Abstract
Novel trityl-nitroxide biradicals were synthesized and exhibited enhanced sensitivity and stability for rapid and simultaneous measurement of redox status and oxygenation by electron paramagnetic resonance spectroscopy.
Stable biradicals have attracted considerable attention as building blocks for organic-based magnetic materials.1 They also found applications in the fields of dynamic nuclear polarization (DNP)2 and living free radical polymerization.3 To date, most stable biradicals consist of two identical radical moieties, but mixed biradicals have also exhibited unique physico-chemical properties and found applications for sensing NO,4 pH5 and redox status6 in biological systems.
Nitroxides have been shown to be useful for in vivo assessment and imaging of redox status by monitoring their conversion rate to the corresponding diamagnetic hydroxylamine using magnetic resonance (MRI) and electron paramagnetic resonance imaging (EPRI) techniques.7 Unfortunately, rapid in vivo reduction with loss of signal, greatly limits the application of nitroxides as redox and oxygen probes. Moreover, the relatively broad EPR triplet spectra of nitroxides also limits the obtainable sensitivity and image resolution for EPR/EPRI analysis. Recently, tetrathiatriarylmethyl trityl radicals have shown high biostability and narrow singlet EPR signal at physiological pH thereby providing 10-fold higher sensitivity and 100-fold improved time resolution for EPRI applications as compared to nitroxides.8 The trityl radical linewidth is oxygen sensitive allowing its application as biomedical EPR oximetry probe. However, due to the inherent inertness of trityl radicals to biological reductants such as ascorbate or glutathione,9 their application as redox probe has been limited.
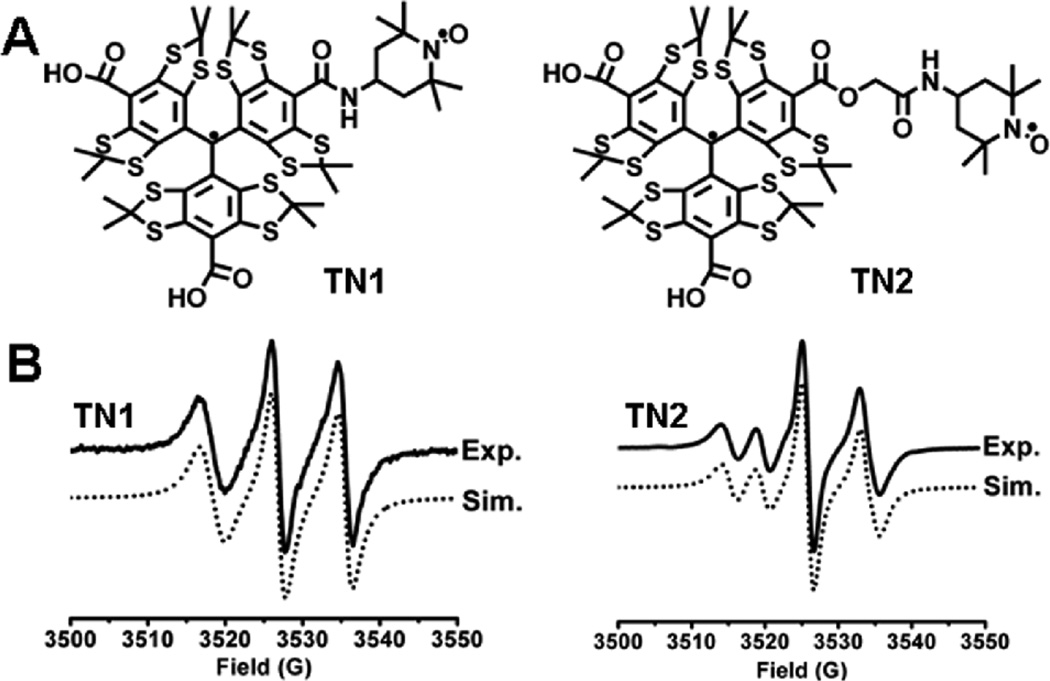
In a typical experiment involving nitroxides, the redox status of a system is usually monitored by spin quenching mechanism (i.e., measurement of the signal decay of the nitroxide). In this work, the strong intramolecular spin exchange interaction between the nitroxide and trityl radicals was exploited to allow measurements of redox status based on new signal formation from the trityl radical. The trityl-nitroxide biradicals exhibit a very broad EPR signal that can be transformed into a much sharper singlet signal upon reduction of the nitroxide moiety to the diamagnetic hydroxylamine. In contrast to the monoradical nitroxide where the relatively broad EPR signal is decreased during reduction, the narrow linewidth, enhanced signal intensity and stability of the trityl can be utilized to assess the redox status as well as oxygentation of the system under investigation. We now report the synthesis of two trityl-nitroxide biradicals TN1 and TN2 with varying linker chain length (Fig. 1A, see synthesis in Supporting Information (SI)) as new probes for the simultaneous measurement of redox metabolism and oxygenation.
