Abstract
Mast cells are thought to be sensitive to mechanical forces, for example, coughing in asthma or pressure in “physical urticarias”. Conversion of mechanical forces to biochemical signals could potentially augment antigenic signaling. Studying the combined effects of mechanical and antigenic cues on mast cells and other hematopoietic cells has been elusive. Here, we present an approach using a modified atomic force microscope cantilever to deliver antigenic signals to mast cells while simultaneously applying mechanical forces. We developed a strategy to concurrently record degranulation events by fluorescence microscopy during antigenic triggering. Finally, we also measured the mechanical forces generated by mast cells while antigen receptors are ligated. We showed that mast cells respond to antigen delivered by the AFM cantilever with prompt degranulation and the generation of strong pushing and pulling forces. We did not discern any relationship between applied mechanical forces and the kinetics of degranulation. These experiments present a new method for dissecting the interactions of mechanical and biochemical cues in signaling responses of immune cells.
Keywords: Mast cell, degranulation, atomic force microscopy, mechanotransduction
INTRODUCTION
Mast cells play an important role in the innate and adaptive immune responses, secreting numerous preformed mediators and cytokines that can recruit as well as polarize other elements of the immune system. Many of these elements are contained in granules, which are released (“degranulated”) upon triggering by a variety of stimuli, most notably crosslinking of the FcεRI by ligation of pre-bound IgEs with antigens. This crosslinking sets in motion a signaling cascade that results in calcium influx and cytoskeletal rearrangements, allowing secretory granules to fuse with the plasma membrane and release their cargo.
We still do not fully understand how the cytoskeleton is altered after crosslinking of FcεRI. Previous literature has suggested there is a need for a large calcium influx through CRAC channels to stimulate the disassembly of the cortical, filamentous actin cytoskeleton [1]. It is believed that cortical actin can act as a barrier to vesicle fusion [2].
Mast cells are best known for their role in mucosal allergic inflammation, in asthma, and in allergen-induced urticaria where allergen binding to IgE crosslinks the FcεRI receptors, leading to degranulation and immunopathogenic release of histamine and other mediators of inflammation and tissue remodeling. Mast cells have also been implicated in the so-called “physical urticarias,” wherein patients display classic ‘wheal-and-flare’ responses due to physical stimuli such as temperature changes and mechanical pressure [3]. We and others hypothesize that mast cells are able to directly sense these physical stimuli, convert them into a biochemical signal, and initiate degranulation. In the context of asthma, the relevant mechanical forces could come from coughing [4].
Previous studies have shown that mast cells are mechanically sensitive, and that mechanical stimulation can induce degranulation [5, 6]. These previous works have the disadvantage of using a bulk assay of mast cell responses, thus losing information at the single cell level. In addition, snapshots taken before and after degranulation lose information on the dynamic behavior of the cells.
Atomic Force Microscopy (AFM) allows us to apply a precise and localized mechanical stimulation to a cell of interest. AFM has been used to demonstrate the mechanosensitivity of a variety of cells, including osteoblasts [7, 8]. Limitations of this kind of stimulation by AFM may be in the amount of force that can be applied to a cell by the cantilever (higher forces might require the use of stiffer cantilevers), by the shape of the cantilever tip that comes into contact with the cell (risking, for example, puncture with sharp tips, or removal from the substrate if pushed too hard). By chemically linking biomolecules to the AFM cantilever, we can also apply a chemical signal to the cell as well. AFM has been used in studying the material properties of biological materials and obtaining high-resolution topographical information of biological structures. However, many of these studies have been performed on fixed (nonliving) samples, thus overlooking dynamic responses.
We applied AFM to studying the mechanosensitivity of mast cells. By chemically attaching antigen molecules to the AFM cantilever, we offered mast cells a precise mechanical as well as biochemical cue concurrently. In this paper, we describe this novel platform and expect this approach can be used in further studies to develop mast cell inhibiting compounds by quantitatively examining the threshold of activation of mast cells.
