ABSTRACT
Amidst growing concerns over the spread of antibiotic-resistant Staphylococcus aureus strains, the identification of alternative therapeutic molecules has become paramount. Previously, we utilized a Caenorhabditis elegans–S. aureus screening platform to identify potential anti-infective agents from a collection of natural extracts and synthetic compounds. One of the hits obtained from the screen was the aqueous extract of Orthosiphon stamineus leaves (UE-12) that enhanced the survival of infected nematodes without interfering with bacterial growth. In this study, we used a fluorescent transgenic reporter strain and observed that the repressed expression of the lys-7 defense gene in infected nematodes was restored in the presence of UE-12. Analysis of a selected panel of PMK-1 and DAF-16-regulated transcripts and loss-of-function mutants in these pathways indicates that the protective role of UE-12 is mediated via the p38 MAP kinase and insulin-like signaling pathways. Further analysis of a panel of known bioactive compounds of UE-12 proposed eupatorin (C18H16O7) as the possible candidate active molecule contributing to the anti-infective property of UE-12. Taken together, these findings strongly suggest that the O. stamineus leaf extract is a promising anti-infective agent that confers an advantage in survival against S. aureus infection by modulating the immune response of the infected host.
Keywords: C. elegans, S. aureus, O. stamineus, Anti-infective, Immunomodulation
INTRODUCTION
Staphylococcus aureus is a leading human pathogen associated with high mortality and morbidity in a wide spectrum of hospital- and community-acquired infections (Klevens et al., 2007; Grundmann et al., 2010). Wound infections, severe skin infections, sepsis and metastatic infections of many organ systems make up typical infections caused by this pathogen (Archer, 1998). Since the introduction of penicillin, the deployment of virtually all antibiotics has been followed by the evolution of clinically significant antibiotic resistance (Perez et al., 2008). This phenomenon begs for fresh approaches to identifying anti-infectives with novel modes of action other than targeting pathogen viability to quell bacterial resistance.
Recently, the genetically tractable nematode Caenorhabditis elegans has been used extensively in the modeling of infectious diseases (Sifri et al., 2005). Although C. elegans has no adaptive immune system, it has comprehensive systemic immunity. There is growing appreciation that the nematode can serve as a powerful tool in drug discovery including identification of antifungal and antimicrobial compounds (Moy et al., 2006; Breger et al., 2007). To date, a wide and still expanding range of pathogens have been reported to infect C. elegans including the human pathogen S. aureus (Sifri et al., 2003; Wu et al., 2010; JebaMercy et al., 2011). S. aureus kills C. elegans via accumulation of large numbers of live bacteria within the intestinal tract (Sifri et al., 2003; Irazoqui et al., 2010) and several virulence determinants known to be important in mammalian pathogenesis are also required for full pathogenicity against nematodes (Sifri et al., 2003).
Previously, we successfully established a liquid-based C. elegans–S. aureus anti-infective screen platform that identified not only substances with anti-bacterial properties, but also hits that did not interfere with bacterial viability (Kong et al., 2014). We screened a number of natural extracts and synthetic compounds for anti-infective properties using this C. elegans system and identified two promising hits that protected the worms from both methicillin-susceptible S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA) infection. Both hits did not affect bacterial replication in vitro; on the other hand, both were able to cause a significant reduction in in vivo intestinal bacterial loads (Kong et al., 2014). We therefore postulated that these extracts may act distinctly from conventional antibiotics by modulating or enhancing the host immune system to eradicate the pathogen.
