Abstract
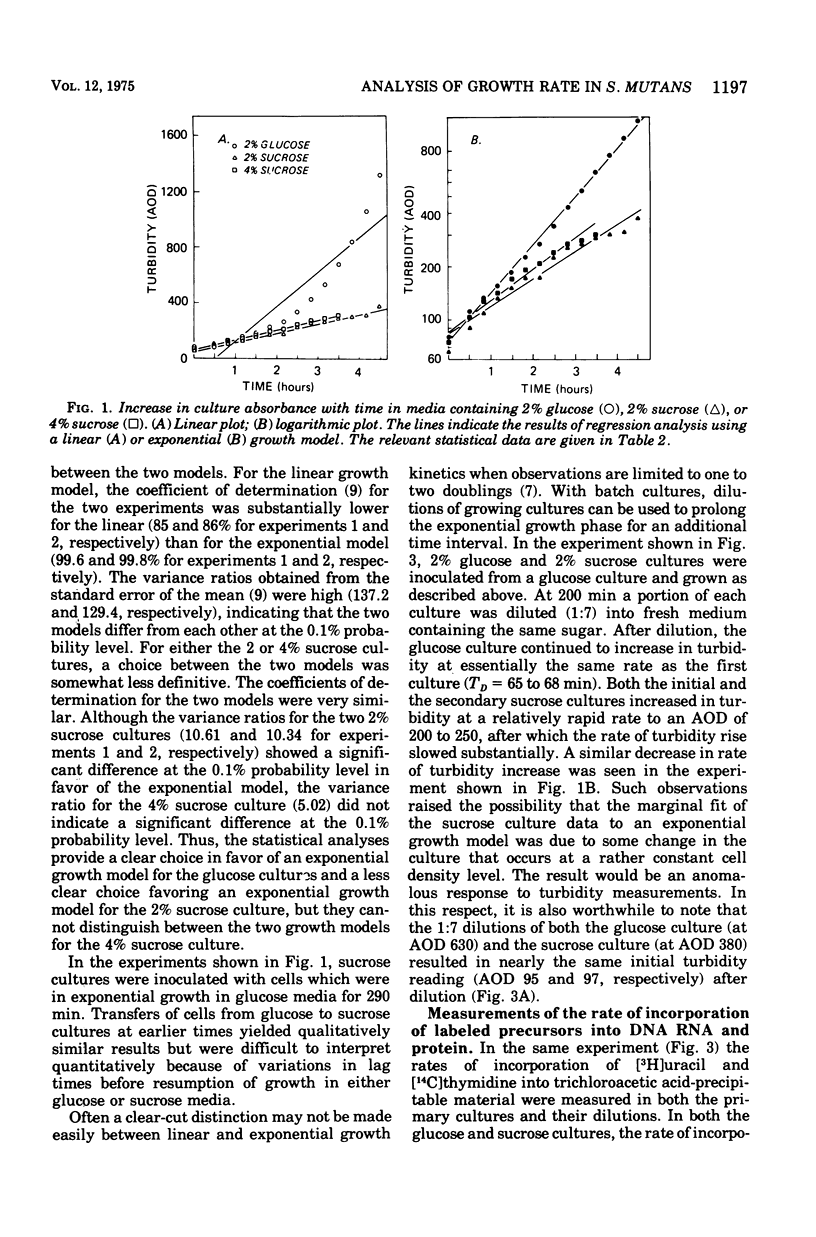
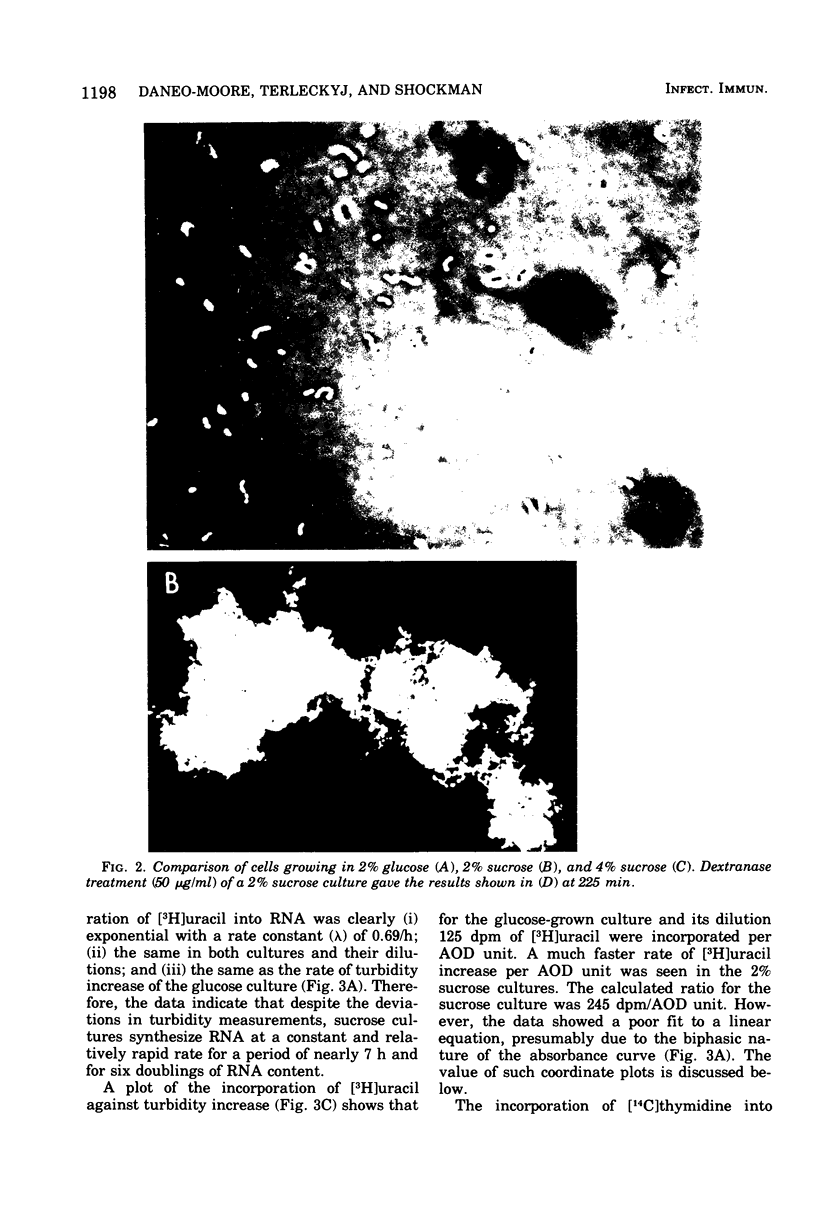
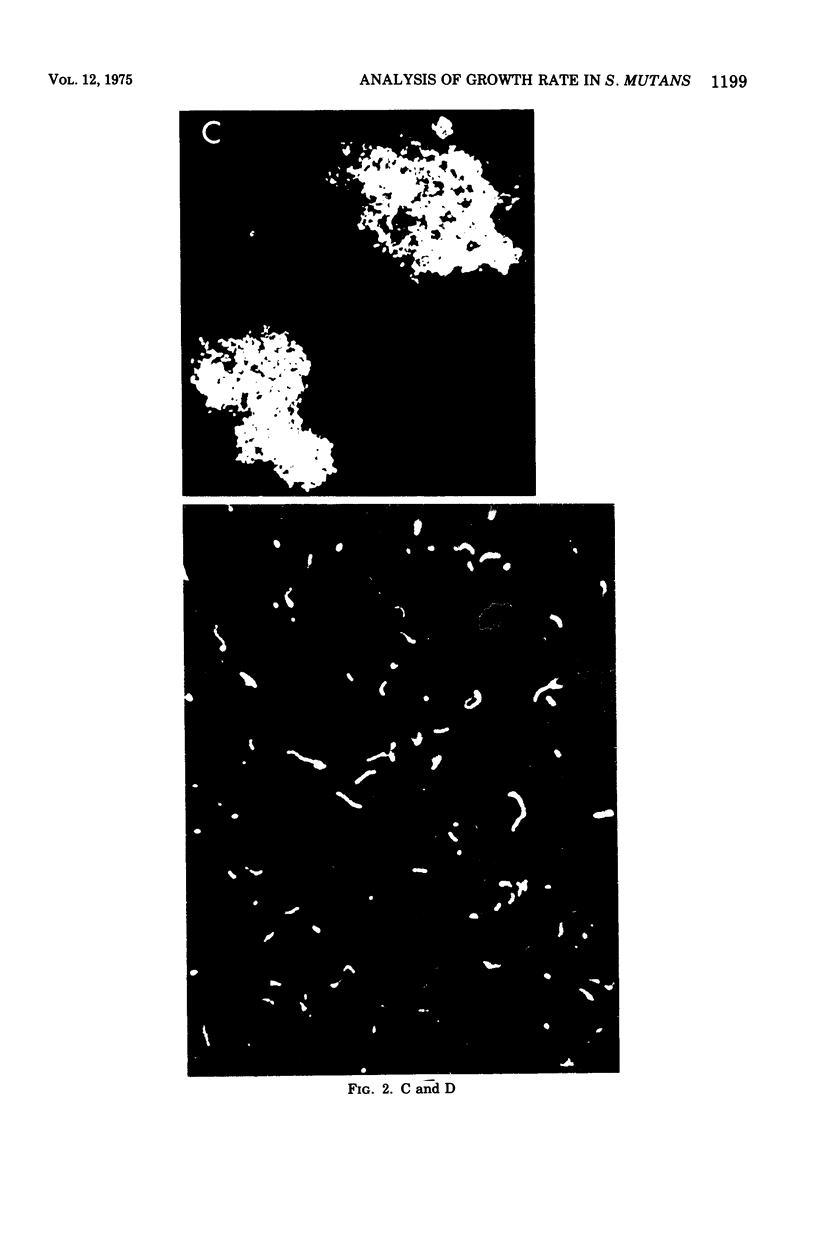
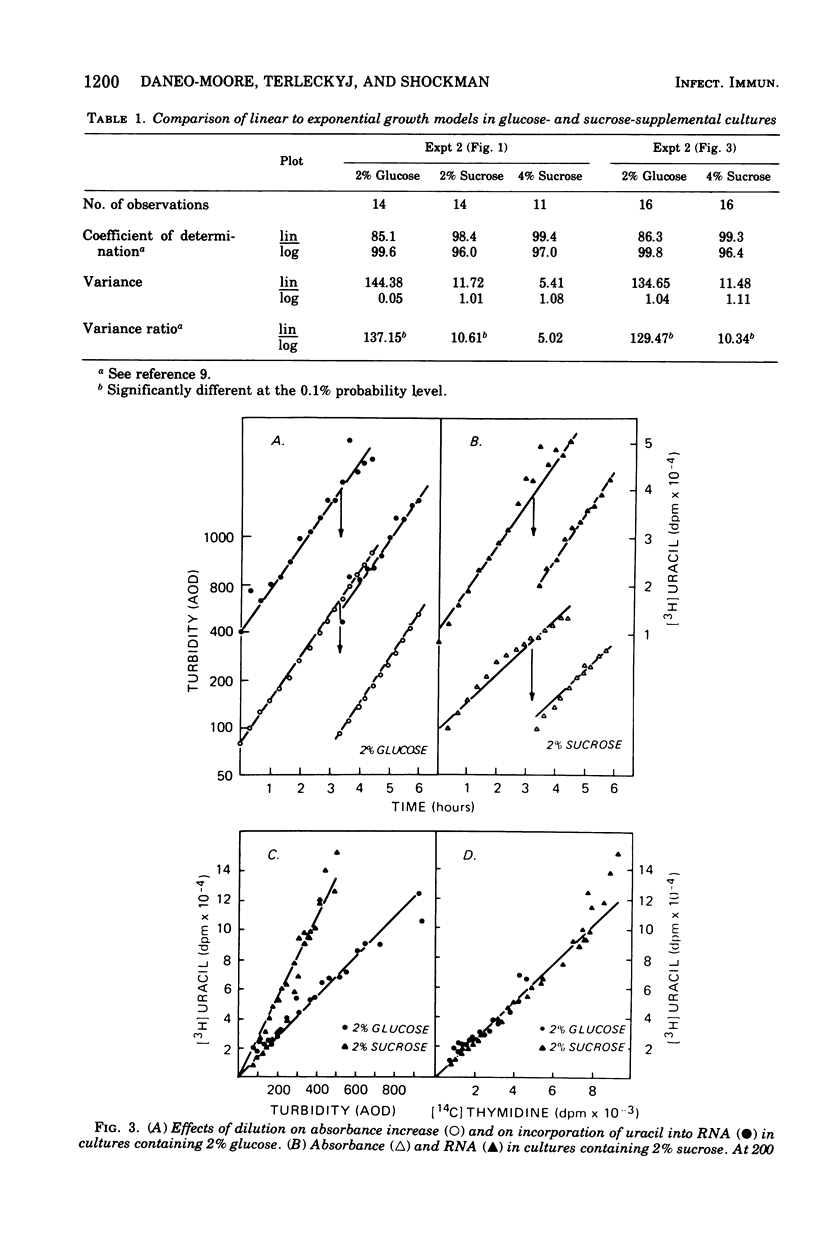
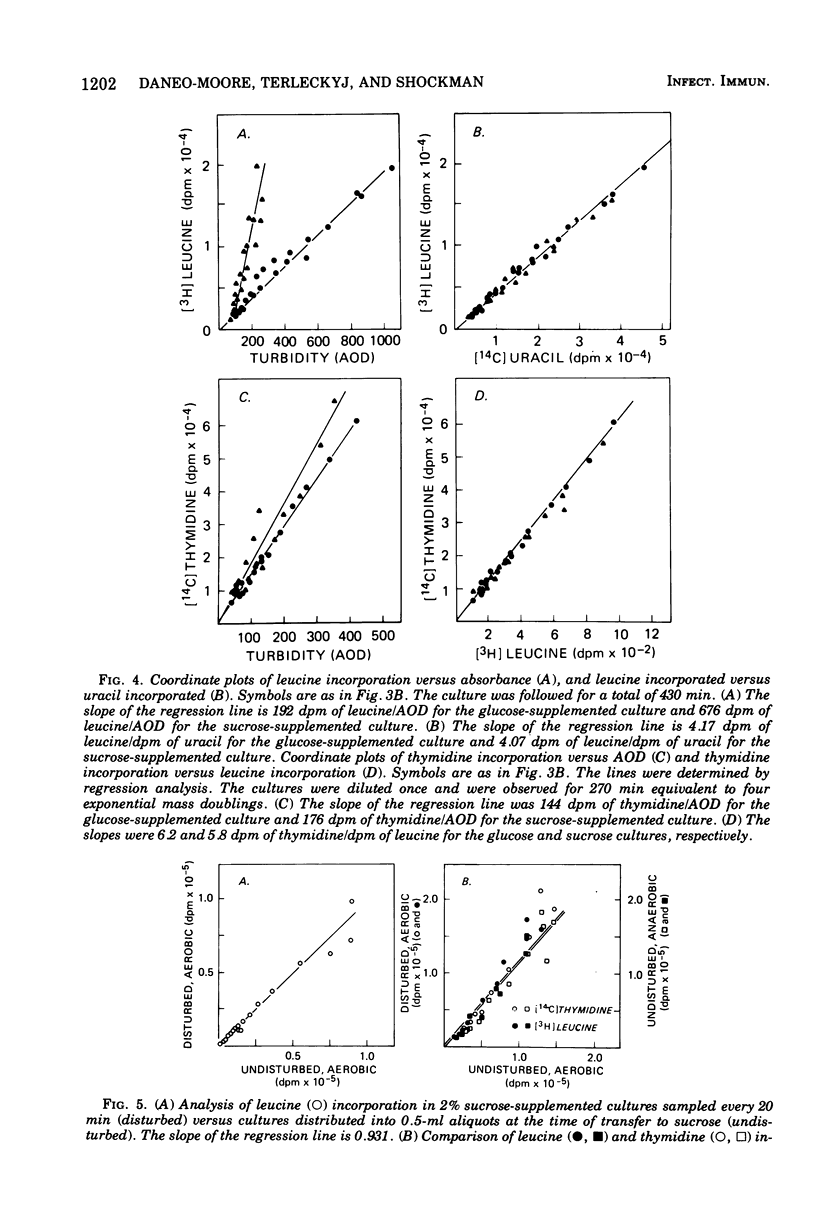
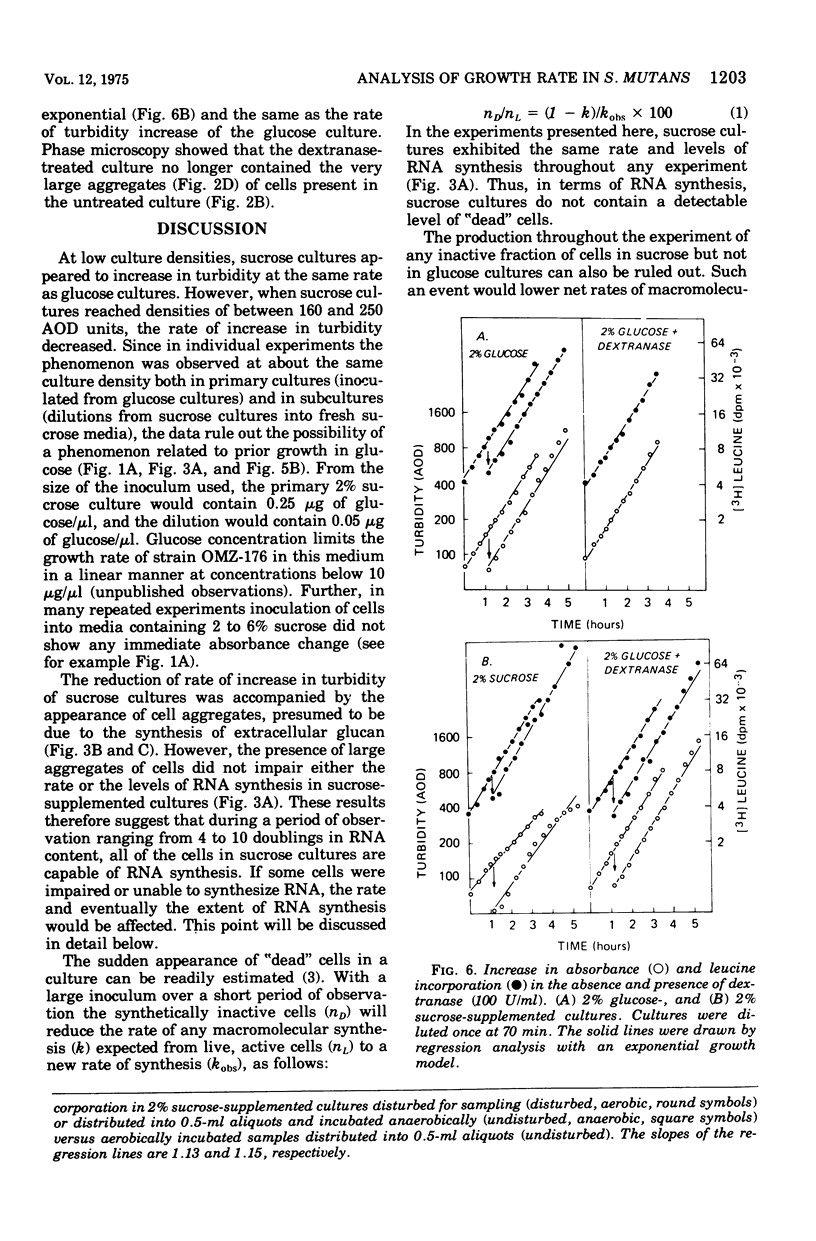
In the presence of sucrose, Streptococcus mutans grows in large glucan-containing aggregates. Because of reports of linear rather than exponential growth of sucrose-grown cultures, the kinetics of growth of sucrose-grown cultures of S. mutans strain OMZ-176 were compared with those of glucose-grown cultures. Culture turbidity measurements indicated that growth of sucrose cultures was slower, did not follow exponential kinetics, and slowed