Abstract
Background: Exercise has positive and negative effects on immune system. Herein, we would like to investigate the effects of incremental aerobic training and fish oil supplementation on the plasma levels of CRP, CPK and IL-17 in trained mice. One of the major roles of immune system is to produce soluble or cellular components that provide the immunity against inflammatory agent. The purpose of this study is to investigate distinct and combine effects of incremental aerobic training and fish oil supplement on plasma levels of IL-17, CPK and CRP in trained male mice.
Methods: Totally, 54 healthy male mice (2 months old, weight= 34±1 grams) were selected. At first 10 mice were killed to determine base line values, the rest of them were randomly divided into four groups, control group (C, n=11), supplement group (S, n=11), training group (T, n=11) and supplement-training group (ST, n=11).The supplement and supplement-training groups were fed with 0.2cc/day fish oil for 8 weeks. Training and supplement-training groups underwent exercise for 5 sessions per week for a period of 8 weeks on animal treadmill. SPSS 16.0 software and multivariate analysis of variance were used for statistical analysis of data
Results: Exercise and fish oil supplement lead to a decrease in CRP levels and subsequently causing a reduction in plasma levels of IL-17 and CK in mice (p<0.05).
Conclusion: Combination of exercise and fish oil can reduce regulate inflammatory response caused by incremental exercise.
Keywords: Fish oil supplement, Inflammation, IL -17, CRP, CPK
Introduction
Exercise has positive and negative effects on immune system, susceptibility to minor illnesses. Regular moderate exercise is also associated with a reduced incidence of infection compared with a completely sedentary state. On the other hand, post exercise immune system dysfunction is mostly pronounced when the exercise is continuous and prolonged (>1.5 h), of moderate to high intensity (55–75% maximum O2 uptake), and performed without food intake (1).
One of the most important functions of immune system is to produce soluble or cellular factors that provide the immunity against inflammatory agents. The balance between omega-3 and omega-6 consumption is important to maintain normal immune response. Some investigators believe that omega-3 fatty acids have a major effect on decreasing inflammation; on the other hand omega-6 fatty acids consumption can lead to tissue damage and susceptibility for inflammatory disease. As omega-3 fatty acids are not synthesized in body, they must be supplied in food. Omega-3 fatty acids exert their biologic effects by changing components and fluidity of cellular membrane lipids bilayer or by directly providing the metabolic substrate. The results of some studies show that increasing consumption of omega-3 fatty acids may increase the body resistance against oxidative stress (2, 3). Eicosapentaenoic acid (EPA) is an unsaturated fatty acid that inhibits the arachidonic acid metabolism and subsequently causes decreased leukotriene 4, prostaglandin E2 and inflammatory cytokines production. Although Docosa-hexaenoicacid (DHA) does not have a direct effect on producing eicosanoids, but it plays an essential anti-inflammatory role by modifying cellular translation factors (4). Although it is believed that the inflammation is beneficent to the host defence system; however, it also acts as an important factor in many chronic diseases such as cancer, cardiovascular disease and diabetes (5). It has been reported that exercise can increase the inflammatory and pre-inflammatory cytokines much like infection or tissue damage (6).
The cytokines, such as interlukin-17(IL-17) act as an intercellular messenger molecules, when attached to the target cell, initiate their biologic effects. Several experimental and clinical studies show that IL-17 family members are involved in specific inflammatory processes. Most published evidence supports a role for IL-17, as a promoter of granulopoiesis, neutrophil accumulation, and neutrophil activation in the lung, joint space, central nervous system, and intestinal tissue. Although IL-17 is a T cell-derived, pro-inflammatory cytokine, but is suggested to be involved in the development of various inflammatory diseases (4, 7), IL-6 secretion (8) and neutrophil activation (9). C-reactive protein (CRP) is an acute phase protein which increases remarkably during infection, inflammation, and tissue damage (8). It is mainly produced and secreted into circulation by liver in response to circulatory inflammatory mediators (10). CRP level is the best indicator to determine tissue damage because CRP increased immediately at early phase of tissue deterioration (11). Recent studies reveal that CRP not only is an inflammatory mediator, but it plays very important role in inflammation (12). The injured muscle also releases protein such as creatinekinase (CK) and myoglobin into blood circulation (11, 13).