In the present study, we extend the use of this C. elegans–S. aureus infection model to dissect the possible underlying mechanism of one of the promising hits obtained i.e. the local plant product, Orthosiphon stamineus leaf extract (henceforth referred to as UE-12), in protecting the host from infection. O. stamineus has been used as folk medicine for centuries in Southeast Asia to treat urinary tract infections, diabetes, hypertension and rheumatoid disease (Ameer et al., 2012). In Europe and Japan, the leaves of O. stamineus are consumed as tea, most commonly known as java tea. As the leaves are non-toxic to humans, the potential of UE-12 in enhancing the host immune system makes this extract an attractive target for the development of a new anti-infective. Through the use of transgenic GFP reporter worms, loss-of-function C. elegans mutants and transcriptome analysis, we unlock the host molecular mechanisms and pathways associated with the activity of UE-12. We also gain insight into the active constituent of UE-12 that contributes to its anti-infective property.
RESULTS
UE-12 restores the repressed expression of the lys-7 defense gene
We had previously identified 14 natural extracts and 14 synthetic compounds that enhanced the survival by at least 2.8-fold relative to the untreated infected control worms (Kong et al., 2014). Of these 28 hits, 7 extracts and 13 compounds rescued the worms from infection by inhibiting S. aureus replication whilst another 7 extracts and 1 compound did not interfere with bacterial growth. We investigated 5 of the 8 hits that did not interfere with bacterial growth, which we term anti-infective candidates. We hypothesized that these anti-infective candidates protected the host from infection, not by direct bacteriostatic or bactericidal effects towards the bacteria, but, act on the host defense system via modulating or stimulating the immune response towards infection.
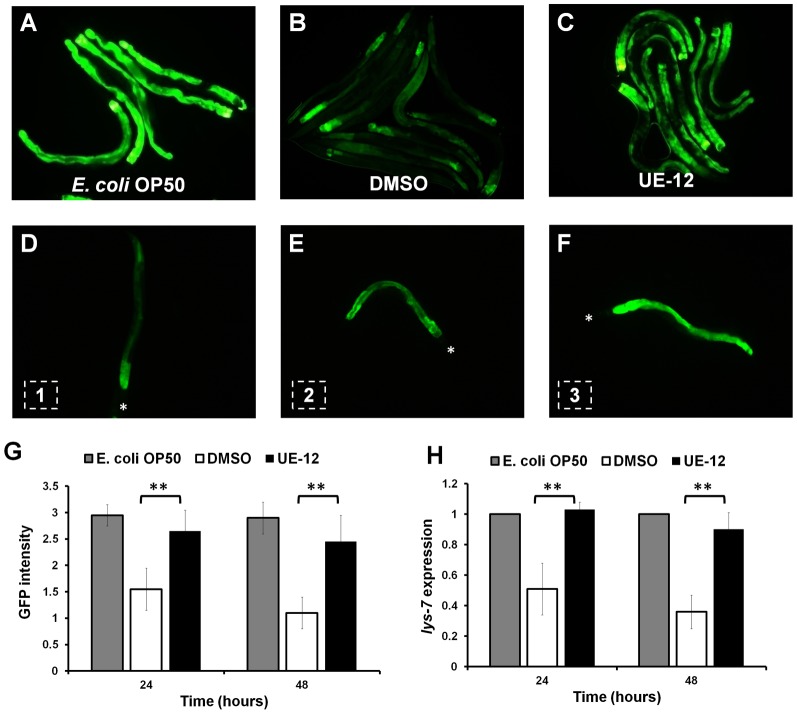
To investigate how the anti-infective candidates affected the C. elegans antimicrobial response, we tested the effect of these candidates on the expression of a host immune effector using transgenic worms carrying a transcriptional GFP reporter for the lys-7 gene. LYS-7 is an enzyme homolog of the antimicrobial lysozyme encoded by the lys4 gene of the amoeboid protozoon Entamoeba histolytica (Leippe, 1999). The transgenic animals were infected by S. aureus in the presence and absence of anti-infective candidates and fluorescence intensity was visualized and compared at 24 and 48 hours post-infection (hpi). As shown in Fig. 1A and Fig. 1B, at 48 hpi, nematodes infected by S. aureus in the absence of anti-infective candidates showed a significant reduction in the overall GFP intensity as compared to the uninfected population fed on the normal food source, Escherichia coli OP50. The uninfected worms showed a consistently strong green fluorescence along the intestine throughout the observation period (Fig. 1A) whereas S. aureus-infected worms exhibited weak fluorescence except at the posterior part of the intestine (Fig. 1B). Interestingly, among the anti-infective candidates tested, the UE-12 extract was able to prevent suppression of GFP expression by S. aureus (Fig. 1C). By contrast, UE-01-4, UE-03-6, UE-11 and UC-10 failed to prevent the suppression of GFP expression by S. aureus (supplementary