MATERIALS AND METHODS
Cells
C57 mast cell line derived from mouse was generously provided by the Galli Lab. Cells were maintained in growth medium comprising DMEM (SigmaAldrich), 10%FBS (Life Technologies), Pen/Strep (Life Technologies), 2 mM L-Glutamine (Life Technologies), 5 μM beta-mercaptoethanol. For sensitization, 106 cells/mL were incubated with 1 μg/mL of anti-DNP-HSA IgE (IgE antibody that recognizes 2,4 dinitrophenol hapten conjugated to human serum albumin, kindly provided by Dr. P. Starkl and Prof. S. J. Galli) overnight at 37 °C.
Construction of pHluorin-LAMP1 and transduction of C57 cells
We prepared a fusion protein of LAMP1 and pHluorin to identify degranulation as the vesicles open to the extracellular space, akin to the strategy used in [9], where the synaptobrevin VAMP was tagged with pHluorin, and in [10], where LAMP1 was fused to pHluorin for studying degranulation of NK cells. Plasmids bearing the cDNA for LAMP1 and pHluorin were obtained through Open Biosystems. The signal sequence was cloned out of LAMP1 and attached to the N-terminus of pHluorin, which was in turn attached to the N-terminus of LAMP1 using Gibson Assembly (New England Biolabs). The fusion construct was simultaneously inserted into backbone of the MSCV retrovirus (AddGene). The final plasmid was confirmed using restriction digest and sequencing.
To package lentiviral particles, MSCV-pHluorin-LAMP1 was transfected into Platinum E cell packaging line (Cell Biolabs, Inc.) using Lipofectamine 2000 (Life Technologies) according to manufacturer’s instructions. After 16 hours, the media was replaced with C57 growth media. After 48 hours, the supernatant containing viruses was harvested and added to C57 cells at 106 cells/mL of supernatant along with 8 μg/mL of polybrene (SigmaAldrich) to increase transduction efficiency. Cells were then centrifuged at 800 × g for 1.5 hours at 32 °C (“spinfection”). This process was repeated three times for the same population of C57 cells to achieve workable transduction efficiencies.
Atomic Force Microscopy
AFM was conducted using Asylum Research MFP3D-Bio system combined with a Nikon Ti-E base. The cantilevers used were HYDRA6R-200N (AppNano). Cantilevers were mounted prior to touching the cell and calibrated using the thermal noise method [11] to obtain the spring constants for individual tips. In the experiments described below, the cantilever was lowered onto cells with the tip positioned approximately at the middle of the mast cell area. The cantilever tip was gently extended toward the cell until a specified force trigger was met, then allowed to dwell in place for 10 min with constant z position. Finally, the cantilever was fully retracted.
To synchronize acquisition of data from the AFM and microscope, we used custom LabView code and a PCI-6115 board (National Instruments) to acquire the analog deflection and Z piezo channels from the AFM controller, digital pulses from the AFM controller corresponding to the start and trigger point of each force curve, and digital pulses from Micro Manager via a DT9816 board (Data Translation) corresponding to frames of the camera. These data were processed and analyzed in Matlab. The analog deflection data was denoised by wavelet decomposition at level 7 with the coif5 wavelet, corresponding to a psuedofrequency of about 800 Hz. We confirmed that our functionalized cantilevers successfully ligated IgEs on the cell surface by noting in every case a strong adhesion force on the cantilever as the cantilever was withdrawn away from the cell surface, corresponding to the breakage of the non-covalent bonds between the antigen on the cantilever and receptors on the mast cell surface.
Cells were washed twice in imaging media and plated onto fibronectin-coated Fluorodishes (World Precision Instruments). The dish was then transferred to a heated stage at 37 °C and the cells allowed to settle for 10 min before touching.
Chemical conjugation of AFM cantilevers
HYDRA6R-200N tips were plasma cleaned and underwent vapor deposition of (3-Aminopropyl) triethoxysilane (APTES, Tokyo Chemical Industry Company) for 30 min. Tips were placed in a chamber with 100 μL of APTES on a hot plate at 100 °C for vapor deposition. Following deposition, tips were cured for 10 min at 110 °C under vacuum.