Immediately after marathon race, the level of CRP does not change, however, after the ultra-marathon race its level increases remarkably. After the end of ultra-marathon race the level of CK increases by 35 times, but after marathon race its levels increases only by 4 times (13). It is also demonstrated that 12 weeks of intensive exercise increased IL-17 levels, but in another group which had moderate exercise no change was observed (14). The effect of exercise along with fish oil supplement consumption on inflammatory mediators’ levels has not been investigated. In this study we investigated the effect of incremental aerobic exercise accompanied with fish oil supplement consumption on IL-17 levels and some inflammatory markers.
Methods
Fifty four adult healthy male mice (age: 2 months old) were used in this study (Table 1). Principles of laboratory animal care (NIH animal care and guide) were followed. This study protocol has been approved by Shiraz Medical Sciences University Ethics Committee of Experimental Animals (Shiraz, Iran).
Table 1. Body mass in various samplings of the 8-week research protocol .
|
Test Time Group |
Pretest weight(gr) |
1
st
Post Test
weight(gr) |
2 nd Post Test weight(gr) | 3 rd Post Test weight(gr) |
4
th
Post Test
weight(gr) |
| Control | 35.7 ± 1.15 | 35.65±1.04 | 35.8 ± 0.83 | 35.5± 0.80 | 35.7± 0.75 |
| Supplement | 35.6± 0.93 | 32.15± 0.78 | 31.75± 0.84 | 30.3± 0.83 | 29.1± 0.74 |
| Training | 35.4± 1.25 | 35.8± 1.10 | 36.3± 1.12 | 36.7± 0.80 | 37.2± 0.70 |
| Supplement+ Training | 35.5± 1.36 | 33.5± 1.18 | 31.2± 1.11 | 30.1± 0.98 | 28.9± 0.82 |
At first 10 mice were killed to determine the base line values and rest of them were divided randomly into four groups, control (C, n=11), supplement (S, n=11), training (T, n=11) and supplement-training (ST, n=11). The mice were kept in separate cages (each cage 6 mice), at ambient temperature of 23°C with a 12-h/12-h light/ dark cycle. The animals had free access to water and pellet chow containing of calcium and phosphorus.
Incremental aerobic exercise was performed using an animal treadmill system which allows 7 mice to run at the same time. In this study, we used a training protocol for treadmill running described by Rico et al., consisted of 5 days per week for 8 weeks (15). The steep grade treadmill incline was used to stimulate high-intensity muscle activity in mice. The mice started exercise trainings to be familiarized with treadmill running for a week. Within the beginning of the familiarization period the speed, steep and running time of treadmill was 5m/s, 0 degree and 10 minutes respectively. At end of the familiarization period the speed, steep and running time of treadmill was increased to 7m/s, 5 degree and 15 minutes respectively. In the principal protocol, running time was gradually increased from 15 min to 30 and 60 min per session for T, ST groups. Treadmill speed increased up to 27m/s with increments of 2-3° to reach a final grade inclination of 18°. The chronological progression in the treadmill speed, grade inclination, and running time are shown in the table 2. Control mice were kept in the cages at the same environmental conditions and inspected daily to control their health.
Table 2. Training protocol (speed, slope, time) during eight weeks of research .
| Days | Variables | 1 st week | 2 nd week | 3 rd week | 4 th week | 5 th week | 6 th week | 7 th week | 8 th week |
| Monday | Speed (m/min) |
10 5 15 |
10 10 15 |
12 15 60 |
13 15 60 |
17 15 60 |
19 15 60 |
22 18 60 |
27 18 60 |
| Slope (degree) | |||||||||
| Time (min) | |||||||||
| Tuesday | Speed (m/min) |
10 5 15 |
10 13 15 |
12 15 60 |
13 15 60 |
17 15 60 |
19 15 60 |
22 18 60 |
27 18 60 |
| Slope (degree) | |||||||||
| Time (min) | |||||||||
| Wednesday | Speed (m/min) |
10 8 15 |
12 13 15 |
12 15 60 |
13 15 60 |
17 15 60 |
19 15 60 |
22 18 60 |
27 18 60 |
| Slope (degree) | |||||||||
| Time (min) | |||||||||
| Thursday | Speed (m/min) |
10 8 15 |
12 15 15 |
12 15 60 |
13 15 60 |
17 15 60 |
19 15 60 |
22 18 60 |
27 18 60 |
| Slope (degree) | |||||||||
| Time (min) | |||||||||
| Friday | Speed (m/min) |
10 10 15 |
12 15 45 |
13 15 60 |
17 15 60 |
19 15 60 |
22 15 60 |
27 18 60 |
27 18 60 |
| Slope (degree) | |||||||||
| Time (min) |
0.2 ml (0.06ml/g) fish oil which contains EPA and DHA was given to two groups of supplementation (S) and supplementation-training (ST) daily for 8 weeks.