material Fig. S1). Next, we quantified the effect of UE-12 treatment on GFP intensity at 100× magnification. Worms were given a score of 1 when only the anterior part of the intestine fluoresced (Fig. 1D), a score of 2 for worms with intermediate fluorescence along the intestine (Fig. 1E) and a score of 3 for worms with intense fluorescence throughout the intestinal tract at 100× magnification (Fig. 1F). Based on the defined criteria, we confirmed that the green fluorescence intensity corresponding to lys-7 expression was significantly higher in the infected worms treated with UE-12 compared to the non-treated infected worms (Fig. 1G, p<0.001, n = 20 worms per treatment).
Fig. 1. UE-12 replenishes the repressed expression of the lys-7 defense gene.
Representative fluorescence micrographs of the population of transgenic plys-7::gfp worms (A) fed on E. coli OP50 (uninfected), (B) infected with S. aureus in the absence of UE-12 (animals were exposed to DMSO only) and (C) infected with S. aureus in the presence of UE-12 (100× magnification). (D,E,F) The criteria used for scoring of plys-7::gfp transgene expression upon S. aureus infection in the presence and absence of extract. Each worm was given a score of 1, 2 or 3 depending on the GFP intensity observed. Asterisks mark the head of the worms. (G) Bars represent the mean ± SD of GFP intensity scores (expressed in arbitrary units) corresponding to the lys-7 expression for each worm (n = 20) according to the criteria presented in panels D, E and F. (H) qRT-PCR analysis of lys-7 mRNA shows significant induction of lys-7 expression in infected animals following treatment with UE-12 at 24 and 48 hpi. **A significant difference between untreated (DMSO) and UE-12-treated worms (p<0.001).
We also quantified the mRNA levels of lys-7 by quantitative real time PCR under the same experimental conditions. Consistent with the GFP data, UE-12 was able to prevent the suppression of lys-7 expression by S. aureus at both 24 and 48 hpi (Fig. 1H, p<0.001). We thus selected UE-12 as our candidate anti-infective agent to elucidate its potential impact on host immunity.
UE-12 confers a survival advantage against S. aureus infection by reducing the number of viable S. aureus in the C. elegans intestine
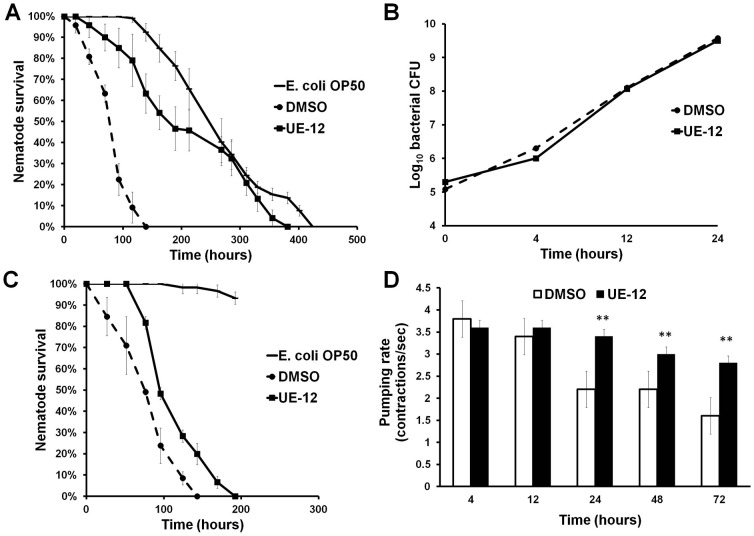
To further evaluate the protective effect of UE-12, we monitored the survival of both UE-12 and DMSO (control)-treated nematodes infected with S. aureus every 24 hours. An additional control group of worms exposed to E. coli OP50 was assayed in parallel. As expected, S. aureus killed the worms significantly faster with a mean time to death (TDmean) of 85.4±2.8 hours compared to the E. coli exposed population (270.9±7.6 hours; p<0.0001). However, in the presence of UE-12, S. aureus-infected worms had an overall survival that was significantly improved with a TDmean of 215.8±9.4 hours (p<0.0001) (Fig. 2A). When the infection was performed using the conventional agar-based C. elegans infection assays, UE-12 was also able to enhance the survival of infected nematodes on the agar medium, albeit to a lesser extent compared to the liquid medium (supplementary material Fig. S2). This difference may be due to inadequate exposure or absorption of UE-12 extract by the host (Zheng et al., 2013). Given that the protection effect was more pronounced in the liquid-based assay, we chose this medium for all the following experiments.
