Abstract
Objective
To examine the association between altered taste perception and nutritional status among hemodialysis patients.
Design
We performed a post-hoc analysis of data from the HEMO Study (n = 1745). Taste perception was assessed at baseline and then updated annually using an item from a quality of life survey, which asked “during the past 4 weeks, to what extent were you bothered by loss of taste?” Responses were categorized as normal taste perception if subjects answered “not at all” or altered taste perception if they reported any degree of bother. Time-updated logistic regression models were used to evaluate predictors of altered taste perception. Time-updated linear regression models were used to examine the association between altered taste perception and indices of nutritional status. Multivariable proportional hazards and Poisson models were used to assess association between altered taste perception and mortality and hospitalization, respectively.
Results
At baseline, 34.6% reported altered taste perception, which was associated with poorer baseline nutritional status. On time-updated analysis, altered taste perception was associated with a persistently higher proportion of subjects requiring enteral nutritional supplements and lower serum albumin, serum creatinine, normalized protein catabolic rate, protein intake, sodium intake, and mid-arm muscle circumference. Altered taste perception at baseline was independently associated with increased all-cause mortality: adjusted HR (95% CI) of 1.17 (1.01–1.37), although not with increased rate of hospitalization.
Conclusion
Altered taste perception was common among prevalent hemodialysis patients and independently associated with poorer indices of nutritional status and increased all-cause mortality.
Keywords: Hemodialysis, taste, nutrition
Introduction
Patients with end-stage renal disease are at risk for impairment in taste perception. Multiple studies have reported an increased frequency of dysgeusias in patients with chronic kidney disease and end-stage renal disease.1–4 Reported taste disturbances include impaired detection of salty taste,3 inability to recognize common foods by taste,1,2 and reporting that certain foods taste metallic or like medication.1 The mechanism of taste alteration is not well understood, but felt to be a consequence of uremia,2 and prevalence is higher among patients with lower native GFR.5 Another potential explanation for taste alteration in hemodialysis patients is zinc deficiency,6 which is commonplace due to high dialytic removal.7
Hemodialysis patients are also at risk for protein-energy malnutrition, which in turn, is potently associated with mortality.8–12 Kidney Dialysis Outcome Quality Initiative (KDOQI) guidelines recommend frequent nutritional assessment of dialysis patients. Currently recommended markers of nutritional status include body mass index, serum albumin, serum creatinine, anthropometric measures, and dietary intake.10,13–15 No one measure taken in isolation is an adequate predictor,16 so combinations of markers are used together in clinical practice, often in conjunction with screening tools that have been validated in the dialysis population.17–19
In previous studies, the prevalence of altered taste perception (altTP) among dialysis patients ranged from 31% to 81%.1,4,20 Disturbances in taste may decrease interest in eating and predispose to a higher risk for nutritional compromise. Despite a high prevalence of altTP, there has been no published research examining the association between altTP and nutritional status among hemodialysis patients. We conducted this post-hoc analysis of the Hemodialysis Study (HEMO)21 in order to examine the association between self-reported altered taste perception and indices of nutritional status. We hypothesized that altTP would be associated with poorer nutritional status as well as increased mortality and hospitalization.
Methods
Study Design
This was a retrospective cohort analysis of data collected in HEMO Study,21,22 which was made available through the National Institute of Diabetes and Digestive Kidney Diseases Data Repository. The study was deemed exempt by the Beth Israel Deaconess Medical Center and Partners Healthcare Institutional Review Boards.
The HEMO Study was a randomized, controlled trial designed to examine the effects of dialysis dose and dialytic membrane flux on clinical outcomes. The study enrolled 1846 adult dialysis patients undergoing thrice-weekly in-center hemodialysis (at one of 15 participating centers in the US) between March 1995 and October 2000; subjects were followed through December 31, 2001. Exclusion criteria included age > 80 years, residual urea clearance > 1.5 ml/min/35 L volume of urea distribution, inability to achieve Kt/V > 1.7, serum albumin < 2.6 g/dl, or serious co-morbid medical conditions (end-stage cardiac, pulmonary, or hepatic disease, malignancy, active infection or unstable angina). We further excluded participants who did not have taste assessment at baseline and those who did not maintain enrollment until the start of at-risk time (day 60 post-randomization; n=101).
Exposures, Outcomes, and Covariates
The exposure considered was self-reported impairment in taste perception, which was recorded at baseline and updated annually. On a quality of life survey, subjects were asked “during the past 4 weeks, to what extent were you bothered by loss of taste?” Allowable responses were “not at all”, “somewhat”, “moderately”, “very much”, and “extremely.” The latter four responses were considered evidence of altTP; the response “not at all” was considered evidence of normal taste perception (nlTP). Of note, TP was based on patient report via the survey without opportunity for objective confirmation.
Age, sex, race, and dialysis vintage were assessed at baseline; age and dialysis vintage were adjusted in time-updated models. Social habits including tobacco, drug, and alcohol use were recorded at baseline and were not reassessed during the study. All other covariates were assessed at baseline and at least annually throughout follow up. Comorbidities included cerebrovascular disease, diabetes, ischemic heart disease, congestive heart failure, and peripheral vascular disease. Dialysis-related covariates included access type, equilibrated-Kt/V and interdialytic weight gain. Equilibrated-Kt/V was calculated using the Daugirdas formula using pre- and 20-minute post dialysis blood urea nitrogen concentrations.23 Laboratory covariates included serum albumin, creatinine, phosphorus, bicarbonate, intact parathyroid hormone, peripheral white blood count, hematocrit, ferritin, and total iron-binding capacity.
