Abstract
The ability to understand and implement calculations required for molarity and dilution computations that are routinely undertaken in the laboratory are essential skills that should be possessed by all students entering an undergraduate Life Sciences degree. However, it is increasingly recognized that the majority of these students are ill equipped to reliably carry out such calculations. There are several factors that conspire against students' understanding of this topic, with the alien concept of the mole in relation to the mass of compounds and the engineering notation required when expressing the relatively small quantities typically involved being two key examples. In this report, we highlight teaching methods delivered via revision workshops to undergraduate Life Sciences students at the University of Nottingham. Workshops were designed to 1) expose student deficiencies in basic numeracy skills and remedy these deficiencies, 2) introduce molarity and dilution calculations and illustrate their workings in a step-by-step manner, and 3) allow students to appreciate the magnitude of numbers. Preworkshop to postworkshop comparisons demonstrated a considerable improvement in students' performance, which attenuated with time. The findings of our study suggest that an ability to carry out laboratory calculations cannot be assumed in students entering Life Sciences degrees in the United Kingdom but that explicit instruction in the form of workshops improves proficiency to a level of competence that allows students to prosper in the laboratory environment.
Keywords: concentration, dilution, mass, molarity, molecular weight, moles, solution
the ability to perform the calculations necessary to accurately prepare solutions and carry out dilutions is a key requirement for all students of life sciences (a grouping that includes, but is not limited to, biology, biochemistry, neuroscience, pharmacology, and physiology), pharmacy, and medicine. Given the importance of these skills and the entry requirement to most undergraduate Life Sciences degrees in the England of an A level in maths and/or chemistry, it should be safely assumed that all undergraduate students possess these relevant skills. This is not the case. In a recent report, Curry (6) stated that “students with A level maths have difficulty with even the simple calculations needed to prepare buffer solutions at a defined salt concentration,” in agreement with another report (5) that identified that “even those (students) with top grades at A-level are woefully ill-equipped to study maths and science at university.” The reasons for this deficit are probably severalfold but almost certainly include 1) dislike or lack of interest in maths, a subject presumed to be difficult, and the worrying institutional acceptance of antipathy to maths shown by most students; 2) the combined difficulties of understanding the concept of the mole and the magnitudes of the associated units, e.g., pico, nano, micro, etc., which requires an understanding of engineering notation; 3) the lack of appropriate context when teaching such methods at secondary school, e.g., directions to make solutions upon which the student has no interest or investment, which are not likely to be viewed as important and result in a lack of involvement; and, perhaps most worryingly of all, 4) “the modulization of A level, whereby there is no interlinking between the different elements of maths, but it is also because there is a race to the bottom at A-level by exam boards competing with each other about the ease with which students can achieve their grades” (5).
A brief description may be expedient for those unfamiliar with the English educational system for children aged 5–18 yr old. The National Curriculum is a set of subjects used by primary and secondary schools so that children learn the same things at the same time, i.e., it is standardized across all English schools. It covers what subjects are taught and the standard that children should reach in each subject (10). Children aged 5–11 yr old attend primary school and progress through year 6. Pupils aged 11–18 yr old attend secondary school and progress from years 7–13. Pupils aged 15–16 yr old in year 11 study between 10 and 11 subjects at the General Certificate of Secondary Education level. In year 12, pupils study four subjects at the AS level, and, dependent on their ability, pupils can then enter year 13 and undertake three subjects at the A2 level. The aggregate marks for the AS and A2 levels comprise the A level mark. Only 37% of 18 yr olds take A levels. University requirements in England for entry into a Life Sciences degree is two A grades and a B grade at the A level (although this can vary among universities and is subject to the widening participation scheme in which pupils from disadvantaged backgrounds are subjected to less strict entry requirements to redress social inequalities), where an A grade is 80% or above (A* are grades of 90% or above in the A2 component) and a B grade is between 70% and 79%. In 2013, 850,752 students took A levels, and of those, 10.2% took maths and 6.1% took chemistry. In A level maths, 43% of pupils achieved A or A* grades and 22.2% achieved B grades, whereas in chemistry, 33.6% achieved A or A* grades and 27.1% achieved B grades (4).