and stopped at lower absorbance values than did glucose-grown cultures. However, measurements of the rates of accumulation of deoxyribonucleic acid (DNA), ribonucleic acid (RNA), and protein using fully equilibrated radioactively labeled precursors of each of these macromolecular species in sucrose and glucose-grown cultures showed that: (i) for glucose cultures the synthesis of each of the three informational molecules occurred at the same exponential rate, which was identical to the rate of turbidity increase; (ii) for sucrose cultures each macromolecular species was synthesized at the same exponential rate and these rates were identical to the rate of increase of turbidity of the glucose-grown culture for periods of up to 7 h. Furthermore, the ratios of DNA to RNA, RNA to protein, and protein to DNA for the sucrose cultures were identical to those for the glucose cultures for up to 10 doublings. From these data it was concluded that in the presence of sucrose S. mutans grows in a balanced fashion at the same exponential rate as it does in glucose. The deviation from an exponential growth model of the absorbance in sucrose cultures was attributed to an optical artifact due to the formation of large glucan-containing aggregates of cells. The addition of dextranase to sucrose cultures resulted in cultures which increased in turbidity at the same exponential rate as glucose-grown cultures, without affecting the rate or extent of macromolecular synthesis.
Full text
PDF




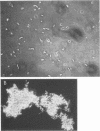
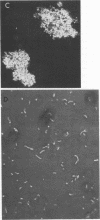
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- CAMPBELL A. Synchronization of cell division. Bacteriol Rev. 1957 Dec;21(4):263–272. doi: 10.1128/br.21.4.263-272.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Fitzgerald R. J. Dextran-induced agglutination of Streptococcus mutans, and its potential role in the formation of microbial dental plaques. J Bacteriol. 1969 May;98(2):341–346. doi: 10.1128/jb.98.2.341-346.