Abstract
Children with 22q11.2 deletion syndrome (22q11DS) present with congenital heart disease (CHD) and high prevalence of psychiatric disorders and neurocognitive deficits. Although CHD has been implicated in neurodevelopment, its role in the neuropsychiatric outcome in 22q11DS is poorly understood. We investigated whether CHD contributes to the high prevalence of psychiatric disorders and neurocognitive impairments in 22q11DS. Fifty-four children ages 8–14 years with 22q11DS and 16 age-matched non-deleted children with CHD participated. They were assessed using semi-structured interviews and a Computerized Neurocognitive Battery. CHD status was assessed using available medical records. Prevalence of psychiatric disorders and cognitive profiles were compared among the groups. There were no significant differences between the prevalence of psychiatric disorders in the 22q11DS with and without CHD. In 22q11DS with CHD, the prevalence rates were 41% anxiety disorders, 37% ADHD and 71% psychosis spectrum. In 22q11DS without CHD, the rates were 33% anxiety disorders, 41% ADHD and 64% psychosis spectrum. In comparison, the non-deleted CHD group had lower rates of psychopathology (25% anxiety disorders, 6% ADHD, and 13% psychosis spectrum). Similarly, the 22q11DS groups, regardless of CHD status, had significantly greater neurocognitive deficits across multiple domains, compared to the CHD-only group. We conclude that CHD in this sample of children with 22q11.2DS does not have a major impact on the prevalence of psychiatric disorders and is not associated with increased neurocognitive deficits. These findings suggest that the 22q11.2 deletion status itself may confer significant neuropsychiatric vulnerability in this population.
Keywords: 22q11.2 deletion syndrome, congenital heart disease, psychiatric disorder, psychosis spectrum, neuropsychology
INTRODUCTION
Initially described as a syndrome by DiGeorge [1965] and determined to be the result of the 22q11.2 microdeletion [Driscoll et al., 1992], 22q11DS is estimated to occur in 1/4,000 live births [Devriendt et al., 1998; Botto et al., 2003]. It is characterized by a host of congenital abnormalities including congenital heart disease (CHD), palatal defects, immune deficiencies and thymic hypoplasia [Ryan et al., 1997; Botto et al., 2003; McDonald-McGinn and Sullivan, 2011]. Most affected individuals have deficits in language, motor, visual-spatial processing, executive function, memory, and social cognition [Golding-Kushner et al., 1984; Gerdes et al., 1999; Bearden et al., 2001; Kiley-Brabeck and Sobin, 2006; De Smedt et al., 2007; Ousley et al., 2007; Van Aken et al., 2007; Goldenberg et al., 2012; Howley et al., 2012]. High prevalence of psychiatric disorders has been associated with the syndrome. Anxiety disorder, major depressive disorder, attention deficit hyperactivity disorder (ADHD) and obsessive-compulsive disorder may emerge in childhood [Swillen et al., 1999; Gothelf et al., 2004; Niklasson et al., 2005; Antshel et al., 2006; Green et al., 2009; Jolin et al., 2009, 2012; Fabbro et al., 2012; Tang et al., 2013] and schizophrenia spectrum features may emerge in adolescence and early adulthood[Murphy et al., 1999; Bassett et al., 2003; Gothelf et al., 2007; Vorstman et al., 2006; Green et al., 2009; Stoddard et al., 2010; Hooper et al., 2013; Tang et al., 2013].
Multiple congenital, psychiatric and neurocognitive phenotypes observed in 22q11DS enable investigation of interactions among genetic vulnerability and congenital anomalies that may impact intrauterine brain development and be associated with psychiatric disorders. CHD is one of the most common defects in 22q11DS and is reported in approximately 60–75% of cases [Marino et al., 1999, 2012], compared to about 6/1,000 live births in the US general population [Hoffman and Kaplan, 2002; Hoffman et al., 2004]. The 22q11.2 deletion is found in increased frequencies in those born with various CHDs such as interrupted aortic arch, truncus arteriosus, conoventricular septal defects and tetralogy of Fallot (TOF) [Agergaard et al., 2012]. With advances in corrective and palliative surgical techniques, the survival rates of infants born with CHDs have dramatically improved [Gilboa et al., 2010; Marino et al., 2012].
Non-deleted individuals with CHDs who survive to infancy or toddler years often have neurodevelopmental delays, while those who survive to pre-school or school age have higher rates of learning difficulties, ADHD, anxiety disorder, and behavioral problems [Rogers et al., 1995; Forbess et al., 2002; Wernovsky et al., 2005; Karsdorp et al., 2007; Shillingford et al., 2008; Kovacs et al., 2009]. A growing literature suggests that severity of CHDs as well as pre- and peri-operative factors are implicated in early brain development and may confer neuropsychiatric vulnerability [Glauser et al., 1990; Fallon et al., 1995; Miller et al., 1995; Bellinger et al., 1999; Marino et al., 2012]. Presently, studies focus primarily on early neurodevelopmental outcomes during infancy or pre-school age. There is a lack of research examining psychiatric disorders in school-aged children or adolescents. Similarly, studies in 22q11DS have been limited to early neurodevelopmental outcomes and have not consistently found a correlation between the CHD status and neurodevelopmental measures [Maharasingam et al., 2003; Gothelf et al., 2004; Swillen et al., 2005; Atallah et al., 2007; Carotti et al., 2008; Cheung et al., 2013]. These studies suggest that 22q11.2 deletion alone may substantially contribute to neurodevelopmental delays and perhaps to neuropsychiatric vulnerability. Neuroimaging studies report that CHDs in 22q11DS correlate with reduction of total cerebral volume and certain cortical regions, and abnormal gyrification, implicating a role for CHDs in neurodevelopment in 22q11DS [Schaer et al., 2009, 2010].
We examined whether the high prevalence of psychiatric disorders in 22q11DS was partly due to effects of CHD on early neurodevelopment. We compared the prevalence of psychiatric disorders, across three clinical groups: 22q11DS with a CHD, 22q11DS without a CHD, and non-deleted with a CHD. We chose the age range of 8–14 years old as many psychiatric disorders are diagnosed during this period in 22q11DS. We also investigated the effect of CHDs on cognition and applied the Computerized Neurocognitive Battery [Gur et al., 2010, 2012]. CNB assesses five cognitive domains commonly impaired in 22q11DS—executive, memory, language, social, and sensorimotor. We hypothesized that 22q11DS with CHDs group would be associated with a higher prevalence of psychiatric disorders and neurocognitive impairments than 22q11DS without a CHD or non-deleted individuals with CHDs.
