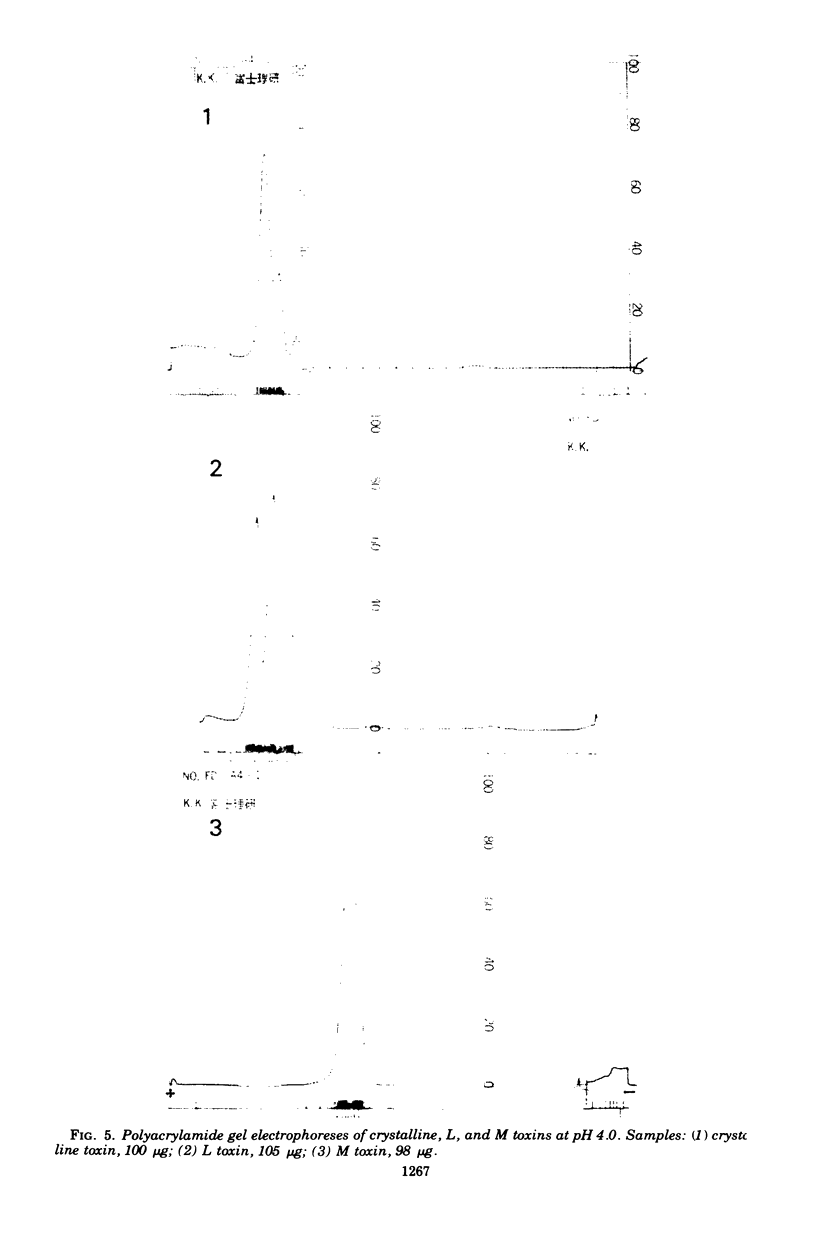
Abstract
Two Clostridium botulinum type A toxic fractions, named large (L) and medium (M) toxins, were eluted from Sephadex G-200. Sucrose density gradient centrifugation resolved L toxin (2.5 X 10(8) to 3.0 X 10(8) mean lethal doses per mg of N) into two fractions, 19S and 16S. The same procedure performed at pH 8resolved it into three fractions; the heavier two were both nontoxic and hemagglutinin positive, and the lightest on (7S) was toxic. M toxin (12S) (4.5 X 10(8) to 5.0 X 10(8) mean lethal doses per mg of N) was homogeneous in electrophoresis and centrifugation at pH 6. The latter procedure performed at pH 8 dmonstrated that it dissociated into uniform 7S components. The nontoxic component of M toxin was free from hemagglutinin. M toxin alone was demonstrated in culture by sucrose density gradient centrifugation at pH 6. Dialysis of the culture supernatant resulted in partial formation of 16S toxin. Centrifugation of the crystalline toxin in 1 MNaCl demonstrated 16S toxin only. The toxic components of L, M, and crystalline toxins were antigenically identical. The nontoxic components of the crystalline and L toxins, consisting of two distinct antigens, were antigenically identical; that of M toxin was identical with one of these two antigens.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroff D. A., Fleck U. Statistical analysis of a rapid in vivo method for the titration of the toxin of Clostridium botulinum. J Bacteriol. 1966 Nov;92(5):1580–1581. doi: 10.1128/jb.92.5.1580-1581.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUFF J. T., KLERER J., BIBLER R. H., MOORE D. E., GOTTFRIED C., WRIGHT G. G. Studies on immunity to toxins of Clostridium botulinum. II. Production and purification of type B toxin for toxoid. J Bacteriol. 1957 May;73(5):597–601. doi: 10.1128/jb.73.5.597-601.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUFF J. T., WRIGHT G. G., KLERER J., MOORE D. E., BIBLER R. H. Studies on immunity to toxins of Clostridium botulinum. I. A simplified procedure for isolation of type A toxin. J Bacteriol. 1957 Jan;73(1):42–47. doi: 10.1128/jb.73.1.42-47.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta B. R., Boroff D. A. Separation of toxin and hemagglutinin from crystalline toxin of Clostridium botulinum type A by anion exchange chromatography and determination of their dimensions by gel filtration. J Biol Chem. 1968 Mar 10;243(5):1065–1072. [PubMed] [Google Scholar]
- Dasgupta B. R., Boroff D. A., Cheong K. Cation-exchange chromatography of Clostridium botulinum type A toxin on amberlite IRC-50 resin at pH 5.55. Biochim Biophys Acta. 1968 Dec 3;168(3):522–531. doi: 10.1016/0005-2795(68)90185-2. [DOI] [PubMed] [Google Scholar]
- Dasgupta B. R., Boroff D. A., Rothstein E. Chromatographic fractionation of the crystalline toxin of Clostridium botulinum type A. Biochem Biophys Res Commun. 1966 Mar 22;22(6):750–756. doi: 10.1016/0006-291x(66)90212-9. [DOI] [PubMed] [Google Scholar]