Figure 1.
(A) Molecular structure of trityl-nitroxide biradicals. (B) Experimental and simulated EPR spectra of TN1 and TN2 in PBS.
As shown in Fig. 1B, the room-temperature EPR spectrum of TN1 shows a well-resolved triplet with line separation of ~9 G, about half the 14N hyperfine splitting of TEMPO (17 G) in aqueous solution. Spectral simulation shows a J-coupling value of >160 G indicating a strong intramolecular spin exchange between the nitroxide and trityl moieties (see SI for procedure in obtaining J-coupling). EPR spectral profile of TN1 is not affected at a concentration range of 10–400 µM (data not shown), further confirming the intramolecular nature of the spin interaction. Interestingly, TN2 afforded a four-line EPR signal due to a weaker intramolecular spin exchange with a J-coupling value of 52.5 ± 0.3 G. Theoretical results at the B3LYP/6–31+G(d,p)//HF/3–21G(d) level of theory show significant spin delocalization on the linker group of the biradical TN1 (see Table S1 in the SI) which implies that the intramolecular spin exchange interaction in TN1 is most likely through-bond. Much lower spin densities were found on the linker of TN2 (see Table S2 in the SI) consistent with the observed lower J-coupling value.
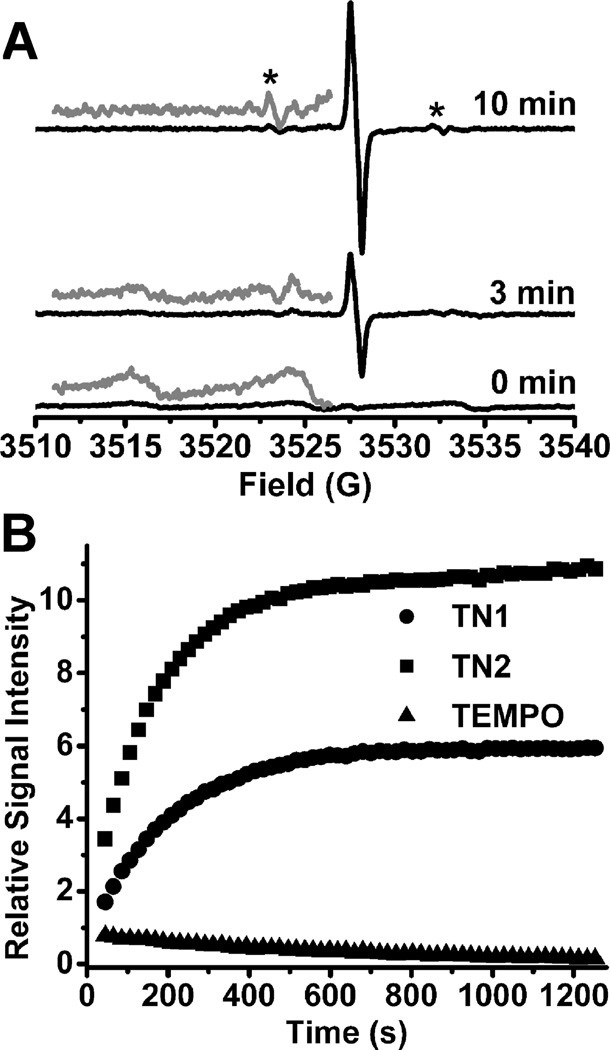
The applicability of these biradicals for EPR measurement of redox status was initially assessed using ascorbic acid. As shown in Fig. 2A, upon addition of ascorbate to a solution of TN1, a relatively sharp singlet signal with a linewidth of 0.65 G appeared and gradually increased due to the conversion of the trityl-nitroxide to trityl-hydroxylamine (TN1-H). Careful measurement of the EPR spectrum with low modulation amplitude (0.05 G) revealed that the singlet signal actually consists of a poorly resolved triplet with a hyperfine splitting constant of 0.22 G (see Fig. S2 in the SI). This additional triplet feature from TN1-H is likely due to the amide-N (I = 1) of the linker group since our theoretical studies show