Anthropometric data considered included estimated dry weight, mid-arm muscle circumference, and triceps skin-fold thickness. Midarm muscle circumference was calculated as arm circumference – (Π * triceps skinfold thickness) (both in cm).24 Measured dietary intake of protein, calories and sodium were also considered; protein and caloric intake was normalized to body weight. Dietary intake was determined by a HEMO Study dietitian using a two-day (one dialysis, one non-dialysis) dietary recall diary, most often on consecutive days. Dietary intake data were converted into nutrient intake using the Nutritionist IV (version 3.5) program. Other nutritional covariates included normalized protein catabolic ratio, appetite assessment, and use of enteral nutritional supplements. Enteral supplements were considered as formulas that contained calories or protein content, but not simple vitamin or mineral preparations. Normalized protein catabolic rate was calculated as 0.0136*([Kt/V]*[(predialysisBUN–20minutes postdialysisBUN)/2]+0.251.25
Statistical Analysis
Baseline data were considered as the latest measurements that preceded day 60 post-randomization. This 60-day period was included to allow for more complete assessment of baseline dietary recall data (which was delayed after randomization in some instances). In time-updated analyses,26,27 data were updated as of the anniversary of this date. Subjects were followed until death, or censoring for transplant, change of modality, change of dialysis unit, or end of study (December 31, 2001).
In baseline cross-sectional analyses, subjects with nlTP and altTP were compared using contingency tables, chi square tests and t-test as dictated by data type. Time-updated logistic regression models were used to explore factors that are associated with altTP. In time-updated models, exposure and covariate status were updated in the analysis at yearly intervals; each subject contributed a separate entry for each year in study. Variance estimates were adjusted to account for clustering of observations within the same patient during different intervals.28 Parallel analyses were conducted in patient-intervals that immediately followed nlTP (ie, response variable representing worsening of taste perception [nlTP→ altTP] versus maintenance of nlTP [nlTP→ nlTP]) and those that immediately followed altTP (ie, response variable representing persistently altered taste perception [altTP→ altTP] versus improvement in taste perception [altTP→ nlTP]). Time-updated linear regression models were used to examine the association between altTP and nutritional status during the following year. Multivariable estimates were adjusted for age, sex, race, vintage, access type, diabetes, congestive heart failure, ischemic heart disease, cerebrovascular disease, and peripheral vascular disease. Effect modification was examined by comparison of models that did and did not include two-way taste perception-by-factor cross product terms by likelihood ratio testing. We examined for, but did not detect, effect modification on the basis of sex; therefore pooled estimates, encompassing males and females, are presented. The analytical form of continuous covariates was guided by assessment of model fit (using AIC) comparing linear, quadratic, and categorical specifications.
The association between baseline altTP and clinical outcomes was examined by Cox proportional hazards regression models. Multivariable models were adjusted for age, sex, race, categorized vintage, access type, diabetes, congestive heart failure, ischemic heart disease, cerebrovascular disease, and peripheral vascular disease. Specification of continuous covariates for these analyses was guided by Martingale residual plots, and comparison of the fit of models in which linear and categorical specifications were used. Hospitalization rates were assessed using multivariable Poisson regression models. All analyses were performed using STATA, versions 9.0 and 10.0MP College Station, TX.
Results
Of the 1846 subjects randomized in the HEMO study, 1745 had baseline data on self-reported taste perception and thus qualified for inclusion in this study. Overall, the mean age of the cohort was 57.8 +/− 14.1 years, 56.2% were female, 63.7% were black, 44.6% were diabetic, 19.6% had a previous stroke, mean serum albumin was 3.6 +/− 0.4 g/dL, and 19.6% were using nutritional supplements. At baseline, 1141 (65.4%) of subjects reported nlTP and 604 (34.6%) reported altTP.
On baseline cross-sectional analysis, subjects with altTP were older, more likely to be female, have ischemic heart disease and dialyze via a graft than subjects with nlTP (Table 1). Additionally, altTP was associated with lower estimated dry weight, serum albumin and creatinine concentrations, normalized protein catabolic rate, reported intake of protein and sodium, increased use of nutritional supplements, and worse self-reported appetite assessment (Table 1).
Table 1.
