Abstract
Background:
Androgenetic alopecia (AGA) is the most common form of hair loss in men and in women. Currently, minoxidil and finasteride are the treatments with the highest levels of medical evidence, but patients who exhibit intolerance or poor response to these treatments are in need of additional treatment modalities.
Objective:
The aim was to evaluate the efficacy and safety of low-level laser therapy (LLLT) for AGA, either as monotherapy or as concomitant therapy with minoxidil or finasteride, in an office-based setting.
Materials and Methods:
Retrospective observational study of male and female patients with AGA, treated with the 655 nm-HairMax Laser Comb®, in an office-based setting. Efficacy was assessed with global photographic imaging.
Results:
Of 32 patients (21 female, 11 male), 8 showed significant, 20 moderate, and 4 no improvement. Improvement was seen both with monotherapy and with concomitant therapy. Improvement was observed as early as 3 months and was sustained up to a maximum observation time of 24 months. No adverse reactions were reported.
Conclusions:
LLLT represents a potentially effective treatment for both male and female AGA, either as monotherapy or concomitant therapy. Combination treatments with minoxidil, finasteride, and LLLT may act synergistic to enhance hair growth.
Keywords: Androgenetic alopecia, concomitant therapy, HairMax Laser Comb®, low level laser therapy, monotherapy
INTRODUCTION
The ability of lasers to induce hair growth was incidentally noted as early as 1967 when Mester et al. used low-level laser therapy (LLLT) to treat cancer in mice with shaved backs.[1] Since then, hypertrichosis has been recognized to be a possible side-effect of laser treatment. First described in 2002 with intense pulsed light therapy,[2] this phenomenon has now been widely acknowledged to occur with an incidence rate ranging from 0.6% to 10% with low fluences and all laser types.[3] It is thought to be the result of suboptimal fluences that are too low to induce thermolysis, but high enough to stimulate follicular growth.
Eventually, LLLT has been developed for the treatment of androgenetic alopecia (AGA). As opposed to other currently marketed systems, the laser comb utilizes hair parting teeth for optimal delivery of laser energy to the exposed scalp. In 2007, the HairMax Laser Comb® (Lexington International, LLC) received 510 (k) clearance from the Food and Drug Administration (FDA) for the treatment of AGA for men, and 2011 for women. This clearance means that the device is considered a moderate-risk medical device by the FDA and is thereby solely screened for safety. The HairMax Laser Comb® has been tested in a company-sponsored study of 110 male patients with the claim of a significant increase in mean terminal hair density when compared to a sham device.[4] Avram and Rogers conducted the first independent blinded study of LLLT and hair growth with seven patients and found that on average, there was a decrease in the number of vellus hairs, an increase in the number of terminal hairs, and an increase in shaft diameter.[5] A consensus written by hair loss experts states that based on anecdotal experience, LLLT, particularly 650-900 nm wavelengths at 5 mW, may be an effective treatment option for patients with AGA.[6] In recent times, Kim et al. reported an increase of hair density with the use of LLLT, when compared to the sham device in a 24-week, randomized, double-blind, sham-device-controlled trial.[7]
To evaluate efficacy of the 655 nm-HairMax Laser Comb® either as monotherapy or as concomitant therapy for treatment of male and female AGA, we performed a retrospective observational study of global photographic assessments of patients in an office-based setting.
MATERIALS AND METHODS
The study design was retrospective and observational. Patients who had purchased a HairMax Laser Comb® between July 2011 and July 2013 for treatment of AGA at the Center for Dermatology and Hair Diseases Prof. Trόeb were retrieved for assessment of global photographic images performed at follow-up visits. Patients on concomitant treatment had been treating with topical minoxidil or oral finasteride for at least 9 months, before starting therapy with the HairMax Laser Comb® . Patients used the HairMax Laser Comb® at home according to instructions 3 times weekly between 8 and 15 min depending on the model purchased (Advanced 7, Lux 9, or Professional 12). Global photographs were performed at 3, 6, 12, and 24 months of treatment follow-up in a standardized manner with a stereotactic camera device of Canfield Scientific Inc., in which the patient's chin and forehead are fixed and on which digital camera and flash device are mounted, ensuring that view and lighting are the same at consecutive visits, thus enabling precise follow-up of the same scalp area of interest with frontal and vertex views. Global photographs were evaluated by two of the authors (AM and RMT), and scored as significant, moderate, or no improvement. In the case of diverging opinions, the inferior score was given.
