Abstract
Background: To investigate thyroid function in preeclamptic patients in comparison with normal pregnant women.
Methods: In this analytical cross-sectional study free Thyroxine (T4), and Thyroid Stimulating Hormone (TSH) levels were measured in 100 preeclamptic patients and were compared with Free T4 and TSH levels in 101 normal pregnant women in their third trimester of pregnancy. Patients with thyroid or other systemic disorders were excluded from this study.
Results: A significant difference in concentration of free T4 levels (0.729 ±0.324 ng/ dl versus 0.929± 0.314 ng/dl, p <0.001) was observed in the preeclamptic group compared with the normotensive group, but the mean TSH level was not significantly different (2.935±1.16 mIU/L versus 2.339±1/15 mIU/L, p = 0.170).
Conclusion: Women who develop preeclampsia are more likely to have lower normal limits of thyroid function during their final weeks of pregnancy.
Keywords: Thyroxin, Hypothyroidism, Preeclampsia, Thyroid stimulating hormone
Introduction
Preeclampsia is associated with a high incidence of maternal and fetal mortality. Currently, there are no reliable, valid and economic screening tests available for predicting this pregnancy related disease (1). Many studies showed a relation between the level of thyroid hormones and development and severity of preeclampsia. These studies concluded that in contrast with the normal pregnant women, there are evidences of hypothyroidism (elevated TSH and decreased T3 and T4) in preeclamptic patients (2). Gonga and Hoffman concluded that trimester-specific Free T4 reference intervals progressively decline with advancing gestation and differ significantly from one another (3). This study examines Free T4 and TSH levels in pregnant women with preeclampsia in comparison with normal women in the third trimester.
Methods
This is an analytic cross-sectional study, done on a group of pregnant women in their third trimester of pregnancy who was referred to Akbarabadi Maternity Hospital between April 2008 and February 2009. The patients were divided into two groups of preeclamptic women and normal pregnant women. The sample size of 100 cases in each group (case and control) was determined based on the Cochran formula with 95% confidence interval. Inclusion criteria were as follows : 1- high blood pressure equal or higher than 140/90 mm/Hg in the sitting position and a proteinuria of equal or greater than 300 mg within 24 - hour urine collection or persistent (1+dipstick) in two random urine sample with an interval of 6 hours. 2- Written consent for participating in the study. Exclusion criteria were: 1- Any history of thyroid disease such as hyper or hypothyroidism or thyroid surgery. 2- Consumption of thyroid related medications 3- Any known systemic disorder or ones which diagnosed during study such as hypo or hyperthyroidism. This study was approved by the ethics committee of Iran University of Medical Sciences. Blood sampling was done from both the case and control groups for measurement of TSH and Free T4 levels. The TSH was measured using DIAPLUS, INC.QI ELISA kit with an intra-assay coefficient of variation (CV) of 5.19-6.54 and the inter-assay CV of 7.36-8.23 and normal range of 0.3-5.1 micro IU/ml. Free T4 was also measured using ELISA KIT (Pishtaz Teb) with an intra-assay CV of 4.14-6.87 and the inter-assay CV of 6.47-7.25 and normal range of 0.7 – 1.4 ng/dl.
Statistical Analysis
To analyze the association between TSH and preeclampsia, odds ratio with 95% CI (confidence interval) were calculated for the TSH levels. In order to describe the continuous and nominal variables, we use statistical indicators of mean, standard deviation and frequency percentage. The data was analyzed using SPSS v.18 software and compared using T. Test, chi–square, and Mann-Whitney statistical tests.
Results
The demographic features of the two groups are shown in Table 1. There were no significant differences between the two groups in the demographic characteristics such as age, gestational age, parity, birth weight, fetal sex and method of delivery. In the control group, age was ranged from 16 to 44 years and the range for the case group was from 17 to 44 years. In the preeclamptic women the gestational age was between 28 to 41 weeks compared with 28 to 40 weeks in the other group. The majority of both groups had the gravidity of 1 and 2 and the parity of one and zero (Table 2). Birth weight in the control group ranged between 800 to 4200 grams compared to 900-4100 grams in the case group. The mean of systolic blood pressure was 150 + 17.22 mm/hg (140 to 220 mm / Hg) and the mean for diastolic blood pressure was 94.4 + 9.16 mm/hg (80 to 130 mm/Hg) in the preeclamptic women. The dipstick test revealed proteinuria of 1+, 2+, 3+ and 4+ in 28%, %22, %27 and %23 of patients respectively. TSH and FT4 levels in both groups are shown in the Table 3. A significant difference in the levels of free T4 (0.729 +0.324 ng/dl versus 0.929+ 0.314 ng/dl, p<0.001) was observed in the preeclamptic group compared with the normotensive group. In contrast, the mean level of TSH (2.935+1.16 mIU/L versus 2.339+1/15 mIU/L, p= 0.170) showed no significant difference (Figures 1 and 2). There was also no significant difference between the levels of systolic and diastolic blood pressure (Table 4).