Fig. 2. UE-12 enhances the survival of S. aureus-infected nematodes without interfering with bacterial growth.
(A) S. aureus infection assay on wild-type N2 nematodes exposed to 200 µg/mL of UE-12 compared to untreated control exposed to solvent (DMSO) only. UE-12 confers significant lifespan extension in infected nematodes (p<0.0001). Graph shows the mean ± SD of six replicates (20 nematodes/replicate) from a representative of three independent assays. (B) UE-12 does not inhibit the growth of S. aureus in the same medium used for C. elegans infection assay, comprising 80% M9 buffer and 20% S. aureus culture. Data are the average of three independent experiments with error bars representing the standard error of the mean. (C) S. aureus infection assay on wild-type N2 nematodes pre-treated with 200 µg/mL of UE-12 for 24 hours compared to untreated control exposed to solvent (DMSO) only. The worms pre-treated with UE-12 were then infected with S. aureus in the absence of extract. The survival curves indicate significantly enhanced survival over time in UE-12-treated worms as compared to the control (p<0.0001). Graph shows the mean ± SD of six replicates (20 nematodes/replicate) from a representative of three independent assays. (D) No significant difference in pharyngeal pumping rates is observed for the first 4 and 12 hours. The pumping rates of untreated animals are significantly lower than the UE-12-treated animals after 24, 48 and 72 hours infection. The bars correspond to mean ± SD of the contractions/second from one representative of two individual replicates. **A significant difference between untreated (DMSO) and treated worms (p<0.001).
We previously showed by disc diffusion and MIC microdilution tests that UE-12 at 2000 µg/ml did not inhibit or limit S. aureus growth in vitro (Kong et al., 2014). To further verify these findings, we examined the growth of S. aureus in the presence (200 µg/ml) and absence of UE-12 based on the number of CFU enumerated under similar conditions as the infection assay. The initial bacterial inoculum was ∼1.5×105 and over time, an increase in the CFU indicated bacterial replication in medium consisting of 20% TSB and 80% M9 buffer confirming that UE-12 did not affect S. aureus growth (Fig. 2B). Hence, we have verified that the enhanced survival of infected worms in the presence of UE-12 extract was not due to the direct killing of S. aureus by UE-12.
To determine if the protective effect conferred by UE-12 is mediated via the host, we pre-treated the worms with UE-12 for 24 hours followed by washing in M9 medium and finally infecting these worms with S. aureus in the absence of UE-12. As shown in Fig. 2C, a significant lifespan extension was also observed in the infected worms pre-treated with UE-12 extract (p<0.0001). The TDmean for UE-12-treated animals was significantly longer (118±4.6 hours) compared to the untreated infected population (83.2±4.6 hours). Nevertheless, the protective effect under these conditions was not as prominent as that observed when worms were continuously exposed to the extract (Fig. 2A), suggesting that continuous exposure to UE-12 is required to achieve the maximal anti-infective effect. This observed difference also proposes that UE-12 may target both pathogen virulence in tandem with host immunity.