Comparison of baseline characteristics between subjects with and without reported alteration in taste.*
| No taste impairment (n =1141)† | Self-reported taste impairment (n =604)† | P-value for difference≠ | |
|---|---|---|---|
|
| |||
| Taste perception | NA | ||
| Not at all | 1141 (100%) | 0 | |
| Somewhat | 0 | 330 (54.6%) | |
| Moderately | 0 | 138 (22.8%) | |
| Very much | 0 | 91 (15.1%) | |
| Extremely | 0 | 45 (7.5%) | |
|
| |||
| Age; years | 57.1 +/− 14.3 | 58.9 +/− 13.7 | 0.02 |
|
| |||
| Female sex | 53.6% | 61.1% | 0.003 |
|
| |||
| Black race | 63.4% | 64.2% | 0.72 |
|
| |||
| Vintage; years | 0.01 | ||
| ≤1 | 23.1% | 22.5% | |
| (1–2] | 25.4% | 24.5% | |
| (2–4] | 19.8% | 26.3% | |
| >4 | 31.7% | 26.7% | |
|
| |||
| Access | 0.01 | ||
| Graft | 57.3% | 62.4% | |
| Fistula | 36.4% | 29.5% | |
| Catheter | 6.3% | 8.1% | |
|
| |||
| Smoking status | 0.27 | ||
| Current smoker | 16.3% | 19.4% | |
| Past smoker | 33.4% | 31.5% | |
| Nonsmoker | 50.2% | 49.2% | |
|
| |||
| Alcohol abuse# | 0.07 | ||
| Current use | 1.0% | 1.2% | |
| Past use | 13.7% | 17.7% | |
| None | 85.4% | 81.1% | |
|
| |||
| Illicit drug abuse | 0.20 | ||
| Current use | 0.5% | 1.2% | |
| Past use | 7.7% | 6.3% | |
| None | 91.8% | 92.5% | |
|
| |||
| Cerebrovascular disease | 18.5% | 21.7% | 0.11 |
|
| |||
| Diabetes | 43.2% | 47.4% | 0.10 |
|
| |||
| Ischemic heart disease | 36.8% | 44.0% | 0.003 |
|
| |||
| Congestive heart failure | 39.0% | 39.6% | 0.82 |
|
| |||
| Peripheral vascular disease | 24.4% | 27.8% | 0.12 |
|
| |||
| Estimated dry weight; kg | |||
| All | 70.3 +/− 14.7 | 67.6 +/− 14.5 | <0.001 |
| Females | 68.1 +/− 15.2 | 65.6 +/− 14.8 | 0.008 |
| Males | 73.0 +/− 13.6 | 70.8 +/− 13.5 | 0.01 |
|
| |||
| Triceps skinfold thickness; mm | |||
| Females | 22.8 +/− 13.9 | 19.9 +/− 10.2 | 0.002 |
| Males | 13.7 +/− 9.7 (n = 1119) | 13.0 +/− 8.6 (n = 590) | 0.52 |
|
| |||
| Midarm muscle circumference; cm | |||
| Females | 24.0 +/− 4.5 | 23.7 +/− 4.2 | 0.20 |
| Males | 25.6 +/− 3.6 (n = 1119) | 25.1 +/− 3.6 (n = 590) | 0.02 |
|
| |||
| Serum albumin; g/dL | 3.66 +/− 0.35 | 3.55 +/− 0.36 | <0.001 |
|
| |||
| Serum creatinine; mg/dL | 10.5 +/− 3.0 | 9.7 +/− 2.7 | <0.001 |
|
| |||
| Serum phosphorus; mg/dL | 5.7 +/− 1.9 (n = 1140) | 5.8 +/− 1.9 (n = 603) | 0.34 |
|
| |||
| Serum bicarbonate; mEq/L | 21 +/− 4 (n = 1139) | 22 +/− 4 (n = 601) | 0.24 |
|
| |||
| Serum intact PTH; pg/mL | 330 +/− 380 (n = 938) | 290 +/− 290 (n = 501) | 0.39 |
|
| |||
| White blood count; K/uL | 6.9 +/− 2.3 (n = 1013) | 7.1 +/− 2.5 (n = 551) | 0.12 |
|
| |||
| Hematocrit; % | 33.6 +/− 4.5 (n = 1140) | 33.8 +/− 4.4 (n = 602) | 0.29 |
|
| |||
| Ferritin; ng/mL | 360 +/− 380 (n = 1009) | 390 +/− 410 (n = 541) | 0.31 |
|
| |||
| TIBC; ug/dL | 230 +/− 60 (n = 934) | 230 +/− 60 (n = 504) | 0.13 |
|
| |||
| eKt/V | 1.50 +/− 0.35 | 1.50 +/− 0.29 | 0.83 |
|
| |||
| Normalized protein catabolic rate | 1.04 +/− 0.26 | 0.99 +/− 0.25 | <0.001 |
|
| |||
| Protein intake; g/kg/d | 0.96 +/− 0.42 (n = 1121) | 0.92 +/− 0.41 (n = 595) | 0.04 |
|
| |||
| Caloric intake; kcal/kg/d | 23.1 +/− 9.4 (n = 1121) | 22.8 +/− 9.8 (n = 595) | 0.26 |
|
| |||
| Sodium intake; mg/day | 2360 +/− 1340 (n = 1122) | 2190 +/− 1150 (n = 595) | 0.05 |
|
| |||
| Interdialytic weight gain; kg | 2.95 +/− 1.30 | 2.81 +/− 1.27 | 0.03 |
|
| |||
| Enteral nutritional supplement use | 17.5% | 23.5% | 0.003 |
|
| |||
| Appetite assessment | <0.001 | ||
| Very good | 36.7% | 16.4% | |
| Good | 40.6% | 33.1% | |
| Fair | 17.3% | 35.3% | |
| Poor | 4.2% | 12.4% | |
| Very poor | 1.2% | 2.8% | |
|
| |||
| Ktv randomization to group 1 | 49.7% | 50.5% | 0.75 |
|
| |||
| Flux randomization to group 1 | 50.1% | 49.8% | 0.91 |
Values expressed as mean +/− standard deviation or proportion.
Except where indicated
Comparisons by t-test and chi square test for continuous and categorical variables, respectively.
Determined by response to survey question “Is there a history of alcohol abuse or does the patient currently abuse alcohol?”