Table 1. Demographic information of preeclamptic vs normal pregnant women.
| Parameter |
Preeclampsia (No=100) |
Control (No=101 ) |
p. value |
| Maternal Age(year) (Mean) | 26.14 + 5.72 | 26.31 + 5.97 | Ns (P=0.947) |
| Gestational Age (week) (Mean) | 35.25 + 3.53 | 35.78 + 2.85 | Ns (P=0.360) |
| Birth weight(gram) (Mean) | 2427 + 771 | 2577 + 693 | Ns* (P=0.190) |
| Neonatal sex | |||
| Male | 46% | 48% | Ns (P=0.157) |
| Female | 54% | 52% | |
| Route of delivery | |||
| NVD | 65% | 68% | Ns** (P=0.122) |
| C/S | 35% | 32% |
*There is not statistically any significant difference between two groups by Mann-Whitney test.
**There is not significant difference between two groups by Pearson-Chi square test.
Table 2. Frequency distribution of gravidity in preeclampsia and normal groups .
| Gravidity | preeclampsia | Normal |
| Frequency | ||
|
1 2 3 4 5 6 7 |
47 29 16 3 2 2 1 |
48 31 15 2 3 1 1 |
| 100 | 101 | |
Table 3. Mean and standard deviation (SD) of FT4 and TSH values in preeclamptic and normal pregnancy groups .
| Parameter | Normal (101) | Preeclampsia (100) | p-value |
| *TSH (IU/ml) (mean ± SD) | 2.34 ± 1.15 | 2.94 ± 1.16 | NS |
| **FT4 (gr / dl) (mean ± SD) | 0.93 ± 0.31 | 0.73 ± 0.32 | 0.0001 |
* There is not statistically significant difference between two groups by Mann-Whitney test (p= 0.170)
** There is statistically significant difference between two groups by Mann-Whitney test.
Figure 1 .
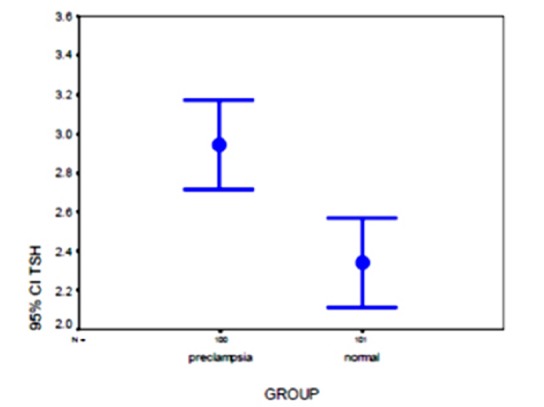
Distribution of TSH values in preeclampsia and normal pregnancy groups.
Figure 2 .

Distribution of FT4 values in preeclampsia and normal pregnancy groups.
Table 4. Correlation coefficient (r) and significance level of severity of hypertension with TSH and FT4 in preeclamptic patients .