RESULTS
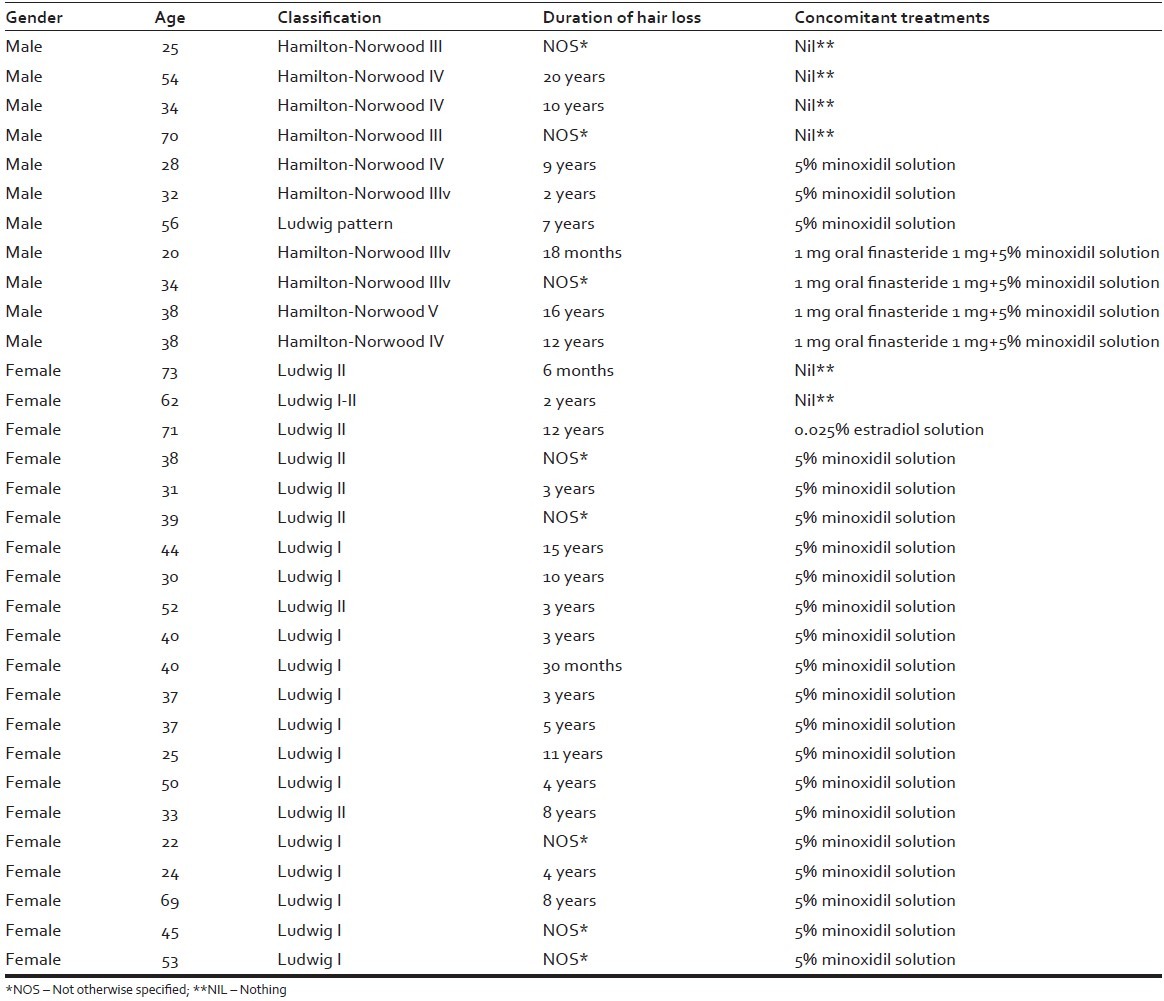
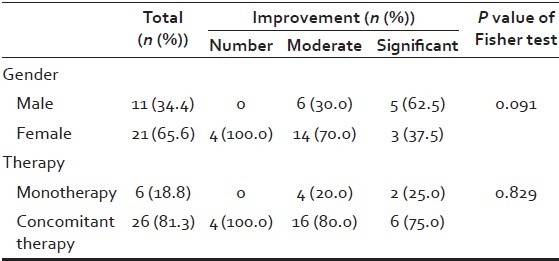
In total, 32 patients with AGA were involved in the study, of which 21 were females, aged 22-73 (mean: 43.6 ± 15.19 standard deviation [SD]), and 11 were males, aged 20-70 (mean: 39 ± 15.01 SD) total mean: 42 ± 15.1 SD. The duration of hair loss in years for men and women was mean 7.1 ± 5.2 SD. The duration of LLLT in months for men and women was mean 8.7 ± 5.2 [Table 1]. The patient characteristics, with respect to gender, age, classification of AGA according to Ludwig and Hamilton-Norwood scales, duration of hair loss, and concomitant treatments are recorded in Table 2.