Over the course of follow up, there was significant crossover between states of nlTP and altTP. Figure 1 displays transitions between taste perception status from baseline assessment through the second year of follow up. Over the entirety of follow up 640 (36.7%) of subjects crossed over at least once; the mean rate of crossover was 0.28 events/patient year.
Figure 1.
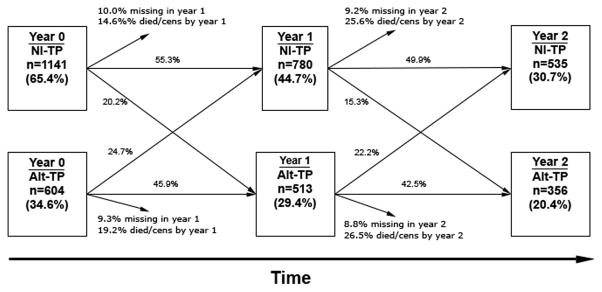
Changes in taste perception over the initial two years of follow up time. [Cens; censored]
We explored predictors of developing altTP versus maintaining nlTP in patient-years that immediately followed one in which taste perception was normal. Predictor variables were considered as the latest recorded measurement in the intervening year. Among factors examined, only dialysis vintage bore an independent association; longer vintages were associated with a decreased likelihood of developing altTP (Table 2). In parallel, we explored predictors of maintaining persistently altTP (versus having improvement in taste perception) in patient-years that immediately followed one in which taste perception was altered. Longer dialysis vintage, congestive heart failure, and remote cerebrovascular disease were associated with a decreased likelihood of maintaining altTP, and inter-current cerebrovascular event was associated with an increased likelihood of maintaining altTP.
Table 2.
Time-updated adjusted associations between predictors and altered taste perception, and change in taste perception.*
| aOR (95% CI) for worsening taste perception (vs persistence of normal taste perception) in setting of normal initial taste perception (n=3207 pt years) | aOR (95% CI) for persistently altered taste perception (vs improving taste perception) in setting of altered initial taste perception (n=2035 pt years) | |
|---|---|---|
| Age; per 10 years | 1.03 (0.97 – 1.10) | 1.00 (0.99 – 1.01) |
| Female | 1.06 (0.89 – 1.27) | 0.95 (0.77 – 1.16) |
| Black race | 1.18 (0.99 – 1.40) | 0.89 (0.73 – 1.10) |
| Vintage; per 1 year longer on dialysis | 0.97 (0.96 – 0.99) | 0.97 (0.95 – 0.99) |
| Catheter as access | 1.15 (0.96 – 1.38) | 0.97 (0.79 – 1.20) |
| Change in access type≠ | 1.11 (0.76 – 1.63) | 1.09 (0.78 – 1.53) |
| Diabetes | 0.99 (0.82 – 1.18) | 1.10 (0.90 – 1.34) |
| Congestive heart failure | 1.01 (0.86 – 1.20) | 0.72 (0.60 – 0.87) |
| Ischemic heart disease | 1.17 (0.98 – 1.39) | 0.99 (0.81 – 1.22) |
| Remote cerebro-vascular disease┼ | 0.89 (0.73 – 1.09) | 0.75 (0.60 – 0.95) |
| Inter-current cerebro-vascular event# | 1.67 (0.94 – 2.98) | 2.28 (1.33 – 3.89) |
| Peripheral vascular disease | 1.09 (0.90 – 1.33) | 0.91 (0.74 – 1.13) |
| Tobacco use | 1.05 (0.89 – 1.24) | 1.01 (0.83 – 1.22) |
| Alcohol abuse | 1.13 (0.89 – 1.42) | 1.18 (0.93 – 1.50) |
| Drug abuse | 0.86 (0.62 – 1.18) | 1.23 (0.85 – 1.77) |
| eKt/V; per 0.1 unit | 0.90 (0.62 – 1.18) | 1.03 (0.97 – 1.04) |
| Change in eKt/V; per 0.1 unit increase from prior# | 1.05 (0.98 – 1.03) | 0.90 (0.96 – 1.02) |
| Ultrafiltration; per 1 L | 0.96 (0.89 – 1.03) | 0.97 (0.89 – 1.05) |
| Change in ultrafiltration; per 1 L increase from prior# | 0.98 (0.90 – 1.07) | 1.02 (0.94 – 1.12) |
All estimates are adjusted for other variables in the table; robust variance estimates used to account for within-subject clustering. Separate, analogous models were fit for patient-intervals beginning with normal and altered taste perception (index year). Predictors were considered as of start of the index year. Outcomes defined based on evolution in taste perception from index year to subsequent year.
In preceding current interval
During the interval leading up to index.
