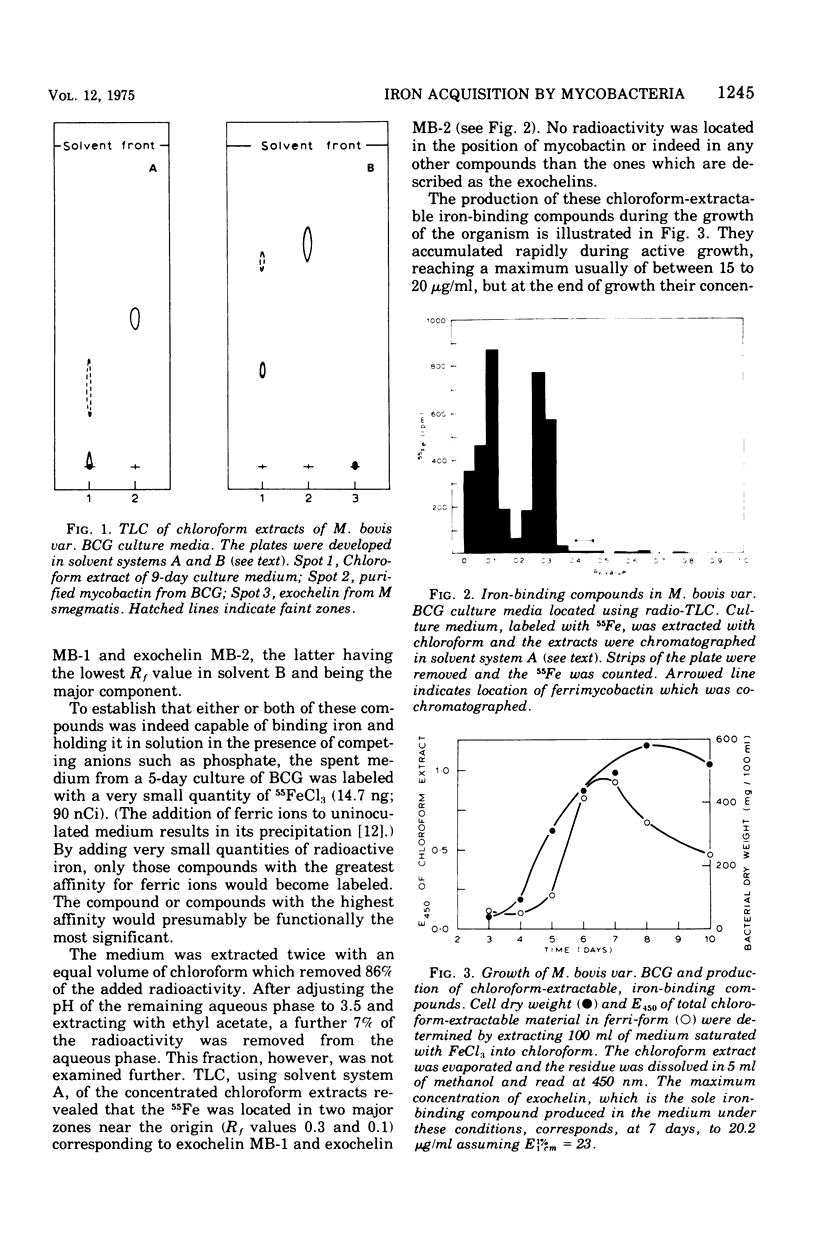
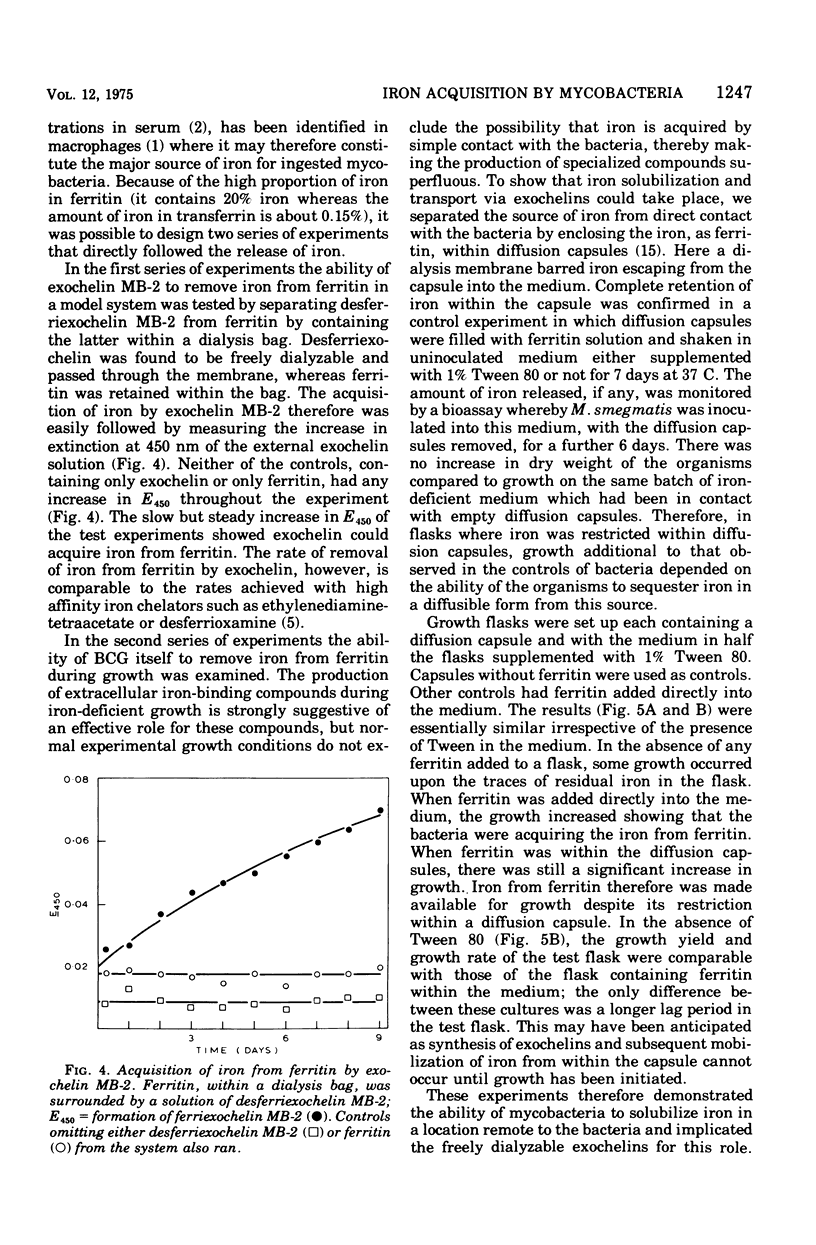
Abstract
Mycobacterium bovis var. BCG was grown under iron-deficient conditions in the presence and absence of 1% Tween 80. Mycobactin, the iron iron ionophore of mycobacteria, was found solely within the bacteria grown in the absence of Tween, but low concentrations (0.75 mug/ml) of it appeared in the medium in the presence of the surfactant. Both types of medium contain agents, named exochelins, which could solubilize iron. 55Fe added to spent culture media was recovered only chelated to these compounds. Two exochelins were detected, isolated, and purified. Neither were precursors or breakdown products of mycobactin. In the desferri-form, exochelin MB-2, the major component, reversed the inhibitory effect of serum on the growth of BCG, and in their ferri-forms exochelins MB-1, MB-2, and MS (from Mycobacterium smegmatis) stimulated the growth of their producing organism in the presence of serum. Exochelin MB-2 could physically remove iron from ferritin, and BCG used ferritin as a source of iron during growth even when ferritin was separated from the bacteria by a dialysis membrane. As solutions of the exochelins were freely dialyzable, whereas solutions of mycobactin, even in Tween, were not, only exochelin could have been active in this experiment. The exochelins are proposed as the functional extracellular iron-binding agents of BCG and other mycobacteria, the role of mycobactin being confined to that of a cell wall iron transporter.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong B. A., Sword C. P. Electron microscopy of Listeria monocytogenes-infected mouse spleen. J Bacteriol. 1966 Mar;91(3):1346–1355. doi: 10.1128/jb.91.3.1346-1355.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamish M. R., Addison G. M., Llewellin P., Hodgkins M., Hales C. N., Jacobs A. Ferritin estimation by a radioimmunoassay technique: serum levels in normal subjects and patients with blood disorders. Br J Haematol. 1972 May;22(5):637–637. [PubMed] [Google Scholar]
