whites (10.0% versus 5.5%) [4, 149]. Early colonoscopy screening (<45 years) is recommended in this group. However, it should be understood that practitioners are poorly trained or informed that they should advocate the use of screening colonoscopy in blacks beginning at the early age of 45 years. Patients who either do not want colonoscopy or do not have access to colonoscopy should be screed by the other recommended modalities [150] that may reduce mortality from CRC as a backup measure to colonoscopy. Individuals, regardless of race or ethnicity, who reside in poorer and underserved communities with lower access to health care do not experience a reduction in CRC incidence compared with more affluent communities; this remains a key barrier to colonoscopy screening [151].
Table 4.
Incidences and death rates for colon and rectal cancer (combined) by race and ethnicity, 2001–2005
| Incidence and death rates | Race and ethnicity (2001–2005) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| All | White | Black | Asian | American Indian | Hispanic | |
| Incidence: male | 61.2 | 58.9 | 71.2 | 48.0 | 46.0 | 47.3 |
| Incidence: female | 44.8 | 43.2 | 54.5 | 35.4 | 41.2 | 32.8 |
| Death: male | 21.0 | 22.1 | 31.8 | 14.4 | 20.5 | 16.5 |
| Death: female | 14.6 | 15.3 | 22.4 | 10.2 | 14.2 | 10.8 |
Rates are per 100,000 and age-adjusted to the 2000 US standard population (adapted from 3)
Restorative proctocolectomy for UC
In patients with UC, RPC and construction of an ileo-anal pouch is, to date, the criterion surgical treatment of choice. The development and refinement of pelvic pouch surgery allows the entire excision of a diseased colon while maintaining transanal continence [24]. Unfortunately, even after surgical treatment with proctocolectomy [with, Fig. 2a or without, Fig. 2b stripping (mucosectomy)], patients are not eliminated from developing neoplasia in the pouch or at anal transition zone. To date, 37 cases of adenocarcinoma of the pouch or outflow tract following RPC for UC have been reported [25, 45–55]. Malignance following RPC appeared after mucosectomy in 23 incidences as well as in 21 patients following stapled anastomosis. In one case, there is no report. The incidence of cancer following RPC with mucosectomy was more than twofold that of cancers following stapled anastomosis. Among the 37 post-RPC adenocarcinoma cases reported here, the indication for the RPC had been neoplasia in 24 (dysplasia, 15; cancer, nine) and no neoplasia in 13. Clearly, it also has been observed that patients with cancer at the original resection had a shorter interval to the development of post-RPC pouch cancer [152]. The median time was 3 years (mean, 4.3; range, 1.3–14 years) in the nine patients who originally had cancer, versus 6.5 years (mean, 7.7; range, 2–18) in the ten patients who originally had low- or high-grade dysplasia and 6.5 (mean, 8.6; range, 2–19) in the six patients who had no neoplasia. Among the 24 patients who had RPC performed on account of cancer or dysplasia, 20% (n=5) of the original lesions had occurred above the rectum.
Fig. 2.
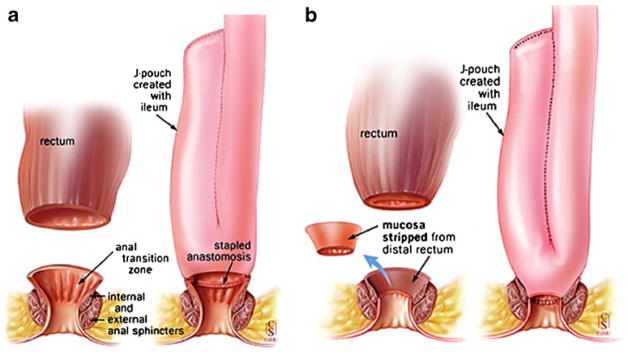
The first restorative proctocolectomy was done at St. Mark’s by Sir Alan Parks in 1978. This is a modified pouch known as “J-shaped pouch” invented by Utsunomyia. a Illustrates J-shaped ileal pouch with anal anastomosis without stripping and b shows J-shaped ileal pouch with anal anastomosis with stripping (mucosectomy). Adapted from 24
A recently published article [33] reviewed 3,203 UC patients who underwent IPAA and identified 11 patients with cancer of the ATZ or pouch body. The cumulative incidence for pouch neoplasia at 5, 10, 15, 20, and 25 years were 0.9, 1.3, 1.9, 4.2, and 5.1, respectively. In the study, it was reported that the prognosis appear poor in these patients with IBD-associated-pouch cancer, as three patients died within a year of adenocarcinoma diagnosis. In the report [33], they observed that a preoperative diagnosis of dysplasia or cancer of colon or rectum was a risk factor for pouch dysplasia or adenocarcinoma.
Indications
One third of UC patients will require surgery [22] because of either disease refractory to medical treatment and/ or dysplasia or cancer (found during screening colonoscopy). Toxic dilatation, perforation and hemorrhage are less common indications [153]. In some cases, retardation of growth in a child or adolescent and in patients receiving high-dose intravenous steroids that have a stool frequency of more than eight times per day on the third treatment day with a C-reactive protein more than 45 mg/L are unlikely to settle. Patients who initially respond but promptly relapse with the reintroduction of diet are also likely to require colectomy. In any of the aforementioned circumstances, conventional surgery or RPC is recommended following an initial subtotal colectomy where the rectum is not suitable for IRA [24]. RPC for UC should always be considered elective [24, 153]. Otherwise, it is customary to instead perform subtotal colectomy with an end ileostomy [15].
Contraindications
Contraindications of RPC for UC may be classified as absolute or relative. The absolute contra-indications are carcinoma in the low rectum requiring a total anorectal excision, incompetent anal sphincter, and emergency presentation. Patients presenting as an emergency should be treated with a colectomy and ileostomy with preservation of the rectum [154]. A period of at least 3 months should elapse before undertaking RPC. The relative contra-indications are PSC and prereproductive females.
Ileal pouch cancer after RPC for UC (vulnerability)
Conventional hand-sewn anastomosis after mucosectomy and double-stapled anastomosis without mucosectomy are the most common anastomosis techniques used [24, 155]. Controversy persists over the techniques of ileo-anal anastomosis (IAA). The majority of surgeons [155] feel that with the double-stapled anastomosis, less anastomotic complications, and superior rectal continence are achieved; however, a cuff of rectal mucosa is retained [35], the main concern and argument from the opponents of the double-stapling technique [15, 53]. It seems clear though, despite controversies, that mucosectomy does not rule out future development of subsequent pouch-related cancer [59, 60]. With the mucosectomy and hand-sewn anastomosis, little to presumably no rectal mucosa is left behind. Microscopic islands of retained rectal mucosa, however, have been reported in 20% of patients [156]. This technique has largely been abandoned in non-high-risk patients replaced by the double-stapled technique, which is said to offer better functional results [24, 157], thus higher possibilities of developing malignancies in the remaining rectal tissue [158, 159] and in the ileal pouch itself [35, 160]. In a recent case report [161], an anal canal mucinous adenocarcinoma with lymph node metastasis has been diagnosed 7 years after ileal pouch excision.
Dysplasia and neoplasia of the pouch mucosa
The incidence of dysplasia in the pouch mucosa has been reported [162]. A study from Karolinska University [163] followed 87 patients, prospectively over a median period of 6.3 years (range, 3 to 14 years). Three patients developed low-grade dysplasia. All had a type C mucosal pattern with chronic pouchitis and all demonstrated DNA aneuploidy, raising the possibility of future malignant transformation. Another study from the same institution [164] noted the development of dysplasia in five of seven of the patients with type C mucosa. Barrett [165] studied 30 UC-RPC patients with regular endoscopic review and multiple biopsies taken from the afferent limb, mid-pouch anteriorly, mid-pouch posteriorly, and the anastomotic area. Four patients demonstrated inflammation; all had mild-to-moderate chronic inflammatory changes, and one had low-grade dysplasia with a background history of chronic pouchitis. Thompson-Fawcett et al. [162] followed 106 high-risk UC-RPC patients, including 29 with Kock ileostomy for more than 14 years, 42 UC-RPC for more than 12 years and 34 patients with chronic pouchitis from a cohort of 1,221 patients. Eleven had a history of dysplasia or cancer in the original proctocolectomy specimen. Thirty-three patients had severe villous atrophy, but only one of the 106 patients had dysplasia. This was multifocal and low-grade. DNA analysis by flow cytometry demonstrated aneuploidy in this patient and in two others [162]. A collaborated study from Sweden and the UK [28] reported long-term mucosal adaptation patterns and the incidence of dysplasia in 40 patients at a mean interval of 30 years, following a Kock continent ileostomy for UC. Type A and type B mucosal patterns, based on the criteria described by Lofberg et al. [164] and Setti Carraro et al. [166] were found in 29 patients, and a type C pattern was observed in 11. There were three cases of dysplasia which was low-grade and found exclusively in the type C group. There was no case of high-grade dysplasia or adenocarcinoma. Due to a significant disagreement among the pathologists in reporting low-grade and indefinite categories of dysplasia, the incidence of indefinite and low-grade dysplasia of 27.5% and 7.5%, reported by one group of pathologists, was reported by the second group to be 7.5% and 5%, respectively. Herline et al. [167] reviewed 222 UC-RPC pouch biopsy specimens from 160 patients for an average follow-up of 8.4 years. From more than 1,800 pouches of surveillance, only one case had focal, low-grade dysplasia. A recent article from Mount Sinai School of Medicine, New York, NY [168], reported adenocarcinoma arising in an ileal pouch 29 years after UC diagnosis and 6 years after RPC.
Pouchoscopies were performed with biopsies taken from the pouch [169] during follow-up of 55 UC-RPC patients for a median of 14 years (range, 10.7–19.8 years). Interestingly, they found no patients with dysplasia in the 440 biopsy samples obtained, and all were negative for p53 antigen [169]. Similar observations were noted in 45 UC-RPC patients for clinical examination and pouch endoscopy, including mucosal biopsies [170]. The duration of their UC until surgery was a median of 6 years (range, 1–28 years) and the median time interval from start of disease until time of follow-up was 24.8 years (range, 17 to 46 years). They did not find high-grade dysplasia nor were invasive carcinomas diagnosed.
The ileal pouch has been shown to undergo histological adaptation to resemble that of colonic mucosa [164]. Few cases of true ileal pouch cancer have been apparently reported [158], arising mostly from the remnant ATZ rather than the ileal pouch itself [35], suggesting that even mucosectomy has clearly not always eliminated this risk [28].
Despite the available controversial published data, it appears that cancer in UC-RPC population, though rare, does happen and seems to relate to the duration of disease rather than to the interval from RPC [158]. In all of the reported cases, the interval from onset of UC was more than 10 years.
Neoplasia of the residual anorectal mucosa
The IAA is made between the ileal pouch and either the anal canal or the lower rectum [171] (Fig. 2). The level depends on whether a manual anastomosis without mucosectomy (Fig. 2a) or a stapled anastomosis with mucosectomy (Fig. 2b) has been performed [24]. In the former technique, the level can be controlled directly by the level at which the mucosectomy is made, although this is more difficult when carrying out a stapled anastomosis [172]. In some patients, there may therefore be a considerable length of anorectal mucosa below the anastomosis, which is considered at risk for developing neoplastic transformation [158]. One recent article from Israel [173] reported that one patient was observed to have cancer cells in random biopsies from the rectal cuff. A decade prior, she had undergone proctocolectomy and IPAA due to T2NO rectal cancer and 28 years prior total colectomy with IRA due to a right-sided T2NO colon cancer.