In order to determine the levels of variables, in each trial, 10 mice were killed for blood sampling (for a total of 5 times including before starting the training protocol, once at each 2 weeks and at the end of the protocol). Blood samples were withdrawn from the tail under intraperitoneal ketamine and xylasine anaesthesia. Serum IL-17 levels were measured by ELISA method using commercial ELISA kits (mouse IL-17- kit-id labs Inc, Hungary) and also CRP was measured by (CRP-kit-neflometry, UK). In order to evaluate local inflammatory state of skeletal muscles, CK activity also was assessed by biochemical technique using UV-Visible spectrophotometer (Shimadzu UV-1601, Japan).
Statistical Analysis
SPPS version 16.0 was used for statistical analysis. Normality of data was analyzed by Kolmogorov–Smirnov test (K–S test). The values are presented as the mean ± standard error of mean (SEM). Multivariate analysis of variance was also used for data analysis. Tukey's HSD Post Hoc Test was used in circumstances which there were significant differences between experimental groups, because of the equality of mice at each group. The 0.05 level of significance was considered.
Results
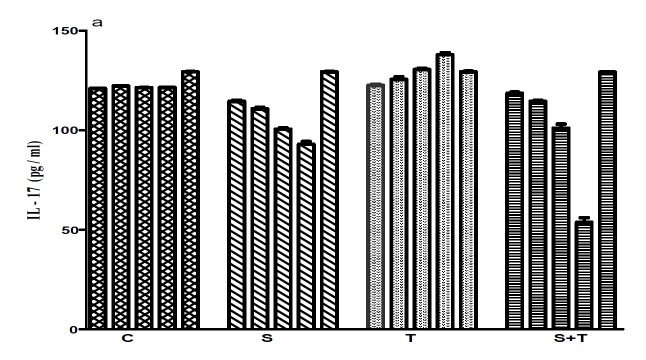
There were no significant differences between the base line values in all groups (Table1, 3). At the end of the study, the results showed a significant difference in IL-17 levels between groups (C: 121±0.52, S: 77±1.29, T: 130±0.91, ST: 130±2.25pg/ml; Figure 1a) (p<0.05). The result of post Hoc test revealed significant differences between the control group with other groups, the training group with the supplement group and also the supplement group with the supplement-training group. The maximum level of IL-17 was seen in the fourth blood sampling of the training group (138±0.65pg/ml), and the minimum level belonged to the fourth sampling of the supplement group (76±0.65pg/ml; Fig. 1.a). There were significant differences for the CRP levels between the two groups of training and supplement-training (T: 2.1±0.59 mg/l, ST: 1.4 ± 0.10 mg/l) and also the other two groups (S:0.25±0.56 mg/l, C: 1.0±0.64mg/l) (p<0.05).
Table 3. IL-17, CRP, CPK plasma levels in 5 samplings. Pretest: baseline, Turn 1. Turn 2,3,4,5: first, second, third, and fourth blood sampling. IL-17(pg/ml); Interleukin 17, CRP; C reactive protein, CPK; creatine phosphokinase Data were shown as Mean ±SD.
