Abstract
Wnt/β-catenin (βcat) signaling is critical for adrenal homeostasis. To elucidate how Wnt/βcat signaling elicits homeostatic maintenance of the adrenal cortex, we characterized the identity of the adrenocortical Wnt-responsive population. We find that Wnt-responsive cells consist of sonic hedgehog (Shh)-producing adrenocortical progenitors and differentiated, steroidogenic cells of the zona glomerulosa, but not the zona fasciculata and rarely cells that are actively proliferating. To determine potential direct inhibitory effects of βcat signaling on zona fasciculata-associated steroidogenesis, we used the mouse ATCL7 adrenocortical cell line that serves as a model system of glucocorticoid-producing fasciculata cells. Stimulation of βcat signaling caused decreased corticosterone release consistent with the observed reduced transcription of steroidogenic genes Cyp11a1, Cyp11b1, Star, and Mc2r. Decreased steroidogenic gene expression was correlated with diminished steroidogenic factor 1 (Sf1; Nr5a1) expression and occupancy on steroidogenic promoters. Additionally, βcat signaling suppressed the ability of Sf1 to transactivate steroidogenic promoters independent of changes in Sf1 expression level. To investigate Sf1-independent effects of βcat on steroidogenesis, we used Affymetrix gene expression profiling of Wnt-responsive cells in vivo and in vitro. One candidate gene identified, Ccdc80, encodes a secreted protein with unknown signaling mechanisms. We report that Ccdc80 is a novel βcat-regulated gene in adrenocortical cells. Treatment of adrenocortical cells with media containing secreted Ccdc80 partially phenocopies βcat-induced suppression of steroidogenesis, albeit through an Sf1-independent mechanism. This study reveals multiple mechanisms of βcat-mediated suppression of steroidogenesis and suggests that Wnt/βcat signaling may regulate adrenal homeostasis by inhibiting fasciculata differentiation and promoting the undifferentiated state of progenitor cells.
The adrenal cortex synthesizes and secretes steroid hormones necessary for life. The adrenal is zonated into distinct steroidogenic layers responsible for the production of steroids under the control of differing physiological stimuli. In the outermost subcapsular layer, the zona glomerulosa (zG), the mineralocorticoid aldosterone is produced under the control of the renin-angiotensin system. The inner zona fasciculata (zF) produces glucocorticoids, such as cortisol in humans and corticosterone in mice that coordinate the mammalian stress response regulated by the hypothalamic-pituitary-adrenal axis.
The static adrenocortical zonation is intriguing given the exceptional dynamicity of the adrenal cortex; cells proliferate at its periphery, differentiate into steroidogenic cells that are displaced centripetally until they eventually turn over at the adrenocortical-medullary boundary. Cell turnover requires replenishment of adrenocortical cells from a somatic stem/progenitor cell pool for maintenance of adrenal tissue. Historically, it has been debated whether each steroidogenic zone contains its own progenitor cell or whether a common adrenocortical progenitor within the periphery of the cortex changes its gene expression pattern during differentiation because it is centripetally displaced throughout life. In recent years, several studies have provided evidence for the latter. Work by King et al (1) demonstrated that sonic hedgehog (Shh)-producing cells that reside within the subcapsular region serve as a common progenitor cell for steroidogenic lineages. Using lineage tracing, relatively undifferentiated Shh-producing cells become steroidogenic cells of both adrenocortical zones over time. Additionally, using an aldosterone synthase-Cre recombinase in cell lineage tracing experiments of zG cells, Freedman et al (2) demonstrated that under normal conditions, all zF cells are derived from zG cells. Collectively, these data suggest unidirectional differentiation of adrenocortical progenitors first to zG cells that then become zF cells as they transit through the cortex. However, how progenitor cells are maintained in the subcapsular region as well as how unidirectional differentiation is regulated is not well understood.
Canonical Wnt signaling is a paracrine signaling pathway implicated in progenitor cell biology in a variety of organ systems, including the adrenal cortex. In the absence of Wnt ligands, the pathway is inactive, resulting from sequestration of the transcriptional regulator β-catenin (βcat) into cytoplasmic destruction complexes composed of axin, adenomatous polyposis coli (APC), glycogen synthase kinase 3β (GSK3β), casein kinase 1α (CKIα), and other components. Phosphorylation of βcat by GSK3β and CKIα results in its ubiquitination by β-transductin-repeat-containing protein (β-TrCP) and subsequent degradation by the proteasome. Wnt ligands binding to Frizzled (Fzd) receptors with low-density lipoprotein receptor-related protein 5/6 coreceptors results in the inhibition of the destruction complex, leading to βcat stabilization and nuclear translocation. βcat then activates Wnt-responsive genes by binding to T cell factor/lymphoid enhancer factor (TCF/LEF) transcription factors at gene enhancers. The Wnt/βcat pathway has been previously implicated in the homeostatic maintenance of the adrenal gland (3). Mice in which βcat is partially knocked out in the adrenal cortex exhibit depletion of adrenocortical cells upon aging, highlighting a critical role for βcat in maintenance of the progenitor cells in the adrenal cortex (3). Wnt signaling maintains adrenocortical progenitors in part through the activation of the βcat target gene, Nr0b1, which encodes dosage-sensitive sex reversal, adrenal hypoplasia congenita critical region on the X chromosome, gene 1 (Dax1) (4). Dax1 is an orphan nuclear receptor implicated in maintaining the pluripotency of embryonic stem cells (5). Dax1-knockout adrenals display enhanced steroidogenesis in early ages with adrenocortical failure at late ages (6), consistent with the precocious differentiation of adrenocortical progenitors in the absence of Dax1 resulting in premature depletion of the progenitor cell pool. Interestingly, Wnt signaling has also recently been implicated in zG cell differentiation. A study by Berthon et al (7) indicates that βcat indirectly promotes aldosterone production by increasing expression of the angiotensin receptor (At1r) and Nr4a1/2 genes, which stimulate the expression of Cyp11b2, the terminal enzyme in aldosterone biosynthesis. It remains unclear whether Wnt signaling influences zF cell differentiation.
In this study, we report that Wnt-responsive cells colocalize with markers of adrenocortical progenitors and differentiated cells of the zG but not the zF. Stimulation of βcat activity in vitro inhibits zF cell differentiation by suppressing steroidogenesis through Sf1-dependent mechanisms as well as through the induction of a new βcat-regulated gene, Ccdc80. Our study provides insight into how Wnt signaling maintains the undifferentiated state of adrenal progenitor cells through inhibition of steroidogenesis. Additionally, our data indicate that βcat inhibits the zF cell phenotype, supporting a role for Wnt signaling in the unidirectional differentiation of adrenal progenitors into zG cells.
Materials and Methods
Mice
All animal experiments were carried out in accordance with protocols approved by the University Committee on Use and Care of Animals at the University of Michigan. Reporter mice used in these studies have been described elsewhere. TCF/Lef:H2B-GFP mice (8) were obtained from The Jackson Laboratory. ShhLacZ mice (9) were obtained from the laboratory of Andrzej Dlugosz at the University of Michigan. Sf1:eGFP mice (10) were obtained from the laboratory of the late Keith Parker.
Immunofluorescence
Adrenals from 6-week-old male Tcf/Lef:H2B-GFP;ShhLacZ mice were fixed for 4 hours with 4% paraformaldehyde, dehydrated in graded ethanol solutions, paraffin-embedded, and sectioned into 5- to 10-μm slices. For bromodeoxyuridine (BrdU) labeling in vivo, mice were ip injected with BrdU labeling reagent (Life Technologies) at 1 mL/100 g body weight, killed 2 hours later, and processed as above. For immunofluorescence analyses, adrenal sections (6 μm) were boiled in 10mM citric acid (pH 2 or pH 6) for 10 minutes followed by 20 minutes cooling for antigen retrieval. Slides were washed with PBS and blocked with 2% nonfat dry milk in PBS with 5% goat serum (Thermo Scientific) for 1 hour followed by primary antibody incubation at 4°C overnight. Slides were washed with Tris-buffered saline with 0.1% Tween, incubated with secondary antibodies for 1 hour at room temperature followed by nuclear counterstaining with 4′,6-diamidino-2-phenylindole (DAPI), and mounted using ProLong Gold antifade reagent (Life Technologies). Slides were cured overnight, and fluorescence microscopy was performed on a Zeiss ApoTome microscope using an AxioCam MRm (Carl Zeiss). Primary antibodies were rabbit α-green fluorescent protein (GFP) 1:200 (Life Technologies), chicken α-LacZ 1:1000 (Abcam), mouse α-proliferating cell nuclear antigen (PCNA) 1:500 (Santa Cruz Biotechnology, Inc), sheep α-BrdU 1:230 (Abcam), mouse α-Cyp11b1 and mouse α-Cyp11b2 1:50 (generous gifts from Dr C. Gomez-Sanchez), and chicken α-GFP 1:1500 (Abcam). Secondary antibodies were goat α-rabbit Alexa Fluor 488, goat α-mouse Dylight 488, donkey α-chicken Dylight 488, goat α-chicken Dylight 549, donkey α-sheep Cy3, goat α-mouse Dylight 649, donkey α-rabbit Dylight 649, all used at 1:800 (all obtained from Jackson ImmunoResearch). For quantification of immunofluorescence data, a total cell count per population (GFP+, LacZ+, or PCNA+) was obtained from 3 separate adrenal sections corresponding to distinct adrenal regions. The number of double-positive GFP+/LacZ+, GFP+/PCNA+, or LacZ+/PCNA+ cells was divided by the total number of GFP+, LacZ+, or PCNA+ cells. Data were averaged from 7 different animals. Cell counts are presented in Table 1. The same approach was used for the quantification of 11b2+ cells with GFP+ and LacZ+ cells as presented in Table 2.
Table 1.
Cell Counts for Data Presented in Figure 1C
| Population | Total No. of Cells per Section | No. of Cells Colocalized With |
||
|---|---|---|---|---|
| GFP+ | LacZ+ | PCNA+ | ||
| GFP+ | 1609 ± 195 | 221 ± 39 | 56 ± 9 | |
| LacZ+ | 308 ± 50 | 221 ± 39 | 1 ± 0.4 | |
| PCNA+ | 198 ± 35 | 56 ± 9 | 1 ± 0.4 | |
Table 2.
Cell Counts for Data Presented in Figure 1H
Cell culture and reagents
ATCL7, 293T, and JEG-3 cells were grown under standard conditions at 37°C with 5% CO2. Growth medium for ATCL7 cells was DMEM:F12 containing 2.5% fetal bovine serum (FBS), 2.5% horse serum, 100 U/mL penicillin/streptomycin, 1% insulin-transferrin-selenium X (all obtained from Life Technologies). For treatment experiments, cells were grown in low-serum media (same as normal growth media except with 0.025% FBS and 0.025% horse serum). The 293T and JEG-3 cells were grown in DMEM with 10% FBS and 100 U/mL penicillin/streptomycin. 6-Bromoindirubin-3′-oxime (BIO), ACTH (human, rat N-terminal fragment 1–24), and dimethylsulfoxide (DMSO) were obtained from Sigma-Aldrich.