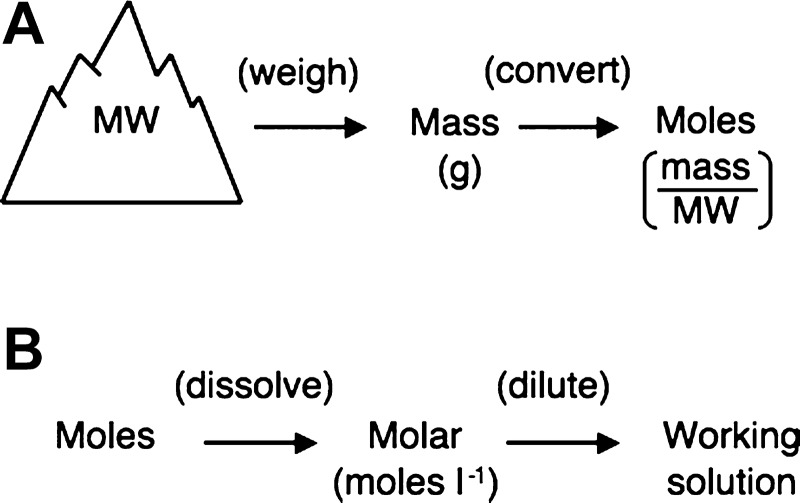
The concept of the mole is probably the major stumbling block to students' full understanding of the themes raised in this report. The mole is an International Systems of Units (SI) definition base unit of the amount of a substance (17). The International Committee for Weights and Measures definition of a mole is “the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon 12; its symbol is ‘mol’.” (17). In addition, the definition of the mole also determines the value of the universal constant that relates the number of entities to the amount of a substance for any sample. This constant is called the Avogadro constant (17). These rather dry statements may not inspire the average student, but rephrasing these definitions in a form that relates the mole to molecular weight (MW) and mass describes the mole in terms that can be readily understood. Thus, defining 1 mol of a chemical as equal to its MW (in g) and, by logical extension, that any given mass of compound divided by its MW gives the number of moles of the compound defines the mole relative to two properties that students should be familiar with: MW and mass. Standard laboratory scales measure compounds in terms of mass, necessitating interconversion between mass and moles. If a mass of a compound, containing a defined number of moles, is dissolved in a solvent, the resulting solution contains the same number of moles, but convention dictates that the solution is now defined in terms of molarity (in M). Molarity is the SI unit of concentration where a 1 M solution contains 1 mol of a solute per liter of solvent (17); hence, 1 M is synonymous with 1 mol/l. The relationships among mass, moles, and molarity are shown in Fig. 1.
Fig. 1.
Relationships between key definitions and units. All compounds have a molecular weight (MW) determined by their chemical composition. A: the mass of the compound is converted to the equivalent number of moles by dividing the mass of the compound by its MW. B: the mass of compound dissolved in a solute is expressed as a molarity, i.e., the number of moles per liter of solution. Stock solutions can be diluted to yield working solutions.
The units used in such calculations can be a source of confusion, since the (usually) small quantities that are involved in everyday laboratory calculations are expressed using engineering notation, a version of scientific notation. In these calculations, the units describing the variables are thus expressed in terms of moles, grams, or liters and scaled appropriately using engineering notation. In scientific notation, numbers are expressed in the form of a × 10b, where a is a number between 1 and 9.9− and b is the exponent and describes the power to which 10 is raised. In engineering notation, a is a number between 1 and 999.9− and the exponent is divisible by 3. Thus, 0.00003 is 3 × 10−5 expressed in scientific notation, whereas it is 30 × 10−6 in engineering notation. Engineering notation is more convenient for our purposes since units of measure are generally preceded by a standard SI prefix [e.g., milli (×10−3), micro (×10−6), nano (×10−9), pico (×10−12), etc.].
Example Calculations
One of the most basic tasks in the laboratory is to make a solution of a desired volume at a desired concentration from dry chemicals, where a known mass of a dry chemical is dissolved in a solvent and made up to the desired volume. Standard practice in these procedures is to dissolve the dry compound in a volume of solution 80–90% of the desired volume and, once the chemical has completely dissolved, make up to the final desired volume in a volumetric flask (1). The key concept to understand in this type of calculation is the relationship between mass and moles described above. One mole of a compound is equal to the MW (in g) of the compound. For example, the MW of glucose is 180.16; thus, 1 mol glucose weighs 180.16 g, 45.04 g glucose contains 0.25 mol glucose, etc. Two common laboratory calculations are described below, which one would expect students entering a Life Sciences degree to understand and be capable of carrying out with confidence.
Laboratory calculation 1: preparing a stock solution.
How would one make up 200 ml of a 4 M glucose solution? The first step in calculating molarity is to understand what 4 M glucose means. The symbol “M” is an abbreviation for moles per liter (mol/l). As the MW of glucose is 180.16, converting moles to mass simply requires multiplication of the number of moles by MW, as follows:
Hence, 720.64 g glucose, made up to 1 liter, constitutes a 4 M solution, i.e., 4 mol/l. However, only 200 ml (0.2 l) is required; thus, the mass required must be scaled appropriately, determined by what fraction of a liter is desired.
Consequently, the final step is to multiply the mass required by the volume, as follows (this is the relationship illustrated in Fig 1A rearranged to isolate the variable, mass):
Combining both steps results in the following relationship, which is used in all similar calculations:
| (1) |
Some chemicals are hydrated, i.e., are bound to water molecules (e.g., MgSO4·7H2O), which complicates calculations. This topic has been previously described in detail elsewhere (1).
Laboratory calculation 2: dilution of stock solutions.