| variables | Control group | Supplement group | Training group | Supplement-training group | ||||||||||||||||
| time point 1 | time point 2 | time point 3 | time point 4 | time point 5 | time point 1 | time point 2 | time point 3 | time point 4 | time point 5 | time point 1 | time point 2 | time point 3 | time point 4 | time point 5 | time point 1 | time point 2 | time point 3 | time point 4 | time point 5 | |
| IL-17 |
117 ± 0.645 |
119 ± 0.654 |
118 ± 0.325 |
119 ± 0.564 |
121 ± 0.526 |
116 ± 0.654 |
114 ±. 645 |
9 ± 0.854 |
76 ± 0.645 |
77 ± 1.29 |
120 ± .654 |
126±.645 |
132 ± 1.33 |
138 ± 0.645 |
130 ± 0.913 |
117 ± 0.654 |
115 ± 0.654 |
105 ± 0.654 |
114 ± 2.16 |
130 ± 2.25 |
| CRP |
0.6 ± 0.11 |
1.1 ± 0.10 |
1.3 ± 0.11 |
0.90 ± 0.12 |
1.0 ± 0.65 |
0.8 ± 0.11 |
1.2 ± 0.59 |
0.9 ± 0.85 |
0.25 ± 0.11 |
0.25 ± 0.57 |
0.9 ± 0.11 |
1.3±0.11 |
2.5 ± 0.59 |
2.1 ± 0.11 |
2.1 ± 0.59 |
0.7 ± 0.11 |
2.1 ± 0.52 |
2.4 ± 0.26 |
1.4 ± 0.56 |
1.4 ± 0.10 |
| CPK |
86 ± 2.52 |
135 ± 3.251 |
121 ± 4.125 |
122 ± 5.36 |
123 ± 4.30 |
81 ± 4.32 |
86 ± 5.05 |
98 ± 4.04 |
57 ± 3.47 |
58 ± 3.11 |
81 ± 5.34 |
133±6.54 |
140 ± 2.28 |
127 ± 7.78 |
203 ± 2.96 |
84 ± 3.52 |
122 ± 2.36 |
120 ± 4.25 |
123 ± 5.21 |
142 ± 4.21 |
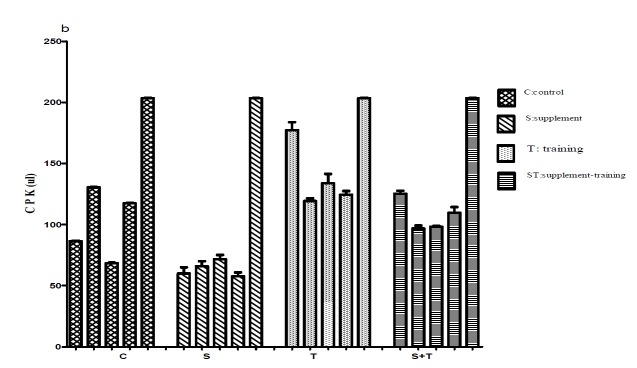
Fig. 1.
Comparison IL-17(pg/ml), CPK(ul), CRP(mg/l) concentrations in 5 samplings between groups
a .
b .
c .
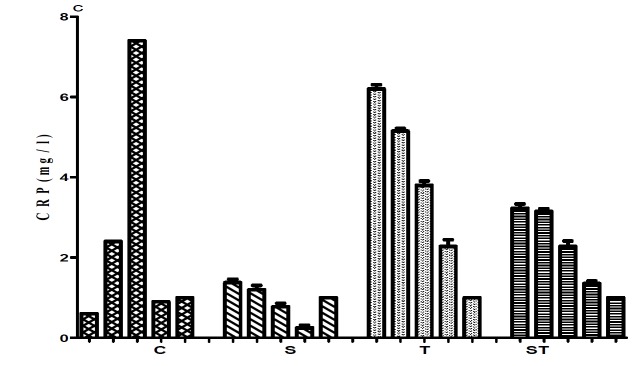
The maximum level of CRP belonged to the training and the supplement-training groups in the third sampling (2.5±0.6 ml/l and 2.4±0.26 mg/l respectively), and the minimum level belonged to the supplement group in fourth and fifth samplings (0.25±0.11, 025±0.57mg.l respectively)(Fig. 1.b). We also observed that there were significant differences in CK levels between the supplement (58±3.11ul) and other groups (C: 123± 4.3, T: 203±2.96, ST: 142 ±4.21 ul; Table 3) (p<0.05). Our results showed that there were significant differences between the supplement group and other groups and also between the training group and the supplement-training group. As shown in Fig. 1.c, maximum level of CK was seen in training group in fifth sampling (203±2.96u/l), and the minimum level belonged to the fourth sampling of the supplement group (57.75± 3.47u/l; Fig. 1c).
Discussion
The results of the present study show that fish oil supplement consumption accompanied with incremental aerobic exercise significantly modulate inflammatory response by suppressing IL-17, CRP and CK that are usually increased following intensive exercise (16). These variables are involved in regulating immune responses and inflammation (17). Although preinflammatory cytokines production is necessary for immune defence, but over production of these cytokines may lead to inflammation and subsequently causing skeletal muscle damage, weakness and increased risk of infection (11). Some researchers believe that the levels of IL-17 may be a useful biochemical index to determine the produced acute inflammation in skeletal muscles (10).