- Hauschild A. H., Hilsheimer R. Heterogeneity of Clstridium botulinum type A toxin. Can J Microbiol. 1968 Jul;14(7):805–807. doi: 10.1139/m68-133. [DOI] [PubMed] [Google Scholar]
- Kitamura M., Sakaguchi S., Sakaguchi G. Purification and some properties of Clostridium botulinum type-E toxin. Biochim Biophys Acta. 1968 Oct 21;168(2):207–217. doi: 10.1016/0005-2795(68)90144-x. [DOI] [PubMed] [Google Scholar]
- Kozaki S., Sakaguchi S., Sakaguchi G. Purification and some properties of progenitor toxins of Clostridium botulinum type B. Infect Immun. 1974 Oct;10(4):750–756. doi: 10.1128/iai.10.4.750-756.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWENTHAL J. P., LAMANNA C. Characterization of botulinal hemagglutination. Am J Hyg. 1953 Jan;57(1):46–59. doi: 10.1093/oxfordjournals.aje.a119562. [DOI] [PubMed] [Google Scholar]
- LOWENTHAL J. P., LAMANNA C. Factors affecting the botulinal hemagglutination reaction, and the relationship between hemagglutinating activity and toxicity of toxin preparations. Am J Hyg. 1951 Nov;54(3):342–353. doi: 10.1093/oxfordjournals.aje.a119491. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamanna C., Eklund H. W., McElroy O. E. Botulinum Toxin (Type A); Including a Study of Shaking with Chloroform as a Step in the Isolation Procedure. J Bacteriol. 1946 Jul;52(1):1–13. [PMC free article] [PubMed] [Google Scholar]
- Lamanna C., McElroy O. E., Eklund H. W. The Purification and Crystallization of Clostridium botulinum Type A Toxin. Science. 1946 May 17;103(2681):613–614. doi: 10.1126/science.103.2681.613. [DOI] [PubMed] [Google Scholar]
- Lamanna C., Sakaguchi G. Botulinal toxins and the problem of nomenclature of simple toxins. Bacteriol Rev. 1971 Sep;35(3):242–249. doi: 10.1128/br.35.3.242-249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamanna C., Spero L., Schantz E. J. Dependence of time to death on molecular size of botulinum toxin. Infect Immun. 1970 Apr;1(4):423–424. doi: 10.1128/iai.1.4.423-424.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi I., Sakaguchi G. Purification of Clostridium botuliunum type F progenitor toxin. Appl Microbiol. 1974 Dec;28(6):923–928. doi: 10.1128/am.28.6.923-928.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Sakaguchi G., Oishi I., Kozaki S., Sakaguchi S., Kitamura M. Molecular structures and biological activities of Clostridium botulinum toxins. Jpn J Med Sci Biol. 1974 Apr;27(2):95–99. [PubMed] [Google Scholar]
- WAGMAN J., BATEMAN J. B. Botulinum type A toxin: properties of a toxic dissociation product. Arch Biochem Biophys. 1953 Aug;45(2):375–383. doi: 10.1016/s0003-9861(53)80014-7. [DOI] [PubMed] [Google Scholar]
- WAGMAN J., BATEMAN J. B. The behavior of the bolulinus toxins in the ultracentrifuge. Arch Biochem Biophys. 1951 May;31(3):424–430. doi: 10.1016/0003-9861(51)90158-0. [DOI] [PubMed] [Google Scholar]