Immunocytochemistry
ATCL7 cells were plated at a density of 105 cells per well on chamber slides (Lab-Tek). After 24 hours, cells were treated with 0.5μM BIO or DMSO overnight and fixed for 15 minutes with 4% paraformaldehyde followed by PBS washes and permeabilization with 0.1% IGEPAL for 3 minutes at room temperature. Cells were blocked using 2% nonfat dry milk in PBS with 5% normal goat serum (Thermo Scientific) for 1 hour, followed by overnight incubation with rabbit α-βcat 1:500 (Santa Cruz Biotechnologies) at 4°C. Slides were washed with PBS, incubated with goat α-rabbit Alexa Fluor 488 secondary antibody 1:800 (Jackson ImmunoLabs) for 1 hour at room temperature, followed by nuclear counterstaining with DAPI and mounted using ProLong Gold antifade reagent (Life Technologies). Fluorescence microscopy was performed using an Optiphot-2 microscope (Nikon) with an Olympus DP-70 camera and software.
ATCL7 cell treatments and electroporation
For BIO experiments, cells were plated at a density of 2.5 × 105 cells per well in 6-well dishes and treated with 0.5μM BIO for 12 hours, followed by 100nM ACTH stimulation for 6 hours. For conditioned media (CM) experiments, cells were plated as above and treated with CM for 18 hours, followed by 100nM ACTH stimulation for 6 hours. For electroporation of ATCL7 cells, an Amaxa Cell Line Nucleofector R Kit (Lonza) was used following the manufacturer's protocol. Briefly, 106 cells per transfection were trypsinized, resuspended in solution R with supplement 1, and mixed with 1 μg pcDNA 3.1 empty vector, 1 μg βcatS33Y, and/or 1 μg dnTCF4E expression constructs. Cell mixtures were electroporated using program T-20, plated in 4 wells of a 12-well dish, and harvested 24 hours later. For luciferase assays, cells were electroporated with reporter constructs (1 μg) and allowed to adhere to tissue culture dishes for 24 hours before BIO treatment.
Cell fractionation and immunoblotting
For whole-cell lysates, cells were harvested with RIPA buffer (Promega) containing protease inhibitors (Sigma-Aldrich) and PhosSTOP (Roche), rocked on ice for 20 minutes, scraped, and sonicated briefly. For immunoblotting, 1/10 of cell lysates (20 μg) were subjected to SDS-PAGE and transferred onto polyvinylidene difluoride membranes. Membranes were blocked in Odyssey blocking buffer (LI-COR) 1:1 with PBS. Primary antibodies were diluted in Tris-buffered saline with 0.1% Tween with 5% BSA (Roche) and incubated at 4°C overnight. Primary antibodies were rabbit α-Sf1 1:1000 (custom), mouse α-active βcat 1:500 (EMD Millipore), rabbit α-βcat 1:2000 (Santa Cruz Biotechnologies Inc), mouse α-β-actin 1:5000 and mouse α-FlagM2 1:2000 (Sigma-Aldrich), rabbit α-myc 1:1000 (Bethyl Laboratories, Inc), and rabbit α-phosphorylated Creb (pCreb) (Ser133), and rabbit α-Creb all used at 1:1000 (all obtained from Cell Signaling). Cell fractionation was achieved following the nuclear extraction protocol using the nuclear extract kit (Active Motif). For immunoblotting, equivalent proportions of each fraction were analyzed.
RNA extraction and quantitative RT-PCR
After specified treatments, cells were harvested using the RNeasy Mini Plus kit (QIAGEN), and 1 μg RNA was converted to cDNA using the iScript Supermix reverse transcriptase kit (Bio-Rad) following the manufacturer's instructions. Quantitative real-time PCR was carried out using 10 ng of cDNA, 1 μM of specific primers (see Table 3) and Power SYBR Green reagent (Applied Biosystems) on an ABI 7300 thermocycler (Applied Biosystems). Gene expression was normalized to Rplp0 mRNA abundance and calculated using the ΔΔ cycle threshold method.
Table 3.
Primer Sets for qRT-PCR
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| Rplp0 | GAAACTGCTGCCTCACATCCG | GCTGGCACAGTGACCTCACACG |
| Axin2 | GCAGGAGCCTCACCCTTC | TGCCAGTTTCTTTGGCTCTT |
| Cyp11b1 | GCCATCCAGGCTAACTCAAT | CATTACCAAGGGGGTTGATG |
| Star | AAGGCTGGAAGAAGGAAAGC | CCACATCTGGCACCATCTTA |
| Cyp11a1 | AAGTATGGCCCCATTTACAGG | TGGGGTCCACGATGTAAACT |
| Mc2r | GTAAGTCAACGGCAAACACC | GTGTCATTGGTGTGTTCATACG |
| Sf1 | CGCTGTCCCTTCTGCGGCTT | AGCACGCACAGCTTCCAGGC |
| Ccdc80 | AGGCATGCAATTTTGGTCTGC | ACATCTTCCCGCTCAACGAT |
Chromatin immunoprecipitation
ATCL7 cells were plated at a density of 6 × 106 cells per 150-mm dish per treatment for 12 hours. Cells were treated with 0.5μM BIO or DMSO in low-serum media for 12 hours followed by stimulation with 100nM ACTH for 6 hours. Cells were cross-linked with 1% formaldehyde with rocking at room temperature for 10 minutes. The reaction was quenched by incubation with 0.125M glycine with rocking at room temperature for 10 minutes. Cells were washed 3 times with ice-cold PBS. Cell membranes were disrupted for 15 minutes at 4°C in nuclear extraction buffer containing 25mM HEPES (pH 7.9), 5mM KCl, 0.5mM MgCl2, 1mM dithiothreitol, 1% IGEPAL, and protease inhibitors (Sigma-Aldrich). Nuclei were isolated from cytoplasmic protein by centrifugation at 1000g for 5 minutes at 4°C. Nuclei were lysed in a buffer containing 1% sodium dodecyl sulfate, 10mM EDTA, and 50mM Tris-HCl (pH 8.1) for 10 minutes on ice. Lysates were sonicated at 100% amplitude, with 20 × 30-second pulses using a Q500 sonicator with a 3-inch cup horn (Qsonica, Llc) to shear genomic DNA between 200 and 400 bp. Sonicated lysates were cleared by centrifugation at 14 000g for 10 minutes at 4°C and diluted 1:10 in a chromatin immunoprecipitation (ChIP) dilution buffer containing 0.01% SDS, 1.1% Triton X-100, 1.2mM EDTA, 16.7mM Tris-HCl (pH 8.1), and 167mM NaCl. The diluted lysates were precleared for 1 hour at 4°C with protein G Dynabeads (Life Technologies) prepared in ChIP dilution buffer with 200 μg/mL BSA and 200 μg/mL glycogen (both from Roche), and 30 μg chromatin per treatment was immunoprecipitated overnight at 4°C using 3 μg Sf1 antibodies (custom) or rabbit IgG (Santa Cruz Biotechnologies, Inc). Immunoprecipitates were recovered for 1 hour at 4°C with protein G Dynabeads as prepared above. Precipitates were washed for 5 minutes each with the following buffers: low-salt buffer (0.1% SDS, 1% Triton X-100, 2mM EDTA, 20mM Tris-HCl [pH 8.1], and 150mM NaCl), high-salt buffer (0.1% SDS, 1% Triton X-100, 2mM EDTA, 20mM Tris-HCl [pH 8.1], and 500mM NaCl), LiCl buffer (0.25 M LiCl, 1% IGEPAL, 1% deoxycholate, 1mM EDTA, and 10mM Tris-HCl [pH 8.1]), and Tris-EDTA (pH 8.0) buffer. Immunoprecipitates were eluted from Dynabeads with two 15-minute incubations in elution buffer (1% SDS, 0.1M NaHCO3). Cross-links were reversed by incubation at 65°C for 4 hours in the presence of 200mM NaCl and 1 μg ribonuclease A (Roche). Protein was digested by incubation for 30 minutes at 65°C in the presence of 10mM EDTA, 40mM Tris-HCl (pH 6.5) and 40 μg proteinase K (Roche). DNA fragments were purified using a PCR purification kit (QIAGEN) according to manufacturer's instructions. Purified DNA fragments were analyzed by qPCR on an ABI 7300 thermocycler (Applied Biosystems) using Power SYBR Green reagent (Applied Biosystems). Primer pairs used for ChIP-qPCR are listed in Table 4.
Table 4.
Primer Sets for ChIP-qPCR
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| Star | AGAGGGTCAAGGATGGAATGATT | CAGTCTGCTCCCTCCCACC |
| Cyp11a1 | GGGAGGTCACCGCTCCATCAGC | GCCAGCATACTGTCCCCACGACT |
| Mc2r | AACCAACCAGACATCCCCTGCC | CAAGGACCTCTCCTCCCACAAAGC |
Corticosterone measurement
Experimental media from treated cells were collected at the time of cell harvest and stored at −20°C. The corticosterone content of the media samples was determined using a mouse and rat corticosterone ELISA kit (ALPCO Diagnostics). Assays were conducted following the manufacturer's instructions except standard curves were prepared in the cell culture medium. Protein concentration of corresponding cell lysates was assessed using a bicinchoninic acid assay kit (Bio-Rad) according to manufacturer's instructions. Corticosterone abundance was normalized to protein concentration and shown as fold change over Vehicle-treated samples.
Adrenal digestion and cell sorting
Ten adrenals per genotype per sort were minced and digested by incubation in DMEM:F12 containing 0.1% collagenase (Roche), 0.01% deoxyribonuclease I (Roche) for 1 hour at 37°C. A single-cell suspension was obtained after mechanical dispersion, filtration through a 40-μm nylon cell strainer, and centrifugation at 320g for 5 minutes followed by resuspension in sterile PBS containing 10% cosmic calf serum and 10 μg/mL propidium iodide (Sigma-Aldrich). In total 10 000 to 50 000 viable GFP-positive cells were isolated via fluorescence-activated cell sorting (FACS) using a FACSAria III cell sorter (BD Biosciences). Cells were sorted directly into RLT buffer, and RNA was harvested using an RNeasy micro kit (QIAGEN).
Microarray analysis
We measured cell transcript abundances in 2 experiments using Affymetrix mouse gene ST 1.1 strip arrays with 28 944 probe sets. In both cases, robust multichip average algorithms were used to measure log2-transformed transcript abundances. In the in vivo experiment, RNA from 4 independent sorts per genotype was used. cDNA was prepared according to the NuGen WT-Pico V2 kit protocol from 5 ng total RNA. Biotinylated single-stranded cDNA was prepared from 3 μg cDNA using the Encore biotin module (NuGEN). We used a 2-sample t test to compare the 2 conditions in this dataset. One TCF/Lef:H2B-GFP sample was discarded for quality control reasons. For the in vitro experiment, RNA from 4 pairs of DMSO or 0.5 μM BIO-treated ATCL7 cells was used. Biotinylated cDNA was prepared using an Ambion WT expression kit from 250 ng total RNA (Affymetrix). The data were modeled using paired T tests for each probe set. The array data and statistical analysis have been deposited in NCBI's Gene Expression Omnibus (GEO) (11) and are accessible through GEO series accession numbers GSE53971 and GSE53981.