In some studies, the effect of training intensity on IL-17 as a major factor has been considered and the results showed that the levels of this cytokine increased in group that had strenuous training, but in another group no change was observed (14). A similar pattern was also observed in our study with the increase in aerobic training (increase in speed, steep and time of training) (Fig.1.A). In contrast to the result of this study, Golzari et al (2010) found that applying compound exercise program (including warming up, stretch trainings, aerobic trainings, strength trainings and relaxation trainings at the end of each session) did not increase IL-17 levels, and even in some cases, some decrease was observed which was attributed to the intensity of training (18). In our study, the finding can be due to adaptation of animals to exercise. On the other hand, levels of this cytokine in the supplement group are lower than other groups at the end of 8 week exercise. These two conflicting result in both groups of training and supplement may be due to the supplement consumption. However, the difference between two groups of supplement and training must be considered. Despite supplement consumption, the level of this cytokine in the supplement-training group is higher than the supplement group. The levels of IL-17 decreased in the third sampling of the ST group, but a significant increase was observed in fourth and fifth samplings that may be related to the amount of supplement consumption that was not enough to suppress inflammation induced by incremental exercise.
Distance of running must be considered as well. As the intensity and time of exercise increases, the level of this cytokine also increases.
Duzova et al (2009) have reported that strenuous exercises increase the IL-17 production, but the moderate exercises don’t increase the IL-17 production (14). Possible involved mechanism could be releasing pre-inflammatory cytokines following strenuous exercise, which makes anti-inflammatory cytokines production such as IL-2, IL-6 and IL-10. It seems that sequence of production of pre-inflammatory and anti-inflammatory cytokines is the reason of production of IL-17 by blood and skeletal muscle peripheral leucocytes (14, 19). Intensity and duration of exercise is an important factor that contributes in increasing IL-17 production. In accordance to recent result the finding showed that omega-3 supplement consumption can somehow prevent the increase of this cytokine.
The normal level of CRP is about 0.8-5 mg/l in adults, but it may be increased during infection or physical activity by 100 times, because the CRP production depend on various agents such as cytokine production (13), as it's observed that omega-3 consumption for 8 weeks decreases CRP levels(20). Lakka et el (2005) reported that 6 gms EPA consumption per day for 8 weeks decreases CRP level from 11 mg/l to 8 mg/l (probably due to IL-6 decreasing) (21). In Fig. 1.b, we can observe that CRP levels are within normal range in all groups. The level of CRP in both supplement and control groups is lower than other groups and in the supplement group at least two samplings (4th and 5th blood samplings) showed the lowest rate. Thus according to the results published in earlier studies, the duration of supplement consumption is an essential factor. In our study, the level of this protein was decreased after 8 weeks, but in some previous studies contrasting results has been observed (22, 23). Additionally, there is a non-significant relation between CRP and DHA levels (23), and the fact that 4 grams of fish oil consumption per day doesn’t change CRP levels in obese subjects (22).
Eight weeks of incremental aerobic training in our training group increased the CRP level. Thus, it can be suggested that inflammatory tendency increased in this group. Kim et al (2009) investigated the effect of distance in a marathon (42.195 km) and ultra-marathon (200 km) on this protein levels, and they found that after marathon race, the CRP level doesn't change, but after a day its level increased 3.4 times, and after 4 days it was recovered to its basal level. They also found that after ultra-marathon race, the CRP level was increased by 40 times, and remained nearly at noticed range up to 6 days after race (13).
The CRP changes in the supplement-training group are noticeable in our study. There was an increase in the supplement-training group similar to the training group in first three samplings, but in the second four weeks, the level of CRP gradually decreased. It can be suggested that fish oil supplement consumption to some extent can downregulate exercise induced inflammatory response, consequently decreasing CRP secretion.