MATERIALS AND METHODS
Study Population
The sample was drawn from a prospective study, Brain-Behavior and Genetic Studies of the 22q11DS, at the University of Pennsylvania and Children’s Hospital of Philadelphia (CHOP) [Tang et al., 2013]. All individuals ≥8 years old and with the confirmed diagnosis of 22q11DS were eligible for the study. They were recruited from the “22q and You Center” at CHOP and through social media nationally. Inclusion criteria were: age ≥8, ability to provide informed consent/assent, English proficiency, ambulatory and stable medical status, and estimated IQ >70 by Wide Range Achievement Test IV (WRAT-IV) [Wilkinson and Robertson, 2006]. Exclusion criteria were: pervasive developmental disorder per medical records or mental retardation (IQ < 70), medical or neurological disorders that may affect brain function (e.g., uncontrolled seizures, head trauma, CNS tumor, and infection) or visual performance (e.g., blindness). For the present study, data from enrolled participants within the larger study were selected based on age (8–14 years old). Demographically matched, non-deleted CHD participants were recruited from the department of cardiothoracic surgery at CHOP and underwent identical assessment procedures as the 22q11DS participants.
Of the 54 participants with 22q11DS, 43 had the typical 3 MB pair deletion and 6 participants had 1.5–1.7 MB deletions; deletion size was unknown for five participants. Half of the participants (n = 27) with 22q11DS were classified as having a CHD (22q-CHD) and the other half were classified as not having a CHD (22q-noCHD). The 16 non-deleted CHD (CHD-only) participants had a confirmed CHD with surgical repair and were matched to the deleted groups in age, gender, and race. They were screened and confirmed to have no deletion in the chromosome 22q11.2 region or other chromosomal abnormalities. Data from 48 demographically matched, typically developing (TD) participants without known genetic syndromes, CHD, other medical conditions, or psychopathology were drawn from our existing cohort [Gur et al., 2012] and served as a comparison group for the Computerized Neurocognitive Battery analysis.
Cardiac phenotypes, other vascular anomalies and history of cardiac surgery were determined by review of available medical records by a board certified pediatric cardiologist (E.G.). All study participants were assessed for CHD status. CHD was defined as having intra-cardiac defect at birth and included TOF, truncus arteriosus, interrupted aortic arch, ventricular septal defect, atrial septal defect, biscuspid aortic valve and post-natal persistent patent ductus arteriosus. Other vascular anomalies such as vascular ring, right aortic arch with mirror-image branching of brachiocephalic vessels, and aberrant origin subclavian artery were also noted but not designated as a CHD due to their minimal effect on circulatory efficiency.
All procedures were approved by the Institutional Review Boards of University of Pennsylvania and CHOP. Informed consent/assent was obtained from each participant and accompanying parent at the time of initial evaluation.
Clinical Assessment
Psychopathology was assessed with the Kiddie-Schedule for Affective Disorders and Schizophrenia [Kaufman et al., 1997], Structured Interview for Prodromal Syndromes (SIPS) [Miller et al., 2003], and the psychotic and mood diagnoses modules of the Structured Clinical Interview for DSM-IV (Modules C and D) [First and Gibbon, 2004]. Due to time considerations, only sections for ADHD, mood disorders, generalized anxiety, separation anxiety, OCD, and substance-related disorders from Kiddie-Schedule for Affective Disorders and Schizophrenia were included. For assessment of subthreshold and threshold psychotic symptoms, portions of the Kiddie-Schedule for Affective Disorders and Schizophrenia psychosis section were integrated into the SIPS. As detailed previously [Tang et al., 2013], positive, negative and disorganized symptoms were rated on a 7-point scale. They were designated as typical (0 = “absent,” 1 = “questionably present,” and 2 = “mild”), clinically significant but subthreshold (3 = “ moderate,” 4 = “moderately severe,” and 5 = “severe but not psychotic”), or psychotic (6 = “severe and psychotic”). Prodrome diagnoses were given for at least one positive symptom rated ≥3 or at least two negative and/or disorganized symptoms rated ≥3. Psychosis spectrum refers to prodrome diagnoses based on SIPS and psychotic disorders.
The participants’ overall disease burden and function was rated using the SIPS Global Assessment of Function (GAF) [Miller et al., 2003]. Current reading level was estimated using the WRAT-IV reading segment, and serves as an estimate of IQ.
Participants were examined by a psychiatrist and semi-structured interviews were administered by supervised research assistants. Participants age 8–10 were assessed with parent interviews alone and age 11–14 with independent proband and collateral interviews. Each interview information and medical records were integrated into a summary and presented for a case conference where consensus diagnosis was reached by at least two doctoral-level clinicians (J.Y., M.E.C., and R.E.G).
As detailed previously [Gur et al., 2010, 2012], the CNB evaluates accuracy (number of correct responses) and speed (response time to correct responses) on the following domains: Executive—abstraction and mental-flexibility, attention, working memory; Episodic memory—verbal memory, face memory, spatial memory; Complex cognition—language, nonverbal reasoning, spatial processing; Social cognition—emotion identification, emotion differentiation, age differentiation; Praxis speed—motor speed and sensorimotor speed.
Data Analysis
Clinical data were analyzed with SAS version 9.3 (SAS Institute Inc., Cary, NC). Categorical variables were analyzed using Fisher’s Exact test while continuous variables were analyzed with single-factor analysis of variance. The z-scores of CNB speed and accuracy were calculated based on the TD participants [Gur et al., 2012]. Missing CNB values (0.48% of CHD-only, 8.83% of 22q-noCHD and 6.12% of 22q-CHD) were excluded from the corresponding analysis. The Z-scores less than four standard deviation from mean of demographically matched TD group were set to a floor value of −4 to reduce undue influence of outliers. The Z-scores for each cognitive domain were calculated by averaging the Z-scores of the corresponding CNB measures (i.e., Executive function = abstraction and mental-flexibility, attention, working memory; memory = verbal memory, face memory, spatial memory; complex cognition = language, non-verbal learning, spatial processing; Social cognition = emotional identification, emotional differentiation, age differentiation; praxis speed = sensorimotor, motor). The Z-scores were analyzed using linear mixed effects model approach. Each analysis included fixed effects for group, performance (accuracy or speed) and interaction terms. Random effect for subject was included and accounts for the repeated measurements from each subject. When the group effect was significant, performance effect was further analyzed in a pairwise comparison among different groups. Subsequently, two-tailed t-test with equal variance was performed for each CNB measure. For most analysis, variances were equal between the comparison groups and exceptions were noted in Table III. Cohen’s d effect size was also calculated for each CNB measure.
TABLE III.