spin density distribution on the amide-N of TN1-H. In the presence of ascorbate, the trityl signal intensity increases with incubation time, whereas the broad signal due to the biradical decreases. After 10 min, the trityl signal intensity reaches its maxima and the broad biradical signal completely disappeared. Disappearance of the biradical spin coupling enables one to visualize the 13C hyperfine splitting from the three aromatic rings (Fig. 2A) (see Fig. S4 in the SI for the simulated spectrum). Addition of K3Fe(CN)6 after reduction by ascorbate restores the original biradical signal with loss of the sharp trityl signal (data not shown). Moreover, UV-vis absorbance of the trityl moiety at 464 nm did not change after addition of ascorbate (see Fig. S5 in the SI) indicating the stability of the trityl in the presence of ascorbate consistent with our prior results.9 A similar behaviour was observed for the formation of TN2-H but with a narrower singlet signal having a linewidth of 0.3 G. The second order rate constant for the reaction of ascorbate with TN1 was found to be slightly faster with k2 = 4.14 ±0.14 M−1s−1 compared to TN2 with k2 = 3.48 ± 0.09 M−1s−1 (see Fig.S7–S10 in the SI). Treatment of TN1 and TN2 with ascorbate showed increase in signal intensity due to the trityl-hydroxylamine formation while TEMPO alone showed a decrease in signal intensity (Fig. 2B). The EPR signal intensities of TN1-H and TN2-H are approximately 70 and 130 times higher, respectively, than TEMPO after 20 min. Double integration of the observed EPR signals for TN2-H and TN1-H were found to be similar indicating that the difference in their EPR signal intensities is mainly due to the difference in their intrinsic linewidths in air.
Figure 2.
(A) EPR spectra obtained by reaction of TN1 (10 µM) with ascorbic acid (400 µM) in PBS (50 mM, pH 7.4). Gray lines shows the enlarged (5×) portion of the spectrum; (*) indicates the 13C hyperfine splittings. (B) Plot of EPR signal intensity as a function of time. For biradicals the singlet signals due to trityl radicals were monitored while the middle peak was monitored for TEMPO. Spectra were recorded with 5 mW microwave power and 0.2 G modulation amplitude.