Inflamed anorectal mucosa following a stapled anastomosis may be symptomatic in up to 25% of patients [174], indicating that residual mucosa, the so called “strip proctitis” or “cuffitis,” may be clinically important. The longitudinal length of this segment will vary according to the level of the ileo-anal anastomosis. It may be referred to as the ATZ. Thompson-Fawcett et al. [175] showed considerable variation in the position and extent of the ATZ both from individual to individual and, to some extent, within the same individual. In almost all patients, the ileo-anal anastomosis will be more proximal, leaving a varying length of columnar epithelium. When referring to the epithelium below the ileo-anal anastomosis, the term ATZ is, perhaps, not accurate and should be replaced by the phrase “residual anorectal mucosa.” Thus, the risk of dysplasia or cancer occurring below the ileo-anal anastomosis after both a stapled and manual-sewn anastomosis is up to 16% [176, 177].
Incidence of neoplasia of the residual anorectal mucosa
In a study from St. Mark’s Hospital [178] on the incidence of dysplasia, the earliest histologic manifestation of cancer process, in the mucosectomy specimen taken from the anorectal stump during RPC of 118 patients with UC, 12 (10%) had dysplasia in the colon specimen. Dysplasia in the anorectal mucosa was found in three patients. There was a positive correlation between this and the presence of carcinoma in the operative specimen and the duration of disease. Observations from Cleveland Clinic Foundation [179] reported a significant incidence of dysplasia in the ATZ of UC-RPC patients. Low-grade dysplasia was found in 3.1% of patients and had developed at a median period of 16 months, post-operatively. No association was found, however, between dysplasia and the duration of UC, the use of double-stapled versus single-stapled technique or the distance of the anastomosis from the pectineal line. A similar study from the same institution [180] later reported the incidence and natural history of dysplasia of the ATZ in UC-RPC patients after a median follow-up of 77 months. Dysplasia developed in seven (3%) of 210 patients. High-grade dysplasia was seen in one patient, and the risk of dysplasia was significantly increased in patients with prior cancer or dysplasia in the colon. In a larger series [181], again from the same institution, 289 patients were followed by regular examinations and biopsies of the ATZ. Dysplasia was found in eight (4.5) patients at a median period of 9 months post-RPC. High-grade dysplasia was seen in two patients, one with a history of chronic pouchitis and the other with preoperative dysplasia in the colon. All eight of the patients with dysplasia were either followed closely or underwent mucosectomy with pouch advancement and re-anastomosis via an endo-anal approach. No patient developed carcinoma. There was no association between the occurrence of dysplasia and gender, age, preoperative disease duration, or extent of colitis; however, dysplasia was significantly associated with cancer or dysplasia in the colon or rectum in the proctocolectomy specimen. Indisputably, dysplasia in the ATZ, though rare, warrants long-term surveillance in these patients.
Scientists from John Radcliffe Hospital, in the UK [177], assessed the risk of dysplasia and the presence of aneuploidy in the columnar cuff epithelium after stapled IAA in 113 patients with UC. The reported mean follow-up after pouch formation was 2.5 years. Successful columnar cuff biopsies were performed in 93% of patients, and no patient with dysplasia was found. Two biopsy specimens from one patient showed aneuploidy. Another study [182], also from the UK, reviewed 135 UC-RPC patients for a median of 56 months, and the median interval from the diagnosis of disease was 8.8 years. There was no evidence of either dysplasia or carcinoma in the anorectal mucosa up to 12 years after surgery. As a result, they suggested that cuff surveillance in the first decade after stapled RPC, in the absence of dysplasia or carcinoma in the original colectomy specimen, may be unnecessary.
Risk factors for neoplastic transformation
There are six observed risk factors for neoplastic transformation following RPC for UC, namely: dysplasia or cancer in the operative specimen; interval from the diagnosis of UC; type C mucosal changes; extraintestinal manifestations (EIM) and manual versus stapled anastomotic technique.
Pouchitis
Pouchitis is inflammation of the ileal pouch [15, 25]. Pouchitis is the most common long-term complication of patients following RPC and IPAA. Thirty-two percent of patients with an ileo-anal pouch have had at least one episode of pouchitis [15]. Symptoms of pouchitis are often similar to those of ulcerative colitis, including diarrhea, abdominal pain, bleeding, fever, joint pain, and increased stool frequency. In UC, the prevalence of pouchitis varies widely from less than 7% to as high as 44%. Histology review suggests that chronic inflammation of the pouch might be associated with pouch adenocarcinoma [33]. Because pouch cancer surveillance biopsies revealed significant inflammation, there is unconfirmed speculation that chronic mucosal inflammation may predispose RPC patients to developing dysplasia or cancer [33, 183].
Dysplasia or cancer in the operative specimen
Most observations have shown that the incidence of dysplasia following RPC is increased with a preoperative history of dysplasia or carcinoma or a finding of the same in the original operative specimen [162]. Twenty-two of 41 (54%) of the patients, who were reported to have developed cancer in the anorectal mucosa or in the ileo-anal pouch, had dysplasia or cancer that was identified before RPC or that was found subsequently in the operative specimen. This appears to be the likely, important predisposing risk factor for the transformation and/or development of neoplasia following RPC.
Interval from the diagnosis of UC
The studies [184] have shown that the risk of colorectal cancer in patients with long-standing UC increases with time and is related to the anatomical extent of the disease in the operative specimen [185]. Pouch-related cancer did not occur in any of the patients before 10 years from the onset of UC, and the median interval was 20 years. The interval from RPC (median, 5 years; range, 1.3 to 18 years) has a greater variance, and it seems likely that the time of onset of the disease is the vital interval when considering surveillance.
Type C mucosal change
A type C mucosal pattern of the pouch mucosa is observed to be associated with chronic pouchitis and also with dysplasia and aneuploidy. While Setti Carraro et al. [166] observed that these patients can be identified within months of RPC by histopathological examination of the biopsy material, others [162] did not demonstrate an association between chronic pouchitis and dysplasia, and furthermore, some have not found dysplasia at all in patients undergoing pouch surveillance [186].
Extraintestinal manifestations
The association between type C changes and EIM, including PSC and their apparent relationship with dysplasia, suggests that these patients are at a higher risk of developing pouch-related neoplastic transformation [164].
Manual versus stapled anastomotic technique
To date, 25 patients with carcinoma in the anorectal mucosa [33, 53] following RPC for UC have been reported. Out of these, 14 occurred after stapled anastomosis without mucosectomy [44, 53, 187] and nine after mucosectomy and hand-sewn anastomosis (in two cases, the technique was not described), indicating that carcinoma can occur after either form of anastomosis. The cumulative risk of carcinoma in the anorectal mucosa from prospective life table analysis is lacking. It is possible that patients who had received a stapled anastomosis [161, 178, 182, 188] are at significant risk in the long-term where there is residual rectal mucosa, particularly if dysplasia was present preoperatively.
Surveillance for neoplastic transformation
Recommended guidelines have recently been developed at St. Mark’s Hospital [27] to provide consistent evidence-based care by the pouch specialist practitioners. The obvious challenging problem is the wide inclusion of patients with different quality of surveillance. Surveillance using “flexible-pouchoscopy” is recommended to be undertaken every 5 years and annually in those at high risk of neoplastic transformation [158]. Delaini et al. [189] suggest that intense follow-up and research-based evidence is obligatory in maintaining RPC as the “criterion standard” procedure for UC.
Despite the fact that RPC risk surveillance is costly [158], the good news is that the incidence of carcinoma in the ileal pouch or anorectal mucosa appears to be rare during the first 10 years after RPC. It is not clear as to whether there is a metaplasia–dysplasia–carcinoma sequence [54, 170] following RPC or whether there is simply increased risk of sporadic cancer in the ileal pouch of certain, susceptible individuals. The literature, however, suggests that patients with preoperative neoplastic transformation, type C pouch mucosal changes, PSC, and antecedent dysplasia/carcinoma are at higher vulnerability of developing dysplasia or invasive adenocarcinoma than the normal population undergoing RPC. These features merit concern and follow-up. Both the pouch and the anorectal mucosa should be monitored by endoscopic biopsy [158]. Initial biopsies from the pouch should be taken at 6 to 12 months post-operatively to identify the appearance of type C changes. From 10 years onward from the onset of disease, pouchoscopy with multiple biopsies and anorectal mucosal biopsies should be considered. This should be repeated annually from 10 years from the onset of UC.
Alternative forms of surveillance have been proposed by Elkowitz et al. [190] including the p53 over expression and aneuploidy of biopsies, in addition to histopathological assessment for dysplasia. A method of surveillance of the anorectal mucosa using high-magnification chromatoscopic pouchoscopy has been described [46]. The method gives an accurate assessment of anorectal surface microanatomy, permitting accurate biopsy targeting. It is possible that these approaches may become part of future surveillance.
Summary
CRC, the most lethal complication of IBD, is the culmination of a complex sequence of molecular and histologic derangements of the colon epithelium that are initiated and at least partially sustained by prolonged long-standing chronic inflammation. Dysplasia, the earliest histologic manifestation of this process, plays vital role in colon cancer prevention by providing the first clinical alert that this sequence is under way and by serving as endpoint in colonoscopic surveillance of patients at high risk for CRC. The diagnosis and grading of dysplasia in endoscopic surveillance biopsies play a decisive role in the management of patients with IBD. The selection of appropriate endpoint for surgical intervention, when dysplasia is diagnosed, requires striking a balance between the risks of potentially unnecessary surgery and those of progression to incurable cancer where intervention is delayed. Unfortunately, even after thorough considerations and pelvic pouch surgery, some patients still get pouch-related cancer [33]. This may happen because: (1) following either mucosectomy or stapled anastomosis, pouch cancer may still occur; (2) this cancer can occur after RPC performed for UC or CC either with or without neoplasia; and (3) this complication is seen whether or not the initial cancer or dysplasia had involved the rectum. A negative pathology report may not mean that the patient is free of future pouch dysplasia or malignancy. Although the current risk of developing ileal pouch cancer is rare, it is apparently increasing in frequency. Those patients with long-standing pouchitis are at higher risk of developing adenocarcinoma at the sight of inflammation in the ileal pouch. Therefore, routine surveillance, as often as every 6 months to a year, is warranted in such patients.
Conclusion
Prolonged chronic inflammation seems, partially, to be the precursor of IBD-associated CRC. Genetic predisposition linked to heritable factors increase susceptibility of IBD-associated CRC. Neoplastic lesion(s) in patients with functional pouches is real. The risk does not limit to the IBD patients with precolectomy dysplasia or cancer. Pouch-related cancers after RPC, particularly in patients with long-standing pouchitis, proctitis, and cuffitis are susceptible and not absolutely preventable for developing pouch neoplasia. The prognosis of pouch adenocarcinoma appears to be poor. Although surveillance has been widely embraced and broadly implemented current endoscopic surveillance modality seems to be inadequate to detect dysplasia preceding pouch adenocarcinoma. The greatest promise for cancer prevention in IBD lies largely in expanding our, thus far, limited knowledge of the molecular pathogenetic relationships between neoplasia and chronic inflammation pathways.
A vast majority of minority patients do not receive standard CRC testing in urban safety-net primary care settings. This first illustrates the need to empower providers to communicate with patients regarding scheduling and completing the recommended CRC testing. The second is the need to actively involve patients in self-management practices that allow them to accept the benefits of early testing and adhere to recommended tests. Unfortunately, this is still a big problem in primary health care disparities.