Pairwise Analysis of Cognitive Domains Assessed in Computerized Neurocognitive Battery
| Groups A vs. group B | Measurea | Df | Accuracy
|
Speed
|
||||
|---|---|---|---|---|---|---|---|---|
| F or tb | P-value | Cohen’s dc | F or tb | P-value | Cohen’s dc | |||
| 22q-CHD vs. 22q-noCHD | ||||||||
| Overall | 1, 51 | 1.70 | 0.20 | — | 3.55 | 0.06 | — | |
| Executive function | 51 | 0.72 | 0.47 | 0.20 | −0.78 | 0.44 | −0.21 | |
| Memory | 50 | 1.02 | 0.31 | 0.28 | −1.35 | 0.18 | −0.38 | |
| Complex cognition | 51 | 0.39 | 0.70 | 0.11 | 0.55 | 0.59 | 0.15 | |
| Social cognition | 50 | 1.88 | 0.07 | 0.52 | −0.74 | 0.47 | −0.20 | |
| Praxis speed | 52 | −0.79 | 0.44 | −0.21 | ||||
| 22q-CHD vs. CHD-only | ||||||||
| Overall | 1, 41 | 28.96 | <0.001d | — | 1.39 | 0.24 | — | |
| Executive function | 41 | −2.35 | 0.02d | −0.74 | −0.64 | 0.53 | −0.20 | |
| Memory | 40 | −4.41 | <0.001d | −1.40 | −0.61 | 0.54 | −0.19 | |
| Complex cognition | 41 | −4.52 | <0.001d | −1.43 | 1.87 | 0.07 | 0.59 | |
| Social cognition | 40 | −4.12 | <0.001d | −1.31 | −2.20 | 0.03d | −0.70 | |
| Praxis speed | 41 | −1.14 | 0.26 | −0.36 | ||||
| 22q-CHD vs. TD | ||||||||
| Overall | 1, 73 | 62.06 | <0.001d | — | 7.48 | <0.01d | — | |
| Executive functione | 73 | −5.08 | <0.001d | −1.22 | −1.22 | 0.23 | −0.29 | |
| Memory | 72 | −5.67 | <0.001d | −1.38 | −1.95 | 0.05 | −0.48 | |
| Complex cognition | 73 | −6.11 | <0.001d | −1.47 | 0.53 | 0.60 | 0.13 | |
| Social cognitione | 72 | −5.60 | <0.001d | −1.36 | −1.63 | 0.11 | −0.40 | |
| Praxis speed | 73 | −1.48 | 0.14 | −0.36 | ||||
| 22q-noCHD vs. CHD-only | ||||||||
| Overall | 1, 40 | 42.47 | <0.001d | — | 0.02 | 0.9 | — | |
| Executive function | 40 | −2.96 | <0.01d | −0.94 | 0.09 | 0.93 | 0.03 | |
| Memorye | 40 | −4.94 | <0.001d | −1.57 | 0.54 | 0.59 | 0.17 | |
| Complex cognition | 40 | −5.21 | <0.001d | −1.66 | 1.38 | 0.17 | 0.44 | |
| Social cognition | 40 | −6.38 | <0.001d | −2.03 | −1.38 | 0.18 | −0.44 | |
| Praxis speed | 41 | −0.41 | 0.68 | −0.13 | ||||
| 22q-noCHD vs. TD | ||||||||
| Overall | 1, 72 | 89.81 | <0.001d | — | 0.14 | 0.71 | — | |
| Executive functione | 72 | −6.03 | <0.001d | −1.47 | −0.18 | 0.86 | −0.04 | |
| Memory | 72 | −6.64 | <0.001d | −1.62 | 0.02 | 0.98 | 0.01 | |
| Complex cognition | 72 | −6.82 | <0.001d | −1.66 | −0.14 | 0.89 | −0.03 | |
| Social cognition | 72 | −8.51 | <0.001d | −2.07 | −0.47 | 0.64 | −0.11 | |
| Praxis speed | 73 | −0.43 | 0.67 | −0.10 | ||||
| CHD-only vs. TD | ||||||||
| Overall | 1, 62 | 0.09 | 0.76 | — | 0.32 | 0.57 | — | |
| Executive function | 62 | −1.30 | 0.20 | −0.38 | −0.30 | 0.77 | −0.09 | |
| Memory | 62 | −0.04 | 0.97 | −0.01 | −0.77 | 0.44 | −0.22 | |
| Complex cognition | 62 | 0.12 | 0.91 | 0.03 | −1.79 | 0.08 | −0.52 | |
| Social cognition | 62 | 0.23 | 0.82 | 0.07 | 1.71 | 0.09 | 0.49 | |
| Praxis speed | 62 | 0.14 | 0.89 | 0.04 | ||||
Each cognitive domain is a composite score of respective CNB tasks (Executive function = abstraction and mental-flexibility, attention and working memory; Memory = verbal memory, face memory, and spatial memory; Complex cognition = language, non-verbal learning and spatial processing; Social cognition = emotional identification, emotional differentiation and age-differentiation; Praxis speed = sensorimotor and motor).
Linear mixed effects model was used to assess the group effect across all measures and subsequently, each cognitive domain was analysized in a pairwise group fashion, using the two-tailed, equal variance t-test.
Cohen’s d effect size was calculated by dividing the mean difference (mean of Group A—mean of Group B) by pooled standard deviation; negative value indicates worse performance.
Significance with P < 0.05.
Analysis without equal variance. TD, typically developing. Df, degrees of freedom.
RESULTS
Study Population
Demographic information for the three groups is presented in Table I. The age range for the CHD-only group was 9–13 years old while it was 8–14 for the 22q11DS groups. Overall, 60% of participants were male and 40% were female. A higher ratio of males was present in the 22q11DS groups compared to CHD-only group. The majority of participants across groups were Caucasians. There was no statistically significant difference in the distributions of age, gender, race, or parental education among the groups. WRAT-IV and GAF scores were significantly higher for the CHD-only group compared to 22q11DS groups. There were no significant differences in WRAT-IV and GAF scores between the 22q11DS groups.
TABLE I.
Characteristics of Study Participants With CHD-Only, 22q-noCHD and 22q-CHD
| Parameters | All groups | CHD-only | 22q-noCHD | 22q-CHD | P-value |
|---|---|---|---|---|---|
| n | 70 | 16 | 27 | 27 | NS |
| Age, mean (SD) | 11.36 (1.92) | 10.81 (1.28) | 11.37 (2.24) | 11.67 (1.88) | |
| Gender | NS | ||||
| Male (%) | 42 (60) | 7 (44) | 18 (67) | 17 (63) | |
| Female (%) | 28 (40) | 9 (56) | 9 (33) | 10 (37) | |
| Race | NS | ||||
| Caucasian (%) | 59 (84) | 13 (81) | 23 (85) | 23 (85) | |
| African American (%) | 6 (8) | 2 (13) | 2 (7) | 2 (7) | |
| Othersa (%) | 5 (7) | 1 (6) | 2 (7) | 2 (7) | |
| WRAT-IVb, mean (SD) | 94.1 (14.9) | 105.6 (13.5) | 90.8 (12.6) | 90.6 (14.8) | 0.001 |
| Parent educationc, mean (SD) | |||||
| Mother | 15 (2.3) | 16 (2.0) | 14 (2.3) | 15 (2.4) | NS |
| Father | 14 (3.0) | 16 (2.2) | 14 (2.8) | 15 (3.5) | NS |
| GAFd, mean (SD) | 69.25 (15.70) | 84.18 (08.67) | 64.44 (14.41) | 65.22 (15.00) | <0.001 |
P-values for age, reading level, parent education, and GAF are determined by single-factor ANOVA; gender and race by Fisher’s Exact test. NS designates non-significance (P > 0.05).
Includes Hispanics, Asians and mixed race individuals.
Wide Range Achievement Test IV.