Silanized tips were then bathed in a solution of sulfo-LC-SPDP (Thermo Scientific) at 200 μM in PBS for 30m at 25 °C. After washing in PBS, tips were then bathed in a 10 μg/mL solution of DNP-HSA in PBS overnight at 25 °C. The tips were gently immersed in PBS prior to use.
Fluorescence Imaging
All fluorescence images presented were collected using epi-fluorescence excitation from a halogen lamp light source (Sutter). The Chroma 49002 - ET - EGFP (FITC/Cy2) filter cube was used for excitation and emission of pHluorin and Fluo-4. Images were collected using an intensified CCD camera (XR/MEGA-10, Stanford Photonics). Images were processed using custom code written with MATLAB (MathWorks, Inc.) object recognition software.
For calcium flux experiments, C57 cells were loaded with Fluo-4 (Life Technologies) at 0.25 μM for 20 min at 37 °C. Cells were then washed twice and plated onto a Delta-T dish (Bioptechs), then allowed to settle and equilibrate at 37 °C on the stage. To stimulate with antigen, a solution of DNP-HSA in imaging media was equilibrated at 37 °C and was introduced manually by pipette such that the final concentration of antigen was 100 ng/mL.
RESULTS
pHluorin indicator for degranulation
To study the responsiveness to antigen of our C57 cell line, we sensitized the cells overnight with IgE anti-HSA-DNP at 1 μg/mL overnight, then plated on fibronectin coated dishes. Soluble HSA-DNP antigen was utilized as the biochemical trigger. To observe the increase in intracellular calcium that accompanies crosslinking of FceRI, we loaded the cells with the calcium sensitive dye Fluo-4. Sensitized cells strongly fluxed Ca2+ (Fig. 1a and 1b). As expected, un-sensitized cells did not show calcium flux upon addition of antigen (Fig. 1c). We noted that both sensitized and un-sensitized cells show continuous, transient, low-level calcium fluctuations (Fig. 1b and 1c).
Fig. 1.

a) Wide field time-lapse imaging of IgE-sensitized C57 mast cells loaded with calcium sensitive dye Fluo-4 stimulated with soluble DNP-HSA at 100 ng/mL. Antigen was added at 30 s. b) Fluo-4 versus time for two independent cells (red and blue) after stimulation with DNP-HSA. Fluorescence was normalized to intensity at time 0. c) Same plot showing responses of two un-sensitized C57 cells after stimulation with DNP-HSA.
Confident that our model system responded in an antigen and IgE-specific manner, we next sought to visualize degranulation events directly. We constructed a genetically encoded fluorescent reporter of vesicle fusion by linking the cDNA of a pH-sensitive variant of GFP pHluorin to the N-terminal cDNA of lysosomal associated membrane protein 1 (LAMP1). LAMP1 is preferentially trafficked to endosomes, and the acidic interior of granules quenches pHluorin fluorescence. Upon vesicle fusion, the acidity of the granule is neutralized by the extracellular environment, dequenching pHluorin, and leading to a local increase in fluorescence. This mechanism allows us to observe spatial and temporal information on individual vesicle fusion events (called “blips” in this paper). Over time, the LAMP1-pHluorin spreads laterally from the site of docking with the membrane broadly across the surface of the cell. We tested our LAMP1-pHluorin fusion protein by retrovirally transducing the construct into mast cells, sensitizing them with antigen as above, then stimulating the mast cells with soluble HSA-DNP while imaging them continuously. We observed, as expected, the appearance of pHluorin-containing vesicles on the cell surface after stimulation (Fig. 2).
Fig. 2.

Wide-field imaging of IgE-sensitized C57 cells transduced with pHluorin-LAMP1 construct. Cells stimulated with 100 ng/mL DNP-HSA. Fluorescence intensity of pHluorin reporter is shown in false-color.
Biochemical and mechanical stimulation of mast cells using AFM
Atomic force microscopy allows for precise sensing and application of force on the piconewton scale. We took advantage of this functionality of AFM to apply a localized biochemical signal with precise temporal and spatial resolution. We accomplished this goal by covalently attaching an allergen to the cantilever. In this case, we utilized the chemical crosslinker sulfo-LC-SPDP to attach HSA-DNP to an APTES-coated silicon cantilever. Sulfo-LC-SPDP reacts with the cantilever-bound APTES through its sulfo-NHS ester, while reacting with the free cysteine-34 of HSA through its pyridyldithiol group.