In the earlier study, we further demonstrated a marked reduction in bacterial loads and no evidence of colonization in the gut of UE-12-treated infected nematodes at 12 and 24 hpi (Kong et al., 2014). To rule out the possibility that the lower intestinal burden was due to UE-12 decreasing pharyngeal pumping rates and thus, lowering uptake of bacteria, we determined pharyngeal pumping rates of S. aureus-infected animals in the presence of UE-12. As had been previously reported (Sifri et al., 2003; JebaMercy et al., 2011), as the infection continued, there was a time-dependent decline in pumping rates of untreated infected animals (Fig. 2D). Specifically, foraging activities and pharyngeal pumping in S. aureus-infected worms appeared to be normal for the first 16 to 20 hours, but progressively decreased after 24 and 48 hpi. UE-12-treated infected worms continued to feed normally at 4 and 12 hours following infection and continued to maintain significantly higher pumping rates at 24, 48 and 72 hours compared to untreated worms (p<0.001) (Fig. 2D). Thus, the low number of intestinal bacteria could not be attributed to a decrease in bacteria uptake. Instead, other in vivo factor(s) may be responsible for the suppression in bacterial loads within the host intestine.
UE-12 does not affect the basal lifespan and reproduction of the worms
Next, we asked if UE-12 is able to extend the basal lifespan of the worm leading to better survival during an infection by comparing the lifespan of uninfected C. elegans in the presence and absence of UE-12. Treatment with 200 µg/mL UE-12 was begun at the young adult stage in the presence of heat-killed E. coli OP50 to exclude any possible effect of UE-12 on the bacteria. Both treated and untreated animals had a mean lifespan of 22–23 days at 25°C in liquid medium (p>0.01) (Fig. 3A), indicating that the enhanced survival of S. aureus-infected worms exposed to UE-12 is unlikely to be a simple consequence of any lifespan extending property of UE-12. No shortening of lifespan was noted in UE-12-treated animals, indicating that UE-12 at this concentration (200 µg/mL) did not exert any toxic effect on the adult worms.
Fig. 3. UE-12 does not affect the basal lifespan and reproduction of the worms.
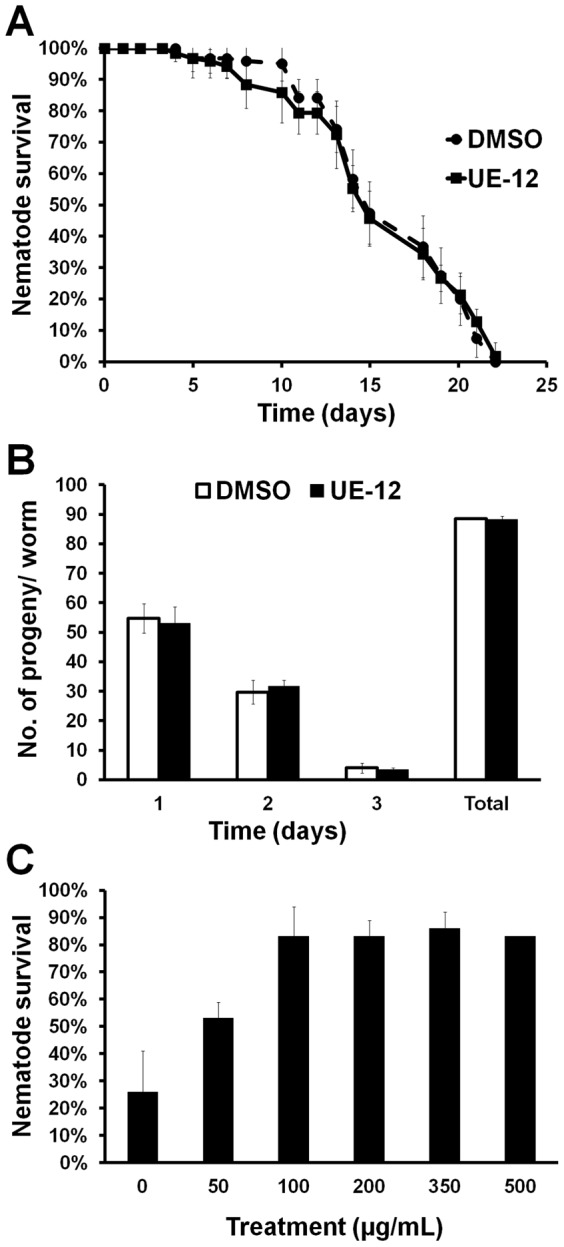