Number of years completed in a formal school.
Global assessment of function.
CHD Phenotype
The 22q-CHD group represents seven different types of CHD (Table II), while only three different variants of CHD (TOF, ventricular septal defect, and interrupted aortic arch) constituted the CHD phenotype in the CHD-only group. TOF was the most common defect found in both groups. It constituted 38% and 26% of CHD variants in CHD-only and 22q-CHD group, respectively. In the CHD-only group, ventricular septal defect was the next most common defect (56%) while in 22q-CHD group, interrupted aortic arch was the second most common defect (22%). The difference in the distribution pattern of CHD variants was statistically significant (P < 0.05).
TABLE II.
CHD Phenotype of Participants With CHD-Only, 22q-noCHD, and 22q-CHD
| CHD-only | 22q-noCHD | 22q-CHD | P-value | |
|---|---|---|---|---|
| CHD phenotype | 0.02 | |||
| Tetralogy of fallot (%) | 6 (38) | — | 7 (26) | |
| Interrupted aortic arch (%) | 1 (6) | — | 6 (22) | |
| Ventricular septal defect (%) | 9 (56) | — | 2 (7) | |
| Truncus arteriosus (%) | 0 | — | 5 (19) | |
| Patent ductus arteriosus (%) | 0 | — | 4 (15) | |
| Atrial septal defect (%) | 0 | — | 2 (7) | |
| Biscuspid aortic valve (%) | 0 | — | 1 (4) | |
| Other vascular anomaliesa | NS | |||
| yes (%) | — | 11 (40) | 19 (70) | |
| no (%) | — | 8 (30) | 5 (19) | |
| unknown (%) | 16 (100) | 8 (30) | 3 (11) | |
| CHD surgery (%) | 16 (100) | — | 21 (78) | NS |
P-values are determined by Fisher’s exact test. NS designates non-significance (P > 0.05).
Includes vascular ring, right aortic arch with mirror-image branching of brachiocephalic vessels, and aberrant origin subclavian artery.
The majority of 22q11DS participants also had other vascular anomalies (Table II). Forty percent of 22q-noCHD and 70% of 22q-CHD groups had vascular anomalies while these diagnoses were unavailable in the CHD-only group. All of the CHD-only participants had cardiac surgery while 78% of 22q-CHD participants had surgery. This difference was not statistically significant. Six 22q-CHD participants (one with atrial septal defect, one with biscuspid aortic valve and four with post-natal persistent patent ductus arteriosus) did not have cardiac surgery.
Psychopathology in Association With CHD
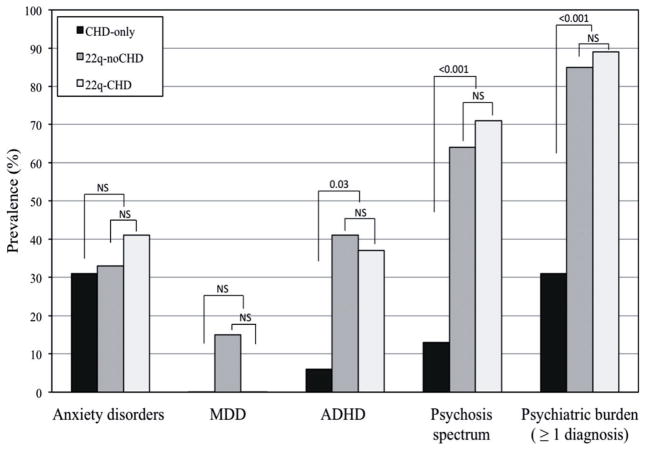
To examine the association between CHD and psychopathology in 22q11DS, the prevalence of psychiatric disorders was compared among the CHD-only, 22q-noCHD and 22q-CHD groups (Fig. 1). Significant differences were present for ADHD, psychosis spectrum and overall psychiatric burden between the CHD-only and 22q11DS groups. Forty-one percent and 37% in 22q-noCHD and 22q-CHD group, respectively, had ADHD compared to 6% in CHD-only group. Sixty-four percent of 22q-noCHD and 71% of 22q-CHD had a psychosis spectrum diagnosis compared to only 13% in CHD-only group. Prodrome diagnosis constituted the majority (94% of 22q-noCHD and 85% of 22q-CHD) of psychosis spectrum (Supplementary Table I). The majority of 22q11DS participants (85% in 22q-noCHD and 89% in 22q-CHD), regardless of CHD status, had at least one psychiatric diagnosis while only 31% of CHD-only group had one or more diagnosis. The differences in prevalence of ADHD, psychosis spectrum or overall psychiatric burden within the 22q11DS groups were not significant. There was no significant difference for anxiety disorders and major depressive disorder among the three groups.
FIG. 1.
Prevalence of psychiatric disorders in CHD-only, 22q-noCHD and 22q-CHD. Anxiety disorder, anxiety disorder-not otherwise specified, generalized anxiety disorder, separation anxiety disorder, and obsessive-compulsive disorder. MDD, major depressive disorder. ADHD, attention deficient hyperactivity disorder. Psychosis spectrum, positive and negative prodrome diagnosis based on Structured Interview for Psychosis Risk Syndrome and psychotic disorders. Psychiatric burden, at least one psychiatric diagnosis. P-values are determined by Fisher’s Exact test. NS designates non-significance (P > 0.05).
Because the six participants without cardiac surgery were unlikely to have had the same degree of hemodynamic instability as those who had undergone surgery, the analyses were repeated after excluding these participants. The significant effects remained unaltered.
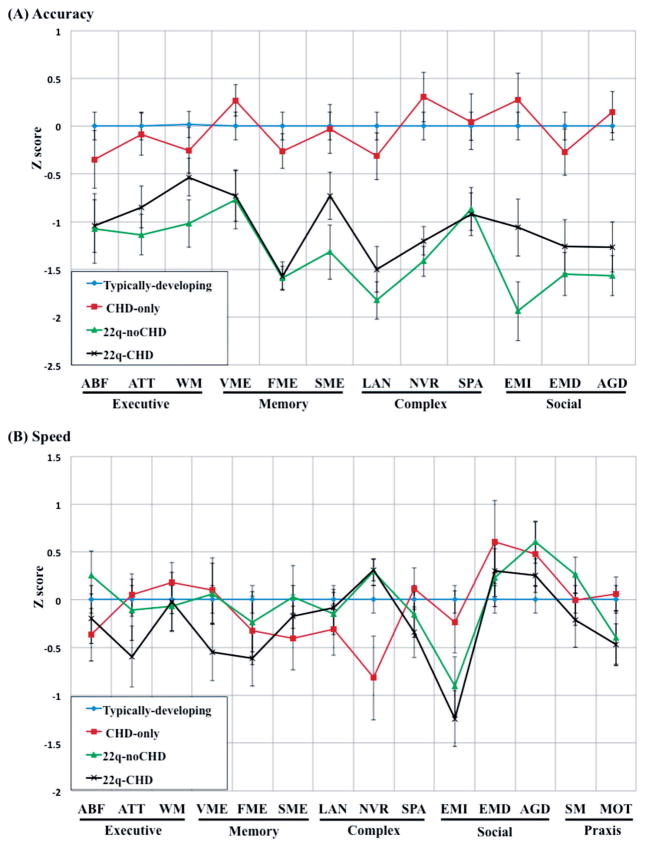
Computerized Neurocognitive Battery (CNB)
Figure 2 presents the Z-scores of (A) accuracy and (B) speed of each CNB measure for each group, with results of significance testing presented in Table III. Overall, both 22q11DS groups performed significantly worse in accuracy than the CHD-only group across all measures. The overall pattern for accuracy was similar between the 22q11DS groups with the exception of emotion-identification, in which the 22q-noCHD performed worse than the 22q-CHD group. A significant overall effect, across all four groups, was present for group (F = 40.39, df = 3, 113 and P < 0.0001), measure (F = 4.86, df = 11, 113 and P < 0.0001), and group × measure interaction term (F = 2.0, df = 33, 113 and P = 0.004). There was no significant difference in accuracy across all cognitive domains between 22q-noCHD and 22q-CHD (Table III). The 22q11DS groups showed significant impairment across most of the domains compared to the CHD-only group (effect size ranged from −0.74 to −2.03) and TD group (effect size ranged from −1.22 to −2.07). There was no significant difference for all measures in accuracy between the CHD-only and TD group (Supplementary Table II).