Evidence showed that intensive exercise may lead to muscular damage at microscopic level, and finally resulting in inflammation. As noticed, when exercise causes muscle damage, the activity of CK increases in blood. Creatine Kinase is usually considered as reliable biomarker in determining muscle damage (24).The effect of two types of exercise (Eccentric and concentric) on some anti-inflammatory indexes has shown a significant relation exists between IL-6 increase and muscle damage measured by CK levels. In another study it was distinguished that carrying out 3 times 90 minutes - exercise training along with carbohydrate supplement cause a significant decrease at second and third times, but no change was observed at first time (25). In another study, the effect of sprint and endurance exercises on CPK level were also investigated. The results showed that there is a direct relation between the intensity and duration of exercise and CPK levels (26). In the present study the lowest level of CPK in different phases was observed in the supplement group suggesting the least muscle damage in this group. Comparing CPK levels in two supplement - training and training groups distinguishes that the CPK increase in the supplement- training group has been decreased by supplement consumption whereas in the training group the CPK level has not been decreased. The results in the training group shows that if increasing CPK is considered as an index for showing inflammation, it can be said that increased CPK levels reflects the micro-tears of skeletal muscle damage in mice. A recent study revealed that the time period of supplementation is a critical cause for changes in second and third blood samplings. (27)The extent and range of injury in different types of exercise modality is dependent on various factors such as duration, intensity, the type of exercise, gender and the level of subject’s physical fitness (13, 26, 27).
Conclusion
Fish oil supplement consumption downregulates incremental aerobic training induced IL-17 plasma levels increase. On the other hand, the incremental exercise activity causes muscle damage. Moreover, production of these IL-6 and IL-17 lead to CRP secretion which increases CK secretion. As shown in the present study, a direct and significant correlation was observed between the changes of CRP and CK (18). The results of present study should be confirmed by forthcoming studies that investigate the role of fish oil supplement on IL-17 and its role in acute inflammatory process of skeletal muscle that takes place with exercise.
Cite this article as: Alizadeh H, Bazgir B, Daryanoosh F, Koushki M, Sobhani V. Effect of aerobic exercise and fish oil supplements on plasma levels of inflammatory indexes in mice. Med J Islam Repub Iran 2014 (17 Feb). Vol. 28:6.
References
- 1.Gleeson M. Immune function in sport and exercise. J Appl Physiol. 2007;103:693–9. doi: 10.1152/japplphysiol.00008.2007. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal BB SS, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–21. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 3.Pischon THF, Rexrode KM, Girman CJ, Manson JE, Rimm EB. Inflammation, the metabolic syndrome, and risk of coronary heart disease in women and men. Atherosclerosis. 2008;197(1):392–9. doi: 10.1016/j.atherosclerosis.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sigal LH. Interleukins of current clinical relevance (Part I) J of Clinical Rheumatology. 2004;10(6):353–9. doi: 10.1097/01.rhu.0000147138.11053.e4. [DOI] [PubMed] [Google Scholar]
- 5.Shishehbor MH, Bhatt DL, Topol EJ. Using C-reactive protein to assess cardiovascular disease risk. Cleve Clan J Med. 2003;70(7):634–40. doi: 10.3949/ccjm.70.7.634. [DOI] [PubMed] [Google Scholar]
- 6.Kawaguchi M, Adachi M, Oda N, Kokubu F, Huang SK. IL-17 Cytokine family. J Allergy Clin Immunol. 2004;114(6):1265–73. doi: 10.1016/j.jaci.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Susumu Nakae AN, Katsuko Sudo , Yoichiro Iwakura . Suppression of Immune Induction of Collagen-Induced Arthritis in IL-17-Deficient Mice. Journal of Immunology. 2003;17(11):6173–7. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 8.Larry C. Borish JWS Cytokines and chemokines. J Allergy Clin Immunol. 2003;(111):S461–S75. doi: 10.1067/mai.2003.108. [DOI] [PubMed] [Google Scholar]