Table 1.
Improvement of alopecia in relation to the variables: Age, duration of hair loss, and duration of LLLT
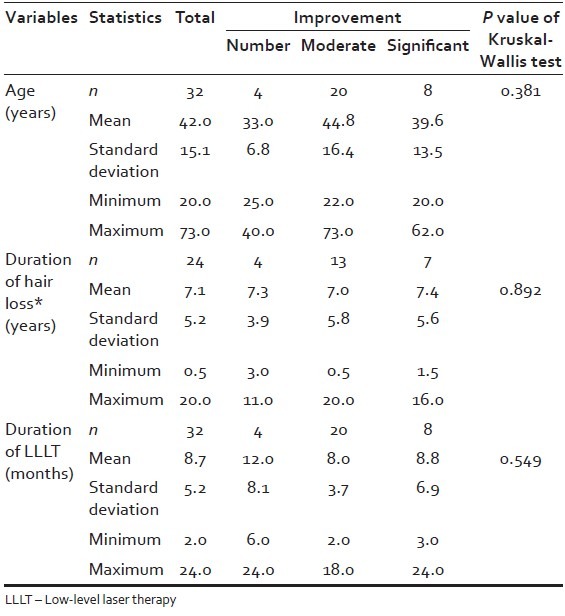
Table 2.
Patient characteristics
The results for the scoring of the global photographic assessment in relation to treatment duration with the HairMax Laser Comb® are demonstrated in Table 3. In summary, eight patients (three female, five male) showed significant improvement, 20 patients (14 female, six male) moderate improvement, and four patients (four female, zero male) no improvement [Figure 1]. Of 32 patients, the HairMax Laser Comb® was used as monotherapy in six patients (two female, four male), and as a concomitant therapy in 26 patients (19 female, seven male). In the monotherapy group, two patients (one female, one male) showed significant improvement [Figure 2], four patients (one female, three male) moderate improvement, and zero patients no improvement [Table 3]. In the concomitant therapy group, six patients (two female, four male) showed significant improvement [Figures 3 and 4], 16 patients (13 female, three male) moderate improvement, and four patients (four female, zero male) no improvement. There was no statistical significant difference between LLLT monotherapy and concomitant therapy with either minoxidil and/or finasteride (P = 0.829), and regarding male or female AGA (P = 0.091) [Table 4].
Table 3.
Scoring of global photographic assessment in relation to treatment duration

Figure 1.
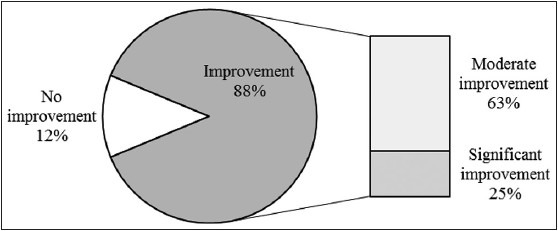
Graphic summary of results
Figure 2.
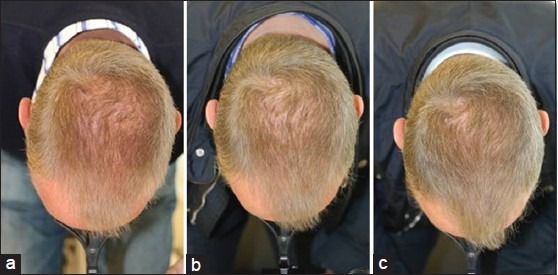
Monotherapy in a 54-year-old male (a) Before treatment, and improvement after (b) 6 months, and (c) 12 months of low-level laser therapy
Figure 3.