FIG. 2.
Computerized neurocognitive battery profile in CHD-only, 22q-noCHD and 22q-CHD. ABF, abstraction, mental-flexibility; ATT, attention; WM, working memory; WME, verbal memory; FME, face memory; SME, spatial memory; LAN, language; NVR, non-verbal learning; SPA, spatial processing; EMI, emotion identification; EMD, emotion differentiation; AGD, age differentiation; SM, sensorimotor; MOT, motor.
For speed, the overall patterns were similar among the three groups (Fig. 2). The 22q-CHD group had decreased speed in attention, verbal memory, face memory, spatial-processing, emotion-identification, and sensorimotor relative to the other three groups; the 22q11DS groups were slower than the CHD-only group in emotion-identification and motor speed; the 22q11DS groups and CHD-only group were faster than TD group in emotion-differentiation and age-differentiation; the CHD-only group was slower than 22q11DS groups in abstraction and mental-flexibility, spatial memory, language and non-verbal learning.
There was no significant overall effect, across the four groups, for group (F = 2.69, df = 3, 114 and P = 0.05); however, there was a significant effect for measure (F = 3.82, df = 13, 114 and P < 0.0001) and group x measure interaction term (F = 1.94, df = 39, 114 and P = 0.004) across the four groups. There was no significant difference in speed for all domains between 22q-noCHD and 22q-CHD (Table III). For most measures, there was no significant difference between the 22q11DS groups and CHD-only or TD group (Supplementary Table II). Exceptions were: the 22q11DS groups were significantly slower in non-verbal learning and emotion-identification, compared to CHD-only; they were also slower in face memory, emotion-identification and age-differentiation, compared to TD only; the CHD-only group was significantly slower than TD group only in non-verbal learning.
The analysis for the 22q-CHD group was repeated after excluding the six participants without cardiac surgery. The results remained the same except that the accuracy of spatial memory became significant (from P = 0.07 to P = 0.04) when compared between the 22q-CHD and CHD-only group.
DISCUSSION
Individuals with 22q11DS have high neuropsychiatric and medical burdens. CHD is one of the most common medical conditions in 22q11DS and has been independently implicated in early neurodevelopmental delays in non-deleted populations. We hypothesized that in 22q11DS, CHDs may potentiate the neuropsychiatric burden manifested later in development. We therefore examined the association between CHDs and neuropsychiatric outcomes in school-age children with 22q11DS. We included a 22q-noCHD comparison group to control for the 22q11.2 deletion background and examine the effect of CHD alone.
We found that individuals with 22q11DS are at increased risk for psychiatric disorders and neurocognitive deficits regardless of CHD status. The majority of participants with 22q11DS had at least one psychiatric diagnosis. In agreement with other studies [Gothelf et al., 2004, 2007; Green et al., 2009; Niklasson et al., 2009; Jolin et al., 2012; Tang et al., 2013], they had high prevalence of ADHD, anxiety disorders and psychosis spectrum, which was most common. The majority of psychosis spectrum individuals exhibited subthreshold symptoms rather than threshold psychotic disorders. Our rate of subthreshold psychosis was higher than previously reported and this may reflect ascertainment bias, variability in assessment methods and the instability of psychosis phenomenon in developing children. SIPS ratings have not been validated in developmentally delayed children. Although it is likely sensitive in detecting subthreshold symptoms in children, the SIPS ratings may not be specific enough and a portion of subthreshold symptoms observed is likely better accounted for by developmental challenges and high level of anxiety in 22q11DS population. We plan to address this issue with a larger sample of 22q11DS in the future.
Notably, the prevalence rates of all psychiatric disorders evaluated were comparable between the two 22q11DS groups. ADHD and psychosis spectrum were significantly higher in the 22q11DS groups than in the non-syndromic CHD-only group, but there was no such contrast for anxiety disorders or major depressive disorder. This lack of a CHD effect on psychopathology in 22q11DS could be due to several possibilities. The neurodevelopmental vulnerability conferred by 22q11.2 deletion may dominate and obscure the effect of CHD. These attenuated effects of CHD are detectable by neuroimaging studies [Schaer et al., 2009, 2010]. However, while these studies demonstrate effect of CHD on brain morphology, it is unclear how they correlate with neuropsychiatric outcomes. CHD is one of many modifying factors involved in the development of psychiatric disorders in 22q11DS. Sample size and time limitations currently preclude investigation of other potential risk factors such as additional co-occurring medical conditions, family history of mental illness and psychosocial stressors. We are likewise unable to account for potential protective factors, such as early mental health utilization, consistent medical care and supportive family environment. The effects of CHD observed in early neurodevelopment may be compensated later in development. Previous studies examining non-deleted school-age children with CHD showed higher rates of learning difficulties, ADHD, anxiety, and behavioral issues and suggest that the CHD effect may persist into later in development [Karsdorp et al., 2007; Shillingford et al., 2008; Marino et al., 2012]. However, it is difficult to draw a conclusion due to the cross-sectional nature and variability of assessment tools used in these studies.
We observed no difference in the rates of anxiety disorder and major depressive disorder among the CHD-only and 22q11DS groups. The prevalence of major depressive disorder was relatively low in our 22q11DS sample compared to other studies [Fabbro et al., 2012; Jolin et al., 2012] and may reflect ascertainment and assessment bias. In agreement with other studies [Karsdorp et al., 2007; Spijkerboer et al., 2008; Marino et al., 2012], anxiety disorders were elevated in the CHD-only group relative to the expected rate in the general population; thus, the prevalence of anxiety disorders is comparable to the levels observed in the 22q11DS groups.