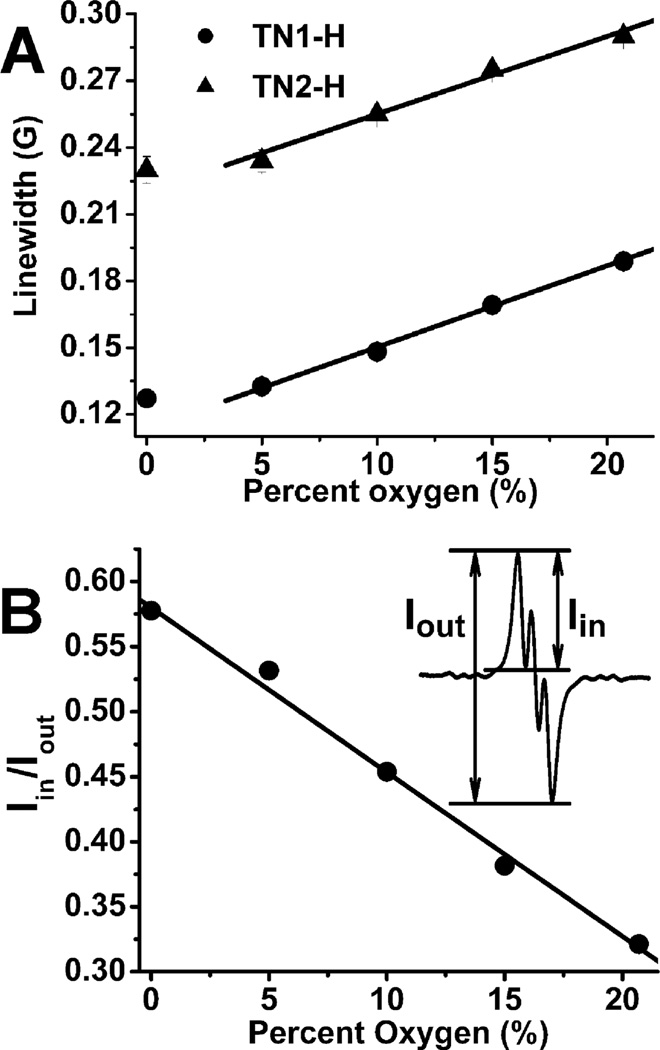
Fig. 3A shows the peak-to-peak linewidth response of TN1-H and TN2-H to O2 giving sensitivities of 3.68 ± 0.08 mG/%O2 and 3.50 ± 0.06 mG/%O2, respectively, at O2 concentration above 5%. However, under anaerobic conditions, the linewidths deviate from linearity similar to our previous observation for the other trityl radicals such as OX063 and CT-03.10 Since the components of the triplet EPR signals of TN1-H partially overlap with each other, the ratio of the first peak intensity (Iin) to the total peak intensity (Iout) was obtained10 (Note: the first peak intenisty, Iin, was obtained by subtracting the positive peak intensity from the negative peak intensity of the first peak while the total peak intensity, Iout, was obtained by subtracting the first positive peak intensity from the last negative peak, see Fig. 3B for illustration). Fig. 3B demonstrates better sensitivity and linearity of the peak intensity ratio (Iin/Iout) with varying O2 concentrations compared to linewidth measurements as shown in Fig. 3A since the ratio of EPR signal peak amplitudes is proportional to the square of the ratio of the EPR linewidths.10 Therefore, TN1-H could be a more advantageous O2 probe than other trityl radicals such as OX063 and CT-03 since the latter compounds only use EPR linewidths to measure O2 concentrations.8–10 Also, worth noting is that the Iin/Iout response to O2 is reversible allowing potential real-time monitoring of oxygenation (see Fig. S13 in the SI).
Figure 3.
(A) Plot of the linewidth of the trityl-hydroxylamine TN1-H and TN2-H as a function of O2 concentration. (B) Plot of Iin/Iout for TN1-H as a function of O2%. TN1-H and TN2-H were generated by mixing the biradical (50 uM) with ascorbate (2 mM) in PBS (50 mM, pH 7.4). See more details in the supporting information.