In situ hybridization
A 386-bp fragment in the 3′-untranslated region of the murine Ccdc80 gene was amplified using the primers 5′-TCCAGTTTCCCCTTCATCTG-3′ and 5′-TTGTGTCTATCCAGATGTTCTACGA-3′. The generated fragment was subsequently cloned into the pCR 2.1TOPO vector (Invitrogen) following the manufacturer's protocol. The Ccdc80 digoxigenin-labeled RNA probe was synthesized using the digoxigenin RNA labeling kit (Roche). In situ hybridization analysis was performed as previously described (12, 13).
Plasmid constructs and mutagenesis
For the Ccdc80-luciferase construct, −1957 to +814 surrounding the transcription start site of murine Ccdc80 was PCR-amplified from genomic DNA using Phusion high-fidelity DNA polymerase (New England BioLabs) and the following primers: forward, 5′-AAAAGCTAGCAGCTGGTTGTGGGATGGATG-3′, adding an NheI site; and reverse, 5′-TGTAAGATCTACTTGGGGACGCAGAGGGGGTATAAT-3′, adding a BglII site. The PCR product was subcloned into the pGL3-Basic vector (Promega) using the engineered NheI and BglII sites. Core elements of TCF binding sites were mutated from AA(A/G)A to CGCT by site-directed mutagenesis using Calbiochem KOD Hot Start polymerase reagents (EMD Millipore) according to the manufacturer's instructions. Primer sets used for site-directed mutagenesis are listed in Table 5.
Table 5.
Primers Sets for Site-Directed Mutagenesis of Ccdc80-Luciferase Construct
| Mutation | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| Tcf site 1 | GGCTCTCATACTGCGAGAAGCCGCTACTTTACCAGCTGAGG | CCTCAGCTGGTAAAGTAGCGGCTTCTCGCAGTATGAGAGCC |
| Tcf site 2 | CCATGGGCTTTTCCAACCCAGAACTCCGCTCATAAAGCAGGCAG | CTGCCTGCTTTATGAGCGGAGTTCTGGGTTGGAAAAGCCCATGG |
| Tcf site 3 | CGCTCGGAAACCGCTGGACTCGGCCAGACTGCAGG | CCTGCAGTCTGGCCGAGTCCAGCGGTTTCCGAGCG |
| Tcf site 4 | CCTCATGCCTGCCGCCTGTAAATCCGCTCTGGATTTCCTGC | GCAGGAAATCCAGAGCGGATTTACAGGCGGCAGGCATGAGG |
The underlined letters represent the mutations introduced to abrogate transcription factor binding.
Luciferase assays
The 293T cells were plated at a density of 2.5 × 104 cells per well into 24-well plates and incubated for 24 hours. For BIO treatment experiments, cells were transiently transfected with 25 ng empty vector pcDNA3.1 construct or 25 ng Sf1 expression construct, 100 ng Star-luciferase reporter construct and 30 ng Renilla-luciferase reporter construct using Fugene HD (Roche). At 24 hours after transfection, cells were treated with 2μM BIO or DMSO for 24 hours and then harvested. For βcatS33Y experiments, cells were transiently transfected with 25 or 125 ng empty vector pcDNA3.1 construct and/or 25ng Sf1 expression construct and/or 100ng βcatS33Y expression construct with 100 ng Star-luciferase reporter construct and 30 ng Renilla-luciferase reporter construct using Fugene HD (Roche). Cells were harvested 48 hours after transfection. For Ccdc80-luciferase experiments, 293T cells were transiently transfected with 100 ng specific Ccdc80-luciferase reporter constructs and 30 ng Renilla-luciferase reporter construct using Fugene HD (Roche), and 24 hours later, cells were treated with 2μM BIO or DMSO for 24 hours and then harvested. Cells were lysed with passive lysis buffer and luciferase assays were performed using the dual-luciferase reporter assay system (Promega) according to manufacturer's instructions on a Veritas microplate luminometer (Turner BioSystems).
Preparation of CM
The 293T cells were plated at a density of 2 × 106 cells per 100-mm dish and transfected with 19 μg Ccdc80-myc or 19 μg empty vector pcDNA 3.1 construct using Fugene HD (Roche) in low-serum media, and 24 hours later, CM were collected and centrifuged at 1000g for 5 minutes to pellet floating cells and debris, sterile-filtered through 0.22-μm filters, aliquoted, and frozen at −80°C. Thawed CM were mixed 1:1 with fresh ATCL7 low-serum growth media just before use in experiments.
Statistical analysis
Unless otherwise noted, data in figures are from repeated experiments with 1 sample per condition per experiment, with the means for each condition and the SD for each condition with main experiment effects removed being shown. The analyses are from two-way ANOVA procedures on log-transformed data with terms for each condition and each experiment, which in the simplest case of 2 conditions are paired t tests. Commonly, pairwise contrasts comparing pairs of conditions were tested, but occasional tests of interactions between 2 treatments required comparing the difference of differences in pairs of conditions. Tests with P values <.05 were considered significant.
Results
Wnt-responsive cells in the adult adrenal cortex are a heterogeneous population in vivo
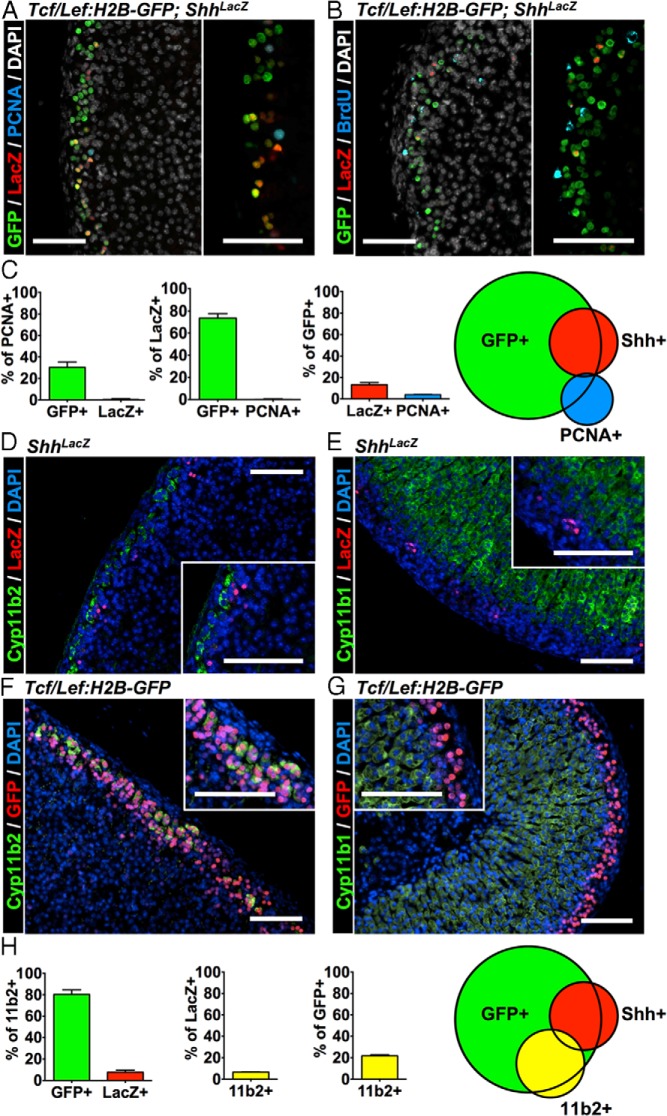
The subcapsular adrenal cortex is the location of both 1) cells that are activated in response to signals from canonical Wnt ligands (3) and 2) cells that produce the ligand Shh (1). The peripheral cortex is also the primary location where adrenocortical cells proliferate (14). Because Shh-producing cells primarily serve as relatively undifferentiated progenitor cells for the steroidogenic cortex (1) and Wnt signaling participates in the maintenance of the adrenocortical progenitor pool (3), we sought to delineate any shared identity of Wnt-responsive and Shh-producing cells and determine the mechanisms by which Wnt/βcat signaling contributes to homeostasis of the adult adrenal cortex. We hypothesized that Wnt-responsive cells would be identical to the Shh-producing progenitors. Additionally, given a role for βcat in the regulation of proliferation during adrenocortical development (3), we speculated that proliferating adrenocortical cells in the adult gland would be Wnt-responsive cells. Therefore, to determine whether Wnt-responsive cells colocalize with markers of adrenocortical progenitors and/or proliferating cells, we crossed the Tcf/Lef:H2B-GFP reporter mice with ShhLacZ reporter mice and performed immunofluorescence on adult adrenal sections. We observed that although many GFP+ (Wnt-responsive) cells do not coexpress LacZ, most LacZ+ (Shh-producing) progenitor cells costain as GFP+ (Wnt-responsive) cells (Figure 1A). Surprisingly, less than 10% of GFP+ (Wnt-responsive) cells colocalize with dividing PCNA+ cells (Figure 1C). This fraction of the GFP+ population constitute less than 30% of the proliferating adrenocortical cells, suggesting that Wnt signaling does not play a primary (cell-autonomous) role in regulating adrenocortical proliferation (Figure 1, A and C). Moreover, less than 1% of LacZ+ (Shh+) cells colocalize with PCNA+ cells, indicating Shh+ progenitor cells are relatively quiescent (Figure 1, A and C). Similar results were obtained using BrdU to mark proliferating cells in Tcf/Lef:H2B-GFP;ShhLacZ mice (Figure 1B). These data suggest that a subset of Wnt-responsive cells are adrenocortical Shh-producing progenitor cells; however, the proliferation of adrenocortical cells during normal maintenance of the adult gland appears to be independent of a direct effect of Wnt signaling.
Figure 1.
The Wnt-responsive population is heterogeneous in vivo. Adrenal glands from 6-week-old male Tcf/Lef:H2B-GFP;ShhLacZ mice were evaluated on paraffin sections. A, Representative coimmunofluorescence image for LacZ, GFP, PCNA, and nuclear counterstain DAPI. The inset on the right is an overlay without DAPI. B, Coimmunofluorescence images for BrdU, GFP, and LacZ staining. Mice were harvested 2 hours after a single BrdU injection. The inset at the bottom is an overlay without DAPI. C, Quantification of data from A from 3 sections each of n = 7 animals; error bars represent SEM. Diagram on the right represents the relative size and overlap of each population graphically. D, Coimmunofluorescence images of Cyp11b2 and LacZ. E, Coimmunofluorescence images of Cyp11b1 and LacZ. F, Coimmunofluorescence images of Cyp11b2 and GFP. G, Coimmunofluorescence images of Cyp11b1 and GFP. H, Quantification of data from D and F from 3 sections each of n = 4 animals; error bars represent SEM. Diagram on the right represents the relative size and overlap of each population graphically. Scale bars, 100 μm.