Acknowledgments
Source of support for this study is *3U54 CA091408 09 S1 (MMC-VICC Cancer Partnership) and the *Research Foundation, American Society of Colon and Rectal Surgeons (ASCRS)-LPG-086.
We acknowledge all the scientists who made contributions to the areas of research that are reviewed here but were not cited owing to space constraints. All authors declare their views to be independent of the research funders.
Footnotes
All the authors substantially not only contributed to conception and design but participated in the acquisition of data, analysis and interpretation of data, and drafting the manuscript.
Competing interests statement The author declares no competing financial interests.
Contributor Information
Amosy E. M’Koma, Email: amkoma@mmc.edu, Department of Biochemistry and Cancer Biology, Meharry Medical College School of Medicine, 1005 Dr. D. B. Todd Jr. Blvd, Nashville, TN 37208-3599, USA. Vanderbilt-Ingram-Cancer Center, Vanderbilt University School of Medicine, Nashville, TN, USA
Harold L. Moses, Vanderbilt-Ingram-Cancer Center, Vanderbilt University School of Medicine, Nashville, TN, USA
Samuel E. Adunyah, Department of Biochemistry and Cancer Biology, Meharry Medical College School of Medicine, 1005 Dr. D. B. Todd Jr. Blvd, Nashville, TN 37208-3599, USA. Vanderbilt-Ingram-Cancer Center, Vanderbilt University School of Medicine, Nashville, TN, USA
References
- 1.Pochapin M. What your doctor nay not tell you about colorectal cancer: New tests, new treatment, new hope. Warner Books; New York: 2004. [Google Scholar]
- 2.NIC. Colon and rectal cancer. 2008 http://www.canncer.gov/cancertopics/types/colon-and-rectal.
- 3.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 4.Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 2008;135(4):1079–1099. doi: 10.1053/j.gastro.2008.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delaunoit T, Limburg PJ, Goldberg RM, Lymp JF, Loftus EV., Jr Colorectal cancer prognosis among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4(3):335–342. doi: 10.1016/j.cgh.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 6.Erdman SE, Poutahidis T. Cancer inflammation and regulatory T cells. Int J Cancer. 2010;127(4):768–779. doi: 10.1002/ijc.25430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erdman SE, Poutahidis T. Roles for inflammation and regulatory T cells in colon cancer. Toxicol Pathol. 2010;38(1):76–87. doi: 10.1177/0192623309354110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin TK, Erdman SH. Double-balloon enteroscopy: pediatric experience. J Pediatr Gastroenterol Nutr. 2010;51(4):429–432. doi: 10.1097/MPG.0b013e3181d2979c. [DOI] [PubMed] [Google Scholar]
- 9.Houghton J, Li H, Fan X, Liu Y, Liu JH, Rao VP, Poutahidis T, Taylor CL, Jackson EA, Hewes C, Lyle S, Cerny A, Bowen G, Cerny J, Moore N, Kurt-Jones EA, Erdman SE. Mutations in bone marrow-derived stromal stem cells unmask latent malignancy. Stem Cells Dev. 2010;19(8):1153–1166. doi: 10.1089/scd.2009.0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt C, Bielecki C, Felber J, Stallmach A. Surveillance strategies in inflammatory bowel disease. Minerva Gastro-enterol Dietol. 2010;56(2):189–201. [PubMed] [Google Scholar]
- 11.Harpaz N, Polydorides AD. Colorectal dysplasia in chronic inflammatory bowel disease: Pathology, clinical implications, and pathogenesis. Arch Pathol Lab Med. Jun;134(6):876–895. doi: 10.5858/134.6.876. [DOI] [PubMed] [Google Scholar]
- 12.Hardy RG, Meltzer SJ, Jankowski JA. ABC of colorectal cancer. Molecular basis for risk factors. BMJ. 2000;321(7265):886–889. doi: 10.1136/bmj.321.7265.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie J, Itzkowitz SH. Cancer in inflammatory bowel disease. World J Gastroenterol. 2008;14(3):378–389. doi: 10.3748/wjg.14.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vleggaar FP, Lutgens MW, Oldenburg B, Schipper ME, Samsom M. British and American screening guidelines inadequate for prevention of colorectal carcinoma in patients with inflammatory bowel disease. Ned Tijdschr Geneeskd. 2007;151(50):2787–2791. [PubMed] [Google Scholar]
- 15.Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126(2):451–459. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Crohn’s BRH. The sigmoidoscopic picture of chronic ulcerative colitis (n0n-specific) Ame J Med Sci. 1925;170:220–228. [Google Scholar]
- 17.Warren S, Sommers SC. Cicatrizing enteritis as a pathologic entity; analysis of 120 cases. Am J Pathol. 1948;24(3):475–501. [PMC free article] [PubMed] [Google Scholar]
- 18.Soderlund S, Granath F, Brostrom O, et al. Inflammatory bowel disease confers a lower risk of colorectal cancer to females than to males. Gastroenterology. May;138(5):1697–1703. doi: 10.1053/j.gastro.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Itzkowitz SH, Present DH. Consensus conference: Colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11(3):314–321. doi: 10.1097/01.mib.0000160811.76729.d5. [DOI] [PubMed] [Google Scholar]
- 20.Barthet M, Gay G, Sautereau D, et al. Endoscopic surveillance of chronic inflammatory bowel disease. Endoscopy. 2005;37(6):597–599. doi: 10.1055/s-2005-861421. [DOI] [PubMed] [Google Scholar]
- 21.Langholz E, Munkholm P, Davidsen M, Binder V. Colorectal cancer risk and mortality in patients with ulcerative colitis. Gastroenterology. 1992;103(5):1444–1451. doi: 10.1016/0016-5085(92)91163-x. [DOI] [PubMed] [Google Scholar]
- 22.Bach SP, Mortensen NJ. Ileal pouch surgery for ulcerative colitis. World J Gastroenterol. 2007;13(24):3288–3300. doi: 10.3748/wjg.v13.i24.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das P, Smith JJ, Tekkis PP, Heriot AG, Antropoli M, John Nicholls R. Quality of life after indefinite diversion/pouch excision in ileal pouch failure patients. Colorectal Dis. 2007;9(8):718–724. doi: 10.1111/j.1463-1318.2007.01216.x. [DOI] [PubMed] [Google Scholar]
- 24.M’Koma AE, Wise PE, Muldoon RL, Schwartz DA, Washington MK, Herline AJ. Evolution of the restorative proctocolectomy and its effects on gastrointestinal hormones. Int J Colorectal Dis. 2007;22(10):1143–1163. doi: 10.1007/s00384-007-0331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bulow S, Bulow C, Vasen H, Jarvinen H, Bjork J, Christensen IJ. Colectomy and ileorectal anastomosis is still an option for selected patients with familial adenomatous polyposis. Dis Colon Rectum. 2008;51(9):1318–1323. doi: 10.1007/s10350-008-9307-3. [DOI] [PubMed] [Google Scholar]
- 26.Parks AG, Nicholls RJ. Proctocolectomy without ileostomy for ulcerative colitis. Br Med J. 1978;2(6130):85–88. doi: 10.1136/bmj.2.6130.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLaughlin SD, Clark SK, Tekkis PP, Ciclitira PJ, Nicholls RJ. Review article: Restorative proctocolectomy, indications, management of complications and follow-up–a guide for gastro-enterologists. Aliment Pharmacol Ther. 2008;27(10):895–909. doi: 10.1111/j.1365-2036.2008.03643.x. [DOI] [PubMed] [Google Scholar]
- 28.Hulten L, Willen R, Nilsson O, Safarani N, Haboubi N. Mucosal assessment for dysplasia and cancer in the ileal pouch mucosa in patients operated on for ulcerative colitis–a 30-year follow-up study. Dis Colon Rectum. 2002;45(4):448–452. doi: 10.1007/s10350-004-6218-9. [DOI] [PubMed] [Google Scholar]
- 29.Paterson CA, Dozois RR. The ileal pouch-anal anastomosis: Success and failure. Chirurgie. 1998;123(6):545–549. doi: 10.1016/s0001-4001(99)80001-4. [DOI] [PubMed] [Google Scholar]
- 30.Neilly P, Neill ME, Hill GL. Restorative proctocolectomy with ileal pouch-anal anastomosis in 203 patients: The Auckland experience. Aust N Z J Surg. 1999;69(1):22–27. doi: 10.1046/j.1440-1622.1999.01464.x. [DOI] [PubMed] [Google Scholar]
- 31.Aziz O, Athanasiou T, Fazio VW, et al. Meta-analysis of observational studies of ileorectal versus ileal pouch-anal anastomosis for familial adenomatous polyposis. Br J Surg. 2006;93 (4):407–417. doi: 10.1002/bjs.5276. [DOI] [PubMed] [Google Scholar]
- 32.Vento P, Lepistö A, Kärkkäinen P, Ristimäki A, Haglund C, Järvinen HJ. Risk of cancer in patients with chronic pouchitis after restorative proctocolectomy for ulcerative colitis. Colorectal Dis. 2009;13(1):58–66. doi: 10.1111/j.1463-1318.2009.02058.x. [DOI] [PubMed] [Google Scholar]
- 33.Kariv R, Remzi FH, Lian L, Bennett AE, Kiran RP, Kariv Y, Fazio VW, Lavery IC, Shen B. Preoperative colorectal neoplasia increases risk for pouch neoplasia in patients with restorative proctocolectomy. Gastroenterology. 2010;139(3):806–812. doi: 10.1053/j.gastro.2010.05.085. [DOI] [PubMed] [Google Scholar]
- 34.Kariv R, Remzi FH, Lian L, et al. Preoperative colorectal neoplasia increases risk for pouch neoplasia in patients with restorative proctocolectomy. Gastroenterology. 2010 Jun 9; doi: 10.1053/j.gastro.2010.05.085. [DOI] [PubMed] [Google Scholar]
- 35.Heppell J, Weiland LH, Perrault J, Pemberton JH, Telander RL, Beart RW., Jr Fate of the rectal mucosa after rectal mucosectomy and ileoanal anastomosis. Dis Colon Rectum. 1983;26 (12):768–771. doi: 10.1007/BF02554744. [DOI] [PubMed] [Google Scholar]
- 36.Soderlund S, Granath F, Brostrom O, et al. Inflammatory bowel disease confers a lower risk of colorectal cancer to females than to males. Gastroenterology. 2010;138(5):1697–1703. doi: 10.1053/j.gastro.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Naugler WE, Sakurai T, Kim S, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317(5834):121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 38.Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317(5834):124–127. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- 39.Tulchinsky H. Colorectal carcinoma in inflammatory bowel disease. Annu Congr Am Soc Colon Rectal Surg. 2010;98:77. [Google Scholar]