Association between taste perception and nutritional indices
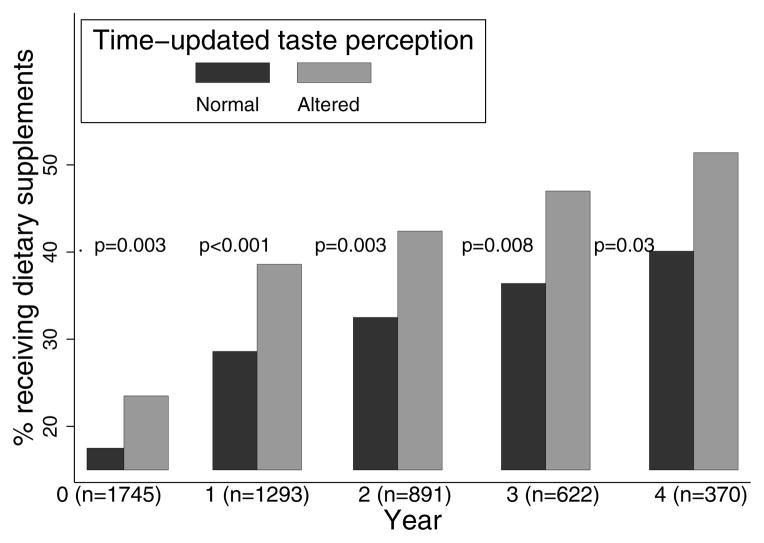
Time-updated linear regression models were used to estimate associations between taste perception (measured at the start of a given interval) and nutritional indices measured during that interval (Table 3). altTP (versus nlTP) was independently associated with lower serum albumin (−0.03 g/dL; p = 0.02), serum creatinine (−0.22 mg/dL; p = 0.009), normalized protein catabolic rate (−0.05; p<0.001), reported protein intake (−0.04 kcal/kg/d; p = 0.006), reported sodium intake (−90 mg/day; p = 0.02), mid-arm muscle circumference (−0.28 cm; p = 0.05), parathyroid hormone levels (−33 pg/ml; p=0.007), and with higher serum ferritin levels (+42 ng/mL; p = 0.05). To examine the interplay between taste and appetite perception, we estimated these associations within strata of self-reported appetite. Estimates within strata defined by normal/impaired appetite were similar to pooled estimates, suggesting that taste perception acts independently of appetite impairment. Also we did not detect effect modification on the basis of self-reported appetite, indicating that the associations between altTP and nutritional outcomes are similar in the setting of normal or impaired appetite. altTP was also associated with increased need for nutritional supplementation (Figure 2).
Table 3.
Adjusted time-updated associations between altered taste perception and nutritional indices as well as components of the malnutrition-inflammation-cachexia syndrome overall and stratified on self-reported alteration in appetite.*
| Adjusted mean difference comparing altTP to nlTP | |||
|---|---|---|---|
| Overall (n=5124 pt-yrs) | No self-reported appetite impairment (n=1367 pt-yrs) | With self-reported appetite impairment (n=3757 pt-yrs) | |
| Normalized protein catabolic rate | −0.05 +/− 0.01 <0.001 |
−0.05 +/− 0.02 0.01 |
−0.04 +/− 0.01 <0.001 |
| p-interaction=0.83 | |||
| Reported caloric intake; kcal/kg/d | −0.54 +/− 0.31 0.08 |
−0.71 +/− 0.66 0.29 |
−0.33 +/− 0.33 0.32 |
| p-interaction=0.43 | |||
| Reported protein intake; g/kg/d | −0.04 +/− 0.01 0.006 |
−0.03 +/− 0.03 0.31 |
−0.03 +/− 0.01 0.04 |
| p-interaction=0.90 | |||
| Reported sodium intake; mg/day | −90 +/− 40 0.02 |
−150 +/− 80 0.05 |
−50 +/− 40 0.23 |
| p-interaction=0.19 | |||
| Interdialytic weight gain; kg | −0.04 +/− 0.04 0.38 |
+ 0.08 +/− 0.09 0.34 |
−0.05 +/− 0.05 0.32 |
| p-interaction=0.26 | |||
| Estimated dry weight; kg | −1.03 +/− 0.58 0.08 |
−0.48 +/− 1.16 0.68 |
−0.70 +/− 0.61 0.25 |
| p-interaction=0.88 | |||
| Midarm muscle circumference; cm | −0.28 +/− 0.14 0.05 |
−0.14 +/− 0.31 0.66 |
−0.23 +/− 0.15 0.14 |
| p-interaction=0.93 | |||
| Triceps skinfold thickness; mm | −0.68 +/− 0.44 0.12 |
−0.57 +/− 0.88 0.52 |
−0.43 +/− 0.46 0.35 |
| p-interaction=0.91 | |||
| Serum albumin; g/dL | −0.03 +/− 0.01 0.02 |
−0.05 +/− 0.02 0.04 |
−0.02 +/− 0.01 0.21 |
| p-interaction=0.35 | |||
| Serum creatinine; mg/dL | −0.22 +/− 0.08 0.009 |
−0.26 +/− 0.17 0.13 |
−0.10 +/− 0.09 0.27 |
| p-interaction=0.30 | |||
| Serum phosphorus; mg/dL | +0.05 +/− 0.06 0.44 |
+0.11 +/− 0.13 0.41 |
+0.05 +/− 0.07 0.49 |
| p-interaction=0.61 | |||
| Serum bicarbonate; mEq/L | +0.07 +/− 0.13 0.61 |
+0.03 +/− 0.27 0.91 |
−0.03 +/− 0.15 0.82 |
| p-interaction=0.77 | |||
| Serum intact PTH; pg/mL | −33 +/− 12 0.007 |
−1 +/− 25 0.97 |
−39 +/− 14 0.004 |
| p-interaction=0.32 | |||
| White blood count; K/uL | +0.09 +/− 0.08 0.26 |
−0.20 +/− 0.16 0.22 |
+0.16 +/− 0.09 0.07 |
| p-interaction=0.03 | |||
| Hematocrit; % | +0.14 +/− 0.14 0.35 |
+0.61 +/− 0.29 0.03 |
+0.10 +/− 0.16 0.55 |
| p-interaction=0.10 | |||
| Ferritin; ng/mL | +42 +/− 17 0.02 |
+14 +/− 27 0.56 |
+39 +/− 20 0.05 |
| p-interaction=0.54 | |||
| TIBC; ug/dL | −1.7 +/− 2.0 0.38 |
−0.2 +/− 3.8 0.96 |
−1.6 +/− 2.2 0.47 |
| p-interaction=0.75 | |||
All analyses adjusted for age, sex, race, vintage, access type, and diabetes, congestive heart failure, ischemic heart disease, cerebrovascular disease, and peripheral vascular disease; variance estimates adjusted for within-subject clustering. Negative coefficients indicate a lower value for altTP relative to nlTP. P-interaction measures the significance of the difference in effect estimates between subjects with normal appetite versus those with impaired appetite.