In addition to progenitor cells, the peripheral subcapsular region contains differentiated steroidogenic cells of the zG that express Cyp11b2, the terminal enzyme in aldosterone production. Although previous reports have indicated that Shh-producing cells are relatively undifferentiated and infrequently colocalize with Cyp11b2+ cells (1), we sought to determine any potential overlap between these populations using immunofluorescence. We find that approximately 6% to 7% of LacZ+ cells colocalize with Cyp11b2+ cells (Figure 1, D and H) and do not colocalize with Cyp11b1+ cells (Figure 1E), indicating that a relatively small proportion of Shh-producing cells are differentiated zG cells. Because a substantial proportion of GFP+ (Wnt-responsive) cells do not costain with LacZ+ cells, we asked whether Wnt-responsive cells also colocalize with Cyp11b2+ cells. We found that ∼80% of Cyp11b2+ cells colocalized with ∼20% of GFP+ cells (Figure 1, F and H). In contrast, differentiated Cyp11b1+ cells of the zF were not GFP+ cells (Figure 1G). Collectively, these data indicate that adrenocortical Wnt-responsive cells constitute a heterogeneous population, containing Shh+ progenitor cells and differentiated Cyp11b2+ cells of the zG.
Adrenocortical cell model of βcat signaling
The mutually exclusive expression of GFP (Wnt-responsive cell) and Cyp11b1 (zF cell) suggest that Wnt signaling may serve to prevent or repress the differentiation of progenitor cells into Cyp11b1+-expressing cells of the zF. We therefore initiated an in vitro investigation of the effects of Wnt/βcat signaling on steroidogenesis in a Cyp11b1-expressing zF cell line. The Wnt gain-of-function mutations present in most adrenocortical cell lines (ie, H295A/R and HAC15) necessitate genetic or pharmacologic loss-of-function experiments that are fraught with an inability to achieve adequate suppression of Wnt signaling, making interpretation of results extremely difficult. Moreover, the reduced cell viability induced by even the partial reduction of βcat expression in the H295R cell line is consistent with oncogene addiction and hampers data interpretation (15). We therefore opted to use the ATCL7 adrenocortical cell line derived from adrenocortical tumors of mice that harbor SV40 large T antigen driven by an Sf1-responsive promoter (16). Importantly, these cells do not have any gain-of-function mutations in βcat, and hence the Wnt signaling pathway is not basally active but can be stimulated pharmacologically, reflecting more accurately the physiological inducibility of this signaling pathway (Figure 2A). Treatment of ATCL7 cells with the GSK3β inhibitor BIO induces βcat stabilization and nuclear translocation (Figure 2, A and B) that results in transcriptional activation of the canonical Wnt-responsive gene Axin2 (Figure 2C), indicating that ATCL7 cells serve as an excellent model of adrenocortical Wnt/βcat-signaling in vitro.
Figure 2.
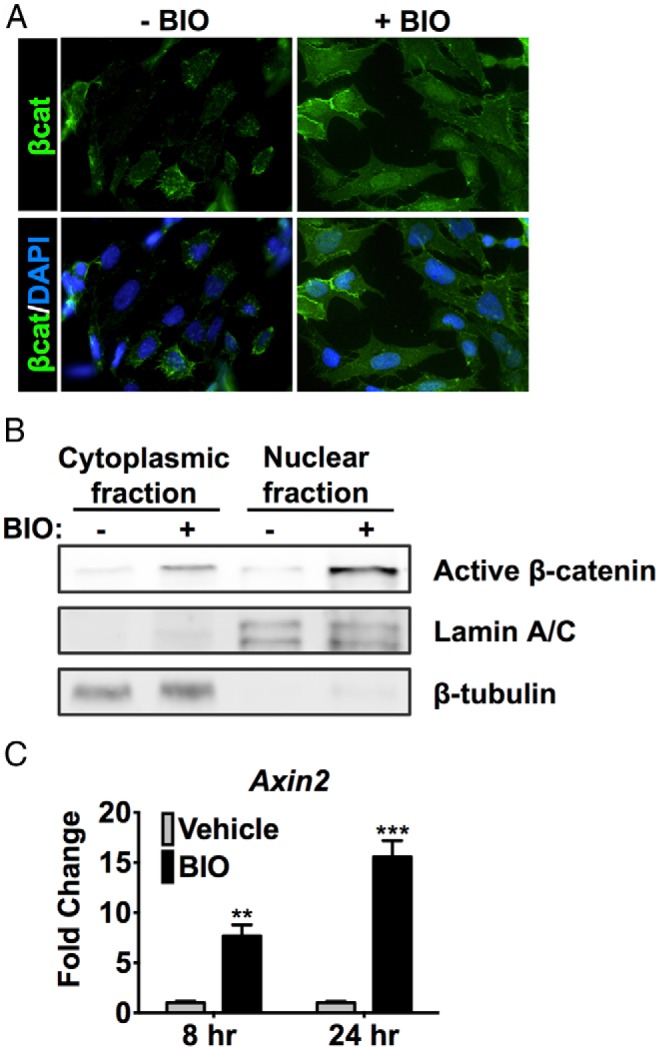
Adrenocortical cell model of βcat activity. ATCL7 cells were treated with 0.5μM BIO or vehicle (DMSO) in low-serum media for 24 hours. A, Subcellular localization of βcat was assessed using immunocytochemistry with βcat antibodies. B, ATCL7 cells were separated into nuclear and cytoplasmic fractions. Fractionation of βcat was determined by SDS-PAGE and immunoblotting. Nuclear lamin A/C and cytoplasmic β-tubulin served as loading and fractionation controls. C, qRT-PCR assessing Axin2 expression 8 and 24 hours after treatment. Values represent the mean ± SD, where vehicle-treated cells were normalized to 1 (n = 4). **, P < .005; ***, P < .0005.
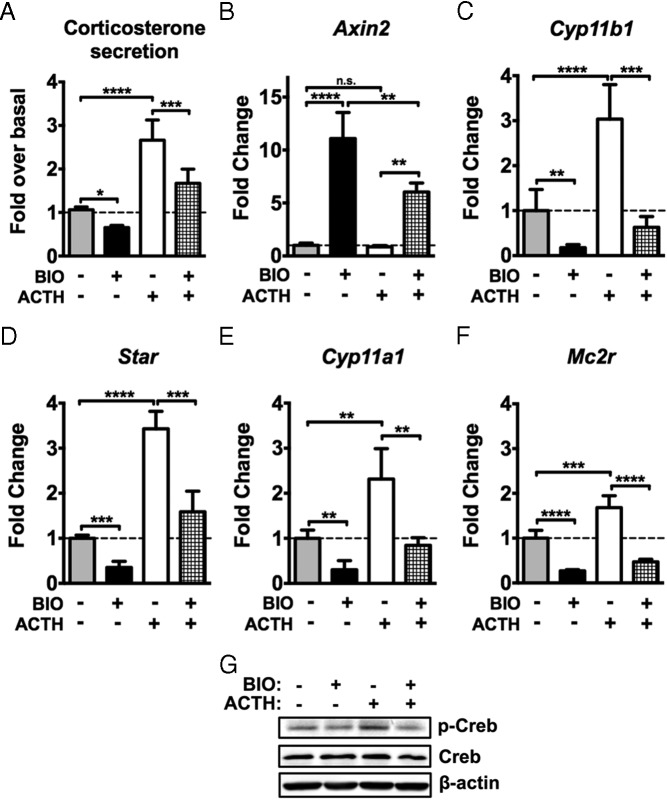
Induction of βcat activity suppresses zF steroidogenesis in vitro
Given that Wnt-responsive cells in the adrenal cortex constitute both progenitor cells and differentiated cells of the mineralocorticoid-producing zG that express Cyp11b2 (and specifically not the differentiated cells of the glucocorticoid-producing zF that express Cyp11b1), we examined whether induction of βcat activity represses the steroidogenic function of zF-like ATCL7 adrenocortical cells. ATCL7 cells do not express Cyp11b2 and therefore do not produce aldosterone. However, they do express the full complement of enzymes involved in production of corticosterone, consistent with a zF cell phenotype. Upon induction of βcat activity with BIO treatment, basal and ACTH-induced corticosterone release was significantly reduced (Figure 3A). We therefore assessed whether reduction of corticosterone secretion was due to altered expression of steroidogenic enzymes. Treatment of ATCL7 cells with BIO resulted in downregulation of the basal and ACTH-induced expression of the steroidogenic genes Cyp11a1, Star, and most importantly, Cyp11b1 as measured by quantitative RT-PCR (qRT-PCR) (Figure 3, C–E). Additional reduction in ACTH receptor (Mc2r) gene expression and concomitant downstream signaling through the protein kinase A pathway was also reduced after BIO treatment, as reflected in reduced levels of pCreb under basal and ACTH-stimulated conditions (Figure 3, F and G).
Figure 3.
Stimulation of βcat activity inhibits basal and ACTH-induced zF steroidogenesis in vitro. ATCL7 cells were pretreated with 0.5μM BIO or vehicle for 12 hours in low-serum media followed by 100nM ACTH stimulation for 6 hours and harvested. A, Harvested media were subjected to ELISA to measure corticosterone release (n = 4). Experimental values were calculated as nanograms corticosterone per milligram protein and normalized to double vehicle-treated cells. B–F, qRT-PCR assessment of changes in expression of Axin2 (B), Cyp11b1 (C), Star (D), Cyp11a1 (E), and Mc2r (F). Values represent the mean ± SD, where vehicle-treated cells were normalized to 1 (n = 4). ****, P < .00005; ***, P < .0005; **, P < .005; *, P < .05; n.s., not significant. G, Protein level changes were assessed by immunoblotting for pCreb (Ser133), total Creb, and β-actin (n = 3, representative data shown).