C57 cells expressing pHluorin-LAMP1 were plated on fibronectin-coated dishes and placed under an Asylum MFP-3D AFM system while being imaged using epi-fluorescence in wide-field mode (Fig. 3a). Using the “force curve” functionality of the AFM, we engaged the HSA-DNP-coated cantilever to a mast cell that was transfected with pHluorin-LAMP1. We targeted the approximate center of the mast cell. Upon reaching a force trigger of 600 pN, the cantilever was then held with constant z-position for 10 min (called “dwelling” on the surface of the cell). Contacted cells showed evidence of degranulation within ~30 seconds of force triggering. “Blips” of fluorescence, indicating sudden contact of the vesicle with the extracellular environment and sudden dequenching of the pHluorin in that vesicle, continued to appear well into the 10 min imaging session (Fig. 3b).
Fig. 3.
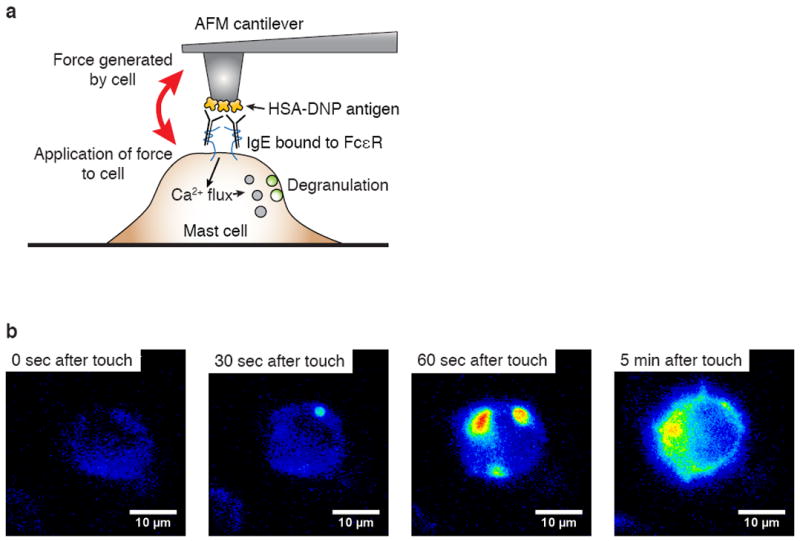
a) Schematic illustrating setup of AFM cantilever for localized stimulation of mast cells and for monitoring responses. b) Wide-field time lapse imaging of IgE sensitized C57 cells transduced with pHluorin-LAMP1 construct (false colored). DNP-HSA coated AFM cantilever tip was brought into contact with the mast cell surface in the middle of the cell area with a force trigger of 600 pN and held with constant z-position for 10 min.
In comparing the number of vesicle fusion events over time, we noticed two distinct degranulatory behaviors (Fig. 4). First, we observed “anaphylactic-type” degranulation, in which the blips appear rapidly within the first 5 min after triggering and then slow down in the rate of appearance. Second, we observed a slower kinetic phenotype in which a small number of fusion events appear steadily over a much longer timescale (“piecemeal”). Whether these differences apply to primary mast cells is not yet known, but notably, fast and slow variations in the kinetics of degranulation have been described previously both in mast cells [12] and in basophils [13].
Fig. 4.

Summarization of the kinetics of degranulation for cells contacted at force triggers of a) 200 pN, b) 600 pN, and c) 2 nN. Degranulation is measured in accumulated “blips” (pHluorin dequenching events). Each color is an independent mast cell.
To test the role of mechanical force as a mechanical adjuvant to biochemical signaling we repeated the same experiment with different cantilevers and different C57 mast cells using engagement forces of 200, 600 pN and 2 nN. Comparing the kinetic profiles of degranulation between the different force triggers used reveals no obvious relationship between the force applied and the rate of degranulation (Fig. 4 and Fig. 5). These data show that antigen-mediated activation of the mast cells in our model is not dependent on external mechanical forces. To assess the effect of purely mechanical stimulation in the absence of antigen, we contacted the cells using uncoated cantilevers, dwelling on the surface as in the experiments with antigen. We consistently observed no response in terms of degranulation (not shown). Thus, mechanical force alone cannot induce degranulation in our model system.