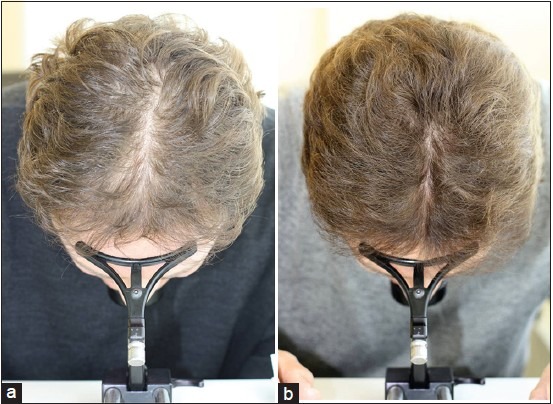
Concomitant treatment with topical 5% minoxidil in a 55-year-old male adding on low-level laser therapy (LLLT) to 4 year pretreatment with 5% topical minoxidil solution (a) Before, and (b) After 3 months of added LLLT
Figure 4.
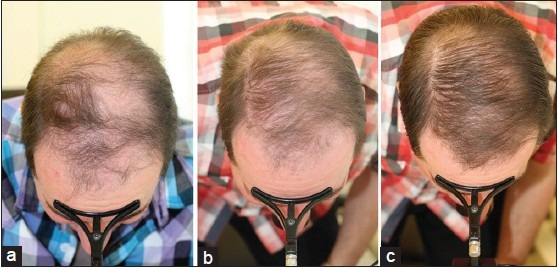
Concomitant treatment with topical 5% minoxidil and 1 mg oral finasteride in a 34-year-old male (a) Before, (b) After 9 months treatment with 1 mg oral finasteride and topical 5% minoxidil solution bid, and (c) After 3 months after adding on low-level laser therapy
Table 4.
Comparative assessment of efficacy between monotherapy and concomitant for male and female androgenetic alopecia
Treatment was well tolerated and no serious adverse events were reported.
DISCUSSION
Androgenetic alopecia is the most common form of hair loss in men and in women. Currently, topical 2% and 5% minoxidil solution and 1 mg oral finasteride are the treatments with the highest levels of medical evidence,[8] but patients who exhibit intolerance or poor response to these treatments are in need of additional treatment modalities. Although low-level energy lasers have been therapeutically used in medicine for photobiostimulation in a variety of indications more than 30 years,[9] it has only recently found the attention of the scientific community for the treatment of AGA.[6,10,11]
We have chosen the 655 nm-HairMax Laser Comb® for several reasons: First, it represents the device with the most clinical study reports regarding its efficacy,[4,5,12] secondly, the cost of the device is affordable, and thirdly, the device is simple enough for patients to use at home. Finally, the fact that the device is safe, for which it received 510 (k) clearance from the FDA for the treatment of AGA, was also an important consideration.
Our study demonstrates clinical efficacy of the device for treatment of male and female AGA, both as monotherapy and as concomitant therapy, in terms of clinically relevant improvement of appearance of hair. Of 32 patients, eight patients (25%) showed significant improvement, and 20 patients (62.5%) showed moderate improvement in global photographic assessments. The effect was observed as early as 3 months of treatment, and was sustained up to a maximum observation time of 24 months. The technology appears to work better for some than for others, and predictive factors which will most benefit from LLLT are to be determined. It seems though, that patients with intermediate alopecia (Hamilton-Norwood III and IV, and Ludwig I and II, respiratory) respond best, since effective photobiostimulation depends on a minimum of hair for effective photobiostimulation, and on a maximum of hair for the laser beam to reach the scalp without absorption or interference from existing hairs.
The hypothesized mechanisms of action of LLLT are increased adenosine tri-phosphate (ATP) production, modulation of reactive oxygen species (ROS), and induction of transcription factors. The proposed cellular chromosphere responsible for the effect of visible light is cytochrome c oxidase (COX) with absorption peaks in the near infrared, and mitochondria the likely site for the initial effects. It is believed that LLLT displaces nitric oxid from COX allowing an influx of oxygen to bond to COX and progress forward in the respiratory process to ATP production and ROS signaling. These effects in turn lead to increased cellular proliferation, modulation in levels of cytokines, growth factors and inflammatory mediators, and increased tissue oxygenation. While the effects of these biochemical and cellular changes have broadly been studied in both animal models and clinical studies with patients, and have shown benefits in diverse conditions, such as increased healing in chronic wounds, improvements in sports injuries and carpal tunnel syndrome, pain reduction in arthritis and neuropathies, and amelioration of damage after heart attacks, stroke, nerve injury and retinal toxicity,[7,9] the effects on hair growth stimulation have only recently gained the attention of the scientific community.