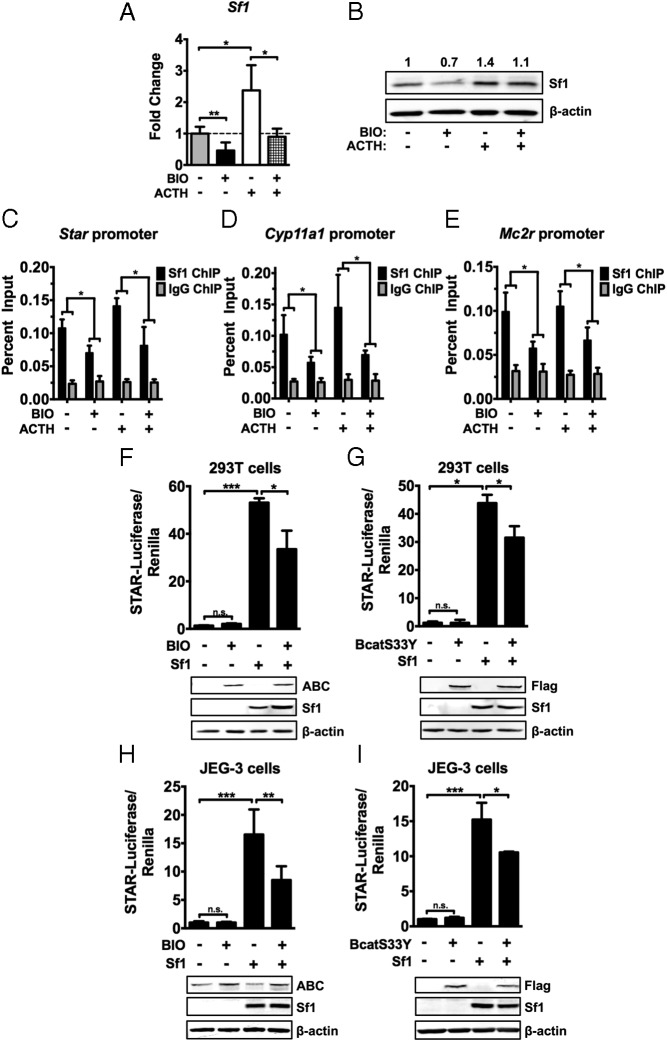
BIO-induced reduction of steroidogenesis is due to effects on Sf1
The above data suggest that the effects of Wnt activation on steroidogenesis may be mediated by primary effects on Mc2r expression and subsequent signaling effects on steroidogenic enzyme expression and corticosterone secretion. However, because Mc2r and most steroidogenic enzymes are direct Sf1 target genes, we investigated whether the suppression of steroidogenesis by BIO could be in part mediated by direct effects on Sf1 expression or Sf1 transcriptional activity. Stimulation of βcat in ATCL7 cells with BIO resulted in a marked decrease in Sf1 expression as measured by qRT-PCR and immunoblotting (Figure 4, A and B). We performed ChIP experiments with antibodies directed against Sf1 to examine whether reduction in Sf1 expression results in less Sf1 occupancy at target promoters. Sf1 was markedly reduced at the promoters of Star, Cyp11a1, and Mc2r under basal and ACTH-stimulated conditions (Figure 4, C–E). We next assessed whether BIO treatment decreased Sf1 transcriptional activity independent of changes in Sf1 levels. Stimulation of the heterologous 293T cell line with BIO resulted in a reduction of Star-luciferase activity in the presence of equivalent amounts of Sf1 (Figure 4F). These results were recapitulated using βcatS33Y, a mutant βcat that is constitutively active (17) (Figure 4G), indicative of a βcat-dependent inhibitory effect of BIO on Sf1-mediated transcription. Similar results were obtained in JEG-3 cells. Stimulation of βcat activity with BIO or transfection of βcatS33Y reduced the Star-luciferase activity induced by Sf1 (Figure 4, H and I). These results demonstrate that, in addition to modulating Sf1 levels, induction of βcat activity interferes with the ability of Sf1 to transactivate steroidogenic promoters.
Figure 4.
βcat activity affects Sf1 levels, promoter occupancy, and transcriptional activity. ATCL7 cells were pretreated with 0.5μM BIO or vehicle for 12 hours in low-serum media followed by 100nM ACTH stimulation for 6 hours and harvested. A, Changes in Sf1 mRNA levels were assessed by qRT-PCR. Values represent the mean ± SD (n = 4), where vehicle-treated cells were normalized to 1. B, Sf1 protein levels were assessed by immunoblot (n = 4, representative data shown). Quantification of changes in Sf1 protein level is normalized to β-actin levels. C–E, Changes in Sf1 DNA occupancy was measured by ChIP assays using Sf1 antisera. Immunoprecipitates were analyzed by qPCR using primers for the proximal promoters of Star (C), Cyp11a1 (D), and Mc2r (E). Preimmune IgG antisera were used as a negative control. Experimental values are normalized to 2% input (n = 4). *, P < .05. F, 293T cells were transfected with Star-luciferase reporter construct, Renilla-luciferase internal control construct, and empty vector or Sf1 expression construct for 24 hours. Transfected cells were treated with 2μM BIO or vehicle for 24 hours before passive lysis. Luciferase activity was measured and normalized to Renilla luciferase for transfection efficiency. Values represent the mean ± SD (n = 4). Protein levels of active βcat (ABC), Sf1, and β-actin were measured by immunoblotting (lower panels, n = 4, representative data shown). G, 293T cells were transfected with Star-luciferase reporter construct, Renilla-luciferase internal control construct, and empty vector, Sf1, or flag-tagged βcatS33Y alone or in combination for 48 hours before passive lysis. Luciferase activity was measured and normalized to Renilla luciferase for transfection efficiency. Values represent the mean ± SD (n = 4). Protein levels of Flag-βcatS33Y, Sf1, and β-actin were measured by immunoblotting (lower panels, n = 4, representative data shown). H, JEG-3 cells were transfected as in F and stimulated with 1μM BIO or vehicle for 24 hours before passive lysis. I, JEG-3 cells were transfected as in G (n = 4). ***, P < .0005; **, P < .005; *, P < .05.
Gene expression profiling of Wnt-responsive cells in vitro and in vivo reveals novel genes regulated by Wnt/βcat signaling in adrenocortical cells
It remains possible that βcat additionally activates (as opposed to inhibits) genes that influence steroidogenesis through Sf1-independent mechanisms. Therefore, to determine genes induced by Wnt/βcat signaling in vitro, 4 paired samples of BIO or vehicle-treated ATCL7 cells were subjected to gene expression profiling with Affymetrix mouse gene ST 1.1 strip arrays. We sought to identify genes induced by βcat stimulation in vitro that were also expressed in Wnt-responsive adrenocortical cells in vivo. To determine the panel of genes more highly expressed in the Wnt-responsive population vs genes expressed throughout the adrenal cortex, we employed 2 different lines of transgenic reporter mice: Tcf/Lef:H2B-GFP mice express GFP in Wnt-responsive cells, whereas Sf1:eGFP mice express GFP in all adrenocortical cells. GFP+ adrenocortical cells were obtained from 6-week-old male Tcf/Lef:H2B-GFP mice and Sf1:eGFP mice independently via FACS. RNA extracted from 4 independent sorts of 10 adrenals per genotype was assessed using Affymetrix mouse gene ST 1.1 strip arrays. Genes highly expressed in the Tcf/Lef:H2B-GFP population compared with the Sf1:eGFP population were intersected with genes upregulated in ATCL7 cells by BIO treatment vs vehicle treatment (Figure 5). Although known Wnt target genes present in other organ systems were captured at the intersection, such as Tnfrsf19 and Nkd1 (18, 19), we focused our efforts on Ccdc80, the gene with the largest fold increase upon BIO treatment that was also expressed in Wnt-responsive cells in vivo.
Figure 5.
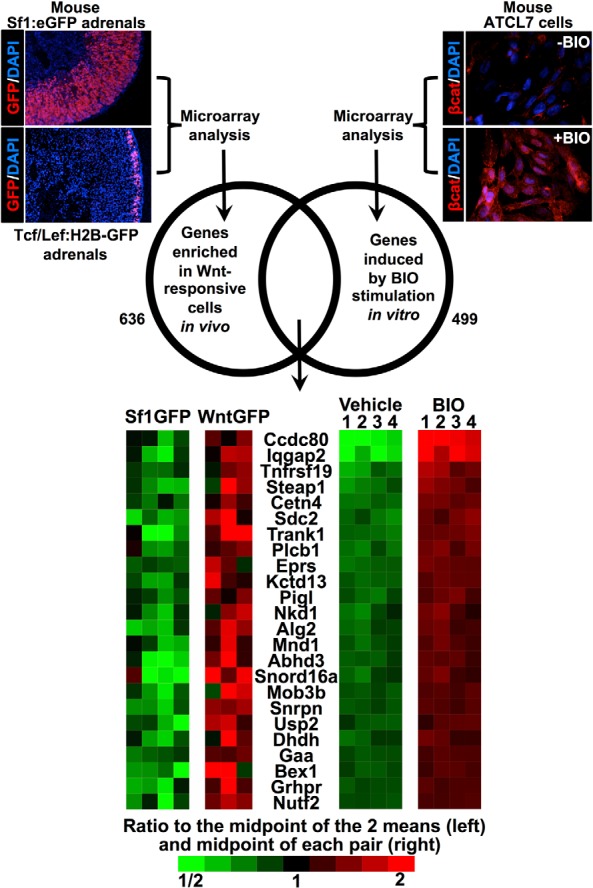
Assessment of the gene expression profile of Wnt-responsive cells in vitro and in vivo. Schematic representation of microarray workflow. Top left, GFP+ adrenocortical cells were obtained from 6-week-old male TCF/Lef:H2B-GFP mice and Sf1:eGFP mice independently via FACS. RNA was extracted from 4 independent sorts per genotype. Using 2-sample t tests, we asked that probe sets give P < .05 and average fold changes of at least 1.5, which selected 636 increased and 907 decreased probe sets in Tcf/Lef:H2B-GFP+ cells, 15% of which were expected to be false-positives based on analysis of datasets where the sample labels were randomly permuted. Top right, ATCL7 cells were treated for 24 hours with 0.5μM BIO or vehicle in low-serum media before harvesting and RNA extraction. Four pairs of treated cells were used for expression profiling. We asked that paired t tests give P < .05 and average fold changes or at least 1.5, which selected 499 upregulated and 746 downregulated probe sets in BIO-treated cells, of which we expect 0.1% to be false-positives based on analysis of datasets where sample labels were permuted within the pairs of samples. The intersection of upregulated probe sets in the 2 experiments was 25 probe sets, representing the 24 distinct genes shown in the heat map on the bottom.