Similar to the findings in psychopathology, we did not observe significant differences in neurocognitive profiles between the 22q11DS groups. Moreover, 22q11DS groups showed a robust reduction in accuracy compared to both CHD-only and TD individuals, suggesting that the observed neurocognitive impairments in 22q11DS are not attributable to cardiac anomalies and their sequelae. 22q11DS groups were particularly impaired in social cognition domain, performing poorly in both accuracy and speed. CHD-only group also performed poorly in social cognition but only in speed. This difference in the type of impairment suggests that different brain regions could be affected in these groups. Notably, our neurocognitive data showed no clear deficit in the CHD-only group compared to the TD group, suggesting that the effect of CHD or heart surgery status may not persist into school-age. However, the type of CHD and severity of complications related to surgery may affect the cognitive outcomes. Due to a relatively small sample size, we could not match the study groups to specific CHD.
Limitations
Previous studies have suggested that the complexity of CHD and extent of hemodynamic compromise measured by perinatal and perioperative hypoxia in non-syndromic population is correlated with worsening developmental delays and functional outcomes [Karsdorp et al., 2007; Marino et al., 2012]. In the current study, we did not measure these aspects of CHD and therefore could not investigate their relationship to neuropsychiatric outcome. It will be important to consider such measures in future studies.
Because the study focused on brain-behavior measures, we included individuals without significant intellectual disabilities and the results may not be generalized to patients with more marked impairment. Similarly, the age range of 8–14 precludes generalization to individuals with the deletion younger or older than the selected range. Notably, the pattern of neurocognitive deficits observed in this sample is similar to that reported in younger children and in older adolescents and young adults.
CONCLUSION
Although we cannot rule out subtle contributions of CHD to psychopathology due to a relatively small sample size, the similar outcomes within the 22q11DS groups suggest that the effect of CHD is obscured by the more prominent risk associated with 22q11DS itself. Other modifying factors are at play in the emergence of psychiatric disorders and longitudinal cohort studies will allow identification of such factors in this vulnerable population.
Supplementary Material
Acknowledgments
Grant sponsor: NIH Grants; Grant numbers: MH087626, MH087636; Grant sponsor: Additional Support; Grant numbers: K08 MH079364, HD 070454.
The authors are grateful to all of participants and their families. We thank Emily Wilkins, Catherine Conroy, Omar Abbas, and Amy Cassidy of Neuropsychiatry section at the University of Pennsylvania for the administration of semi-structured interviews and Adam Savitt for CNB validation and data preparation. We also thank Alice Bailey and Nathaniel Meyers of the “22q and You” Center for scheduling participants’ visits and retrieval of medical records. We finally thank Colleen Franconi and Meghan McNamara of the Human Genetics Division at the Children’s Hospital of Philadelphia for handling of blood samples and coordinating shipments to NIMH repository.
Footnotes
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- Agergaard P, Olesen C, Østergaard JR, Christiansen M, Sørensen KM. The prevalence of chromosome 22q11.2 deletions in 2,478 children with cardiovascular malformations. A population-based study. Am J Med Genet Part A. 2012;158A:498–508. doi: 10.1002/ajmg.a.34250. [DOI] [PubMed] [Google Scholar]
- Antshel KM, Fremont W, Roizen NJ, Shprintzen R, Higgins AM, Dhamoon A, Kates WR. ADHD, major depressive disorder, and simple phobia are prevalent psychiatric conditions in youth with velocardiofacial syndrome. J Am Acad Child Adolesc Psychiatry. 2006;45(5):596–603. doi: 10.1097/01.chi.0000205703.25453.5a. [DOI] [PubMed] [Google Scholar]
- Atallah J, Joffe AR, Robertson CM, Leonard N, Blakley PM, Nettel-Aguirre A, Sauve RS, Ross DB, Rebeyka IM. Two-year general and neurodevelopmental outcome after neonatal complex cardiac surgery in patients with deletion 22q11.2: A comparative study. J Thorac Cardiovasc Surg. 2007;134(3):772–779. doi: 10.1016/j.jtcvs.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Bassett AS, Chow EW, AbdelMalik P, Gheorghiu M, Husted J, Weksberg R. The schizophrenia phenotype in 22q11 deletion syndrome. Am J Psychiatry. 2003;160(9):1580–1586. doi: 10.1176/appi.ajp.160.9.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, Woodin MF, Wang PP, Moss E, McDonald-McGinn D, Zackai E, Emanuel B, Cannon TD. The Neurocognitive phenotype of the 22q11.2 deletion syndrome: Selective deficit in visual-spatial memory. J Clin Exp Neuropsychol. 2001;23(4):447–464. doi: 10.1076/jcen.23.4.447.1228. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, Wypij D, Kuban KC, Rappaport LA, Hickey PR, Wernovsky G, Jonas RA, Newburger JW. Developmental and neurological status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation. 1999;100(5):526–532. doi: 10.1161/01.cir.100.5.526. [DOI] [PubMed] [Google Scholar]
- Botto LD, May K, Fernhoff PM, Correa A, Coleman K, Rasmussen SA, Merritt RK, O’Leary LA, Wong LY, Elixson EM, Mahle WT, Campbell RM. A population-based study of the 22q11. 2 deletion: Phenotype, incidence, and contribution to major birth defects in the population. Pediatrics. 2003;112(1):101–107. doi: 10.1542/peds.112.1.101. [DOI] [PubMed] [Google Scholar]
- Carotti A, Digilio MC, Piacentini G, Saffirio C, Di Donato RM, Marino B. Cardiac defects and results of cardiac surgery in 22q11. 2 deletion syndrome. Dev Disabil Res Rev. 2008;14(1):35–42. doi: 10.1002/ddrr.6. [DOI] [PubMed] [Google Scholar]
- Cheung EN, George SR, Andrade DM, Chow EW, Silversides CK, Bassett AS. Neonatal hypocalcemia, neonatal seizures, and intellectual disability in 22q11.2 deletion syndrome. Genet Med. 2013 doi: 10.1038/gim.2013.71. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smedt B, Devriendt K, Fryns J-P, Vogels A, Gewillig M, Swillen A. Intellectual abilities in a large sample of children with Velo–Cardio–Facial syndrome: An update. J Intellect Disabil Res. 2007;51(9):666–670. doi: 10.1111/j.1365-2788.2007.00955.x. [DOI] [PubMed] [Google Scholar]