(A) UE-12 does not prolong or shorten the basal lifespan of uninfected worms. The graph represents the mean ± SD of six replicates (20 nematodes/replicate) from a representative of three independent assays. (B) Daily and cumulative reproduction output was determined from 10 individual worms. UE-12 does not influence reproduction in C. elegans. The error bars represent the standard error of the mean. (C) S. aureus infection assay of wild-type nematodes exposed to different concentrations of UE-12. Enhanced survival is observed in UE-12 at all concentrations tested (50, 100, 200, 350 and 500 µg/mL) in a dose dependent manner at 96 hpi. The bars show the mean ± SD for percentage nematode survival from one representative of two individual replicates.
We also examined if treatment with UE-12 affected C. elegans reproduction by measuring egg-laying and progeny production of UE-12-treated animals. We found that the daily and total reproductive output of the worms did not differ significantly between UE-12-treated and untreated animals (p>0.01) (Fig. 3B). In addition, we did not observe any abnormality in the fecundity of the eggs and development of the progeny into different larva stages. This suggests that UE-12 did not affect the reproduction ability of the worms nor was there any toxic effect on the progeny.
Finally, we showed that UE-12 promoted the survival of S. aureus-infected nematodes in a dose-dependent manner maxing out at 100 µg/mL (Fig. 3C). The continued positive treatment effects at 500 µg/mL suggest that even at this concentration, UE-12 is not toxic to the worms.
UE-12 promotes worm survival via the p38 MAP kinase and insulin-like signaling pathways
Previous studies have shown that three major highly conserved signal transduction pathways, the nsy-1/sek-1/pmk-1 p38 MAP kinase pathway, the daf-2/daf-16 insulin/IGF1 pathway and the dbl-1/TGF-β signaling pathway, play significant roles in the survival of the host during infection (Kim et al., 2002; Mallo et al., 2002; Garsin et al., 2003). Using readily available C. elegans mutants for these three major signaling pathways, we asked if any of these pathways mediate the protective effect of UE-12 against S. aureus infection. We hypothesized that if a specific pathway is required for the protective effect of UE-12, then UE-12 will be unable to promote survival of the associated mutant following infection.