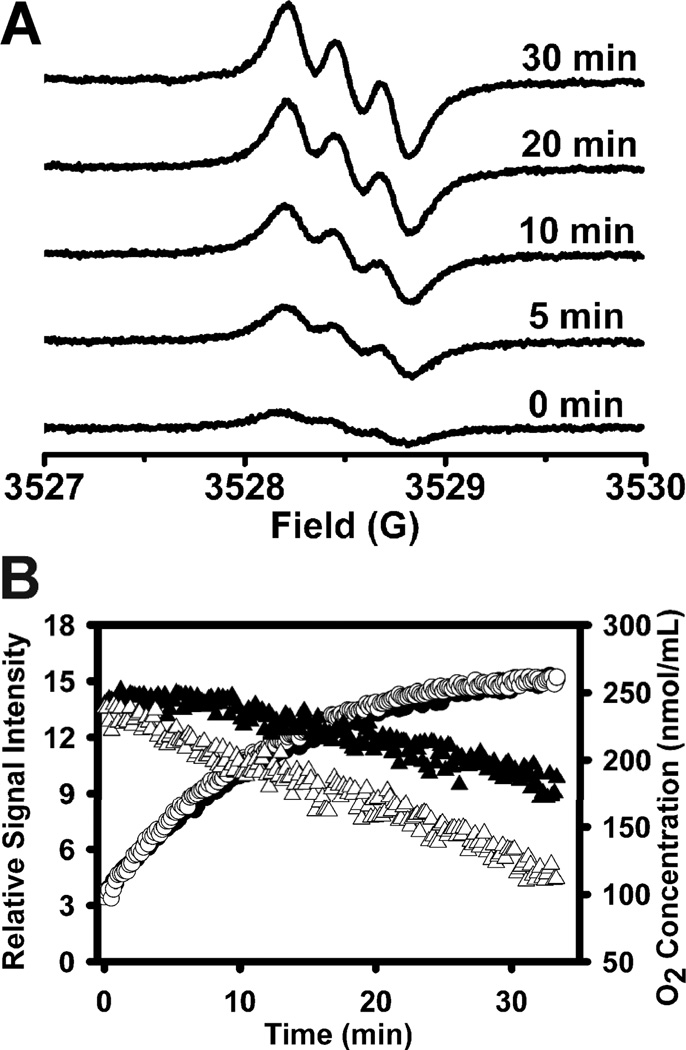
TN1 was utilized to investigate O2 consumption and redox status in fresh rat liver homogenate in order to demonstrate its application as dual probe for the simultaneous measurement of redox status and oxygenation. As shown in Fig. 4A, a weak and broad triplet signal was initially observed and in the presence of endogenous reducing agents, increase in signal intensity and sharper linewidth were observed due to the formation of the reduced form, TN1-H. Fig. 4B shows plots of the EPR signal intensity and O2 concentration as a function of time with the initial reduction rate of TN1 being 1.47 ± 0.03 µmol/min/mg protein and the O2 consumption rate being 1.18 ± 0.03 nmol/min/mg protein. Addition of succinate substrate to stimulate mitochondrial respiration leads to a much faster O2 consumption rate of 2.03 ± 0.02 nmol/min/mg protein but only a slight increase in the initial reduction rate of TN1 (1.60 ± 0.04 µmol/min/mg protein).
Figure 4.
(A) Time-dependent EPR spectra obtained by incubating TN1 (50 µM) with fresh rat liver homogenate (1.8 mg protein/mL) in the presence of succinate (50 mM) in a sealed capillary. (B) Variations of EPR signal double integration (circle) and O2 concentration (triangle) with time in the presence (unfilled) or absence (filled) of succinate. Spectra were recorded with 5 mW microwave power and 0.03 G modulation amplitude.
In summary, our preliminary results show that the newly synthesized trityl-nitroxide biradicals provide an opportunity for the design of new probes that exploit the sensitivity of nitroxides to measure redox status, along with the narrow singlet signal and stability of the trityl for enhanced sensitivity and speed of detection as well as potential for greatly increased resolution EPR imaging. This new probe design has great potential in enabling the simultaneous EPR measurement of redox status and oxygenation in a wide variety of chemical and biological systems.