- Brown K. A., Ratledge C. Iron transport in Mycobacterium smegmatis: ferrimycobactin reductase (nad(p)h:ferrimycobactin oxidoreductase), the enzyme releasing iron from its carrier. FEBS Lett. 1975 May 1;53(2):262–266. doi: 10.1016/0014-5793(75)80033-0. [DOI] [PubMed] [Google Scholar]
- Dognin J., Girardet J. L., Chapron Y. Etude polarographique de la mobilisation du fer de la ferritine. Biochim Biophys Acta. 1973 Feb 28;297(2):276–284. [PubMed] [Google Scholar]
- Golden C. A., Kochan I., Spriggs D. R. Role of mycobactin in the growth and virulence of tubercle bacilli. Infect Immun. 1974 Jan;9(1):34–40. doi: 10.1128/iai.9.1.34-40.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan I., Cahall D. L., Golden C. A. Employment of tuberculostasis in serum-agar medium for the study of production and activity of Mycobactin. Infect Immun. 1971 Aug;4(2):130–137. doi: 10.1128/iai.4.2.130-137.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan I., Pellis N. R., Golden C. A. Mechanism of Tuberculostasis in Mammalian Serum III. Neutralization of Serum Tuberculostasis by Mycobactin. Infect Immun. 1971 Apr;3(4):553–558. doi: 10.1128/iai.3.4.553-558.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan I. The role of iron in bacterial infections, with special consideration of host-tubercle bacillus interaction. Curr Top Microbiol Immunol. 1973;60:1–30. doi: 10.1007/978-3-642-65502-9_1. [DOI] [PubMed] [Google Scholar]
- Macham L. P., Ratledge C. A new group of water-soluble iron-binding compounds from Mycobacteria: the exochelins. J Gen Microbiol. 1975 Aug;89(2):379–382. doi: 10.1099/00221287-89-2-379. [DOI] [PubMed] [Google Scholar]