- 9.Hiroshi Hoshino ML, Margareta S, Lötvall J, Bengt-Eric S, Lindén A. Increased elastase and myeloperoxidase activity associated with neutrophil recruitment by IL-17 in airways in vivo. J Allergy Clin Immunol. 2000;(105):143–9. doi: 10.1016/s0091-6749(00)90189-1. [DOI] [PubMed] [Google Scholar]
- 10.Shoelson SE, LJa GA. Inflammation and insulin resistance. J Clin Invest. 2006;(116):1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomedicine and Pharmacotherapy. 2006;(60):502–7. doi: 10.1016/j.biopha.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 12.Peace JM, Suzuki K, Wilson G, Harder M, Nosaka K, Mackinnon L, Coombes JS. Exercise-induced muscle damage, plasma cytokines, and markers of neutrophil activation. Medicine Science in Sports Exercise. 2005;37(5):737–45. doi: 10.1249/01.mss.0000161804.05399.3b. [DOI] [PubMed] [Google Scholar]
- 13.Kim HJ, Lee Lee, YH YH, Kim Kim, CK CK. Changes in serum cartilage oligomeric matrix protein (COMP), plasma CPK and plasma hs-CRP in relation to running distance in a marathon (42195 km) and an ultra-marathon (200 km) race. Eur J Appl Physiol. 2009;105(5):765–70. doi: 10.1007/s00421-008-0961-x. [DOI] [PubMed] [Google Scholar]
- 14.Duzova H, Karakoc Y, Hanifi MT, Yilmaz ZD, Kilinc E. Effects of acute moderate and strenuous exercise bouts on IL-17 production and inflammatory response in trained rats. Journal of Sports Science and Medicine. 2009;(8):219–24. [PMC free article] [PubMed] [Google Scholar]
- 15.Rico H, Gervas J.J, Hernandez E. R, Seco C, Villa LF, Revilla M. Sanchez-Atrio A Effects of alprazolam supple-mentation on vertebral and femora l bone mass in rats on strenu-ous treadmill training exercise. Calcified Tissue International. 1999;65(2):139–42. doi: 10.1007/s002239900672. [DOI] [PubMed] [Google Scholar]
- 16.Duzova HKY, Hanifi MT, Yilmaz ZD, Kilinc EJ. Effects of acute moderate and strenuous exercise bouts on IL-17 production and inflammatory response in trained rats. ournal of Sports Science and Medicine. 2009;8:219–24. [PMC free article] [PubMed] [Google Scholar]
- 17.Keller P, Penkowa M, Keller CA, Steensberg A, Fischer CP, Giralt M. et al. Interleukin-6 receptor expression in contracting human skeletal muscle: regulating role of IL-6. The FASEB Journal. 2005;19(9) doi: 10.1096/fj.04-3278fje. [DOI] [PubMed] [Google Scholar]
- 18.Golzari Z, Shabkhiz F, Soudi S, Kordi MR, Hashemi SM. Combined exercise training reduces IFN-γ and IL-17 levels in the plasma and the supernatant of peripheral blood mononuclear cells in women with multiple sclerosis. Int Immuno- pharmacol. 2010;10(11):1415–9. doi: 10.1016/j.intimp.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Dong CCSH. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 2007;(17):435–40. doi: 10.1038/cr.2007.35. [DOI] [PubMed] [Google Scholar]
- 20.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory disease"s. Am J Clin Nutr. 2008;83(6):1505S–19S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 21.Timo A, Lakka H-ML, Tuomo R, Arthur SLeon DC, Rao James S. et al. Effect of exercise training on plasma levels of C-reactive protein in healthy adults: the HERITAGE Family Study. European Heart Journal. 2005;26(19):2018–25. doi: 10.1093/eurheartj/ehi394. [DOI] [PubMed] [Google Scholar]
- 22.Chan DC, Watts GF, Barrett PH, Beilin LJ, Mori TA. Effect of atorvastatin and fish oil on plasma high-sensitivity C-reactive protein concentrations in individuals with visceral obesity. Clan Chem. 2002;(48):877–83. [PubMed] [Google Scholar]
- 23.Kawaguchi M, Adachi M, Oda N, Kokubu F, Huang SK. IL-17 Cytokine family. J Allergy Clin Immunol. 2004;114(6):1265–73. doi: 10.1016/j.jaci.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 24.Howatson G, Van Someren KA. The Prevention and Treatment of Exercise-Induced Muscle Damage. Sports Med. 2008;38(6):483–503. doi: 10.2165/00007256-200838060-00004. [DOI] [PubMed] [Google Scholar]
- 25.T MAJ. The influence of individualizing physical lods on speed, ceratin kinaz activity and lactate dehydrogenize in football players. Biology of Sport. 2008;25(2):135–46. [Google Scholar]
- 26.Andrzejewski MM, Chmura J, Wiacek M, Zubrzycki IZ. The influence of individualizing physical lods on speed, ceratin kinaz activity and lactate dehydrogenize in football players. Biology of Sport. 2008;25(2):135–46. [Google Scholar]
- 27.Rahnama N, Faramarzi M, Gaeini AA. Effects of Intermittent Exercise on Cardiac Troponin I and Creatine Kinase-MB. Int J Prev Med. 2011;2(1):20–3. [PMC free article] [PubMed] [Google Scholar]