Figure 2.
Association between taste perception and use of enteral nutritional supplements at baseline and over the course of follow up. [P-values for difference in need for eneteral supplements based on TP were determined using the chi-squared test.]
Association between taste perception and clinical outcomes
Overall there were 4,359 years of at-risk time, during which 739 subjects died; median follow up time was 2.05 years. Unadjusted and multivariable proportional hazards models were used to estimate the independent associations between altTP and time to death from any cause. The unadjusted and HR (95% CI) for altTP (versus nlTP) was 1.22 (1.05–1.42); following multivariable adjustment the association was modestly attenuated but remained statistically significant: adjusted HR (95% CI) 1.17 (1.01–1.37). Unadjusted and multivariable Poisson regression was used to estimate the association between altTP and the rate of hospitalization: unadjusted IRR (95% CI) was 1.09 (0.99–1.21) and adjusted IRR (95% CI) was 1.03 (0.94–1.12).
Discussion
Nutritional decline is common among hemodialysis patients and malnutrition increases the risk of mortality and other adverse health events. These data suggest that altTP is associated with poorer nutritional status, and may be one pathway through which hemodialysis patients become malnourished. At baseline, nearly 35% of participants in the HEMO Study reported altTP, which considering the selected nature of trial participants (in particular the exclusion of subjects with significant hypoalbuminemia), may be a conservative estimate of its prevalence in the general hemodialysis population. On baseline analysis, altTP (compared to nlTP) was associated with poorer nutritional indices. Time-updated analyses revealed that altTP (compared to nlTP) was associated with lower serum albumin, serum creatinine, normalized protein catabolic rate, protein intake, sodium intake, and mid-arm muscle circumference, and a greater need for enteral nutritional supplements. Additionally, altTP was independently associated with a 17% greater risk of death, though not with increased hospitalization rate.
One plausible mechanism by which altTP may influence nutritional outcomes is through effects on appetite. An analysis by Burrowes et al.29 examined self-reported appetite impairment with clinical outcomes among the HEMO Study population and found significant associations with hospitalization rates, although the adjusted analysis did not show an association with all-cause mortality. Our data indicate a strong association between altTP and appetite impairment. Interestingly, however, we observed a number of subjects with altTP who displayed no appetite disturbance and vice versa. In addition, the observed associations between altTP and nutritional outcomes were similar among subjects with normal and impaired appetite, suggesting that the two function independently of one another.
There are multiple nutritional screening tools available for the detection of patients at high nutritional risk, and there is ongoing research focused on development and validation of additional nutritional assessment tools.30,31 It is believed that protein energy malnutrition acts synergistically with inflammation to affect outcomes.8,32–35 altTP was associated with many components of Kalantar’s malnutrition—inflammation complex such as serum albumin, serum creatinine, normalized protein catabolic rate, parathyroid hormone and ferritin. Given these associations in addition to altTP’s association with all-cause mortality, altTP could potentially provide another facet of nutritional assessment among hemodialysis patients.
The HEMO Study provided a unique data set in which to study self-reported taste alteration as it was a prospective study with extensive and detailed data collection over years of follow-up. Some limitations of this study bear note. Generalizability of findings may be limited due to the rigorous eligibility criteria imposed by the source trial. Specifically, given the exclusion of subjects with significant hypoalbumemia (albumin<2.6 g/dL), further studies are needed to confirm findings in those at very high nutritional risk. In addition, generalization to patients who are very elderly, obese, and those with end-stage cardiac, hepatic, pulmonary disease, and malignant disease is not warranted.
Another limitation of this study is the subjective nature of the exposure and its lack of validation with respect to an objective gold standard. Subjects were asked at yearly intervals if they were “bothered” by alteration in taste in the last month. The wording of the question may not be ideal in that subjects who had taste alteration, but were not bothered by it, may have answered “no.” No attempt was made to confirm the presence or severity of taste alteration versus an objective standard. Therefore, findings may apply specifically to subjects with more significant and bothersome dysgeusia than to subjects with milder symptomatology. However, the ease of assessment of altTP might prove a strength in terms of clinical adoption as a risk indicator, because these data would suggest that elevated risk can be inferred even using only patient self-report. Nonetheless, additional study using objective measures is needed to confirm findings and to determine whether impairment of particular aspects of tastes (eg, bitter, sweet) may bear different prognostic significance.