| Blood Pressure | TSH level | FT4 level |
| Systolic Blood pressure | r : 0.09 | r : 0.04 |
| P.V: 0.371 | P.V: 0.72 | |
| Diastolic Blood pressure | r : 0.10 | r :0.04 |
| P.V: 0.30 | P.V: 0.72 |
Discussion
This study showed that levels of FT4 in the preeclamptic pregnancies is less than normal pregnancies. With due regard to the negative impacts of preeclampsia on pregnancy, any change may be important. Damage of endothelial cells and vasospasm are major pathologies during preeclampsia and eclampsia. Torry showed that angiogenesis is required for early implantation and placentation and it is likely that human gestation is dependent upon at least three temporally different vascular processes: (a) adequate uterine angiogenesis at the time of implantation, (b) development and expansion of the placental-villous-vasculature soon after implantation and (c) remodeling of the maternal uterine circulation near the maternal–fetal interface (4). Consequently, it is possible that the disruption of these early vascular events may contribute to the pathology of conditions like preeclampsia or intra uterine growth retardation of the fetus. Both problems displayed impaired vascular function of the fetal and maternal compartments (5-6). Endothelial activation/dysfunction is a central pathogenic feature in women with preeclampsia, which is a multiple system disorder during human pregnancy (7-8). In preeclampsia, endovascular invasion of the cytotrophoblats remains superficial. Concerning the impaired trophoblast invasion in preeclampsia, it has also to be considered that impaired invasion not only concerns invasion depth per se, but also the extension of this deep invasion from the central towards the more lateral spiral arteries of the placental bed (9-10). It has been found that the concentration of Vascular Endothelial Growth Factor Receptor 1 (VEGFR-1) was elevated prior to clinical diagnosis; so it could be used as a predictive marker (11). Increased circulating VEGER-1 concentration in preeclamptic women were associated with decreased circulating levels of free VEGF and PIGF, leading to an anti-angiogenic state and causing endothelial cell dysfunction (12-14). Among the best characterized of the circulating placental angiogenic proteins are Vascular Endothelial Growth Factor (VEGF) and Placental Growth Factor (PGF), both of which interact with the family of VEGF receptors. The placenta is also the source of multiple circulating anti-angiogenic proteins. Among these, the soluble isoform of VEGF receptor, soluble Fms-like tyrosine kinase (sFlt-1), and soluble endoglin (sEng), which is a coreceptor for transforming growth factor beta, are best described at present. Elevated concentrations of sFlt-1 and soluble endoglin with simultaneously reduced concentrations of PGF have been shown to precede the development of preeclampsia symptoms (15). Placental syncytiotrophoblasts and in particular syncytial knots were identified as a major source of sFlt1 and sEng production. Syncytial knots are induced by placental hypoxia and are noted predominantly in preeclamptic placentas (16). Even though sFlt1 and Endoglin (Eng) are major components of the disease, other placental molecules are certainly involved. For instance, the Hypoxia Inducible Factor (HIF) complex increases the production of the potent vasoconstrictor Endothelin-1 and reduces that of Nitric oxide (NO) vasodilatator, which contributes to the elevation of blood pressure and activation of the coagulation pathway (17).
There is strong evidence that TSH can act as a tissue specific angiogenesis in physiological and pathological conditions. Thus, increased levels of VEGF and TSH protein correlated with each other. TSH up regulates VEGF expression in vivo and vitro (18). Khadem compared 40 normal pregnant women and 40 cases of preeclampsia in third trimester of pregnancy. Her study does not support the hypothesis that changes in FT3, FT4 and TSH levels could be a possible etiology of preeclampsia (19). Our study observed that FT4 concentration in preeclamptic patients is lower significantly (p<0.001) in comparison with normal pregnancies but the increase in TSH level in these patients wasn’t significantly different (although it was higher) in comparison with normal pregnancy (p<0.17). This can be also due to the qualitative and not quantitative measurement. It can be suggested that women who develop preeclampsia are more likely to have lower normal limits of thyroid function during the final weeks of their pregnancies. Our study did not show any significant difference between the levels of systolic and diastolic blood pressure and the concentration of TSH and FT4. Study of maternal thyroid function and detection of lower normal limits in pregnant women especially in their third trimester may concern us about development of preeclampsia.
In conclusion, women who develop preeclampsia are more likely to have lower normal limits of thyroid function during the final weeks of their pregnancies. Therefore, in addition to the recommended thyroid function screening in first trimester of pregnancy, its follow up within the third trimester of pregnancy is recommended.
Acknowledgments
We would like to appreciate the financial support of the research committee of Iran University of medical sciences.
Cite this article as: Raoofi Z, Jalilian A, Shabani Zanjani M, Parvar SP, Parvar SP. Comparison of thyroid hormone levels between normal and preeclamptic pregnancies. Med J Islam Repub Iran 2014 (12 Feb). Vol. 28:1.
References
- 1. Cunnigham F, Leveno KJ, Bloom SL, Hauth JC, Gilstrap LC, Wenstrom KD, Williams obstetrics. 22nd ed. Mac Graw Hill; 2010; 725.