Supplementary Material
Footnotes
† Electronic Supplementary Information (ESI) available: Synthesis, experimental details, and calculated spin density distribution. See DOI: 10.1039/b000000x/
Notes and references
- 1.(a) Lahti PM, editor. Magnetic Properties of Organic Materials. New York: Marcel Dekker; 1999. [Google Scholar]; (b) Kahn O, editor. Molecular Magnetism. VCH; Cambridge, UK: 1993. [Google Scholar]; (c) Miller JS, Drillon M, editors. Magnetism: Molecules to Materials. I–IV. Weinheim: Wiley-VCH; 2001–2003. [Google Scholar]; (d) Rajca A. Chem. Rev. 1994;94:871. [Google Scholar]; (e) Inoue K, Iwamura H. Angew. Chem., Int. Ed. Engl. 1995:34–927. [Google Scholar]; (f) Shultz DA, Farmer GT. J. Org. Chem. 1998;63:6254. doi: 10.1021/jo980562p. [DOI] [PubMed] [Google Scholar]
- 2.(a) Hu KN, Yu HH, Swager TM, Griffin RG. J. Am. Chem. Soc. 2004;126:10844. doi: 10.1021/ja039749a. [DOI] [PubMed] [Google Scholar]; (b) Song CS, Hu KN, Joo CG, Swager TM, Griffin RG. J. Am. Chem. Soc. 2006;128:11385. doi: 10.1021/ja061284b. [DOI] [PubMed] [Google Scholar]; (c) Dane EL, Maly T, Debelouchina GT, Griffin RG, Swager TM. Org. Lett. 2009;11:1871. doi: 10.1021/ol9001575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Hill NL, Braslau R. Macromolecules. 2005;38:9066. [Google Scholar]; (b) Lizotte JR, Anderson SG, Long TE. J. Polym. Sci., A: Polym. Chem. 2004;42:1547. [Google Scholar]; (c) Huang WL, Chiarelli R, Charleux B, Rassat A, Vairon JP. Macromolecules. 2002;35:2305. [Google Scholar]
- 4.Marx L, Rassat A. Angew. Chem.-Int. Edit. 2000;39:4494. doi: 10.1002/1521-3773(20001215)39:24<4494::aid-anie4494>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 5.Ishiguro K, Ozaki M, Sekine N, Sawaki Y. J. Am. Chem. Soc. 1997;119:3625. [Google Scholar]
- 6.Roshchupkina GI, Bobko AA, Bratasz A, Reznikov VA, Kuppusamy P, Khramtsov VV. Free Radic. Biol. Med. 2008;45:312. doi: 10.1016/j.freeradbiomed.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Berliner LJ, Fujii H, Wan XM, Lukiewicz SJ. Magn. Reson. Med. 1987;4:380. doi: 10.1002/mrm.1910040410. [DOI] [PubMed] [Google Scholar]; (b) Zweier JL, Kuppusamy P. Proc. Natl. Acad. Sci. U. S. A. 1988;85:5703. doi: 10.1073/pnas.85.15.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kuppusamy P, Chzhan M, Vij K, Shteynbuk M, Lefer DJ, Zweier JL. Proc. Natl. Acad. Sci. U. S. A. 1994;91:3388. doi: 10.1073/pnas.91.8.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Kuppusamy P, Li HQ, Ilangovan G, Cardounel AJ, Zweier JL, Yamada K, Krishna MC, Mitchell JB. Cancer Res. 2002;62:307. [PubMed] [Google Scholar]; (e) He GL, Kutala VK, Kuppusamy P, Zweier JL. Free Radic. Biol. Med. 2004;36:665. doi: 10.1016/j.freeradbiomed.2003.11.024. [DOI] [PubMed] [Google Scholar]; (f) Hyodo F, Matsumoto K, Matsumoto A, Mitchell JB, Krishna MC. Cancer Res. 2006;66:9921. doi: 10.1158/0008-5472.CAN-06-0879. [DOI] [PubMed] [Google Scholar]; (g) Utsumi H, Yamada K, Ichikawa K, Sakai K, Kinoshita Y, Matsumoto S, Nagai M. Proc. Natl. Acad. Sci. U. S. A. 2006;103:1463. doi: 10.1073/pnas.0510670103. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Swartz HM, Khan N, Khramtsov VV. Antioxid. Redox Signal. 2007;9:1757. doi: 10.1089/ars.2007.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Reddy TJ, Iwama T, Halpern HJ, Rawal VH. J. Org. Chem. 2002;67:4635. doi: 10.1021/jo011068f. [DOI] [PubMed] [Google Scholar]; (b) Liu YP, Villamena FA, Sun J, Xu Y, Dhimitruka I, Zweier JL. J. Org. Chem. 2008;73:1490. doi: 10.1021/jo7022747. [DOI] [PubMed] [Google Scholar]; (c) Liu YP, Villamena FA, Sun J, Wang TY, Zweier JL. Free Radic. Biol. Med. 2009;46:876. doi: 10.1016/j.freeradbiomed.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu YP, Villamena FA, Zweier JL. Chem. Commun. 2008:4336. doi: 10.1039/b807406b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bobko AA, Dhimitruka I, Eubank TD, Marsh CB, Zweier JL, Khramtsov VV. Free Radic. Biol. Med. 2009;47:654. doi: 10.1016/j.freeradbiomed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.