- Devriendt K, Fryns J-P, Mortier G, Van Thienen MN, Keymolen K. The annual incidence of DiGeorge/velocardiofacial syndrome. J Med Genet. 1998;35(9):789–790. doi: 10.1136/jmg.35.9.789-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGeorge AM. Discussions on a new concept of the cellular basis of immunology. J Pediatr. 1965;67:907. [Google Scholar]
- Driscoll DA, Budarf ML, Emanuel BS. A genetic etiology for DiGeorge syndrome: Consistent deletions and microdeletions of 22q11. Am J Hum Genet. 1992;50(5):924–933. [PMC free article] [PubMed] [Google Scholar]
- Fabbro A, Rizzi E, Schneider M, Debbane M, Eliez S. Depression and anxiety disorders in children and adolescents with velo-cardio-facial syndrome (VCFS) Eur Child Adolesc Psychiatry. 2012;21(7):379–385. doi: 10.1007/s00787-012-0273-x. [DOI] [PubMed] [Google Scholar]
- Fallon P, Aparicio JM, Elliott MJ, Kirkham FJ. Incidence of neurological complications of surgery for congenital heart disease. Arch Dis Child. 1995;72(5):418–422. doi: 10.1136/adc.72.5.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Gibbon M. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II) In: Hilsenroth MJ, Segal DL, editors. Comprehensive handbook of psychological assessment. Vol. 2, Personality assessment. Hoboken, NJ: John Wiley and Sons; 2004. pp. 134–143. [Google Scholar]
- Forbess JM, Visconti KJ, Hancock-Friesen C, Howe RC, Bellinger DC, Jonas RA. Neurodevelopmental outcome after congenital heart surgery: Results from an institutional registry. Circulation. 2002;106(12):I95–102. [PubMed] [Google Scholar]
- Gerdes M, Solot C, Wang PP, Moss E, LaRossa D, Randall P, Goldmuntz E, Clark BJ, Driscoll DA, Jawad A, Emanuel BS, McDonald-McGinn DM, Batshaw ML, Zackai EH. Cognitive and behavior profile of preschool children with chromosome 22q11.2 deletion. Am J Med Genet. 1999;85(2):127–133. [PubMed] [Google Scholar]
- Gilboa SM, Salemi JL, Nembhard WN, Fixler DE, Correa A. Mortality resulting from congenital heart disease among children and adults in the United States, 1999 to 2006. Circulation. 2010;122(22):2254–2263. doi: 10.1161/CIRCULATIONAHA.110.947002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauser TA, Rorke LB, Weinberg PM, Clancy RR. Congenital brain anomalies associated with the hypoplastic left heart syndrome. Pediatrics. 1990;85(6):984–990. [PubMed] [Google Scholar]
- Goldenberg PC, Calkins ME, Richard J, McDonald-McGinn D, Zackai E, Mitra N, Emanuel B, Devoto M, Borgmann-Winter K, Kohler C, Conroy CG, Gur RC, Gur RE. Computerized neurocognitive profile in young people with 22q11.2 deletion syndrome compared to youths with schizophrenia and at-risk for psychosis. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(1):87–93. doi: 10.1002/ajmg.b.32005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding-Kushner KJ, Weller G, Shprintzen RJ. Velo-cardio-facial syndrome: Language and psychological profiles. J Craniofac Genet Dev Biol. 1984;5(3):259–266. [PubMed] [Google Scholar]
- Gothelf D, Presburger Gadi D, Levy A, Nahmani M, Burg M, Berant L, Blieden Y, Finkelstein A, Frisch A, Apter A, Weizman A. Genetic, developmental, and physical factors associated with attention deficit hyperactivity disorder in patients with velocardiofacial syndrome. Am J Med Genet B Neuropsychiatr Genet. 2004;126B:116–121. doi: 10.1002/ajmg.b.20144. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Feinstein C, Thompson T, Gu E, Penniman L, Van Stone E, Kwon H, Eliez S, Reiss AL. Risk factors for the emergence of psychotic disorders in adolescents with 22q11.2 deletion syndrome. Am J Psychiatry. 2007;164(4):663–669. doi: 10.1176/ajp.2007.164.4.663. [DOI] [PubMed] [Google Scholar]
- Green T, Gothelf D, Glaser B, Debbane M, Frisch A, Kotler M, Weizman A, Eliez S. Psychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11.2 deletion) syndrome. J Am Acad Child Adolesc Psychiatry. 2009;48(11):1060–1068. doi: 10.1097/CHI.0b013e3181b76683. [DOI] [PubMed] [Google Scholar]
- Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, Brensinger C, Gur RE. A cognitive neuroscience based computerized battery for efficient measurement of individual differences: Standardization and initial construct validation. J Neurosci Methods. 2010;187(2):254–262. doi: 10.1016/j.jneumeth.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, Loughead J, Connolly JJ, Qui H, Mentch FD, Abou-Sleiman PM, Hakonarson H, Gur RE. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology. 2012;26(2):251–265. doi: 10.1037/a0026712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- Hoffman JI, Kaplan S, Liberthson RR. Prevalence of congenital heart disease. Am Heart J. 2004;147(3):425–439. doi: 10.1016/j.ahj.2003.05.003. [DOI] [PubMed] [Google Scholar]
- Hooper SR, Curtiss K, Schoch K, Keshavan MS, Allen A, Shashi V. A longitudinal examination of the psychoeducational, neurocognitive, and psychiatric functioning in children with 22q11.2 deletion syndrome. Res Dev Disabil. 2013;34(5):1758–1769. doi: 10.1016/j.ridd.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley SA, Prasad SE, Pender NP, Murphy KC. Relationship between reaction time, fine motor control, and visual-spatial perception on vigilance and visual-motor tasks in 22q11.2 deletion syndrome. Res Dev Disabil. 2012;33(5):1495–1502. doi: 10.1016/j.ridd.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Jolin EM, Weller RA, Jessani NR, Zackai EH, McDonald-McGinn DM, Weller EB. Affective disorders and other psychiatric diagnoses in children and adolescents with 22q11.2 Deletion Syndrome. J Affect Disord. 2009;119(1):177–180. doi: 10.1016/j.jad.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Jolin EM, Weller RA, Weller EB. Occurrence of affective disorders compared to other psychiatric disorders in children and adolescents with 22q11. 2 deletion syndrome. J Affect Disord. 2012;136(3):222–228. doi: 10.1016/j.jad.2010.11.025. [DOI] [PubMed] [Google Scholar]
- Karsdorp PA, Everaerd W, Kindt M, Mulder BJ. Psychological and cognitive functioning in children and adolescents with congenital heart disease: A meta-analysis. J Pediatr Psychol. 2007;32(5):527–541. doi: 10.1093/jpepsy/jsl047. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryna N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kiley-Brabeck K, Sobin C. Social skills and executive function deficits in children with the 22q11.2 deletion syndrome. Appl Neuropsychol. 2006;13(4):258–268. doi: 10.1207/s15324826an1304_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs AH, Saidi AS, Kuhl EA, Sears SF, Silversides C, Harrison JL, Ong L, Colman J, Oechslin E, Nolan RP. Depression and anxiety in adult congenital heart disease: Predictors and prevalence. Int J Cardiol. 2009;137:158–164. doi: 10.1016/j.ijcard.2008.06.042. [DOI] [PubMed] [Google Scholar]