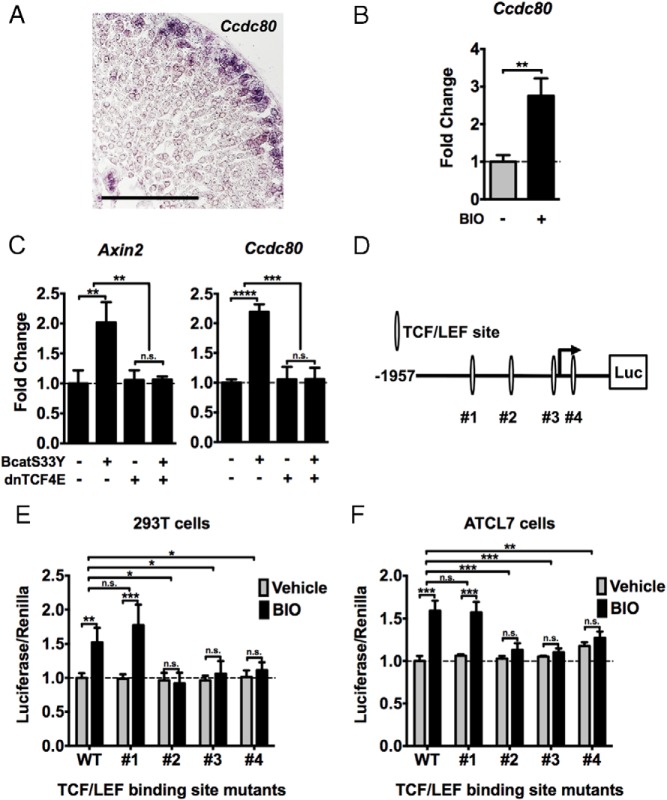
Ccdc80 is a βcat-regulated gene in adrenocortical cells
Given that Ccdc80 was enriched in both datasets, we hypothesized that Ccdc80 was a bona fide direct target gene of Wnt/βcat signaling expressed in the periphery of the adrenal cortex. In situ hybridization on adult mouse adrenal sections revealed that Ccdc80 expression is restricted to the subcapsular region of the gland (Figure 6A), where Wnt-responsive cells reside. Treatment of ATCL7 cells with BIO induced Ccdc80 expression, consistent with our gene expression profiling (Figure 6B). To determine whether the induction of Ccdc80 was dependent on a transcriptionally competent βcat/TCF complex, we electroporated ATCL7 cells with βcatS33Y alone or in combination with a dominant-negative TCF (dnTCF4E). Because transfection of ATCL7 cells is very inefficient, we compared changes in Ccdc80 expression with induction of Axin2. Forced expression of βcatS33Y in ATCL7 cells increased Ccdc80 expression, commensurate with an increase in Axin2 levels (Figure 6C). This increase was abrogated in the presence of dnTCF4E, suggesting regulation of Ccdc80 expression by a βcat/TCF complex. Sequence analysis of the promoter region of Ccdc80 with MatInspector (20) revealed 4 potential TCF/LEF binding sites within a 2-kb region encompassing the transcription start site (Figure 6D). We constructed a series of luciferase reporters containing this region of the Ccdc80 promoter with each TCF site individually mutated. These constructs were transfected into 293T cells (Figure 6E) or electroporated into ATCL7 cells (Figure 6F). As with endogenous Ccdc80 expression, treatment of 293T and ATCL7 cells with BIO induced activity of Ccdc80-luciferase (Figure 6, E and F). Although mutation of putative TCF site 1 did not suppress the induction of Ccdc80-luciferase activity, mutation of each individual TCF site (2, 3, or 4) abolished BIO-induced luciferase activity of these constructs (Figure 6, E and F). These data indicate that Ccdc80 is a direct βcat/TCF-regulated gene and that 3 putative TCF sites are each essential for maximal Wnt-mediated transcriptional activation of Ccdc80.
Figure 6.
Ccdc80 is a novel βcat-regulated gene in adrenocortical cells. A, Section in situ hybridization for Ccdc80 expression in the adult mouse adrenal. B, ATCL7 cells were treated with 0.5μM BIO or vehicle for 18 hours in low-serum media and harvested. Changes in Ccdc80 expression were assessed by qRT-PCR. Values represent the mean ± SD, where vehicle-treated cells were normalized to 1 (n = 4). **, P < .005. C, ATCL7 cells were electroporated with empty vector, βcatS33Y, or dnTCF4E alone or in combination. At 48 hours after transfection, cells were harvested and changes in Axin2 expression (left) and Ccdc80 expression (right) were measured by qRT-PCR. Values represent the mean ± SD (n = 4), where vehicle-treated cells were normalized to 1. D, Diagram of Ccdc80-luciferase reporter construct; −1957 to +814 upstream of the transcription start site of Ccdc80 was cloned into pGL3b luciferase reporter plasmid. Four putative Tcf/Lef binding sites identified using Genomatix MatInspector are labeled #1 to #4. E, 293T cells were transfected with wild-type Ccdc80-luciferase construct or constructs with each individual Tcf binding site mutated (#1–4) and Renilla luciferase internal control construct for 24 hours. Cells were treated with 2μM BIO or vehicle for 24 hours before passive lysis. Luciferase activity was measured and normalized to Renilla luciferase for transfection efficiency. Values represent the mean ± SD (n = 4), where wild-type vehicle-treated cells were normalized to 1. F, ATCL7 cells were electroporated with wild-type Ccdc80-luciferase construct or constructs with each individual Tcf binding site mutated (#1–4) and Renilla luciferase internal control construct for 24 hours. Cells were treated with 0.5μM BIO or vehicle for 24 hours before passive lysis. Luciferase activity was measured and normalized to Renilla luciferase for transfection efficiency. Values represent the mean ± SD, where wild-type vehicle-treated cells were normalized to 1 (n = 4). ***, P < .0005; **, P < .005; *, P < .05; n.s., not significant.
Ccdc80 suppresses steroidogenesis in vitro
We identified a novel βcat-regulated gene in adrenocortical cells, Ccdc80. Although little is known about the function of Ccdc80, it has been recently shown to be a secreted protein implicated in preadipocyte differentiation and extracellular matrix biology, including the potentiation of growth factor/growth factor receptor interaction (21, 22). Because βcat signaling activates Ccdc80 expression and suppresses Sf1-mediated zF cell steroidogenesis, we sought to determine whether Ccdc80 also contributed to Wnt-mediated steroidogenic inhibition in adrenocortical cells. We first generated Ccdc80-containing conditioned media to confirm its identity as a secreted protein and to allow for an evaluation of its role as an extracellular regulator of adrenocortical steroidogenesis. Immunoblot analysis revealed that Ccdc80-myc expressed in 293T cells is present in both cell lysates and collected media. Ccdc80-myc migrates on SDS-PAGE at ∼110 kDa, with multiple higher molecular weight bands likely due to its glycosylation (Figure 7A). Treatment of ATCL7 cells with Ccdc80-containing CM resulted in a reduction in basal and ACTH-induced corticosterone secretion in comparison with treatment of cells with CM from 293T cells transfected with an empty vector (Figure 7B). As in our previous experiments with βcat stimulation, treatment with Ccdc80-CM resulted in a significant decrease in expression of Star, Cyp11a1, and Cyp11b1 (Figure 7, C–E). Given that treatment of ATCL7 cells with Ccdc80-CM partially phenocopies the induction of βcat in these cells, we assessed βcat levels and transcriptional activity. Treatment with Ccdc80-CM does not induce βcat stability or Axin2 expression (Figure 7, H and I). These data indicate that extracellular Ccdc80 does not induce Wnt signaling in a paracrine or autocrine fashion, and therefore the suppression of steroidogenesis by Ccdc80 does not appear to be due to feed-forward stimulation of the Wnt pathway. Additionally, in contrast to what we observed upon stimulation of βcat activity, treatment of ATCL7 cells with Ccdc80-CM did not affect expression of Mc2r or Sf1 and alter the levels of pCreb (Figure 7, F–I). Together these data indicate that Ccdc80 is likely suppressing steroidogenesis through mechanisms distinct from signaling events initiated through active βcat.
Figure 7.
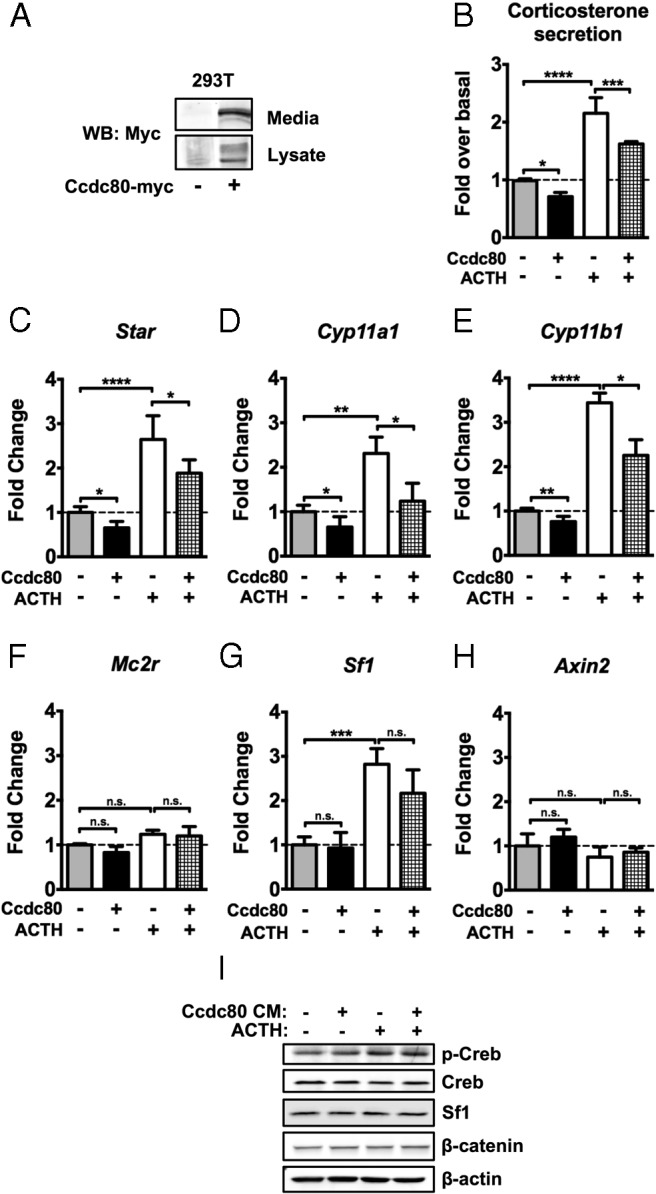
Ccdc80 suppresses basal and ACTH-induced zF steroidogenesis in vitro. A, 293T cells were transfected with Ccdc80-myc or empty vector for 24 hours and harvested. Media were collected at the time of harvest. Concentrated CM and cell lysates were subjected to immunoblotting for Myc. B, ATCL7 cells were treated with Mock or Ccdc80-CM derived as in A for 18 hours followed by 100nM ACTH stimulation for 6 hours and harvested. Media collected at harvest were subjected to ELISA to measure corticosterone release (n = 4). Experimental values were calculated as nanograms corticosterone per milligram protein and normalized to untreated cells. C–H, qRT-PCR assessment of changes in expression of Star (C), Cyp11a1 (D), Cyp11b1 (E), Mc2r (F), Sf1 (G), and Axin2 (H) (n = 4). ****, P < .00005; ***, P < .0005; **, P < .005; *, P < .05; n.s., not significant. I, Protein level changes were measured by immunoblotting for pCreb (Ser133), total Creb, Sf1, βcat, and β-actin (n = 3, representative data shown).