Fig. 5.

Representative traces of the mechanical forces generated by mast cells following forceful contact with antigen-coated AFM cantilever. Degranulation is also shown, as measured in accumulated “blips” (pHluorin dequenching events). Three different force triggers when contacting the cells with antigen were tested to assess the sensitivity of mast cells to mechanical forces while undergoing receptor ligation. a and b) 200 pN; c and d) 600 pN; e and f) 2 nN.
To evaluate the forces generated by the mast cells during antigenic ligation, we measured the deflection of the cantilever continuously during dwells. This deflection can be directly translated to force by multiplying by the spring constant of the cantilever. Previous studies have shown that fibroblasts can generate forces up to 10 nN/um2 [14], however, forces exerted by lymphocytes and other hematopoietic cells are much less studied. We observed that sensitized mast cells engaged by antigen are capable of exerting both pulling and pushing forces on the order of nanonewtons (Fig. 5), comparable to the forces generated by cardiomyocytes [15]. Second, we observed that the responses of some mast cells included large forceful fluctuations and these fluctuations were associated with emphatic degranulatory responses (measured by pHluorin dequenching). In contrast, cells contacted with uncoated, control cantilevers did not generate any significant forces (not shown). Furthermore, there were a few, rare experiments noted where degranulation was unaccompanied by forceful movements (Fig. 5e) or where forceful movements were not accompanied by degranulation (Fig. 5f). In addition, we saw no obvious correlation between force generated by the mast cells during antigenic contact and the force applied to the mast cell at the time of contact (Fig. 5). These results demonstrate that the degranulation we observe largely coincides with generation of mechanical forces by the mast cells.
DISCUSSION
In this paper we present a method for delivering a biochemical and mechanical stimulus simultaneously with spatial and temporal precision. We examined mast cells as a model system for studying cellular responses to AFM-delivered stimulation due to their dramatic and easily-studied degranulation response.
The method presented here can be extended to studying many other single cell systems where precise localization of biochemical stimuli is needed. For example, stimulation of T cells using cantilevers coated with peptide-MHC is possible (M.A. Bruce, M.J. Butte, unpublished). Because the AFM allows us to measure the mechanical forces generated by cells upon ligation of cell-surface receptors, this approach allowed us to study the cytoskeletal changes that occur downstream of receptor ligation in mast cells. We showed here, for example, that mast cells generate mechanical forces upon antigenic triggering of the FcεRI. It is possible these mechanical forces are important for the cytoskeletal reorganization that accompanies degranulation [16].
Other modes of the AFM are equally interesting to apply in this context: in this study we set the cantilever at a fixed z-height during contact with the cell; however, the AFM is also able to dynamically adjust the z-height of the cantilever to match mechanical forces generated by the cell. In effect, such a “force clamp” could be used to completely neutralize the mechanical forces generated by the cell against the cantilever. This setup would be tremendously useful to uncover the role of mechanical force in receptor-ligand interactions, which has been proposed for the T cell receptor [17]. In vivo, cells are subject to a variety of mechanical forces. The mechanisms for sensing these forces, converting them to biochemical cues, and generating responses are just beginning to be appreciated. The method we have described will be useful in understanding the interactions of mechanical and biochemical cues in cellular signaling.
Acknowledgments
We are grateful to the assistance of Dr. Philipp Starkl and Prof. Stephen J. Galli for providing reagents and guidance. M.J.B. is supported by NIH/NIAID (K08 AI079268), NSF (CBET-1264833), the Stanford Child Health Research Institute, and the Center for Probing the Nanoscale, an NSF NSEC (PHY-0830228). M.A.B. is supported by the Stanford Graduate Fellowship.