Stock solutions are a convenient and space-saving way of storing solutions: it is not necessary to make up a new solution each time the solution is required; rather, a volume of an existing stock solution is diluted in an appropriate volume of solvent to produce the working solution. Practically, this is easier and quicker than making up a new solution from dry chemicals each time and ensures reliability as the same stock solution can be used repeatedly. Dilution of a stock solution is a comparatively simple calculation based on proportion, the concept of which should be readily understandable. In these types of calculations, there are four variables
1. The concentration of the stock solution (in mol/l)
2. The volume of the stock solution (in liters)
3. The desired concentration of the solution (in mol/l)
4. The desired volume of the solution (in liters)
The relationship between these variables is as follows:
In this relationship, the second variable is the unknown, which is calculated by rearranging the equation as follows:
| (2) |
Assessment of Student Ability to Carry Out Routine Molarity and Dilution Calculations
In our daily interactions with first-year neuroscience students, it is evident that the majority are incapable of successfully carrying out the calculations described above. The Pharmacy degree at the University of Nottingham afforded an opportunity to compare the proficiency of students in carrying out these calculations, since whereas the neuroscience students receive no instruction on laboratory-based calculations, all first-year pharmacy students (as part of the Professional Skills 1: Introduction to Pharmacy Practice module, which is designed to develop students' appreciation of basic elements of pharmacy practice via didactic lectures and workshops with a key component being training students to undertake numeric tasks of relevance to pharmacy) attend revision workshops that include calculations on molarity, dilutions, and drug dosage. We therefore carried out an ad hoc test of 55 first-year pharmacy students and 35 first-year neuroscience students. Students had no prior knowledge of the test and were allowed 20 min to complete the test. The test comprised the following four questions, which were designed to test student understanding of the relationship between moles and mass and dilution of stock solutions, the most likely calculations that neuroscience students will be required to carry out in their undergraduate laboratory studies:
1. What mass of glucose is required to make up 200 ml of a 4 M glucose solution? (MW glucose = 180)
2. What volume of a 2 M stock solution is required to make up 200 ml (0.2 liters) of a 50 mM (0.05 mol/l) working solution?
3. What concentration of a stock solution of sodium lactate is required such that injection of 200 μl (200 × 10−6 liters) of the stock solution into a rat with a blood volume of 19.5 ml (1.95 × 10−2 liters) results in a blood lactate concentration of 10 mM (10 × 10−3 mol/l)?
4. What is 90 mg/dl glucose expressed in moles per liter?
The results of the test (shown in Fig. 2A) demonstrated that pharmacy students performed far better than neuroscience students, with only 7.4% of pharmacy students but 42.9% of neuroscience students achieving no correct answers. The cumulative index of the number of correct answers (where the x-axis indicates the accumulated number of correct scores, such that 3 indicates students who achieved 0, 1, 2 or 3 correct answers) showed a clear disparity between the two cohorts of students (Fig. 2B). Further analysis of the data was carried out to determine if pharmacy students achieved significantly higher marks than neuroscience students. The percentage of students who correctly answered questions 1–4 are plotted for each cohort in Fig. 3A. A Mann-Whitney test yielded a P value of 0.026 with sum ranks of 25 (pharmacy students) and 11 (neuroscience students), indicating a significant difference. We next sought to determine if neuroscience students who possessed A levels in chemistry and/or maths achieved higher marks than those lacking the qualification (Fig. 3B). A Friedman test revealed no significant difference (P = 0.80), implying the possession of such qualifications was no predictor of performance.
Fig. 2.
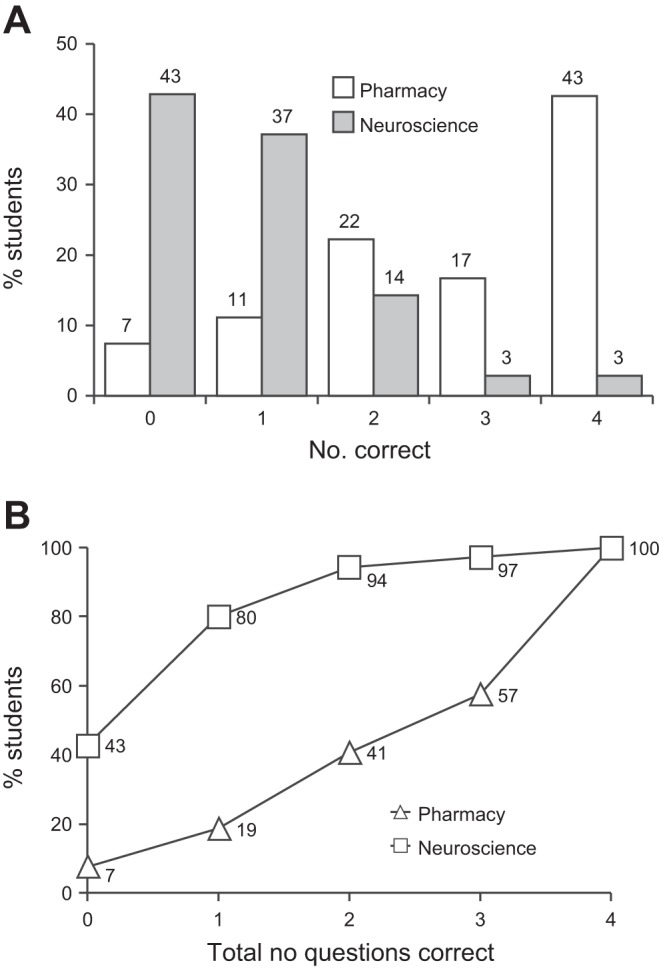
Summary of student scores in questionnaires designed to test competency in molarity/dilution calculations. A: number of students achieving 0–4 questions correct for both first-year pharmacy and neuroscience students. B: cumulative scores for students scoring 0–4 questions correct clearly showing the superior performance of pharmacy students compared with neuroscience students. Values adjacent the symbols indicate scores rounded to the nearest percentage point.