Discussion
The current study aimed to characterize the cells in the mouse adrenal cortex that participate in Wnt-mediated organ homeostasis. In this report, we demonstrate that the Wnt-responsive cells in the peripheral cortex of the adrenal gland are indeed a heterogeneous population containing both relatively undifferentiated Shh-producing progenitor cells and differentiated mineralocorticoid-producing cells of the zG. We also find that a small proportion of Shh-secreting cells are differentiated cells of the zG. It remains possible that a fraction of differentiated Cyp11b2+, LacZ+ and/or Cyp11b2+, GFP+ cells are no longer Shh-producing or Wnt-responsive, yet appear so due to persistence of the reporter proteins in these transgenic mouse models. However, a recent report implicating Wnt signaling in zG biology (7) provides further support to our observation that a portion of Wnt-responsive cells are Cyp11b2-expressing cells of the zG. Nonetheless, we were surprised at the observation that the Wnt-responsive cells constitute only a small fraction of the proliferating adrenocortical cells in the adult mouse under normal conditions. These results were unanticipated given the increased adrenocortical cell proliferation observed in the adrenals of mice with genetic gain of Wnt pathway function vs the decreased proliferation present in mice with loss of Wnt pathway activation (3, 23–25). It has remained unclear whether the Wnt-responsive cells or perhaps their descendants are the bona fide proliferating cells in these mutant adrenal cortices. It is plausible that during simple homeostatic organ maintenance, Wnt signaling contributes indirectly to adrenocortical proliferation through upregulation of paracrine factors that promote cell proliferation in a non–cell-autonomous fashion and/or that during organ regrowth after injury repair (as opposed to organ maintenance), Wnt-responsive cells contribute more directly to the proliferating pool of adult adrenocortical cells.
Previous studies have highlighted the role of both βcat and the βcat target genes Dax1 and inhibin-α (Inhα) in the maintenance of the undifferentiated state of adrenocortical progenitors (Dax1) and the fidelity of an adrenal vs a gonadal fate of these progenitors (Inhα) (3, 6, 26). Additionally, adrenals from mice with adrenocortical-specific knockout of APC, a component of the βcat destruction complex, exhibit cell accumulation at the cortical-medullary boundary that express Sf1, stabilized βcat and Dax1, but not markers of differentiated zG or zF cells (23). Collectively, these findings demonstrate that βcat maintains the undifferentiated state of adrenocortical progenitors through suppression of steroidogenesis and restriction of differentiation to an adrenal fate. However, the interpretation of these data is challenging in light of a recent study supporting a stimulatory role of adrenocortical Wnt signaling in zG differentiation through indirect regulation of aldosterone synthesis (7). DeltaCat mice engineered to express a genetically modified constitutively active βcat partially phenocopy mice with adrenocortical-specific knockout of APC, with aberrant cell accumulation at the cortical-medullary boundary. These cells express Sf1 and stabilized βcat; however, a subset displays ectopic expression of Cyp11b2, resulting in overt hyperaldosteronism (24). Supporting such a role of Wnt signaling in aldosterone production in the zG is the loss of secreted Wnt inhibitors recently observed in aldosterone-producing adenomas (7). In our current study, we now report that stimulation of βcat reduces steroidogenic enzyme expression and corticosterone secretion in zF-like cells. Our molecular data suggest that βcat signaling may actively prevent or repress zF cell differentiation of progenitor cells. This is supported by our observation that Wnt-responsive cells do not overlap with Cyp11b1+ cells in vivo. Additionally, deltaCat adrenals that have stabilized βcat throughout the adrenal cortex have fewer zF cells and lower Cyp11b1 expression compared with wild-type animals (24). The combined data support an emerging model whereby Wnt signaling in the adrenal cortex serves to inhibit fasciculata-specific steroidogenesis (differentiation) and facilitate glomerulosa-specific steroidogenesis.
An interesting aspect of our study is that the repressive effect of βcat signaling on fasciculata steroidogenesis is partially mediated through effects on Sf1 expression and activity. Here we find that stimulation of βcat activity results in downregulation of Sf1 expression and decreased occupancy of Sf1 from steroidogenic promoters. Although transcriptional regulation of Sf1 expression in the adult adrenal is not well-characterized, it is plausible that βcat can directly repress the Nr5a1 gene through conserved Tcf/Lef binding sites upstream of its transcription start site (Zubair M and Hammer GD, unpublished observation), or that βcat can activate genes that negatively regulate Sf1 expression. Nonetheless, independent of changes in Sf1 expression, we find that in heterologous cell lines, βcat reduces the ability of Sf1 to transactivate steroidogenic promoters. Additionally, in a fasciculata cell line, stimulation of βcat decreases expression of a number of steroidogenic enzymes including Cyp11b1 that is responsible for glucocorticoid production and defines the differentiated state of these cells. Because Sf1 has been shown to actually repress the expression of Cyp11b2 that is responsible for mineralocorticoid production (27), it is plausible that the observed Wnt-mediated facilitation of zG fate (7, 24) involves in part the release of Sf1-mediated repression of Cyp11b2. Similarly, in the developing gonad, the roles of Wnt signaling as an activator vs repressor of Sf1 activity are temporally and spatially distinct. In the ovary, βcat blocks Sf1-mediated testis differentiation by removing Sf1 from the Tesco enhancer of Sox9 (28). Given that Sf1 and βcat physically interact and synergistically coactivate a subset of genes involved in progenitor cell biology (Dax1 and Inhα) (4, 29–31), a model emerges whereby Wnt pathway activation potentially titrates Sf1 away from the zF promoters of steroidogenic genes to coregulate genes involved in progenitor cell biology with subsequent activation of zG differentiation.
In this report, we also unveil a unique Sf1-independent mechanism of Wnt-mediated suppression of zF steroidogenesis. In search of Wnt-activated targeted genes, Ccdc80 emerged as a novel βcat-regulated gene that encodes a secreted protein in the peripheral adrenal cortex. Ccdc80 was originally cloned as Urb, a gene upregulated in brown adipose tissue in bombesin-receptor-3 knockout mice (32) and subsequently shown to be downregulated in adipose tissue in obese mouse models vs wild-type mice (33). The exact role for Ccdc80 in adipose differentiation is unclear because both overexpression and knockdown of Ccdc80 resulted in suppression of preadipocyte differentiation (21). Several studies on the chicken ortholog of Ccdc80, equarin, have revealed that it is critical for lens differentiation through two functional outputs of heparan sulfate proteoglycan binding: promoting cell adhesion and facilitating fibroblast growth factor (FGF) signaling (22, 34). Little is known about the role of the extracellular matrix and cell adhesion in steroidogenesis or the adrenal progenitor niche. A role for FGF signaling in the adrenal cortex has only recently been appreciated. Guasti et al (35) found expression of FGF ligands and receptors in both the adrenal capsule and the adrenal cortex. Global Fgfr2IIIb-knockout adrenals exhibit adrenal hypoplasia by embryonic day 15.5 in part due to reduced proliferation in both the adrenal capsule and cortex, reflecting a role for FGF signaling in adrenal development. Any potential roles of the extracellular matrix and signaling pathways in Ccdc80 regulation of adrenal homeostatic maintenance will be an important area of future research, as will potential roles of Ccdc80 on zG function.
In summary, we propose a model in which the fate of adrenocortical progenitor cells (maintenance vs differentiation) is regulated by temporal and spatial integration of paracrine and endocrine stimuli (Figure 8). Our data, in combination with other studies, suggest that Wnt signaling is primarily maintaining the undifferentiated state of adrenal progenitors, yet priming them to become zG cells upon endocrine differentiation signals (angiotensin II/ACTH). In this model, paracrine signals (Wnt) would keep progenitor cells undifferentiated (through expression of Dax1) (6) yet primed to differentiate directly to a zG cell (through expression of the angiotensin receptor [At1r] and Nr4a1/2 genes) (7), whereas zF differentiation is actively repressed (by effects on Sf1 and through Ccdc80, as shown in this study). In times of greater physiological need for increased steroid production, endocrine signals could override paracrine inputs and promote differentiation and steroidogenesis. Evidence for such phenomena are supported in this study, where ACTH stimulation reduces the induction of βcat signaling, reflected in a decrease of Axin2 levels induced by BIO (Figure 3) and in other studies, where angiotensin II stimulation of H295R cells decreases the expression of βcat target genes Axin2 and Lef1 (7). How paracrine and endocrine signals are integrated or override one another to dictate the fate of adrenal progenitors remains unclear but likely involves thresholds and temporal regulation. Freedman et al (2) demonstrated that, under normal conditions, all fasciculata cells are derived from glomerulosa cells. Stem and progenitor cells in multiple organ systems engage differently depending on pathological perturbation or injury repair vs normal homeostatic maintenance (36, 37). Indeed, ACTH clears transcriptional activators from the Dax1 promoter, thereby shutting off this gene (38). It remains possible that under great physiological need to replenish zF cells, such as regrowth after dexamethasone-induced atrophy of the zF, a portion of progenitor cells could differentiate directly into zF cells without a zG intermediate. Future studies of dynamic models of injury repair will be necessary to further define the diverse cellular populations engaged in the complex process of adrenal homeostasis.
Figure 8.
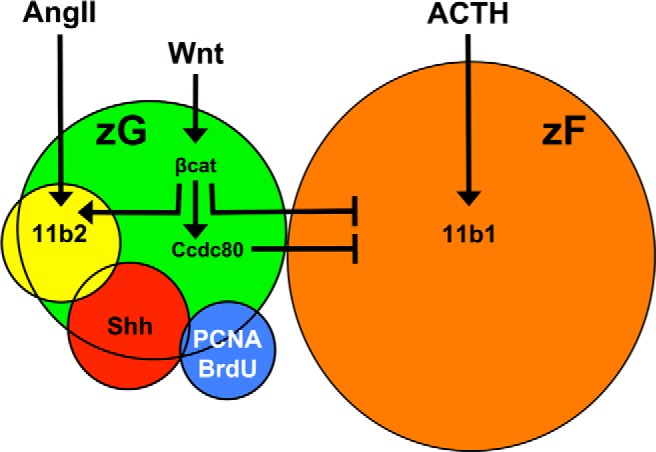
Integration of Wnt and endocrine signals specify zG vs zF gene expression. The Wnt-responsive population (green) is heterogeneous, consisting of differentiated Cyp11b2-expressing cells (yellow), Shh-producing progenitor cells (red), and few actively proliferating cells (blue) but no differentiated Cyp11b1-expressing cells of the zF (orange). Angiotensin II (AngII) and ACTH promote steroidogenesis in the zG and zF, respectively, defining the differentiated state of the two adrenocortical cell types. Wnt signals stimulate βcat, which inhibits the transcription of zF-associated steroidogenic genes and transcriptionally activates Ccdc80, a secreted protein that in turn also inhibits zF-associated steroidogenic gene expression. The rules that govern the balance the paracrine (Wnt/Ccdc80) and endocrine (ACTH/AngII) signals in this model are predicted to involve temporal and spatial cues. Coupled with the known role of βcat in the regulation of pro-zG genes, these data collectively support a role of Wnt signaling in facilitating the unidirectional centripetal differentiation of adrenocortical progenitor cells. Specifically, Wnt-mediated inhibition of zF-associated steroidogenesis, maintenance of the progenitor cell pool, and priming of zG cell fate assures the differentiation of adrenocortical progenitor cells into Cyp11b2-expressing zG cells before becoming Cyp11b1-expressing zF cells as supported by recent work. See Discussion for details.