- 40.Stern H, Walfisch S, Mullen B, McLeod R, Cohen Z. Cancer in an ileoanal reservoir: A new late complication? Gut. 1990;31 (4):473–475. doi: 10.1136/gut.31.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Negi SS, Chaudhary A, Gondal R. Carcinoma of pelvic pouch following restorative proctocolectomy: Report of a case and review of the literature. Dig Surg. 2003;20(1):63–65. doi: 10.1159/000068855. [DOI] [PubMed] [Google Scholar]
- 42.Bell SW, Parry B, Neill M. Adenocarcinoma in the anal transitional zone after ileal pouch for ulcerative colitis: Report of a case. Dis Colon Rectum. 2003;46(8):1134–1137. doi: 10.1007/s10350-004-7293-7. [DOI] [PubMed] [Google Scholar]
- 43.Kotanagi H, Kon H, Iida M, Ito M, Koyama K. Adenocarcinoma at the site of ileoanal anastomosis in Crohn’s disease: Report of a case. Dis Colon Rectum. 2001;44(8):1210–1213. doi: 10.1007/BF02234646. [DOI] [PubMed] [Google Scholar]
- 44.Sequens R. Cancer in the anal canal (transitional zone) after restorative proctocolectomy with stapled ileal pouch-anal anastomosis. Int J Colorectal Dis. 1997;12(4):254–255. doi: 10.1007/s003840050100. [DOI] [PubMed] [Google Scholar]
- 45.Puthu D, Rajan N, Rao R, Rao L, Venugopal P. Carcinoma of the rectal pouch following restorative proctocolectomy. Report of a case. Dis Colon Rectum. 1992;35(3):257–260. doi: 10.1007/BF02051019. [DOI] [PubMed] [Google Scholar]
- 46.Iwama T, Kamikawa J, Higuchi T, et al. Development of invasive adenocarcinoma in a long-standing diverted ileal J-pouch for ulcerative colitis: Report of a case. Dis Colon Rectum. 2000;43(1):101–104. doi: 10.1007/BF02237251. [DOI] [PubMed] [Google Scholar]
- 47.Botterill ID, Sagar PM. Adenocarcinoma in the residual outflow tract after incomplete excision of a failed ileoanal pouch: A late complication of unrecognized Crohn’s colitis: Report of a case. Dis Colon Rectum. 2002;45(8):1112–1115. doi: 10.1007/s10350-004-6370-2. [DOI] [PubMed] [Google Scholar]
- 48.Laureti S, Ugolini F, D’Errico A, Rago S, Poggioli G. Adenocarcinoma below ileoanal anastomosis for ulcerative colitis: Report of a case and review of the literature. Dis Colon Rectum. 2002;45(3):418–421. doi: 10.1007/s10350-004-6194-0. [DOI] [PubMed] [Google Scholar]
- 49.Vieth M, Grunewald M, Niemeyer C, Stolte M. Adenocarcinoma in an ileal pouch after prior proctocolectomy for carcinoma in a patient with ulcerative pancolitis. Virchows Arch. 1998;433(3):281–284. doi: 10.1007/s004280050248. [DOI] [PubMed] [Google Scholar]
- 50.Hyman N. Rectal cancer as a complication of stapled IPAA. Inflamm Bowel Dis. 2002;8(1):43–45. doi: 10.1097/00054725-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 51.Baratsis S, Hadjidimitriou F, Christodoulou M, Lariou K. (691) Adenocarcinoma in the anal canal after ileal pouch-anal anastomosis for ulcerative colitis using a double stapling technique: Report of a case. Dis Colon Rectum. 45(5):687. doi: 10.1007/s10350-004-6268-z. discussion 691–682. [DOI] [PubMed] [Google Scholar]
- 52.Hassan C, Zullo A, Speziale G, Stella F, Lorenzetti R, Morini S. Adenocarcinoma of the ileoanal pouch anastomosis: An emerging complication? Int J Colorectal Dis. 2003;18(3):276–278. doi: 10.1007/s00384-002-0452-1. [DOI] [PubMed] [Google Scholar]
- 53.Rotholtz NA, Pikarsky AJ, Singh JJ, Wexner SD. Adenocarcinoma arising from along the rectal stump after double-stapled ileorectal J-pouch in a patient with ulcerative colitis: The need to perform a distal anastomosis. Report of a case. Dis Colon Rectum. 2001;44(8):1214–1217. doi: 10.1007/BF02234647. [DOI] [PubMed] [Google Scholar]
- 54.Heuschen UA, Heuschen G, Autschbach F, Allemeyer EH, Herfarth C. Adenocarcinoma in the ileal pouch: Late risk of cancer after restorative proctocolectomy. Int J Colorectal Dis. 2001;16(2):126–130. doi: 10.1007/s003840000276. [DOI] [PubMed] [Google Scholar]
- 55.Pedersen ME, Rahr HB, Fenger C, Qvist N. Adenocarcinoma arising from the rectal stump eleven years after excision of an ileal J-pouch in a patient with ulcerative colitis: Report of a case. Dis Colon Rectum. 2008;51(7):1146–1148. doi: 10.1007/s10350-008-9238-z. [DOI] [PubMed] [Google Scholar]
- 56.Ota H, Yamazaki K, Endoh W, et al. Adenocarcinoma arising below an ileoanal anastomosis after restorative proctocolectomy for ulcerative colitis: Report of a case. Surg Today. 2007;37 (7):596–599. doi: 10.1007/s00595-006-3452-x. [DOI] [PubMed] [Google Scholar]
- 57.Chia CS, Chew MH, Chau YP, Eu KW, Ho KS. Adenocarcinoma of the anal transitional zone after double stapled ileal pouch-anal anastomosis for ulcerative colitis. Colorectal Dis. 2008;10(6):621–623. doi: 10.1111/j.1463-1318.2007.01402.x. [DOI] [PubMed] [Google Scholar]
- 58.Rodriguez-Sanjuan JC, Polavieja MG, Naranjo A, Castillo J. Adenocarcinoma in an ileal pouch for ulcerative colitis. Dis Colon Rectum. 1995;38(7):779–780. doi: 10.1007/BF02048042. [DOI] [PubMed] [Google Scholar]
- 59.Knupper N, Straub E, Terpe HJ, Vestweber KH. Adenocarcinoma of the ileoanal pouch for ulcerative colitis–a complication of severe chronic atrophic pouchitis? Int J Colorectal Dis. 2006;21(5):478–482. doi: 10.1007/s00384-005-0063-8. [DOI] [PubMed] [Google Scholar]
- 60.Walker M, Radley S. Adenocarcinoma in an ileoanal pouch formed for ulcerative colitis in a patient with primary sclerosing cholangitis and a liver transplant: Report of a case and review of the literature. Dis Colon Rectum. 2006;49(6):909–912. doi: 10.1007/s10350-006-0517-2. [DOI] [PubMed] [Google Scholar]
- 61.Bentrem DJ, Wang KL, Stryker SJ. Adenocarcinoma in an ileal pouch occurring 14 years after restorative proctocolectomy: Report of a case. Dis Colon Rectum. 2003;46(4):544–546. doi: 10.1007/s10350-004-6597-y. [DOI] [PubMed] [Google Scholar]
- 62.Lee SW, Sonoda T, Milsom JW. Three cases of adenocarcinoma following restorative proctocolectomy with hand-sewn anastomosis for ulcerative colitis: A review of reported cases in the literature. Colorectal Dis. 2005;7(6):591–597. doi: 10.1111/j.1463-1318.2005.00794.x. [DOI] [PubMed] [Google Scholar]
- 63.Sagar P. Adenocarcinoma in a pouch without a preceeding history of dysplasia. Colorectal Dis. 2006;8(6):526–527. doi: 10.1111/j.1463-1318.2006.01054.x. [DOI] [PubMed] [Google Scholar]
- 64.Branco BC, Sachar DB, Heimann TM, Sarpel U, Harpaz N, Greenstein AJ. Adenocarcinoma following ileal pouch-anal anastomosis for ulcerative colitis: Review of 26 cases. Inflamm Bowel Dis. 2009;15(2):295–299. doi: 10.1002/ibd.20609. [DOI] [PubMed] [Google Scholar]
- 65.Ault GT, Nunoo-Mensah JW, Johnson L, Vukasin P, Kaiser A, Beart RW., Jr Adenocarcinoma arising in the middle of ileoanal pouches: Report of five cases. Dis Colon Rectum. 2009;52 (3):538–541. doi: 10.1007/DCR.0b013e318199effe. [DOI] [PubMed] [Google Scholar]
- 66.Rubin DT, Parekh N. Colorectal cancer in inflammatory bowel disease: Molecular and clinical considerations. Curr Treat Options Gastroenterol. 2006;9(3):211–220. doi: 10.1007/s11938-006-0040-5. [DOI] [PubMed] [Google Scholar]
- 67.Eaden J. Review article: Colorectal carcinoma and inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20(Suppl 4):24–30. doi: 10.1111/j.1365-2036.2004.02046.x. [DOI] [PubMed] [Google Scholar]
- 68.Harpaz N, Talbot IC. Colorectal cancer in idiopathic inflammatory bowel disease. Semin Diagn Pathol. 1996;13(4):339–357. [PubMed] [Google Scholar]
- 69.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: A meta-analysis. Gut. 2001;48 (4):526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rubio CA, Befrits R, Ljung T, Jaramillo E, Slezak P. Colorectal carcinoma in ulcerative colitis is decreasing in Scandinavian countries. Anticancer Res. 2001;21(4B):2921–2924. [PubMed] [Google Scholar]
- 71.Loftus EV., Jr Epidemiology and risk factors for colorectal dysplasia and cancer in ulcerative colitis. Gastroenterol Clin North Am. 2006;35(3):517–531. doi: 10.1016/j.gtc.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 72.Choi PM, Zelig MP. Similarity of colorectal cancer in Crohn’s disease and ulcerative colitis: Implications for carcino-genesis and prevention. Gut. 1994;35(7):950–954. doi: 10.1136/gut.35.7.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Svrcek M, Cosnes J, Beaugerie L, et al. Colorectal neoplasia in Crohn’s colitis: A retrospective comparative study with ulcerative colitis. Histopathology. 2007;50(5):574–583. doi: 10.1111/j.1365-2559.2007.02663.x. [DOI] [PubMed] [Google Scholar]
- 74.Rubio CA, Befrits R. Colorectal adenocarcinoma in Crohn’s disease: A retrospective histologic study. Dis Colon Rectum. 1997;40(9):1072–1078. doi: 10.1007/BF02050932. [DOI] [PubMed] [Google Scholar]
- 75.Canavan C, Abrams KR, Mayberry J. Meta-analysis: Colorectal and small bowel cancer risk in patients with Crohn’s disease. Aliment Pharmacol Ther. 2006;23(8):1097–1104. doi: 10.1111/j.1365-2036.2006.02854.x. [DOI] [PubMed] [Google Scholar]
- 76.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323(18):1228–1233. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 77.Karlen P, Lofberg R, Brostrom O, Leijonmarck CE, Hellers G, Persson PG. Increased risk of cancer in ulcerative colitis: A population-based cohort study. Am J Gastroenterol. 1999;94 (4):1047–1052. doi: 10.1111/j.1572-0241.1999.01012.x. [DOI] [PubMed] [Google Scholar]
- 78.Lindberg J, Stenling R, Palmqvist R, Rutegard J. Early onset of ulcerative colitis: Long-term follow-up with special reference to colorectal cancer and primary sclerosing cholangitis. J Pediatr Gastroenterol Nutr. 2008;46(5):534–538. doi: 10.1097/MPG.0b013e31815a98ef. [DOI] [PubMed] [Google Scholar]