Fig. 3.
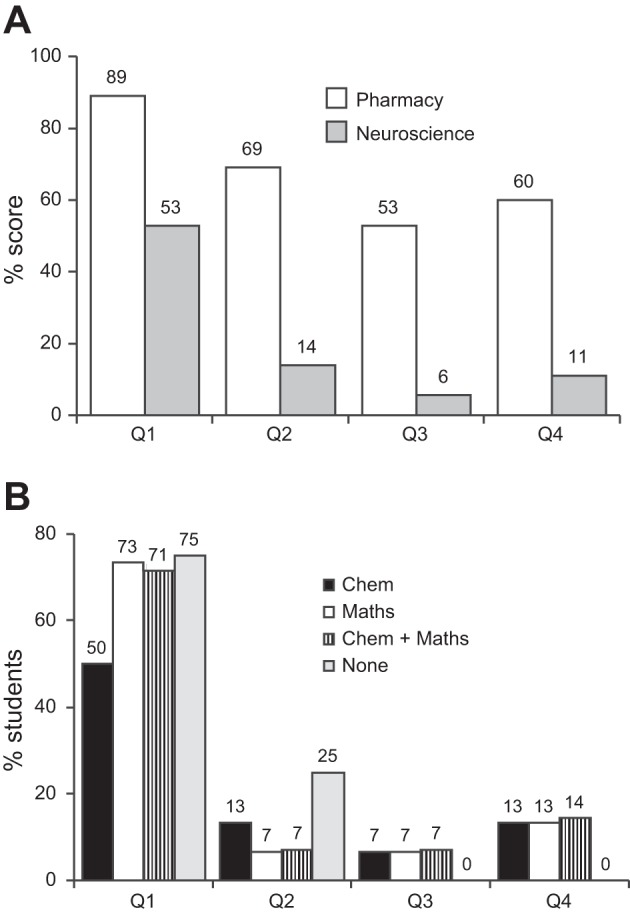
Question-by-question comparison of pharmacy and neuroscience student performance. A: in all four questions, a higher percentage of pharmacy students achieved the correct answer compared with neuroscience students. B: comparison of neuroscience students based on A level performance demonstrated no advantage for students possessing A level chemistry and/or maths compared with students lacking these qualifications.
Workshops
Given the varying backgrounds of students entering undergraduate Life Science degrees in England, an expectation of equivalent numeracy skills and their application to the field of chemistry is unrealistic. Thus, a series of revision workshops based on those delivered to pharmacy students were given to neuroscience students to bring all students to an acceptable level of understanding and application in numeracy and pharmaceutical calculations, i.e., those calculations involved with molarities and dilutions.
The course of workshops we describe here requires concessions from both academic staff and students. Students must be willing to acknowledge deficiencies despite top grades at A level and be prepared for further study, and academic staff must be prepared to accept student limitations and expend the time and effort required to run such workshops in a nonjudgmental manner.
As the first part of the workshop, students were given a self-assessment calculation exercise (appendix a). Students were informed that a key part of the course was to ensure that all first-year students had a good grounding in such calculations for their later studies and that the aim of these workshops was to ensure all students achieve the same fundamental level. Students were instructed to carry out the 25 questions in 33 min without the aid of electronic calculators. Students were informed that the results from the assessment would be known only to them and the instructor and that using data from all students would allow instructors to focus on areas that students struggle with most. After this workshop was completed, the results of the questions were described in a clear manner to ensure that students understood the key steps/rules involved in the calculations. Several days later, students underwent a similar workshop (appendix b) under similar conditions in which molarity and dilution calculations were introduced to reinforce the basic arithmetical skills underlying calculations. As before, the solutions to the questions were described in detail. Based on the student performance in this workshop, a selection process divided the students into one of the following three groups based on numeric ability:
1. Students who did all 20 questions in 30 min and got no more than 2 questions wrong.
2. Students who did all 20 questions but took longer than 30 min or got more than 2 questions wrong or students who managed all but 1 or 2 questions.
3. Students who could not manage to do all 20 questions and/or felt that they needed additional support.
Each group then carried out the pharmaceutical calculation workshop (APPENDIX C), which involved more complex calculations concerning molarities. The solutions to the questions were described in detail until students were confident that they could comfortably carry out the calculations. There was no time limit to this, and the session ended when all students in the group were confident of their abilities. Ultimately, the goal of these workshops was to 1) highlight where deficiencies lay and address these deficiencies, 2) allow students to understand definitions and rearranging equations, and 3) get a feel for the magnitude of numbers.
Postworkshop performance.
After students had completed the three workshops and were confident of their abilities, they took a test comprising the following four questions. Students had 20 min to complete the test, and calculators were allowed.
1. What mass of sodium chloride is required to make up 250 ml of a 40 mM solution? (MW sodium chloride = 58.4)
2. What volume of a 5 M stock solution is required to make up 250 ml (0.25 liters) of a 150 mM (0.15 mol/l) working solution?