- Pirt S. J. The diffusion capsule, a novel device for the addition of a solute at a constant rate to a liquid medium. Its application to metabolic regulation. Biochem J. 1971 Jan;121(2):293–297. doi: 10.1042/bj1210293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RATLEDGE C., WINDER F. G. The accumulation of salicylic acid by mycobacteria during growth on an iron-deficient medium. Biochem J. 1962 Sep;84:501–506. doi: 10.1042/bj0840501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratledge C., Hall M. J. Influence of metal ions on the formation of mycobactin and salicylic acid in Mycobacterium smegmatis grown in static culture. J Bacteriol. 1971 Oct;108(1):314–319. doi: 10.1128/jb.108.1.314-319.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratledge C., Macham L. P., Brown K. A., Marshall B. J. Iron transport in Mycobacterium smegmatis: a restricted role for salicylic acid in the extracellular environment. Biochim Biophys Acta. 1974 Nov 4;372(1):39–51. doi: 10.1016/0304-4165(74)90071-3. [DOI] [PubMed] [Google Scholar]
- Ratledge C., Marshall B. J. Iron transport in Mycobacterium smegmatis: the role of mycobactin. Biochim Biophys Acta. 1972 Aug 18;279(1):58–74. doi: 10.1016/0304-4165(72)90241-3. [DOI] [PubMed] [Google Scholar]
- Ratledge C., Snow G. A. Isolation and structure of nocobactin NA, a lipid-soluble iron-binding compound from Nocardia asteroides. Biochem J. 1974 May;139(2):407–413. doi: 10.1042/bj1390407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow G. A. Mycobactins: iron-chelating growth factors from mycobacteria. Bacteriol Rev. 1970 Jun;34(2):99–125. doi: 10.1128/br.34.2.99-125.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A. J., Snow G. A. Isolation of mycobactinss from various mycobacteria. The properties of mycobactin S and H. Biochem J. 1969 Mar;111(5):785–792. doi: 10.1042/bj1110785. [DOI] [PMC free article] [PubMed] [Google Scholar]