- 79.Mir-Madjlessi SH, Farmer RG, Easley KA, Beck GJ. Colorectal and extracolonic malignancy in ulcerative colitis. Cancer. 1986;58(7):1569–1574. doi: 10.1002/1097-0142(19861001)58:7<1569::aid-cncr2820580731>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 80.Sugita A, Sachar DB, Bodian C, Ribeiro MB, Aufses AH, Jr, Greenstein AJ. Colorectal cancer in ulcerative colitis. Influence of anatomical extent and age at onset on colitis-cancer interval. Gut. 1991;32(2):167–169. doi: 10.1136/gut.32.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gupta RB, Harpaz N, Itzkowitz S, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: A cohort study. Gastroenterology. 2007;133(4):1099–1105. doi: 10.1053/j.gastro.2007.08.001. quiz 1340–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eaden J, Abrams K, Ekbom A, Jackson E, Mayberry J. Colorectal cancer prevention in ulcerative colitis: A case-control study. Aliment Pharmacol Ther. 2000;14(2):145–153. doi: 10.1046/j.1365-2036.2000.00698.x. [DOI] [PubMed] [Google Scholar]
- 83.Mahadevan U. Evaluation of activity in ulcerative colitis (UC)—who is right: The clinician, endoscopist or pathologist? Gastroenterology. 1998;14(4 Suppl 1):1030–1031. [Google Scholar]
- 84.Gomes P, du Boulay C, Smith CL, Holdstock G. Relationship between disease activity indices and colonoscopic findings in patients with colonic inflammatory bowel disease. Gut. 1986;27(1):92–95. doi: 10.1136/gut.27.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rhodes JM. Ulcerative colitis extent varies with time but endoscopic appearances may be deceptive. Gut. 2001;49(3):322–323. doi: 10.1136/gut.49.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nuako KW, Ahlquist DA, Mahoney DW, Schaid DJ, Siems DM, Lindor NM. Familial predisposition for colorectal cancer in chronic ulcerative colitis: A case-control study. Gastroenter-ology. 1998;115(5):1079–1083. doi: 10.1016/s0016-5085(98)70077-0. [DOI] [PubMed] [Google Scholar]
- 87.Askling J, Dickman PW, Karlen P, et al. Family history as a risk factor for colorectal cancer in inflammatory bowel disease. Gastroenterology. 2001;120(6):1356–1362. doi: 10.1053/gast.2001.24052. [DOI] [PubMed] [Google Scholar]
- 88.Mendeloff AI, Monk M, Siegel CI, Lilienfeld A. Some epidemiological features of ulcerative colitis and regional enteritis. A preliminary report. Gastroenterology. 1966;51(5):748–756. [PubMed] [Google Scholar]
- 89.Wright JP, Froggatt J, O’Keefe EA, et al. The epidemiology of inflammatory bowel disease in Cape Town 1980–1984. S Afr Med J. 1986;70(1):10–15. [PubMed] [Google Scholar]
- 90.Wright JP, Marks IN, Jameson C, Garisch JA, Burns DG, Kottler RE. Inflammatory bowel disease in Cape Town, 1975–1980. Part I. Ulcerative colitis. S Afr Med J. 1983;63(7):223–226. [PubMed] [Google Scholar]
- 91.Rogers BH, Clark LM, Kirsner JB. The epidemiologic and demographic characteristics of inflammatory bowel disease: An analysis of a computerized file of 1400 patients. J Chron Dis. 1971;24 (12):743–773. doi: 10.1016/0021-9681(71)90087-7. [DOI] [PubMed] [Google Scholar]
- 92.Kurata JH, Kantor-Fish S, Frankl H, Godby P, Vadheim CM. Crohn’s disease among ethnic groups in a large health maintenance organization. Gastroenterology. 1992;102(6):1940–1948. doi: 10.1016/0016-5085(92)90317-r. [DOI] [PubMed] [Google Scholar]
- 93.Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: A population-based study. Cancer. 2001;91(4):854–862. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 94.Ahmadi A, Polyak S, Draganov PV. Colorectal cancer surveillance in inflammatory bowel disease: The search continues. World J Gastroenterol. 2009;15(1):61–66. doi: 10.3748/wjg.15.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kornfeld D, Ekbom A, Ihre T. Is there an excess risk for colorectal cancer in patients with ulcerative colitis and concomitant primary sclerosing cholangitis? A population based study. Gut. 1997;41(4):522–525. doi: 10.1136/gut.41.4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Triantafillidis JK, Nasioulas G, Kosmidis PA. Colorectal cancer and inflammatory bowel disease: Epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res. 2009;29(7):2727–2737. [PubMed] [Google Scholar]
- 97.Loftus EV, Jr, Harewood GC, Loftus CG, et al. PSC-IBD: A unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54(1):91–96. doi: 10.1136/gut.2004.046615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Allavena P, Garlanda C, Borrello MG, Sica A, Mantovani A. Pathways connecting inflammation and cancer. Curr Opin Genet Dev. 2008;18(1):3–10. doi: 10.1016/j.gde.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 99.Talero E, Sanchez-Fidalgo S, Villegas I, de la Lastra CA, Illanes M, Motilva V. Role of different inflammatory and tumor biomarkers in the development of ulcerative colitis-associated carcinogenesis. Inflamm Bowel Dis. 2010 Aug 18; doi: 10.1002/ibd.21420. [DOI] [PubMed] [Google Scholar]
- 100.M’Koma AE. Proteomic Patterns of Colonic Submucosa Discriminates Inflammatory Colitides. Annual Congress; Hollywood, Florida: The American Society of Colon and Rectal Surgeons; 2009. pp. 47–166. [Google Scholar]
- 101.M’Koma AE. Proteomic analysis of colonic submucosa differentiates Crohn’s and ulcerative colitis. Annual Congress-Digestive Disease Week; Chicago, IL. 2009. p. M1096.p. 600. [Google Scholar]
- 102.M’Koma AE, Seeley EH, Washington MK, Schwartz DA, Muldoon RL, Herline AJ, Wise PE, Caprioli RM. Proteomic profiling of mucosal and submucosal colonic tissues yields protein signatures that differentiate the inflammatory colitides. Inflamm Bowel Dis. 2010 doi: 10.1002/ibd.21442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.M’Koma AE. Human alpha defensins are differentially expressed between the inflammatory colitides. Annual Congress-Digestive Disease Week; New Orleans, LA T1270. 2010. p. 720. [Google Scholar]
- 104.M’Koma AE. Gene expression of colonic submucosa differs between the inflammatory colitides. Annual Congress - The American Society of Colon and Rectal Surgeons; Minneapolis, MN RF6 . 2010. p. 117. [Google Scholar]
- 105.Burstein E, Fearon ER. Colitis and cancer: A tale of inflammatory cells and their cytokines. J Clin Invest. 2008;118(2):464–467. doi: 10.1172/JCI34831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Becker C, Fantini MC, Schramm C, et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21(4):491–501. doi: 10.1016/j.immuni.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 107.Fukata M, Abreu MT. Role of Toll-like receptors in gastrointestinal malignancies. Oncogene. 2008;27(2):234–243. doi: 10.1038/sj.onc.1210908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441(7092):431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 109.Fukata M, Chen A, Vamadevan AS, et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133(6):1869–1881. doi: 10.1053/j.gastro.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen GY, Shaw MH, Redondo G, Nunez G. The innate immune receptor Nod1 protects the intestine from inflammation-induced tumorigenesis. Cancer Res. 2008;68(24):10060–10067. doi: 10.1158/0008-5472.CAN-08-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Engle SJ, Ormsby I, Pawlowski S, et al. Elimination of colon cancer in germ-free transforming growth factor beta 1-deficient mice. Cancer Res. 2002;62(22):6362–6366. [PubMed] [Google Scholar]
- 112.Greten FR, Eckmann L, Greten TF, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118(3):285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 113.Zhang J, Stirling B, Temmerman ST, et al. Impaired regulation of NF-kappaB and increased susceptibility to colitis-associated tumorigenesis in CYLD-deficient mice. J Clin Invest. 2006;116(11):3042–3049. doi: 10.1172/JCI28746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Clevers H. At the crossroads of inflammation and cancer. Cell. 2004;118(6):671–674. doi: 10.1016/j.cell.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 115.Endo Y, Marusawa H, Kou T, Nakase H, Fujii S, Fujimori T, Kinoshita K, Honjo T, Chiba T. Activation-induced cytidine deaminase links between inflammation and the development of colitis-associated colorectal cancers. Gastroenterology. 2008;135(3):889–898. doi: 10.1053/j.gastro.2008.06.091. [DOI] [PubMed] [Google Scholar]
- 116.Abraham C, Cho JH. IL-23 and autoimmunity: New insights into the pathogenesis of inflammatory bowel disease. Annu Rev Med. 2009;60:97–110. doi: 10.1146/annurev.med.60.051407.123757. [DOI] [PubMed] [Google Scholar]
- 117.Langowski JL, Zhang X, Wu L, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442(7101):461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 118.M’Koma AE, Wise PE, Seeley EH, Washington MK, Schwartz DA, Caprioli RM, Muldoon RL, Herline AJ. Human Alpha Defensins are Differentially Expressed Between the Inflammatory Colitides. Annual Congress, Digestive Disease Week; New Orleans, LA, T1270: AGA Institute; 2010. p. 720. [Google Scholar]
- 119.Askling J, Dickman PW, Karlen P, et al. Colorectal cancer rates among first-degree relatives of patients with inflammatory bowel disease: A population-based cohort study. Lancet. 2001;357 (9252):262–266. doi: 10.1016/S0140-6736(00)03612-6. [DOI] [PubMed] [Google Scholar]
- 120.Riegler G, Caserta L, Castiglione F, et al. Increased risk of breast cancer in first-degree relatives of Crohn’s disease patients. An IG-IBD study. Dig Liver Dis. 2006;38(1):18–23. doi: 10.1016/j.dld.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 121.Bertone ER, Giovannucci EL, King NW, Jr, Petto AJ, Johnson LD. Family history as a risk factor for ulcerative colitis-associated colon cancer in cotton-top tamarin. Gastroenterology. 1998;114(4):669–674. doi: 10.1016/s0016-5085(98)70580-3. [DOI] [PubMed] [Google Scholar]
- 122.Yin J, Harpaz N, Souza RF, et al. Low prevalence of the APC I1307K sequence in Jewish and non-Jewish patients with inflammatory bowel disease. Oncogene. 1999;18(26):3902–3904. doi: 10.1038/sj.onc.1202638. [DOI] [PubMed] [Google Scholar]
- 123.Silverberg MS, Clelland C, Murphy JE, et al. Carrier rate of APC I1307K is not increased in inflammatory bowel disease patients of Ashkenazi Jewish origin. Hum Genet. 2001;108 (3):205–210. doi: 10.1007/s004390100474. [DOI] [PubMed] [Google Scholar]