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L. Overall controls on the biosynthesis of ribosomes in growing bacteria. J Theor Biol. 1970 Aug;28(2):201–231. doi: 10.1016/0022-5193(70)90053-6. [DOI] [PubMed] [Google Scholar]
- Koch A. L. Turbidity measurements of bacterial cultures in some available commercial instruments. Anal Biochem. 1970 Nov;38(1):252–259. doi: 10.1016/0003-2697(70)90174-0. [DOI] [PubMed] [Google Scholar]
- Roth G. S., Shockman G. D., Daneo-Moore L. Balanced macromolecular biosynthesis in "protoplasts" of Streptococcus faecalis. J Bacteriol. 1971 Mar;105(3):710–717. doi: 10.1128/jb.105.3.710-717.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAECHTER M., MAALOE O., KJELDGAARD N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Conover M. J., Kolb J. J., Phillips P. M., Riley L. S., Toennies G. LYSIS OF STREPTOCOCCUS FAECALIS. J Bacteriol. 1961 Jan;81(1):36–43. doi: 10.1128/jb.81.1.36-43.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Thompson J. S., Conover M. J. Replacement of Lysine by Hydroxylysine and Its Effects on Cell Lysis in Streptococcus faecalis. J Bacteriol. 1965 Sep;90(3):575–588. doi: 10.1128/jb.90.3.575-588.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Wood W. I., Krichevsky M. I. Linear growth kinetics of plaque-forming streptococci in the presence of sucrose. J Gen Microbiol. 1969 Sep;58(1):125–133. doi: 10.1099/00221287-58-1-125. [DOI] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]