3. What concentration of a stock solution of sodium chloride is required such that injection of 500 μl (500 × 10−6 liters) of the stock solution into a rat with a blood volume of 20 ml (2 × 10−2 liters) results in a blood lactate concentration of 10 mM (10 × 10−3 mol/l)?
4. What is 200 mg/dl calcium (MW = 20) expressed in moles per liter?
To determine the retention of the information delivered in the workshops, students (now second-year neuroscience students) were tested 9 mo after the initial workshops with the same four questions as above (it is a safe assumption that students will have forgotten the answers to the questions). The results (shown in Fig. 4) demonstrated a clear postworkshop improvement in proficiency among students but also an attenuation of performance after 9 mo. A Friedman test demonstrated a significant effect (P = 0.046) between the pretest and posttest, achieving significance. A Mann-Whitney test between pretest and 9-mo posttest revealed a P value of 0.026, implying that students retained the information delivered in the workshops to the extent that their performance was significantly different compared with the pretest results.
Fig. 4.

Neuroscience student performance was improved after students attended the workshops. After students attended the workshops, student performance improved in all four questions, but this improvement was attenuated 9 mo after the workshops.
Discussion
This report is intended to illustrate a method that can lead to improvements in student performance in laboratory calculations, with the information above suitable as a guide to carrying out such calculations. In our experience, many students undertaking undergraduate laboratory-based work do not fully comprehend the concept of the mole and its central role in the calculations required to prepare solutions. The Royal Society for Chemistry has highlighted the “dumbing-down” of science taught at the A level in England as a principal reason for students failing to achieve competency in key fundamental concepts of chemistry (8). Given this deficiency in secondary school teaching, coupled with the assumption of competency at the undergraduate level, students may be ill equipped to prosper in the laboratory environment. Mistakes resulting from an inability to carry out these routine tasks can lead to faulty experiments in which it is extremely difficult to track down the sources of error (e.g., making up artificial cerebrospinal fluid incorrectly in electrophysiology experiments). It can also lead to costly mistakes in which expensive reagents and antibodies are wasted, and, in the case of medical and pharmacy students, mistakes that could potentially be life threatening.
Our study identified two key points. First, students entering Life Sciences degrees in the United Kingdom are ill equipped to carry out routine laboratory calculations, which, given the requirement for maths and/or chemistry at the A level, they would be expected to carry out with ease. Indeed, we would extrapolate our findings from the narrow range of neuroscience students described in this study to apply to students studying physiology, biology, etc. To extrapolate our findings further, although we provide no evidence, it is likely that the issue we identify in this report is relevant to students in all countries progressing to study college and undergraduate degrees. A comparison between pharmacy students and neuroscience students clearly showed a difference in ability, e.g., 42% of neuroscience students achieved no correct answers in the initial molarity questions compared with only 7% of pharmacy students (Fig. 2). The reason for this difference is that not that all pharmacy students possess an A level in chemistry, since 30 of 35 neuroscience students possess the qualification. Indeed, analysis of A levels possessed by neuroscience students showed no advantage for students possessing A level chemistry and/or maths compared with students who had neither qualification (Fig. 3). This corroborates studies that have claimed that there is a lack of adequate rigor of maths in A level science subjects (3, 5). Second, the identified deficits in numeracy can be remedied with a course of workshops based around identifying and then subsequently correcting the deficits. The increased proficiency of pharmacy students is certainly due to the mandatory teaching module attended by all students, a key factor in the basic training of pharmacy students, who must demonstrate professional proficiency at the end of first year in these type of calculation to progress in the course. The improvement in neuroscience student performance after they attended the revision workshops clearly demonstrated their effectiveness (Fig. 4). However, the information was not reliably retained, with performance decreasing 9 mo after the revision session, suggesting that refresher courses should be offered every year.
While it is critical that students understand the theory behind these calculations, such that they can do them with pen and paper, we understand that under laboratory conditions, where multiple, repetitive calculations may be required, available technology in the form of desktop computers and smart phones can offer convenient and time-saving solutions. There are a variety of suitable web-based programs available, which include, but are not limited to, those offered by Sigma-Aldrich (16), Promega (14), GraphPad Software (11), Tocris Bioscience (18), and Functional Biosciences (7). All of the above programs carry out both molarity and dilution calculations except for that of Functional Biosciences, which is limited to dilution calculations. There are websites that contain either calculators (13) or tables for converting between conventional and SI units (9). In addition, there are numerous apps for iPhones/iPads available via iTunes AppStores (search molarity or dilution). These include the following: Molarity (free), DailyCalcs (free), Solutions ($0.99), LabCal (free), LabCalPro ($1.99), chemCalLite (free), iSolutions (free), Molarity Calculator ($1.99), MolarCalc ($1.99), and Lab Solver ($1.99). Some of these programs carry out both molarity and dilution calculations, but some are limited to molarity calculations only (Solutions, LabCalPro, chemCalLite, iSolutions, Molarity Calculator, and MolarCalc). Equivalent Android apps include Scientific Calculators ($0.99), AgileSciTools (free), and Biolegend Lab Tools (free), which are available for download from Android websites. We have written a rigorously tested stand-alone software program that is freely available, runs on Mac, Linux, or Windows operating systems, and requires only one download to run on a desktop computer. The program is prepopulated with commonly used chemicals for ease of use, has a help page that offers succinct descriptions of the calculations available, and is available on request from the corresponding author. In addition, there are a variety of useful books available that cover the topics described within this report in detail, of which the following are recommended: Refs. 2, 12, and 15.