CONCLUSION
From our own observations, we share with other authors the opinion that LLLT represents a safe and potentially effective treatment option for patients with AGA who do not respond or are not tolerant to standard treatment of AGA.[6,7] Moreover, combining LLLT with topical minoxidil solution and oral finasteride may act synergistic to enhance hair growth. Due to the known beneficial effect on wound healing, it is conceivable that LLLT as an adjunctive therapy in hair transplant surgery may also reduce postoperative shedding, reduce healing time, and increase graft patency. The scientific basis for such an approach is given, but there is a need for controlled studies with a higher number of patients to establish an increase in efficacy of combination regimens.[13]
Footnotes
Source of Support: Nil
Conflict of Interest: Ralph M. Trüeb performs consultant activity for Lexington International LLC. This study represents an integral part of Andréia Munck's traineeship in trichology at the Center for Dermatology and Hair Diseases Prof. Trüeb.
REFERENCES
- 1.Mester E, Szende B, Gärtner P. The effect of laser beams on the growth of hair in mice. Radiobiol Radiother (Berl) 1968;9:621–6. [PubMed] [Google Scholar]
- 2.Moreno-Arias G, Castelo-Branco C, Ferrando J. Paradoxical effect after IPL photoepilation. Dermatol Surg. 2002;28:1013–6. doi: 10.1046/j.1524-4725.2002.02101.x. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein EF. Hair growth induced by diode laser treatment. Dermatol Surg. 2005;31:584–6. doi: 10.1111/j.1524-4725.2005.31168. [DOI] [PubMed] [Google Scholar]
- 4.Leavitt M, Charles G, Heyman E, Michaels D. HairMax LaserComb laser phototherapy device in the treatment of male androgenetic alopecia: A randomized, double-blind, sham device-controlled, multicentre trial. Clin Drug Investig. 2009;29:283–92. doi: 10.2165/00044011-200929050-00001. [DOI] [PubMed] [Google Scholar]
- 5.Avram MR, Rogers NE. The use of low-level light for hair growth: Part I. J Cosmet Laser Ther. 2009;11:110–7. doi: 10.1080/14764170902842531. [DOI] [PubMed] [Google Scholar]
- 6.Avram MR, Leonard RT, Jr, Epstein ES, Williams JL, Bauman AJ. The current role of laser/light sources in the treatment of male and female pattern hair loss. J Cosmet Laser Ther. 2007;9:27–8. doi: 10.1080/14764170601134479. [DOI] [PubMed] [Google Scholar]
- 7.Kim H, Choi JW, Kim JY, Shin JW, Lee SJ, Huh CH. Low-level light therapy for androgenetic alopecia: A 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177–83. doi: 10.1111/dsu.12200. [DOI] [PubMed] [Google Scholar]
- 8.Blumeyer A, Tosti A, Messenger A, Reygagne P, Del Marmol V, Spuls PI, et al. European Dermatology Forum (EDF) Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men. J Dtsch Dermatol Ges. 2011;9(Suppl 6):S1–57. doi: 10.1111/j.1610-0379.2011.07802.x. [DOI] [PubMed] [Google Scholar]
- 9.Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng. 2012;40:516–33. doi: 10.1007/s10439-011-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta AK, Daigle D. The use of low-level light therapy in the treatment of androgenetic alopecia and female pattern hair loss. J Dermatolog Treat. 2014;25:162–3. doi: 10.3109/09546634.2013.832134. [DOI] [PubMed] [Google Scholar]
- 11.Ghanaat M. Types of hair loss and treatment options, including the novel low-level light therapy and its proposed mechanism. South Med J. 2010;103:917–21. doi: 10.1097/SMJ.0b013e3181ebcf71. [DOI] [PubMed] [Google Scholar]
- 12.Satino JL, Markou M. Hair regrowth and increased hair tensile strength using Hair Max Laser Comb for low-level laser therapjy. Int J Cosmet Surg Aesthet Dermatol. 2003;5:113–7. [Google Scholar]
- 13.Rajput RJ. Controversy: Is there a role for adjuvants in the management of male pattern hair loss? J Cutan Aesthet Surg. 2010;3:82–6. doi: 10.4103/0974-2077.69016. [DOI] [PMC free article] [PubMed] [Google Scholar]