- 124.Mathy C, Schneider K, Chen YY, Varma M, Terdiman JP, Mahadevan U. Gross versus microscopic pancolitis and the occurrence of neoplasia in ulcerative colitis. Inflamm Bowel Dis. 2003;9(6):351–355. doi: 10.1097/00054725-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 125.von Herbay A, Herfarth C, Otto HF. Cancer and dysplasia in ulcerative colitis: A histologic study of 301 surgical specimen. Z Gastroenterol. 1994;32(7):382–388. [PubMed] [Google Scholar]
- 126.Riegler G, Bossa F, Caserta L, et al. Colorectal cancer and high grade dysplasia complicating ulcerative colitis in Italy. A retrospective co-operative IG-IBD study. Dig Liver Dis. 2003;35 (9):628–634. doi: 10.1016/s1590-8658(03)00380-3. [DOI] [PubMed] [Google Scholar]
- 127.Lennard-Jones JE, Melville DM, Morson BC, Ritchie JK, Williams CB. Precancer and cancer in extensive ulcerative colitis: Findings among 401 patients over 22 years. Gut. 1990;31 (7):800–806. doi: 10.1136/gut.31.7.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Butt JH, Konishi F, Morson BC, Lennard-Jones JE, Ritchie JK. Macroscopic lesions in dysplasia and carcinoma complicating ulcerative colitis. Dig Dis Sci. 1983;28(1):18–26. doi: 10.1007/BF01393356. [DOI] [PubMed] [Google Scholar]
- 129.Greenstein AJ, Slater G, Heimann TM, Sachar DB, Aufses AH., Jr A comparison of multiple synchronous colorectal cancer in ulcerative colitis, familial polyposis coli, and de novo cancer. Ann Surg. 1986;203(2):123–128. doi: 10.1097/00000658-198602000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Levi GS, Harpaz N. Intestinal low-grade tubuloglandular adenocarcinoma in inflammatory bowel disease. Am J Surg Pathol. 2006;30(8):1022–1029. doi: 10.1097/00000478-200608000-00014. [DOI] [PubMed] [Google Scholar]
- 131.Morson BC, Pang LS. Rectal biopsy as an aid to cancer control in ulcerative colitis. Gut. 1967;8(5):423–434. doi: 10.1136/gut.8.5.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lennard-Jones JE, Morson BC, Ritchie JK, Williams CB. Cancer surveillance in ulcerative colitis. Experience over 15 years. Lancet. 1983;2(8342):149–152. doi: 10.1016/s0140-6736(83)90129-0. [DOI] [PubMed] [Google Scholar]
- 133.Eaden JA, Mayberry JF. Guidelines for screening and surveillance of asymptomatic colorectal cancer in patients with inflammatory bowel disease. Gut. 2002;51(Suppl 5):V10–V12. doi: 10.1136/gut.51.suppl_5.v10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Friedman S, Rubin PH, Bodian C, Harpaz N, Present DH. Screening and surveillance colonoscopy in chronic Crohn’s colitis: Results of a surveillance program spanning 25 years. Clin Gastroenterol Hepatol. 2008;6(9):993–998. doi: 10.1016/j.cgh.2008.03.019. quiz 953–994. [DOI] [PubMed] [Google Scholar]
- 135.Greenstein AJ, Sachar DB, Smith H, Janowitz HD, Aufses AH., Jr Patterns of neoplasia in Crohn’s disease and ulcerative colitis. Cancer. 1980;46(2):403–407. doi: 10.1002/1097-0142(19800715)46:2<403::aid-cncr2820460232>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 136.Hamilton SR. Colorectal carcinoma in patients with Crohn’s disease. Gastroenterology. 1985;89(2):398–407. doi: 10.1016/0016-5085(85)90343-9. [DOI] [PubMed] [Google Scholar]
- 137.Connell WR, Talbot IC, Harpaz N, et al. Clinicopathological characteristics of colorectal carcinoma complicating ulcerative colitis. Gut. 1994;35(10):1419–1423. doi: 10.1136/gut.35.10.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stahl TJ, Schoetz DJ, Jr, Roberts PL, et al. Crohn’s disease and carcinoma: Increasing justification for surveillance? Dis Colon Rectum. 1992;35(9):850–856. doi: 10.1007/BF02047872. [DOI] [PubMed] [Google Scholar]
- 139.Rutter MD, Saunders BP, Wilkinson KH, et al. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology. 2006;130(4):1030–1038. doi: 10.1053/j.gastro.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 140.Connell WR, Lennard-Jones JE, Williams CB, Talbot IC, Price AB, Wilkinson KH. Factors affecting the outcome of endoscopic surveillance for cancer in ulcerative colitis. Gastro-enterology. 1994;107(4):934–944. doi: 10.1016/0016-5085(94)90216-x. [DOI] [PubMed] [Google Scholar]
- 141.Itzkowitz SH. Molecular biology of dysplasia and cancer in inflammatory bowel disease. Gastroenterol Clin North Am. 2006;35 (3):553–571. doi: 10.1016/j.gtc.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 142.Colliver DW, Crawford NP, Eichenberger MR, et al. Molecular profiling of ulcerative colitis-associated neoplastic progression. Exp Mol Pathol. 2006;80(1):1–10. doi: 10.1016/j.yexmp.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 143.Collins PD, Mpofu C, Watson AJ, Rhodes JM. Strategies for detecting colon cancer and/or dysplasia in patients with inflammatory bowel disease. Cochrane Database Syst Rev. 2006;(2):CD000279. doi: 10.1002/14651858.CD000279.pub3. [DOI] [PubMed] [Google Scholar]
- 144.Nguyen GC, Torres EA, Regueiro M, et al. Inflammatory bowel disease characteristics among African Americans, Hispanics, and non-Hispanic Whites: Characterization of a large North American cohort. Am J Gastroenterol. 2006;101 (5):1012–1023. doi: 10.1111/j.1572-0241.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- 145.American Cancer Society. Cancer facts and figures for African American 2003–2004. Atlanta, GA: 2003. [Google Scholar]
- 146.ACS. American Cancer Society. Cancer facts and figures for Hispanic/Latino 2003–2005. Atlanta, GA: 2003. [Google Scholar]
- 147.Irby K, Anderson WF, Henson DE, Devesa SS. Emerging and widening colorectal carcinoma disparities between Blacks and Whites in the United States (1975–2002) Cancer Epidemiol Biomark Prev. 2006;15(4):792–797. doi: 10.1158/1055-9965.EPI-05-0879. [DOI] [PubMed] [Google Scholar]
- 148.Bazargan M, Ani C, Bazargan-Hejazi S, Baker RS, Bastani R. Colorectal cancer screening among underserved minority population: Discrepancy between physicians’ recommended, scheduled, and completed tests. Patient Educ Couns. 2009;76(2):240–247. doi: 10.1016/j.pec.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 149.Carethers JM. Racial and ethnic factors in the genetic pathogenesis of colorectal cancer. J Assoc Acad Minor Physicians. 1999;10(3):59–67. [PubMed] [Google Scholar]
- 150.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 151.Hao Y. Treands in colorectal cancer incidence rates by age, race/ethnicity and indices of access to medical care, 1995–2004 (United States) Cancer Causes Control. 2010 doi: 10.1007/s10552-009-9379-y. In Press. [DOI] [PubMed] [Google Scholar]
- 152.Branco BC, Sachar DB, Heimann T, Sarpel U, Harpaz N, Greenstein AJ. Adenocarcinoma complicating restorative proctocolectomy for ulcerative colitis with mucosectomy performed by Cavitron Ultrasonic Surgical Aspirator. Colorectal Dis. 2009;11(4):428–429. doi: 10.1111/j.1463-1318.2008.01651.x. [DOI] [PubMed] [Google Scholar]
- 153.El-Raouf AA, Hak NG, Fathy O, El-Ebidy G, Salah T, El-Hemaly M. Outcome of pouch surgery for ulcerative colitis: Single center experience. Hepatogastroenterology. 2008;55(88):2130–2134. [PubMed] [Google Scholar]
- 154.Vella M, Masood MR, Hendry WS. Surgery for ulcerative colitis. Surgeon. 2007;5(6):356–362. doi: 10.1016/s1479-666x(07)80088-6. [DOI] [PubMed] [Google Scholar]
- 155.Kayaalp C, Nessar G, Akoglu M, Atalay F. Elimination of mucosectomy during restorative proctocolectomy in patients with ulcerative colitis may provide better results in low-volume centers. Am J Surg. 2003;185(3):268–272. doi: 10.1016/s0002-9610(02)01376-4. [DOI] [PubMed] [Google Scholar]
- 156.O’Connell PR, Pemberton JH, Weiland LH, et al. Does rectal mucosa regenerate after ileoanal anastomosis? Dis Colon Rectum. 1987;30(1):1–5. doi: 10.1007/BF02556908. [DOI] [PubMed] [Google Scholar]
- 157.Heald RJ, Allen DR. Stapled ileo-anal anastomosis: A technique to avoid mucosal proctectomy in the ileal pouch operation. Br J Surg. 1986;73(7):571–572. doi: 10.1002/bjs.1800730719. [DOI] [PubMed] [Google Scholar]
- 158.Das P, Johnson MW, Tekkis PP, Nicholls RJ. Risk of dysplasia and adenocarcinoma following restorative proctoco-lectomy for ulcerative colitis. Colorectal Dis. 2007;9(1):15–27. doi: 10.1111/j.1463-1318.2006.01148.x. [DOI] [PubMed] [Google Scholar]
- 159.Koh PK, Doumit J, Downs-Kelly E, et al. Ileo-anal j-pouch cancer: An unusual case in an unusual location. Tech Colo-proctol. 2008;12(4):341–345. doi: 10.1007/s10151-008-0420-z. [DOI] [PubMed] [Google Scholar]
- 160.Schaus BJ, Fazio VW, Remzi FH, Bennett AE, Lashner BA, Shen B. Clinical features of ileal pouch polyps in patients with underlying ulcerative colitis. Dis Colon Rectum. 2007;50(6):832–838. doi: 10.1007/s10350-006-0871-0. [DOI] [PubMed] [Google Scholar]
- 161.Gullberg K, Liljeqvist L. Stapled ileoanal pouches without loop ileostomy: A prospective study in 86 patients. Int J olorectal Dis. 2001;16(4):221–227. doi: 10.1007/s003840100289. [DOI] [PubMed] [Google Scholar]
- 162.Thompson-Fawcett MW, Marcus V, Redston M, Cohen Z, McLeod RS. Risk of dysplasia in long-term ileal pouches and pouches with chronic pouchitis. Gastroenterology. 2001;121(2):275–281. doi: 10.1053/gast.2001.26442. [DOI] [PubMed] [Google Scholar]
- 163.Veress B, Reinholt FP, Lindquist K, Lofberg R, Liljeqvist L. Long-term histomorphological surveillance of the pelvic ileal pouch: Dysplasia develops in a subgroup of patients. Gastroenterology. 1995;109(4):1090–1097. doi: 10.1016/0016-5085(95)90566-9. [DOI] [PubMed] [Google Scholar]
- 164.Lofberg R, Liljeqvist L, Lindquist K, Veress B, Reinholt FP, Tribukait B. Dysplasia and DNA aneuploidy in a pelvic pouch. Report of a case. Dis Colon Rectum. 1991;34(3):280–283. doi: 10.1007/BF02090171. discussion 283–284. [DOI] [PubMed] [Google Scholar]
- 165.Barrett M. Long-term risk of neoplastic changes in ileoanal pouches in patients with ulcerative colitis. Dis Colon Rectum. 1998;41:126. [Google Scholar]