In conclusion, our results indicate that students entering Life Science degrees at English universities are unlikely to be able to satisfactorily carry out the types of routine laboratory calculation involving molarities, dilutions, and drug dosage that academic staff would expect, even if the students possess A grades at A level in maths and/or chemistry. These deficiencies are likely due to the dumbing down of content in maths A level, thus failing to prepare students adequately for the rigors of undergraduate Life Science degree courses. Here, we describe a series of revision workshops that are mandatory for first-year pharmacy students at the University of Nottingham, given the importance of understanding such calculations to their professional proficiency, and demonstrate how introduction of the workshops to poorly performing neuroscience students significantly improved performance in this vital skill.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.L.S., H.K.Q., and A.M.B. analyzed data; J.L.S., H.K.Q., M.J.B., and A.M.B. interpreted results of experiments; J.L.S., H.K.Q., M.J.B., and A.M.B. edited and revised manuscript; J.L.S., H.K.Q., M.J.B., and A.M.B. approved final version of manuscript; M.J.B. and A.M.B. conception and design of research; M.J.B. and A.M.B. drafted manuscript; A.M.B. performed experiments; A.M.B. prepared figures.
Appendix A: QUESTIONNAIRE FOR TESTING BASIC MATHEMATICAL SKILLS
Students were required to complete the test in 40 min without the aid of a calculator.
1. 128 + 273 (Answer: 401)
2. 12.8 + 97.56 (Answer: 110.36)
3. 0.13 + 0.0083 (Answer: 0.1383)
4. 735 − 63 (Answer: 672)
5. 28.3 − 19.65 (Answer: 8.65)
6. 46 × 567 (Answer: 26,082)
7. 4.8 × 6.3 (Answer: 30.24)
8. 8.6 × 3.46 (Answer: 29.756)
9. 250 ÷ 4 (Answer: 62.5)
10. 459 ÷ 3 (Answer: 53)
11. 456 ÷ 32 (Answer: 14.25)
12. + (Answer: )
13. × (Answer: )
14. ÷ (Answer: )
15. Express 0.0045 using standard form (Answer: 4.5 × 10−3)
16. Express 1,653 mg in grams (Answer: 1.653 g)
17. Express 0.045 g in milligrams (Answer: 45 mg)
18. Convert 3 stones 2 lb to kilograms. (Answer: 20 kg; 1 stone = 14 lb, 2.2 lb = 1 kg)
19. Express the following number to three significant figures: 1.37476502 (Answer: 1.37)
20. Express 107 × 10−2 as a power of 10 (Answer: 105)
21. A stock solution containing 4.5% sodium chloride is diluted to make a solution of 0.9% sodium chloride. What is the dilution factor? (Answer: 5)
22. Rearrange the following equation to make m the subject:
[Answer: m = n(6 − k)]
23. Sodium hydroxide has a molecular mass of 40. What concentration will a solution have if 20 g solute is dissolved in water to produce 500 ml solution? (Answer: 1 M)
24. Substance A has a relative molecular mass of 128. How many moles are there in 32 g solid? (Answer: 0.25 mol)
25. Simplify log(2) + log(3) [Answer: log(6)]
Appendix B: QUESTIONNAIRE FOR TESTING MORE ADVANCED MATHEMATICAL SKILLS AND MOLARITY/DILUTION-TYPE CALCULATIONS
1. Express 29,642 mg in grams (Answer: 29.642 g)
2. Express 0.097 g in milligrams (Answer: 97 mg)
3. Sodium hydroxide has a molecular mass of 40. What (molar) concentration will a solution have if 30 mg solute is dissolved in water to produce 125 μl solution? (Answer: 6 M)
4. Substance J has a relative molecular mass of 224. How many moles are there in 28 g solid? (Answer: 0.125 mol)
5. You have 250 ml of a 125 mg/5 ml erythromycin suspension. How many grams of erythromycin are there in 75 ml? (Answer: 1.875 g)
6. A cream contains 3% (wt/wt) diclofenac. How many milligrams of diclofenac are there in 50 g cream? (Answer: 1,500 mg)
7. A prefilled syringe contains 2 ml epinephrine at 1 in 1,000. What volume must be given to provide a dose of 0.3 mg? (Answer: 0.3 ml)
8. How many milliliters of concentrated chloroform water are required to produce 500 ml chloroform water? (Answer: 12.5 ml)
9. How much alimemazine syrup (7.5 mg/5 ml) is required to treat a patient requiring a dose of 15 mg for 3 times/day for 3 days? (Answer: 90 ml)
10. How much castor oil is required to make 450 ml of an emulsion containing 40% (vol/vol) castor oil? (Answer: 180 ml)
11. Complete the formula below for the preparation of 250 ml of a solution of potassium permanganate in water from which a patient can dilute 20 ml up to a total volume of 2,000 ml with water to produce a potassium permanganate solution of 1 in 10,000. (Answer: potassium permanganate = 2.5 g or 2,500 mg; water for preparations = to 250 ml)