Although we attempted to identify potential causes of altTP, the scope of considered variables was limited by available data. In particular, we were unable to assess how circulating levels of zinc, number and type of medications, various uremic toxins and inflammatory mediators (eg, C-reactive protein, adiponectin) may impact taste perception. We were unable to detect an association between urea clearance and altTP. However, our ability to assess this relationship was limited considering that all HEMO Study subjects received at least adequate urea removal. These data should not be extrapolated to indicate that lesser degrees of small molecule clearance do not promote dysgeusia. Nonetheless, it is noteworthy that even in the context of adequate/supra-adequate urea clearance, over 1/3 of participants reported altered taste perception. This indicates that provision of adequate urea clearance, though likely necessary, is not alone sufficient to prevent dysgeusia. More directed study is needed to assess the potential pathogenic role of specific uremic toxins (including those of larger molecular weight). Finally, analyses of predictors were subject to potential survivor bias considering that high-risk subjects may not have survived until detection of altTP in the subsequent year.
Conclusion
In conclusion, these analyses support an association between altTP and poorer nutritional status among prevalent hemodialysis patients as well as an increased risk of all-cause mortality. Future work is needed to delineate if, when and how assessment of taste perception should be integrated into nutritional assessments of hemodialysis patients.
Practical Application
Altered taste perception is common among hemodialysis patients. In this study, self-reported altered taste perception was found to be associated with poorer indices of nutritional status as well as increased all-cause mortality among prevalent hemodialysis patients.
Acknowledgments
The Hemodialysis Study was conducted by the Hemodialysis Study Investigators and supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). This manuscript was not prepared in collaboration with Investigators of the Hemodialysis Study and does not necessarily reflect the opinions or views of the Hemodialysis study or the NIDDK. This work was presented in abstract form at the XVI International Congress on Nutrition and Metabolism in Renal Disease 2012 (June 2012, Honolulu, Hawaii).
References
- 1.Ng K, Woo J, Kwan M, et al. Effect of age and disease on taste perception. J Pain Symptom Manage. 2004;28(1):28–34. doi: 10.1016/j.jpainsymman.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Ciechanover M, Peresecenschi G, Aviram A, Steiner JE. Malrecognition of taste in uremia. Nephron. 1980;26(1):20–22. doi: 10.1159/000181944. [DOI] [PubMed] [Google Scholar]
- 3.Fernstrom A, Hylander B, Rossner S. Taste acuity in patients with chronic renal failure. Clin Nephrol. 1996;45(3):169–174. [PubMed] [Google Scholar]
- 4.Bots CP, Poorterman JH, Brand HS, et al. The oral health status of dentate patients with chronic renal failure undergoing dialysis therapy. Oral Dis. 2006;12(2):176–180. doi: 10.1111/j.1601-0825.2005.01183.x. [DOI] [PubMed] [Google Scholar]
- 5.Rocco MV, Gassman JJ, Wang SR, Kaplan RM. Cross-sectional study of quality of life and symptoms in chronic renal disease patients: the Modification of Diet in Renal Disease Study. Am J Kidney Dis. 1997;29(6):888–896. doi: 10.1016/s0272-6386(97)90463-7. [DOI] [PubMed] [Google Scholar]
- 6.Mahajan SK. Zinc in kidney disease. J Am Coll Nutr. 1989;8(4):296–304. doi: 10.1080/07315724.1989.10720305. [DOI] [PubMed] [Google Scholar]
- 7.Dvornik S, Cuk M, Racki S, Zaputovic L. Serum zinc concentrations in the maintenance hemodialysis patients. Coll Antropol. 2006;30(1):125–129. [PubMed] [Google Scholar]
- 8.Qureshi AR, Alvestrand A, Divino-Filho JC, et al. Inflammation, malnutrition, and cardiac disease as predictors of mortality in hemodialysis patients. J Am Soc Nephrol. 2002;13 (Suppl 1):S28–36. [PubMed] [Google Scholar]
- 9.Combe C, McCullough KP, Asano Y, Ginsberg N, Maroni BJ, Pifer TB. Kidney Disease Outcomes Quality Initiative (K/DOQI) and the Dialysis Outcomes and Practice Patterns Study (DOPPS): nutrition guidelines, indicators, and practices. Am J Kidney Dis. 2004;44(5 Suppl 2):39–46. doi: 10.1053/j.ajkd.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Pifer TB, McCullough KP, Port FK, et al. Mortality risk in hemodialysis patients and changes in nutritional indicators: DOPPS. Kidney Int. 2002;62(6):2238–2245. doi: 10.1046/j.1523-1755.2002.00658.x. [DOI] [PubMed] [Google Scholar]
- 11.Leavey SF, McCullough K, Hecking E, Goodkin D, Port FK, Young EW. Body mass index and mortality in ‘healthier’ as compared with ‘sicker’ haemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2001;16(12):2386–2394. doi: 10.1093/ndt/16.12.2386. [DOI] [PubMed] [Google Scholar]
- 12.Chan M, Kelly J, Batterham M, Tapsell L. Malnutrition (Subjective Global Assessment) Scores and Serum Albumin Levels, but not Body Mass Index Values, at Initiation of Dialysis are Independent Predictors of Mortality: A 10-Year Clinical Cohort Study. J Ren Nutr. 2012 doi: 10.1053/j.jrn.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Kopple JD. National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis. 2001;37(1 Suppl 2):S66–70. doi: 10.1053/ajkd.2001.20748. [DOI] [PubMed] [Google Scholar]