References
- 1.Vig M, DeHaven WI, Bird GS, Billingsley JM, Wang H, Rao PE, et al. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 2008;9(1):89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishida K, Yamasaki S, Ito Y, Kabu K, Hattori K, Tezuka T, et al. Fc{epsilon}RI-mediated mast cell degranulation requires calcium-independent microtubule-dependent translocation of granules to the plasma membrane. J Cell Biol. 2005;170(1):115–26. doi: 10.1083/jcb.200501111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abajian M, Mlynek A, Maurer M. Physical urticaria. Current allergy and asthma reports. 2012;12(4):281–7. doi: 10.1007/s11882-012-0269-0. [DOI] [PubMed] [Google Scholar]
- 4.Mita Y, Dobashi K, Nakazawa T, Mori M. Mechanical fluid flow enhances high-affinity-IgE-receptor-mediated secretion by mast cells adherent to fibronectin. The Journal of asthma : official journal of the Association for the Care of Asthma. 2001;38(5):435–41. doi: 10.1081/jas-100001499. [DOI] [PubMed] [Google Scholar]
- 5.Yang W, Chen J, Zhou L. Effects of shear stress on intracellular calcium change and histamine release in rat basophilic leukemia (RBL-2H3) cells. Journal of environmental pathology, toxicology and oncology : official organ of the International Society for Environmental Toxicology and Cancer. 2009;28(3):223–30. doi: 10.1615/jenvironpatholtoxicoloncol.v28.i3.30. [DOI] [PubMed] [Google Scholar]
- 6.Zhang D, Spielmann A, Wang L, Ding G, Huang F, Gu Q, et al. Mast-cell degranulation induced by physical stimuli involves the activation of transient-receptor-potential channel TRPV2. Physiological research / Academia Scientiarum Bohemoslovaca. 2012;61(1):113–24. doi: 10.33549/physiolres.932053. [DOI] [PubMed] [Google Scholar]
- 7.Charras GT, Horton MA. Single cell mechanotransduction and its modulation analyzed by atomic force microscope indentation. Biophys J. 2002;82(6):2970–81. doi: 10.1016/S0006-3495(02)75638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGarry JG, Maguire P, Campbell VA, O’Connell BC, Prendergast PJ, Jarvis SP. Stimulation of nitric oxide mechanotransduction in single osteoblasts using atomic force microscopy. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2008;26(4):513–21. doi: 10.1002/jor.20515. [DOI] [PubMed] [Google Scholar]
- 9.Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394(6689):192–5. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 10.Rak GD, Mace EM, Banerjee PP, Svitkina T, Orange JS. Natural killer cell lytic granule secretion occurs through a pervasive actin network at the immune synapse. PLoS biology. 2011;9(9):e1001151. doi: 10.1371/journal.pbio.1001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butt HJ, Jaschke M. Calculation of thermal noise in atomic force microscopy. Nanotechnology. 1995;6:1. [Google Scholar]
- 12.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–86. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 13.MacGlashan D., Jr Expression of CD203c and CD63 in human basophils: relationship to differential regulation of piecemeal and anaphylactic degranulation processes. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2010;40(9):1365–77. doi: 10.1111/j.1365-2222.2010.03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelham RJ, Jr, Wang Y. High resolution detection of mechanical forces exerted by locomoting fibroblasts on the substrate. Molecular biology of the cell. 1999;10(4):935–45. doi: 10.1091/mbc.10.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Sun N, Bruce MA, Wu JC, Butte MJ. Atomic force mechanobiology of pluripotent stem cell-derived cardiomyocytes. Plos One. 2012;7(5):e37559. doi: 10.1371/journal.pone.0037559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng Z, Zink T, Chen HY, Walters D, Liu FT, Liu GY. Impact of actin rearrangement and degranulation on the membrane structure of primary mast cells: a combined atomic force and laser scanning confocal microscopy investigation. Biophys J. 2009;96(4):1629–39. doi: 10.1016/j.bpj.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Reinherz EL. The structural basis of αβ T-lineage immune recognition: TCR docking topologies, mechanotransduction, and co-receptor function. Immunological Reviews. 2012;250(1):102–19. doi: 10.1111/j.1600-065X.2012.01161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