12. En-De-Kay Fluotabs contain 1.1 mg sodium fluoride, and 2.2 mg sodium fluoride provides 1 mg fluoride ions. How many tablets would you provide to give a 500-μg dose every other day for 12 wk? (Answer: 42 tablets)
13. You are supplied with a solution of erythromycin of 100 mg/ml. Complete the formula below for the preparation of 280 ml of a solution containing 125 mg/5 ml erythromycin. (Answer: 100 mg/ml erythromycin = 70 ml; water for preparations = 210 ml or “to 280 ml”)
14. You are required to supply 200 g Elocon ointment at 1 to 4. Complete the formula below for such a supply. (Answer: Elocon ointment = 40 g; white soft paraffin = 160 g or “to 200 g”)
15. A child weighing 30 kg is required to receive a dose of 20 mg/kg cefaclor daily in three divided doses. What volume of a 125 mg/5 ml suspension should be given for each dose? (Answer: 8 ml)
16. How many grams of Dermovate ointment are contained in 750 g of a 1 in 5 dilution? (Answer: 150 g)
17. You are required to make up a preparation to the formula below. What is the percentage (in vol/vol) of alcohol in the final preparation? [Answer: 13.5% (vol/vol); 10 ml solution A containing 40% (vol/vol) alcohol; 50 ml solution B, containing 15% (vol/vol) alcohol; 40 ml solution C, containing 5% (vol/vol) alcohol]
18. How many milliliters of water must be added to 100 ml of a 27% (wt/vol) stock solution of sodium chloride to prepare 0.9% (wt/vol) sodium chloride solution? (Answer: 2,900 ml)
19. A patient uses 250 ml of a 1:1,000 solution of a disinfectant footwash, 4 times/day, for 7 days. How many grams of the disinfectant have been used? (Answer: 7 g)
20. A 5-yr-old child weighing 3 stones 2 lb has been prescribed 25 mg tobramycin for 3 times/day. The British National Formulary dose for a child is 3–5 mg/kg daily in divided doses. Is the prescribed dose appropriate? (1 stone = 14 lb, 1 kg = 2.2 lb) (Answer: Yes)
Appendix C: QUESTIONNAIRE FOR TESTING ADVANCED MOLARITY/DILUTION-TYPE CALCULATIONS
1. Express 5,683 mg in grams (Answer: 5.683 g)
2. Express 0.007 g in milligrams (Answer: 7 mg)
3. Sodium hydroxide has a molecular mass of 40. What (molar) concentration will a solution have if 10 g solute is dissolved in water to produce 500 ml solution? (Answer: 0.5 M)
4. Substance J has a relative molecular mass of 132. How many moles are there in 26.4 g solid? (Answer: 0.2 mol)
5. You have 325 ml of 250 mg/5 ml ampicillin suspension. How many grams of ampicillin are there in 80 ml? (Answer: 4 g)
6. A cream contains 0.5% (wt/wt) hydrocortisone. How many milligrams of hydrocortisone are there in 50 g cream? (Answer: 250 mg)
7. A prefilled syringe contains 1 ml epinephrine at 1 in 1,000. What volume must be given to provide a dose of 0.6 mg? (Answer: 0.6 ml)
8. How many milliliters of concentrated peppermint water are required to produce 200 ml of double strength peppermint water? (Answer: 10 ml)
9. How much morphine sulphate solution (10 mg/5 ml) is required to treat a patient requiring a dose of 45 mg for 4 times/day for 7 days? (Answer: 630 ml)
10. Duraphat toothpaste contains sodium fluoride in a concentration that equates to 2,800 ppm of fluoride ions; 1 mg of fluoride ions are provided by 2.2 mg sodium fluoride. What strength [expressed in % (wt/wt)] of sodium fluoride is contained in Duraphat toothpaste? [Answer: 0.616% (wt/wt)]
11. Complete the formula below to produce 600 ml of a solution containing 40 mg/5 ml propranolol. Your stock solution contains 200 mg/5 ml. (Answer: propranolol = 200 mg/5 ml = 120 ml; water for preparations = 480 ml or “to 600 ml”)
12. Complete the formula below for the preparation of 120 ml of a solution of potassium permanganate in water from which a patient can dilute 10 ml up to a total volume of 1,500 ml with water to produce a potassium permanganate solution of 1 in 10,000. (Answer: potassium permanganate = 1.8 g or 1,800 mg; water for preparations = to 120 ml)
13. You are supplied with a solution of 20 mg/ml Oramorph concentrate. Complete the formula below for the preparation of 140 ml of a solution containing 10 mg/5 ml Oramorph. (Answer: Oramorph concentrate = 14 ml; water for preparations = 126 ml or “to 140 ml”)
14. How much almond oil is required to make 750 ml of an emulsion containing 20% (vol/vol) of almond oil? (Answer: 150 ml)
15. You are required to supply 240 g of Propaderm ointment at 1 to 3. Complete the formula below for such a supply. (Answer: Propaderm ointment = 60 g; white soft paraffin = 180 g or “to 240 g”)
16. You add 1 g of coal tar to 50 g of Lassar's paste. How much coal tar (in g) is contained in 10 g of the final product? Quote your answer to three decimal places. Lassar's paste contains 5% (wt/wt) coal tar. (Answer: 0.686 g)