- Maharasingam M, Ostman-Smith I, Pike MG. A cohort study of neurodevelopmental outcome in children with DiGeorge syndrome following cardiac surgery. Arch Dis Child. 2003;88(1):61–64. doi: 10.1136/adc.88.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino B, Digilio MC, Toscano A, Giannotti A, Dallapiccola B. Congenital heart defects in patients with DiGeorge/velocardiofacial syndrome and del22q11. Genet Couns. 1999;10(1):25–33. [PubMed] [Google Scholar]
- Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor J, Mussatto KA, Uzark K, Goldberg CS, Johnson WH, Li J, Smith SE, Bellinger DC, Mahle WT. Neurodevelopmental outcomes in children with congenital heart disease: Evaluation and management: A scientific statement from the American Heart Association. Circulation. 2012;126:1143–1172. doi: 10.1161/CIR.0b013e318265ee8a. [DOI] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Sullivan KE. Chromosome 22q11.2 deletion syndrome (DiGeorge Syndrome/Velocardiofacial syndrome) Medicine. 2011;90(1):1–18. doi: 10.1097/MD.0b013e3182060469. [DOI] [PubMed] [Google Scholar]
- Miller G, Eggli KD, Contant C, Baylen BG, Myers JL. Postoperative neurologic complications after open heart surgery on young infants. Arch Pediatr Adolesc Med. 1995;149(7):764–768. doi: 10.1001/archpedi.1995.02170200054008. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: Predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry. 1999;56(10):940–945. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- Niklasson L, Rasmussen P, Oskarsdóttir S, Gillberg C. Attention deficits in children with 22q.11 deletion syndrome. Dev Med Child Neurol. 2005;47(12):803–807. doi: 10.1017/S0012162205001702. [DOI] [PubMed] [Google Scholar]
- Niklasson L, Rasmussen P, Oskarsdóttir S, Gillberg C. Autism, ADHD, mental retardation and behavior problems in 100 individuals with 22q11 deletion syndrome. Res Dev Disabil. 2009;30(4):763–773. doi: 10.1016/j.ridd.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Ousley O, Rockers K, Dell ML, Coleman K, Cubells JF. A review of neurocognitive and behavioral profiles associated with 22q11 deletion syndrome: Implications for clinical evaluation and treatment. Curr Psychiatry Rep. 2007;9(2):148–158. doi: 10.1007/s11920-007-0085-8. [DOI] [PubMed] [Google Scholar]
- Rogers BT, Msall ME, Buck GM, Lyon NR, Norris MK, Roland JM, Gingell RL, Cleveland DC, Pieroni DR. Neurodevelopmental outcome of infants with hypoplastic left heart syndrome. J Pediatr. 1995;126(3):496–498. doi: 10.1016/s0022-3476(95)70478-7. [DOI] [PubMed] [Google Scholar]
- Ryan AK, Goodship JA, Wilson DI, Philip N, Levy A, Seidel H, Schuffenhauer S, Oechsler H, Belohradsky B, Prieur M, Aurias A, Raymond FL, Clayton-Smith J, Hatchwell E, McKeown C, Beemer FA, Dallapiccola B, Novelli G, Hurst JA, Ignatius J, Green AJ, Winter RM, Bruenton L, Brøndum-Nielsen K, Scambler PJ. Spectrum of clinical features associated with interstitial chromosome 22q11 deletions: A European collaborative study. J Med Genet. 1997;34(10):798–804. doi: 10.1136/jmg.34.10.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaer M, Glaser B, Cuadra MB, Debbane M, Thiran JP, Eliez S. Congenital heart disease affects local gyrification in 22q11.2 deletion syndrome. Dev Med Child Neurol. 2009;51(9):746–753. doi: 10.1111/j.1469-8749.2009.03281.x. [DOI] [PubMed] [Google Scholar]
- Schaer M, Glaser B, Ottet MC, Schneider M, Bach Cuadra M, Debbane M, Thiran JP, Eliez S. Regional cortical volumes and congenital heart disease: A MRI study in 22q11.2 deletion syndrome. J Neurodev Disord. 2010;2(4):224–234. doi: 10.1007/s11689-010-9061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shillingford AJ, Glanzman MM, Ittenbach RF, Clancy RR, Gaynor JW, Wernovsky G. Inattention, hyperactivity, and school performance in a population of school-age children with complex congenital heart disease. Pediatrics. 2008;121(4):e759–e767. doi: 10.1542/peds.2007-1066. [DOI] [PubMed] [Google Scholar]
- Spijkerboer AW, Utens EM, Bogers AJ, Helbing WA, Verhulst FC. A historical comparison of long-term behavioral and emotional outcomes in children and adolescents after invasive treatment for congenital heart disease. J Pediatr Surg. 2008;43:534–539. doi: 10.1016/j.jpedsurg.2007.10.037. [DOI] [PubMed] [Google Scholar]
- Stoddard J, Niendam T, Hendren R, Carter C, Simon TJ. Attenuated positive symptoms of psychosis in adolescents with chromosome 22q11.2 deletion syndrome. Schizophr Res. 2010;118(1):118–121. doi: 10.1016/j.schres.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swillen A, Devriendt K, Legius E, Prinzie P, Vogels A, Ghesquiere P, Fryns JP. The behavioural phenotype in velo-cardio-facial syndrome (VCFS): From infancy to adolescence. Genet Couns. 1999;10(1):79–88. [PubMed] [Google Scholar]
- Swillen A, Feys H, Adriaens T, Nelissen L, Mertens L, Gewillig M, Devriendt K, Fryns JP. Early motor development in young children with 22q.11 deletion syndrome and a conotruncal heart defect. Dev Med Child Neurol. 2005;47(12):797–802. doi: 10.1017/S0012162205001696. [DOI] [PubMed] [Google Scholar]
- Tang SX, Yi JJ, Calkins ME, Whinna DA, Kohler CG, Souders MC, McDonald-McGinn DM, Zackai EH, Emanuel BS, Gur RC, Gur RE. Psychiatric disorders in 22q11.2 deletion syndrome are prevalent but under-treated. Psychol Med. 2013;9:1–11. doi: 10.1017/S0033291713001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aken K, De Smedt B, Van Roie A, Gewillig M, Devriendt K, Fryns JP, Simons J, Swillen A. Motor development in school-age children with 22q11 deletion (velocardiofacial DiGeorge syndrome) Dev Med Child Neurol. 2007;49:210–213. doi: 10.1111/j.1469-8749.2007.00210.x. [DOI] [PubMed] [Google Scholar]
- Vorstman JA, Morcus ME, Duijff SN, Klaassen PW, Heineman-de Boer JA, Beemer FA, Swaab H, Kahn RS, van Engeland H. The 22q11.2 deletion in children: High rate of autistic disorders and early onset of psychotic symptoms. J Am Acad Child Adolesc Psychiatry. 2006;45(9):1104–1113. doi: 10.1097/01.chi.0000228131.56956.c1. [DOI] [PubMed] [Google Scholar]
- Wernovsky G, Shillingford AJ, Gaynor JW. Central nervous system outcomes in children with complex congenital heart disease. Curr Opin Cardiol. 2005;20(2):94–99. doi: 10.1097/01.hco.0000153451.68212.68. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ. Wide range achievement test. 4. Lutz, FL: Psychological Assessment Resources WRAT 4 Introductory Kit; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.