Acknowledgments
We thank Dr C. Carter-Su (University of Michigan, Ann Arbor, MI) for kindly providing the Ccdc80-myc construct and Dr C. Gomez-Sanchez (University of Mississippi Medical Center, Jackson, MS) for kindly providing the Cyp11b1 and Cyp11b2 antibodies. We also thank Dr C. Walczak (University of Michigan, Ann Arbor, MI) for critical reading of the manuscript, reagents, and technical assistance in reporter construct mutagenesis.
This work was supported by National Institutes of Health (NIH) research grants (DK062027 and CA134606 to G.D.H.), NIH Training Grants (Cellular and Molecular Biology T32-GM007315 and Cancer Biology 2T32CA009676-21A1 that supported E.M.W.), and the NIH-funded University of Michigan Comprehensive Cancer Center Grant P30 CA046592 (to R.K.).
Disclosure Summary: The authors have no conflict of interest to declare.
Footnotes
- APC
- adenomatous polyposis coli
- BIO
- 6-bromoindirubin-3′-oxime
- BrdU
- bromodeoxyuridine
- βcat
- β-catenin
- ChIP
- chromatin immunoprecipitation
- CKIα
- casein kinase 1α
- CM
- conditioned media
- DAPI
- 4′,6-diamidino-2-phenylindole
- Dax1
- dosage-sensitive sex reversal, adrenal hypoplasia congenita critical region on the X chromosome, gene 1
- DMSO
- dimethylsulfoxide
- FACS
- fluorescence-activated cell sorting
- FBS
- fetal bovine serum
- GFP
- green fluorescent protein
- FGF
- fibroblast growth factor
- GSK3β
- glycogen synthase kinase 3β
- pCreb
- phosphorylated Creb
- qRT-PCR
- quantitative RT-PCR
- Shh
- sonic hedgehog
- TCF/LEF
- T cell factor/lymphoid enhancer factor
- zF
- zona fasciculata
- zG
- zona glomerulosa
References
- 1. King P, Paul A, Laufer E. Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages. Proc Natl Acad Sci U S A. 2009;106(50):21185–21190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Freedman B, Kempna P, Carlone D, et al. Adrenocortical zonation results from lineage conversion of differentiated zona glomerulosa cells. Dev Cell. 2013;26(6):666–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim AC, Reuter AL, Zubair M, et al. Targeted disruption of beta-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development. 2008;135(15):2593–2602 [DOI] [PubMed] [Google Scholar]
- 4. Mizusaki H, Kawabe K, Mukai T, et al. Dax-1 (dosage-sensitive sex reversal-adrenal hypoplasia congenita critical region on the X chromosome, gene 1) gene transcription is regulated by wnt4 in the female developing gonad. Mol Endocrinol. 2003;17(4):507–519 [DOI] [PubMed] [Google Scholar]
- 5. Khalfallah O, Rouleau M, Barbry P, Bardoni B, Lalli E. Dax-1 knockdown in mouse embryonic stem cells induces loss of pluripotency and multilineage differentiation. Stem Cells. 2009;27(7):1529–1537 [DOI] [PubMed] [Google Scholar]
- 6. Scheys JO, Heaton JH, Hammer GD. Evidence of adrenal failure in aging Dax1-deficient mice. Endocrinology. 2011;152(9):3430–3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berthon A, Drelon C, Ragazzon B, et al. WNT/β-catenin signalling is activated in aldosterone producing adenomas and controls aldosterone production. Hum Mol Genet. 2013;23(4):889–905 [DOI] [PubMed] [Google Scholar]
- 8. Ferrer-Vaquer A, Piliszek A, Tian G, Aho R, Dufort D, Hadjantonakis AK. A sensitive and bright single-cell resolution live imaging reporter of Wnt/β-catenin signaling in the mouse. BMC Dev Biol. 2010;10:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lewis PM, Gritli-Linde A, Smeyne R, Kottmann A, McMahon AP. Sonic hedgehog signaling is required for expansion of granule neuron precursors and patterning of the mouse cerebellum. Dev Biol. 2004;270(2):393–410 [DOI] [PubMed] [Google Scholar]
- 10. Stallings NR. Development of a transgenic green fluorescent protein lineage marker for steroidogenic factor 1. Mol Endocrinol. 2002;16:2360–2370 [DOI] [PubMed] [Google Scholar]
- 11. Barrett T, Wilhite SE, Ledoux P, et al. NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Res. 2013;41(Database issue):D991–D995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mendelsohn C, Batourina E, Fung S, Gilbert T, Dodd J. Stromal cells mediate retinoid-dependent functions essential for renal development. Development. 1999;126(6):1139–1148 [DOI] [PubMed] [Google Scholar]
- 13. Di Giacomo G, Koss M, Capellini TD, Brendolan A, Pöpperl H, Selleri L. Spatio-temporal expression of Pbx3 during mouse organogenesis. Gene Expr Patterns. 2006;6(7):747–757 [DOI] [PubMed] [Google Scholar]
- 14. Zajicek G, Ariel I, Arber N. The streaming adrenal cortex: direct evidence of centripetal migration of adrenocytes by estimation of cell turnover rate. J Endocrinol. 1986;111(3):477–482 [DOI] [PubMed] [Google Scholar]
- 15. Gaujoux S, Hantel C, Launay P, et al. Silencing mutated β-catenin inhibits cell proliferation and stimulates apoptosis in the adrenocortical cancer cell line H295R. PLoS One. 2013;8(2):e55743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ragazzon B, Lefrançois-Martinez AM, Val P, et al. Adrenocorticotropin-dependent changes in SF-1/DAX-1 ratio influence steroidogenic genes expression in a novel model of glucocorticoid-producing adrenocortical cell lines derived from targeted tumorigenesis. Endocrinology. 2006;147(4):1805–1818 [DOI] [PubMed] [Google Scholar]
- 17. Morin PJ, Sparks AB, Korinek V, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275(5307):1787–1790 [DOI] [PubMed] [Google Scholar]
- 18. Qiu W, Hu Y, Andersen TE, et al. Tumor necrosis factor receptor superfamily member 19 (TNFRSF19) regulates differentiation fate of human mesenchymal (stromal) stem cells through canonical Wnt signaling and C/EBP. J Biol Chem. 2010;285(19):14438–14449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zeng W, Wharton KA, Jr, Mack JA, et al. naked cuticle encodes an inducible antagonist of Wnt signalling. Nature. 2000;403(6771):789–795 [DOI] [PubMed] [Google Scholar]
- 20. Cartharius K, Frech K, Grote K, et al. MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics. 2005;21(13):2933–2942 [DOI] [PubMed] [Google Scholar]
- 21. Tremblay F, Revett T, Huard C, et al. Bidirectional modulation of adipogenesis by the secreted protein Ccdc80/DRO1/URB. J Biol Chem. 2009;284(12):8136–8147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Song X, Sato Y, Felemban A, et al. Equarin is involved as an FGF signaling modulator in chick lens differentiation. Dev Biol. 2012;368(1):109–117 [DOI] [PubMed] [Google Scholar]
- 23. Heaton J, Wood M, Kim A, et al. Progression to adrenocortical tumorigenesis in mice and humans through insulin-like growth factor 2 and β-catenin. Am J Pathol. 2012;181(3):1017–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berthon A, Sahut-Barnola I, Lambert-Langlais S, et al. Constitutive beta-catenin activation induces adrenal hyperplasia and promotes adrenal cancer development. Hum Mol Genet. 2010;19(8):1561–1576 [DOI] [PubMed] [Google Scholar]
- 25. Drelon C, Berthon A, Ragazzon B, et al. Analysis of the role of igf2 in adrenal tumour development in transgenic mouse models. PLoS One. 2012;7(8):e44171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Looyenga B, Hammer GD. Origin and identity of adrenocortical tumors in inhibin knockout mice: implications for cellular plasticity in the adrenal cortex. Mol Endocrinol. 2006;20(11):2848–2863 [DOI] [PubMed] [Google Scholar]
- 27. Ye P, Nakamura Y, Lalli E, Rainey WE. Differential effects of high and low steroidogenic factor-1 expression on CYP11B2 expression and aldosterone production in adrenocortical cells. Endocrinology. 2009;150(3):1303–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bernard P, Ryan J, Sim H, et al. Wnt signaling in ovarian development inhibits Sf1 activation of Sox9 via the Tesco enhancer. Endocrinology. 2012;153(2):901–912 [DOI] [PubMed] [Google Scholar]
- 29. Gummow BM, Winnay JN, Hammer GD. Convergence of Wnt signaling and steroidogenic factor-1 (SF-1) on transcription of the rat inhibin alpha gene. J Biol Chem. 2003;278(29):26572–26579 [DOI] [PubMed] [Google Scholar]
- 30. Kennell JA, O'Leary EE, Gummow BM, Hammer GD, MacDougald OA. T-cell factor 4N (TCF-4N), a novel isoform of mouse TCF-4, synergizes with beta-catenin to coactivate C/EBPalpha and steroidogenic factor 1 transcription factors. Mol Cell Biol. 2003;23(15):5366–5375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hossain A, Saunders GF. Synergistic cooperation between the beta-catenin signaling pathway and steroidogenic factor 1 in the activation of the Mullerian inhibiting substance type II receptor. J Biol Chem. 2003;278(29):26511–26516 [DOI] [PubMed] [Google Scholar]
- 32. Aoki K, Sun YJ, Aoki S, Wada K, Wada E. Cloning, expression, and mapping of a gene that is upregulated in adipose tissue of mice deficient in bombesin receptor subtype-3. Biochem Biophys Res Commun. 2002;290(4):1282–1288 [DOI] [PubMed] [Google Scholar]
- 33. Okada T, Nishizawa H, Kurata A, et al. URB is abundantly expressed in adipose tissue and dysregulated in obesity. Biochem Biophys Res Commun. 2008;367(2):370–376 [DOI] [PubMed] [Google Scholar]
- 34. Song X, Sato Y, Sekiguchi K, Tanaka H, Ohta K. Equarin is involved in cell adhesion by means of heparan sulfate proteoglycan during lens development. Dev Dyn. 2013;242(1):23–29 [DOI] [PubMed] [Google Scholar]
- 35. Guasti L, Candy Sze W, McKay T, Grose R, King P. FGF signalling through Fgfr2 isoform IIIb regulates adrenal cortex development. Mol Cell Endocrinol. 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10(3):207–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Krausova M, Korinek V. Wnt signaling in adult intestinal stem cells and cancer. Cell Signal. 2014;26(3):570–579 [DOI] [PubMed] [Google Scholar]
- 38. Gummow BM, Scheys JO, Cancelli V, Hammer GD. Reciprocal regulation of a glucocorticoid receptor-steroidogenic factor-1 transcription complex on the Dax-1 promoter by glucocorticoids and adrenocorticotropic hormone in the adrenal cortex. Mol Endocrinol. 2006;20(11):2711–2723 [DOI] [PubMed] [Google Scholar]