17. You mix 200 g of a 10% (wt/wt) ichthammol ointment, 450 g of 5% ichthammol ointment, and 350 g of white soft paraffin (diluent). What is the percentage of ichthammol in the finished product? [Answer: 4.25% (wt/wt)]
18. How many grams of Synalar ointment are contained in 75 g of a 1 in 5 dilution? (Answer: 15 g)
19. An adult requires 750 mg ciprofloxacin at 2 times/day for 7 days. How much of a 250 mg/5 ml suspension would you provide? (Answer: 210 ml)
20. You are required to make up a preparation to the formula below. What is the percent (vol/vol) of alcohol in the final preparation? [Answer: 38.6% (vol/vol); 50 ml solution A, containing 80% (vol/vol) alcohol; 150 solution B, containing 22% (vol/vol) alcohol; 300 ml solution C, containing 40% (vol/vol) alcohol]
21. How many milliliters of water must be added to 250 ml of a 18% (wt/vol) stock solution of sodium chloride to prepare 0.9% (wt/vol) sodium chloride solution? (Answer: 4,750 ml)
22. You are required to make up a syringe containing glucose for a syringe driver. You have a 100-ml ampoule containing 50% (wt/vol) glucose. How much of this solution must you draw up (and then dilute) to produce 250 ml of a solution containing 100 mg/ml? (Answer: 50 ml)
23. If an antibiotic injection contains 5% (wt/vol) of the drug, how many milliliters of diluent should be added to 5 ml of the injection to prepare a solution containing 5 mg/ml antibiotic? (Answer: 45 ml)
24. A patient uses 50 ml of a 1:1,000 solution of an antiseptic mouthwash at 4 times/day for 7 days. How many grams of the antiseptic have been used? (Answer: 1.4 g)
25. A doctor requires their patient to receive 1 mg/kg body wt of tobramycin sulphate. The patient weighs 9 stone 6 lb. How many milliliters of a 80 mg/2 ml tobramycin sulphate injection should be administered to achieve this dosage? (1 stone = 14 lb, 1 kg = 2.2 lb) (Answer: 1.5 ml)
REFERENCES
- 1. Adams DS. Lab Math: a Handbook of Measurements, Calculations and Other Quantitative Skills for Use at the Bench. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 2003. [Google Scholar]
- 2. Bonner M, Wright D. Practical Pharmaceutical Calculations. Oxford: Radcliffe Publishing, 2008. [Google Scholar]
- 3. Burns J. A-Level Sciences ‘Lack the Maths Students Need’ (online). http://www.bbc.com/news/education-17854008 [9 June 2014].
- 4. Chalabi M. A-Level Results 2013: the Complete Breakdown (online). http://www.theguardian.com/news/datablog/2013/aug/15/a-level-results-complete-breakdown [9 June 2014]
- 5. Collins NJ. Top Universities Forced to Introduce Remedial Maths Classes (online). http://www.telegraph.co.uk/education/educationnews/9420771/Top-universities-forced-to-introduce-remedial-maths-classes.html [9 June 2014].
- 6. Curry S. Figures of merit. Times High Educ 2005: 38–41, 2011. [Google Scholar]
- 7.Functional Biosciences. Dilution Calculator (online). http://functionalbio.com/web/calc.php [9 June 2014].
- 8. Gallagher P. Proposed Science Exams “Plumbed New Depths” says RSC Chief Exec (online). http://www.rsc.org/AboutUs/News/PressReleases/2010/Proposedexams.asp [9 June 2014].
- 9.GLOBALRPh. Conventional Units - International Units (online). http://www.globalrph.com/conv_si.htm [9 June 2014].
- 10.Gov.UK. The National Curriculum (online). https://www.gov.uk/national-curriculum/overview [9 June 2014].
- 11.GraphPad Software. QuickCalcs (online). http://www.graphpad.com/quickcalcs/ [9 June 2014].
- 12. Gregson K. Understanding Mathematics. Nottingham: Nottingham Univ. Press, 2007. [Google Scholar]
- 13.Jay Clinical Services. Clinical Analyte Unit Conversion (online). http://dwjay.tripod.com/conversion.html [9 June 2014].
- 14.Promega. BioMath Calculators (online). http://www.promega.com/resources/tools/biomath-calculators/ [9 June 2014].
- 15. Rees JA, Smith I. Introduction to Pharmaceutical Calculations. London: Pharmaceutical Press, 2010. [Google Scholar]
- 16.Sigma-Aldrich. Web Toolbox - Interactive Calculators, Search Explorers, and Resources (online). http://www.sigmaaldrich.com/technical-service-home/web-tool-box.html [9 June 2014].
- 17. Taylor BN, Thompson A. The International System of Units (SI). Gaithersburg, MD: National Institutes of Standards and Technology, U.S. Department of Commerce, 2008, p. 21–22. [Google Scholar]
- 18.Tocris Bioscience. The Tocris Molarity Calculator (online). http://www.tocris.com/molarityCalculator.php#.U5XsJHaowjw [9 June 2014].