- 14.Leavey SF, Strawderman RL, Jones CA, Port FK, Held PJ. Simple nutritional indicators as independent predictors of mortality in hemodialysis patients. Am J Kidney Dis. 1998;31(6):997–1006. doi: 10.1053/ajkd.1998.v31.pm9631845. [DOI] [PubMed] [Google Scholar]
- 15.Bergstrom J. Nutrition and mortality in hemodialysis. J Am Soc Nephrol. 1995;6(5):1329–1341. doi: 10.1681/ASN.V651329. [DOI] [PubMed] [Google Scholar]
- 16.National Kidney Foundation. Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis. 2000;35(6 Suppl 2):S1–140. doi: 10.1053/ajkd.2000.v35.aajkd03517. [DOI] [PubMed] [Google Scholar]
- 17.Yamada K, Furuya R, Takita T, et al. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am J Clin Nutr. 2008;87(1):106–113. doi: 10.1093/ajcn/87.1.106. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi I, Ishimura E, Kato Y, et al. Geriatric Nutritional Risk Index, a simplified nutritional screening index, is a significant predictor of mortality in chronic dialysis patients. Nephrol Dial Transplant. 2010;25(10):3361–3365. doi: 10.1093/ndt/gfq211. [DOI] [PubMed] [Google Scholar]
- 19.Rambod M, Bross R, Zitterkoph J, et al. Association of Malnutrition-Inflammation Score with quality of life and mortality in hemodialysis patients: a 5-year prospective cohort study. Am J Kidney Dis. 2009;53(2):298–309. doi: 10.1053/j.ajkd.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atkin-Thor E, Goddard BW, O’Nion J, Stephen RL, Kolff WJ. Hypogeusia and zinc depletion in chronic dialysis patients. Am J Clin Nutr. 1978;31(10):1948–1951. doi: 10.1093/ajcn/31.10.1948. [DOI] [PubMed] [Google Scholar]
- 21.Eknoyan G, Beck GJ, Cheung AK, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347(25):2010–2019. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 22.Greene T, Beck GJ, Gassman JJ, et al. Design and statistical issues of the hemodialysis (HEMO) study. Control Clin Trials. 2000;21(5):502–525. doi: 10.1016/s0197-2456(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 23.Daugirdas JT. Estimation of equilibrated Kt/V using the unequilibrated post dialysis BUN. Semin Dial. 1995;8:283–284. [Google Scholar]
- 24.Blumenkrantz MJ, Kopple JD, Gutman RA, et al. Methods for assessing nutritional status of patients with renal failure. Am J Clin Nutr. 1980;33(7):1567–1585. doi: 10.1093/ajcn/33.7.1567. [DOI] [PubMed] [Google Scholar]
- 25.Lightfoot BO, Caruana RJ, Mulloy LL, Fincher ME. Simple formula for calculating normalized protein catabolic rate (NPCR) in hemodialysis (HD) patients (abstract) J Am Soc Nephrol. 1993;4:363. [Google Scholar]
- 26.Habib ZA, Tzogias L, Havstad SL, et al. Relationship between thiazolidinedione use and cardiovascular outcomes and all-cause mortality among patients with diabetes: a time-updated propensity analysis. Pharmacoepidemiol Drug Saf. 2009;18(6):437–447. doi: 10.1002/pds.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ledergerber B, Furrer H, Rickenbach M, et al. Factors associated with the incidence of type 2 diabetes mellitus in HIV-infected participants in the Swiss HIV Cohort Study. Clin Infect Dis. 2007;45(1):111–119. doi: 10.1086/518619. [DOI] [PubMed] [Google Scholar]
- 28.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56(2):645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 29.Burrowes JD, Larive B, Chertow GM, et al. Self-reported appetite, hospitalization and death in haemodialysis patients: findings from the Hemodialysis (HEMO) Study. Nephrol Dial Transplant. 2005;20(12):2765–2774. doi: 10.1093/ndt/gfi132. [DOI] [PubMed] [Google Scholar]
- 30.Beberashvili I, Azar A, Sinuani I, et al. Objective Score of Nutrition on Dialysis (OSND) as an alternative for the malnutrition-inflammation score in assessment of nutritional risk of haemodialysis patients. Nephrol Dial Transplant. 2010;25(8):2662–2671. doi: 10.1093/ndt/gfq031. [DOI] [PubMed] [Google Scholar]
- 31.Oliveira CM, Kubrusly M, Mota RS, Silva CA, Choukroun G, Oliveira VN. The phase angle and mass body cell as markers of nutritional status in hemodialysis patients. J Ren Nutr. 2010;20(5):314–320. doi: 10.1053/j.jrn.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Kalantar-Zadeh K. Recent advances in understanding the malnutrition-inflammation-cachexia syndrome in chronic kidney disease patients: What is next? Semin Dial. 2005;18(5):365–369. doi: 10.1111/j.1525-139X.2005.00074.x. [DOI] [PubMed] [Google Scholar]
- 33.Kalantar-Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis. 2003;42(5):864–881. doi: 10.1016/j.ajkd.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 34.de Mutsert R, Grootendorst DC, Indemans F, et al. Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. J Ren Nutr. 2009;19(2):127–135. doi: 10.1053/j.jrn.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 35.de Mutsert R, Krediet RT. Malnutrition, inflammation and atherosclerosis (MIA-syndrome) in dialysis patients. Ned Tijdschr Geneeskd. 2006;150(37):2023–2027. [PubMed] [Google Scholar]