- 166.Setti Carraro P, Talbot IC, Nicholls RJ. Longterm appraisal of the histological appearances of the ileal reservoir mucosa after restorative proctocolectomy for ulcerative colitis. Gut. 1994;35(12):1721–1727. doi: 10.1136/gut.35.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Herline AJ, Meisinger LL, Rusin LC, et al. Is routine pouch surveillance for dysplasia indicated for ileoanal pouches? Dis Colon Rectum. 2003;46(2):156–159. doi: 10.1007/s10350-004-6517-1. [DOI] [PubMed] [Google Scholar]
- 168.Branco BC, Sachar DB, Heimann TM, Sarpel U, Harpaz N, Greenstein AJ. Adenocarcinoma complicating restorative proctocolectomy for ulcerative colitis with mucosectomy performed by Cavitron Ultrasonic Surgical Aspirator. Colorectal Dis. 2009;11(4):428–429. doi: 10.1111/j.1463-1318.2008.01651.x. [DOI] [PubMed] [Google Scholar]
- 169.Tarroni D. Long-term histological assessment of the ileal reservoir following restorative proctocolectomy for ulcerative colitis and dysplasia. Dis Colon Rectum. 2002;(45):a7. [Google Scholar]
- 170.Borjesson L, Willen R, Haboubi N, Duff SE, Hulten L. The risk of dysplasia and cancer in the ileal pouch mucosa after restorative proctocolectomy for ulcerative proctocolitis is low: A long-term term follow-up study. Colorectal Dis. 2004;6(6):494–498. doi: 10.1111/j.1463-1318.2004.00716.x. [DOI] [PubMed] [Google Scholar]
- 171.Geiger JD, Teitelbaum DH, Hirschl RB, Coran AG. A new operative technique for restorative proctocolectomy: The endorectal pull-through combined with a double-stapled ileo-anal anastomosis. Surgery. 2003;134(3):492–495. doi: 10.1067/s0039-6060(03)00087-4. [DOI] [PubMed] [Google Scholar]
- 172.Seow-Choen F, Ho YH, Goh HS. The ileo-anal reservoir: Results from an evolving use of stapling devices. J R Coll Surg Edinb. 1994;39(1):13–16. [PubMed] [Google Scholar]
- 173.Zmora O, Spector D, Dotan I, Klausner JM, Rabau M, Tulchinsky H. Is stapled ileal pouch anal anastomosis a safe option in ulcerative colitis patients with dysplasia or cancer? Int J Colorectal Dis. 2009;24(10):1181–1186. doi: 10.1007/s00384-009-0744-9. [DOI] [PubMed] [Google Scholar]
- 174.Bernstein CN. Natural history and management of flat and polypoid dysplasia in inflammatory bowel disease. Gastroenterol Clin North Am. 2006;35(3):573–579. doi: 10.1016/j.gtc.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 175.Thompson-Fawcett MW, Warren BF, Mortensen NJ. A new look at the anal transitional zone with reference to restorative proctocolectomy and the columnar cuff. Br J Surg. 1998;85(11):1517–1521. doi: 10.1046/j.1365-2168.1998.00875.x. [DOI] [PubMed] [Google Scholar]
- 176.Schmitt SL, Wexner SD, Lucas FV, James K, Nogueras JJ, Jagelman DG. Retained mucosa after double-stapled ileal reservoir and ileoanal anastomosis. Dis Colon Rectum. 1992;35 (11):1051–1056. doi: 10.1007/BF02252995. [DOI] [PubMed] [Google Scholar]
- 177.Thompson-Fawcett MW, Rust NA, Warren BF, Mortensen NJ. Aneuploidy and columnar cuff surveillance after stapled ileal pouch-anal anastomosis in ulcerative colitis. Dis Colon Rectum. 2000;43(3):408–413. doi: 10.1007/BF02258310. [DOI] [PubMed] [Google Scholar]
- 178.Tsunoda A, Talbot IC, Nicholls RJ. Incidence of dysplasia in the anorectal mucosa in patients having restorative proctoco-lectomy. Br J Surg. 1990;77(5):506–508. doi: 10.1002/bjs.1800770510. [DOI] [PubMed] [Google Scholar]
- 179.Ziv Y, Fazio VW, Sirimarco MT, Lavery IC, Goldblum JR, Petras RE. Incidence, risk factors, and treatment of dysplasia in the anal transitional zone after ileal pouch-anal anastomosis. Dis Colon Rectum. 1994;37(12):1281–1285. doi: 10.1007/BF02257797. [DOI] [PubMed] [Google Scholar]
- 180.Brown CJ, Maclean AR, Cohen Z, Macrae HM, O’Connor BI, McLeod RS. Crohn’s disease and indeterminate colitis and the ileal pouch-anal anastomosis: Outcomes and patterns of failure. Dis Colon Rectum. 2005;48(8):1542–1549. doi: 10.1007/s10350-005-0059-z. [DOI] [PubMed] [Google Scholar]
- 181.Shen B, Remzi FH, Lavery IC, Lashner BA, Fazio VW. A proposed classification of ileal pouch disorders and associated complications after restorative proctocolectomy. Clin Gastro-enterol Hepatol. 2008;6(2):145–158. doi: 10.1016/j.cgh.2007.11.006. quiz 124. [DOI] [PubMed] [Google Scholar]
- 182.Coull DB, Lee FD, Henderson AP, Anderson JH, McKee RF, Finlay IG. Risk of dysplasia in the columnar cuff after stapled restorative proctocolectomy. Br J Surg. 2003;90(1):72–75. doi: 10.1002/bjs.4007. [DOI] [PubMed] [Google Scholar]
- 183.Kariv R, Plesec TP, Gaffney K, et al. Pyloric gland metaplasia and pouchitis in patients with ileal pouch-anal anastomoses. Aliment Pharmacol Ther. Apr;31(8):862–873. doi: 10.1111/j.1365-2036.2010.04249.x. [DOI] [PubMed] [Google Scholar]
- 184.Shelton AA, Lehman RE, Schrock TR, Welton ML. Retrospective review of colorectal cancer in ulcerative colitis at a tertiary center. Arch Surg. 1996;131(8):806–810. doi: 10.1001/archsurg.1996.01430200016003. discussion 810–801. [DOI] [PubMed] [Google Scholar]
- 185.Stahlberg D, Veress B, Tribukait B, Broome U. Atrophy and neoplastic transformation of the ileal pouch mucosa in patients with ulcerative colitis and primary sclerosing cholangitis: A case control study. Dis Colon Rectum. 2003;46(6):770–778. doi: 10.1007/s10350-004-6655-5. [DOI] [PubMed] [Google Scholar]
- 186.Sarigol S, Wyllie R, Gramlich T, et al. Incidence of dysplasia in pelvic pouches in pediatric patients after ileal pouch-anal anastomosis for ulcerative colitis. J Pediatr Gastroenterol Nutr. 1999;28(4):429–434. doi: 10.1097/00005176-199904000-00015. [DOI] [PubMed] [Google Scholar]
- 187.Sierra-Montenegro E, Fernandez-Rivero JM, Villanueva-Saenz E, Pena-Ruiz Esparza JP, Martinez-Hernandez Magro P, Solo-Quirino R. Quality of life after restorative proctocolectomy with ileo-anal J pouch in patients with ulcerative colitis. Cir. 2007;75(6):449–452. [PubMed] [Google Scholar]
- 188.Memon AA, Marks CG. Stapled anastomoses in colorectal surgery: A prospective study. Eur J Surg. 1996;162 (10):805–810. [PubMed] [Google Scholar]
- 189.Delaini GG, Scaglia M, Colucci G, Hulten L. The ileoanal pouch procedure in the long-term perspective: A critical review. Tech Coloproctol. 2005;9(3):187–192. doi: 10.1007/s10151-005-0225-2. [DOI] [PubMed] [Google Scholar]
- 190.Elkowitz D, Daum F, Markowitz J, et al. Risk factors for carcinoma of the pelvic ileal pouch/anal canal in ulcerative colitis. Ann Clin Lab Sci. 2004;34(2):143–149. [PubMed] [Google Scholar]
- 191.Greenwald JE, Ritter D, Tetens E, Rotwein PS. Renal expression of the gene for atrial natriuretic factor. Am J Physiol. 1992;263:F974–F978. doi: 10.1152/ajprenal.1992.263.5.F974. [DOI] [PubMed] [Google Scholar]
- 192.Kern SE, Redston M, Seymour AB, Caldas C, Powell SM, Kornacki S, Kinzler KW. Molecular genetic profiles of colitis-associated neoplasms. Gastroenterology. 1994;107(2):420–428. doi: 10.1016/0016-5085(94)90167-8. [DOI] [PubMed] [Google Scholar]
- 193.Tarmin L, Yin J, Harpaz N, Kozam M, Noordzij J, Antonio LB, Jiang HY, Chan O, Cymes K, Meltzer SJ. Adenomatous polyposis coli gene mutations in ulcerative colitisassociated dysplasias and cancers versus sporadic colon neoplasms. Cancer Res. 1995;55(10):2035–2038. [PubMed] [Google Scholar]
- 194.Redston MS, Papadopoulos N, Caldas C, Kinzler KW, Kern SE. Common occurrence of APC and K-ras gene mutations in the spectrum of colitis-associated neoplasias. Gastroenterology. 1995;108(2):383–392. doi: 10.1016/0016-5085(95)90064-0. [DOI] [PubMed] [Google Scholar]
- 195.Willenbucher RF, Zelman SJ, Ferrell LD, Moore DH, 2nd, Waldman FM. Chromosomal alterations in ulcerative colitis-related neoplastic progression. Gastroenterology. 1997;113 (3):791–801. doi: 10.1016/s0016-5085(97)70173-2. [DOI] [PubMed] [Google Scholar]
- 196.Umetani N, Sasaki S, Watanabe T, Shinozaki M, Matsuda K, Ishigami H, Ueda E, Muto T. Genetic alterations in ulcerative colitis-associated neoplasia focusing on APC, Kras gene and microsatellite instability. Jpn J Cancer Res. 1999;90 (10):1081–1087. doi: 10.1111/j.1349-7006.1999.tb00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Aust DE, Willenbucher RF, Terdiman JP, Ferrell LD, Chang CG, Moore DH, 2nd, Molinaro-Clark A, Baretton GB, Loehrs U, Waldman FM. Chromosomal alterations in ulcerative colitis-related and sporadic colorectal cancers by comparative genomic hybridization. Hum Pathol. 2000;31(1):109–114. doi: 10.1016/s0046-8177(00)80206-3. [DOI] [PubMed] [Google Scholar]
- 198.Aust DE, Terdiman JP, Willenbucher RF, Chew K, Ferrell L, Florendo C, Molinaro-Clark A, Baretton GB, Löhrs U, Waldman FM. Altered distribution of beta-catenin, and its binding proteins E-cadherin and APC, in ulcerative colitis-related colorectal cancers. Mod Pathol. 2001;14(1):29–39. doi: 10.1038/modpathol.3880253. [DOI] [PubMed] [Google Scholar]
- 199.Aust DE, Terdiman JP, Willenbucher RF, Chang CG, Molinaro-Clark A, Baretton GB, Loehrs U, Waldman FM. The APC/beta-catenin pathway in ulcerative colitis-related colorectal carcinomas: a mutational analysis. Cancer. 2002;94(5):1421–1427. doi: 10.1002/cncr.10334. [DOI] [PubMed] [Google Scholar]
- 200.Tomlinson I, Ilyas M, Johnson V, Davies A, Clark G, Talbot I, Bodmer W. A comparison of the genetic pathways involved in the pathogenesis of three types of colorectal cancer. J Pathol. 1998;184(2):148–152. doi: 10.1002/(SICI)1096-9896(199802)184:2<148::AID-PATH986>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 201.Lei J, Zou TT, Shi YQ, Zhou X, Smolinski KN, Yin J, Souza RF, Appel R, Wang S, Cymes K, Chan O, Abraham JM, Harpaz N, Meltzer SJ. Infrequent DPC4 gene mutation in esophageal cancer, gastric cancer and ulcerative colitis-associated neoplasms. Oncogene. 1996;13(11):2459–2462. [PubMed] [Google Scholar]