- 2.Roncaglia N, Avagliano L, Crippa I, Cameroni I, Malberti S, Sala F. Preeclampsia is strongly associated with maternal hypothyroidism. Am J Obstet Gynecol. 2006;195(6):S109. [Google Scholar]
- 3.Gonga Y, Hoffman BR. Free thyroxine reference interval in each trimester of pregnancy determined with the Roche Modular E-170 electro-chemiluminescent. immunoassay Clinical Biochemistry. 2008;41(10–11):902–906. doi: 10.1016/j.clinbiochem.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Torry DS, Hinrichs M, Torry RJ. Determinants of placental vascularity. Am J Reprod Immunol. 2004;(51):257–268. doi: 10.1111/j.1600-0897.2004.00154.x. [DOI] [PubMed] [Google Scholar]
- 5.Spaanderman MEA, Willekes C, Hoeks Hoeks, APG, Ekhart THA, Aardenburg R, Courtar DA. et al. Maternal nonpregnant vascular function correlates with subsequent fetal growth. Am J Obstet Gynecol. 2005;192(2):504–512. doi: 10.1016/j.ajog.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 6.Hwang HS, Maeng YS, Park YW, Koos BJ, Kwon YG, Kim YH. Increased senescence and reduced functional ability of fetal endothelial progenitor cells in pregnancies complicated by preeclampsia without intrauterine growth restriction. Am J Obstet Gynecol. 2008;199(3):259 e1–259 e7. doi: 10.1016/j.ajog.2008.06.060. [DOI] [PubMed] [Google Scholar]
- 7.Hubel CA, Sipose PI, Crockere IP. Endothelial progenitor cells: Their potential role in pregnancy and preeclampsia. Pregnancy Hypertension. 2011;1(1):48–58. doi: 10.1016/j.preghy.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Gu Y, Zhang Y, Lewis DF. Evidence of endothelial dysfunction in preeclampsia: decreased endothelial nitric oxide synthase expression is associated with increased cell permeability in endothelial cells from preeclampsia. Am J Obstet Gynecol. 2004;190(3):817–824. doi: 10.1016/j.ajog.2003.09.049. [DOI] [PubMed] [Google Scholar]
- 9.Pijnenborga R, Vercruyssea L, Hanssensa M, Brosensb I. Endovascular trophoblast and preeclampsia: a reassessment Pregnancy Hypertension: An International Journal of Women's Cardiovascular Health. 2011;1(1):66–71. doi: 10.1016/j.preghy.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 10. Ji L, Brkić J, Liu M, Fu G, Peng C, Wang YL. Placental trophoblast cell differentiation: Physiological regulation and pathological relevance to preeclampsia: Molecular Aspects of Medicine, Accessed online 28 December 2012. [DOI] [PubMed]
- 11.Chaiworapongsa T, Romero R. et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre- eclampsia. J Maternal Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 12.Polliotti BM, Fry AG, Saller DN, Mooney RA, Cox C, Miller RK. Second-trimester maternal serum placental growth factor and vascular endothelial growth factor for predicting severe, early-onset preeclampsia. Obstet Gynecol. 2003;101(6):1266–1274. doi: 10.1016/s0029-7844(03)00338-7. [DOI] [PubMed] [Google Scholar]
- 13.Koga K, Osuga Y, Yoshino O. et al. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol. 2003;(88):2348–2351. doi: 10.1210/jc.2002-021942. [DOI] [PubMed] [Google Scholar]
- 14.Tsatsaris V, Goffin F, Munaut C. et al. Over expression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab. 2003;(88):5555–5563. doi: 10.1210/jc.2003-030528. [DOI] [PubMed] [Google Scholar]
- 15.McElrath TF, Lim KH, Pare E, Edwards JR, Pucci D, Troisi R. et al. Longitudinal evaluation of predictive value for preeclampsia of circulating angiogenic factors through pregnancy. Am J Obstet Gynecol. 2012;207(5):407 e1–407 e7. doi: 10.1016/j.ajog.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Mihran V, Naljayan S, Karumanchi A. New developments in the pathogenesis of preeclampsia. Advances in Chronic Kidney Disease: 2013;20(3):265–270. doi: 10.1053/j.ackd.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chelbi ST, Vaiman D. Genetic and epigenetic factors contribute to the onset of preeclampsia. Molecular and Cellular Endocrinology. 2008;282(1–2):120–129. doi: 10.1016/j.mce.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 18.Reisinger K, Baal N, McKinnon T, Munstedt K, Zygmunt M. The gonadotropins: Tissue-specific angiogenic factors? Mol Cell Endocrinol. 2007;269(1-2):65–80. doi: 10.1016/j.mce.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Khadem N, Ayatollahi H, Vahid Roodsari F, Ayati S, Dalili E, Shahabian M. et al. Comparison of serum levels of Tri-iodothyronine (T3), Thyroxine (T4), and Thyroid- Stimulating Hormone (TSH) in preeclampsia and normal pregnancy. Iranian Journal of Reproductive Medicine, 2012;10(1):47–52. [PMC free article] [PubMed] [Google Scholar]