- 202.Hoque AT, Hahn SA, Schutte M, Kern SE. DPC4 gene mutation in colitis associated neoplasia. Gut. 1997;40(1):120–122. doi: 10.1136/gut.40.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Burmer GC, Rabinovitch PS, Haggitt RC, Crispin DA, Brentnall TA, Kolli VR, Stevens AC, Rubin CE. Neoplastic progression in ulcerative colitis: histology, DNA content, and loss of a p53 allele. Gastroenterology. 1992;103(5):1602–1610. doi: 10.1016/0016-5085(92)91184-6. [DOI] [PubMed] [Google Scholar]
- 204.Yin J, Harpaz N, Tong Y, Huang Y, Laurin J, Greenwald BD, Hontanosas M, Newkirk C, Meltzer SJ. p53 point mutations in dysplastic and cancerous ulcerative colitis lesions. Gastroenterology. 1993;104(6):1633–1639. doi: 10.1016/0016-5085(93)90639-t. [DOI] [PubMed] [Google Scholar]
- 205.Harpaz N, Peck AL, Yin J, Fiel I, Hontanosas M, Tong TR, Laurin JN, Abraham JM, Greenwald BD, Meltzer SJ. p53 protein expression in ulcerative colitis-associated colorectal dysplasia and carcinoma. Hum Pathol. 1994;25(10):1069–1074. doi: 10.1016/0046-8177(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 206.Brentnall TA, Crispin DA, Rabinovitch PS, Haggitt RC, Rubin CE, Stevens AC, Burmer GC. Mutations in the p53 gene: an early marker of neoplastic progression in ulcerative colitis. Gastroenterology. 1994;107(2):369–378. doi: 10.1016/0016-5085(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 207.Chaubert P, Benhattar J, Saraga E, Costa J. K-ras mutations and p53 alterations in neoplastic and nonneoplastic lesions associated with longstanding ulcerative colitis. Am J Pathol. 1994;144(4):767–775. [PMC free article] [PubMed] [Google Scholar]
- 208.Klump B, Holzmann K, Kühn A, Borchard F, Sarbia M, Gregor M, Porschen R. Distribution of cell populations with DNA aneuploidy and p53 protein expression in ulcerative colitis. Eur J Gastroenterol Hepatol. 1997;9(8):789–794. doi: 10.1097/00042737-199708000-00010. [DOI] [PubMed] [Google Scholar]
- 209.Holzmann K, Klump B, Borchard F, Hsieh CJ, Kühn A, Gaco V, Gregor M, Porschen R. Comparative analysis of histology, DNA content, p53 and Ki-ras mutations in colectomy specimens with long-standing ulcerative colitis. Int J Cancer. 1998;76(1):1–6. doi: 10.1002/(sici)1097-0215(19980330)76:1<1::aid-ijc1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 210.Rabinovitch PS, Dziadon S, Brentnall TA, Emond MJ, Crispin DA, Haggitt RC, Bronner MP. Pancolonic chromosomal instability precedes dysplasia and cancer in ulcerative colitis. Cancer Res. 1999;59(20):5148–5153. [PubMed] [Google Scholar]
- 211.Wong NA, Mayer NJ, MacKell S, Gilmour HM, Harrison DJ. Immunohistochemical assessment of Ki67 and p53 expression assists the diagnosis and grading of ulcerative colitis-related dysplasia. Histopathology. 2000;37(2):108–114. doi: 10.1046/j.1365-2559.2000.00934.x. [DOI] [PubMed] [Google Scholar]
- 212.Hussain SP, Amstad P, Raja K, Ambs S, Nagashima M, Bennett WP, Shields PG, Ham AJ, Swenberg JA, Marrogi AJ, Harris CC. Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: a cancer-prone chronic inflammatory disease. Cancer Res. 2000;60(13):3333–3337. [PubMed] [Google Scholar]
- 213.Nathanson JW, Yadron NE, Farnan J, Kinnear S, Hart J, Rubin DT. p53 mutations are associated with dysplasia and progression of dysplasia in patients with Crohn’s disease. Dig Dis Sci. 2008;53(2):474–480. doi: 10.1007/s10620-007-9886-1. [DOI] [PubMed] [Google Scholar]
- 214.Hsieh CJ, Klump B, Holzmann K, Borchard F, Gregor M, Porschen R. Hypermethylation of the p16INK4a promoter in colectomy specimens of patients with longstanding and extensive ulcerative colitis. Cancer Res. 1998;58(17):3942–3945. [PubMed] [Google Scholar]
- 215.Issa JP, Ahuja N, Toyota M, Bronner MP, Brentnall TA. Accelerated age-related CpG island methylation in ulcerative colitis. Cancer Res. 2001;61(9):3573–3577. [PubMed] [Google Scholar]
- 216.Sato F, Harpaz N, Shibata D, Xu Y, Yin J, Mori Y, Zou TT, Wang S, Desai K, Leytin A, Selaru FM, Abraham JM, Meltzer SJ. Hypermethylation of the p14(ARF) gene in ulcerative colitis-associated colorectal carcinogenesis. Cancer Res. 2002;62 (4):1148–1151. [PubMed] [Google Scholar]
- 217.Moriyama T, Matsumoto T, Nakamura S, Jo Y, Mibu R, Yao T, Iida M. Hypermethylation of p14 (ARF) may be predictive of colitic cancer in patients with ulcerative colitis. Dis Colon Rectum. 2007;50(9):1384–1392. doi: 10.1007/10350-007-0302-x. [DOI] [PubMed] [Google Scholar]
- 218.Walsh S, Murphy M, Silverman M, Odze R, Antonioli D, Goldman H, Loda M. p27 expression in inflammatory bowel disease-associated neoplasia. Further evidence of a unique molecular pathogenesis. Am J Pathol. 1999;155(5):1511–1518. doi: 10.1016/S0002-9440(10)65466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ilyas M, Tomlinson IP, Hanby A, Talbot IC, Bodmer WF. Allele loss, replication errors and loss of expression of E-cadherin in colorectal cancers. Gut. 1997;40(5):654–659. doi: 10.1136/gut.40.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wheeler JM, Kim HC, Efstathiou JA, Ilyas M, Mortensen NJ, Bodmer WF. Hypermethylation of the promoter region of the E-cadherin gene (CDH1) in sporadic and ulcerative colitis associated colorectal cancer. Gut. 2001;48(3):367–371. doi: 10.1136/gut.48.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Azarschab P, Porschen R, Gregor M, Blin N, Holzmann K. Epigenetic control of the E-cadherin gene (CDH1) by CpG methylation in colectomy samples of patients with ulcerative colitis. Genes Chromosom Cancer. 2002;35(2):121–126. doi: 10.1002/gcc.10101. [DOI] [PubMed] [Google Scholar]
- 222.van Dekken H, Wink JC, Vissers KJ, Franken PF, Ruud Schouten WJ, Hop WC, Kuipers EJ, Fodde R, Janneke van der Woude C. Wnt pathway-related gene expression during malignant progression in ulcerative colitis. Acta Histo-chem. 2007;109(4):266–272. doi: 10.1016/j.acthis.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 223.Burmer GC, Levine DS, Kulander BG, Haggitt RC, Rubin CE, Rabinovitch PS. c-Kiras mutations in chronic ulcerative colitis and sporadic colon carcinoma. Gastroenterology. 1990;99 (2):416–420. doi: 10.1016/0016-5085(90)91024-z. [DOI] [PubMed] [Google Scholar]
- 224.Meltzer SJ, Mane SM, Wood PK, Resau JH, Newkirk C, Terzakis JA, Korelitz BI, Weinstein WM, Needleman SW. Activation of c-Ki-ras in human gastrointestinal dysplasias determined by direct sequencing of polymerase chain reaction products. Cancer Res. 1990;50(12):3627–3630. [PubMed] [Google Scholar]
- 225.Bell SM, Kelly SA, Hoyle JA, Lewis FA, Taylor GR, Thompson H, Dixon MF, Quirke P. c-Ki-ras gene mutations in dysplasia and carcinomas complicating ulcerative colitis. Br J Cancer. 1991;64(1):174–178. doi: 10.1038/bjc.1991.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lyda MH, Noffsinger A, Belli J, Fenoglio-Preiser CM. Microsatellite instability and Kras mutations in patients with ulcerative colitis. Hum Pathol. 2000;31(6):665–671. doi: 10.1053/hupa.2000.7643. [DOI] [PubMed] [Google Scholar]
- 227.Holzmann K, Weis-Klemm M, Klump B, Hsieh CJ, Borchard F, Gregor M, Porschen R. Comparison of flow cytometry and histology with mutational screening for p53 and Ki-ras mutations in surveillance of patients with long-standing ulcerative colitis. Scand J Gastroenterol. 2001;36(12):1320–1326. doi: 10.1080/003655201317097191. [DOI] [PubMed] [Google Scholar]
- 228.Aust DE, Haase M, Dobryden L, Markwarth A, Löhrs U, Wittekind C, Baretton GB, Tannapfel A. Mutations of the BRAF gene in ulcerative colitis-related colorectal carcinoma. Int J Cancer. 2005;115(5):673–677. doi: 10.1002/ijc.20925. [DOI] [PubMed] [Google Scholar]
- 229.Cartwright CA, Coad CA, Egbert BM. Elevated c-Src tyrosine kinase activity in premalignant epithelia of ulcerative colitis. J Clin Invest. 1994;93(2):509–515. doi: 10.1172/JCI117000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Souza RF, Lei J, Yin J, Appel R, Zou TT, Zhou X, Wang S, Rhyu MG, Cymes K, Chan O, Park WS, Krasna MJ, Greenwald BD, Cottrell J, Abraham JM, Simms L, Leggett B, Young J, Harpaz N, Meltzer SJ. A transforming growth factor beta 1 receptor type II mutation in ulcerative colitis-associated neoplasms. Gastroenterology. 1997;112(1):40–45. doi: 10.1016/s0016-5085(97)70217-8. [DOI] [PubMed] [Google Scholar]
- 231.Brentnall TA, Chen R, Lee JG, Kimmey MB, Bronner MP, Haggitt RC, Kowdley KV, Hecker LM, Byrd DR. Microsatellite instability and K-ras mutations associated with pancreatic adenocarcinoma and pancreatitis. Cancer Res. 1995;55 (19):4264–4267. [PubMed] [Google Scholar]
- 232.Noffsinger AE, Belli JM, Fogt F, Fischer J, Goldman H, Fenoglio-Preiser CM. A germline hMSH2 alteration is unrelated to colonic microsatellite instability in patients with ulcerative colitis. Hum Pathol. 1999;30(1):8–12. doi: 10.1016/s0046-8177(99)90293-9. [DOI] [PubMed] [Google Scholar]
- 233.Fleisher AS, Esteller M, Harpaz N, Leytin A, Rashid A, Xu Y, Liang J, Stine OC, Yin J, Zou TT, Abraham JM, Kong D, Wilson KT, James SP, Herman JG, Meltzer SJ. Microsatellite instability in inflammatory bowel disease-associated neoplastic lesions is associated with hypermethylation and diminished expression of the DNA mismatch repair gene, hMLH1. Cancer Res. 2000;60(17):4864–4868. [PubMed] [Google Scholar]
- 234.Willenbucher RF, Aust DE, Chang CG, Zelman SJ, Ferrell LD, Moore DH, 2nd, Waldman FM. Genomic instability is an early event during the progression pathway of ulcerative-colitis-related neoplasia. Am J Pathol. 1999;154(6):1825–1830. doi: 10.1016/S0002-9440(10)65438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Maia L, Dinis J, Cravo M, Claro I, Baltazar C, Fonseca I, Veloso T, Capelinha AF, Carneiro F, Nobre-Leitão C. Who takes the lead in the development of ulcerative colitisassociated colorectal cancers: mutator, suppressor, or methylator pathway? Cancer Genet Cytogenet. 2005;162(1):68–73. doi: 10.1016/j.cancergencyto.2005.02.017. [DOI] [PubMed] [Google Scholar]