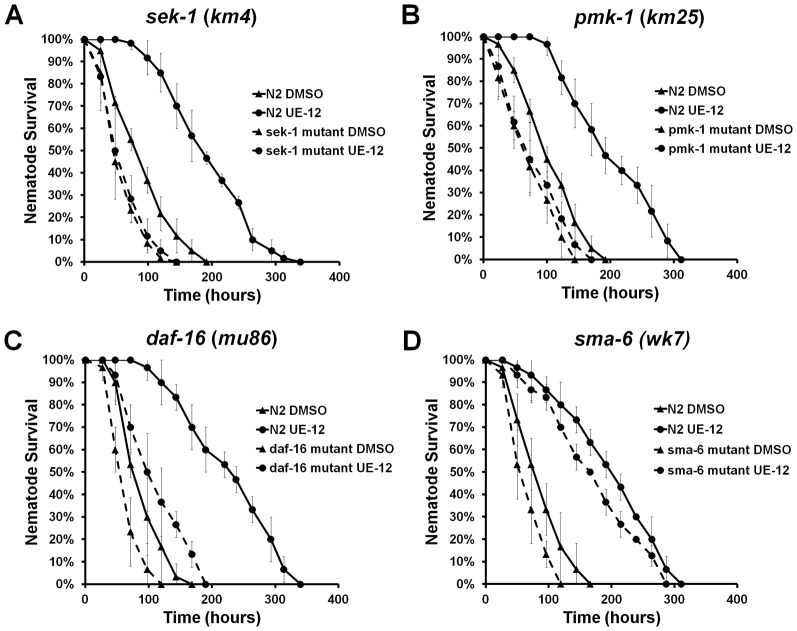
Since we used the liquid based assay rather than the conventional agar-based assay, we first determined the sensitivity of these well-characterized C. elegans mutants to killing by S. aureus in a liquid-based assay. In agreement with reports using an agar-based assay, the sek-1 (km4) mutant is more susceptible to killing by S. aureus in the liquid killing assay (supplementary material Fig. S3A) (Sifri et al., 2003). Additionally, we found that the pmk-1 (km25), daf-16 (mu86) and sma-6 (wk7) mutants were also hypersensitive to S. aureus infection under the same conditions (p<0.0001) (supplementary material Fig. S3B–D). The ability of UE-12 to enhance worm survival was completely abrogated in animals devoid of SEK-1 and PMK-1 (Fig. 4A,B, p>0.05) and partially attenuated in the daf-16 (mu86) null mutant (Fig. 4C, p<0.0001). In contrast, the protective effect of UE-12 was unaffected in the sma-6 (wk7) mutant infected by S. aureus (Fig. 4D, p<0.0001) even though the sma-6 mutation conferred enhanced sensitivity to S. aureus (supplementary material Fig. S3D). This suggests that the SMA/TGF-β pathway is not required for the anti-infective activity of UE-12. On the other hand, failure of UE-12 to protect sek-1 (km4) and pmk-1 (km25) mutants indicates that the p38 MAP kinase signaling pathway is necessary for the anti-infective activity of UE-12. Although a significant lifespan extension was observed in the daf-16 (mu86) mutant infected by S. aureus, the effect was less pronounced than that observed in wild-type C. elegans, suggesting that the positive effect of UE-12 was only partially dependent on the insulin-like signaling pathway.
Fig. 4. UE-12 requires the C. elegans p38 MAP kinase and insulin-like signaling pathways to confer its full protective effect.
The survival curves of wild-type N2 and (A) sek-1 (km4) mutant animals, (B) pmk-1 (km25) mutants, (C) daf-16 (mu86) mutants and (D) sma-6 (wk-7) mutants following treatment with UE-12 or DMSO. The effect of UE-12 is diminished in sek-1 (km4) and pmk-1 (km25) mutants whilst partially attenuated in daf-16 (mu86) mutants. In sma-6 (wk7) mutants, UE-12 demonstrated a similar effect as in wild-type N2 animals (p<0.0001). Data at each time point are the average of six wells with each well containing 20 animals per well. Data are representative of three independent experiments.
UE-12 induces PMK-1 and DAF-16-regulated genes
To gain further insights into how p38 MAP kinase and insulin signaling mediate the protective effects of UE-12, we performed transcriptome analysis on a selected panel of PMK-1 and DAF-16-regulated genes using quantitative real-time PCR. The selected PMK-1-regulated genes comprised mainly presumptive antimicrobial candidates, namely CUB-like genes (C17H12.8, dod-24, F35E12.5 and F55G11.8) (Alper et al., 2007), ShK toxin genes (T24B8.5 and C14C6.5) (Nicholas and Hodgkin, 2004; O'Rourke et al., 2006) and C-type lectin genes (clec-67 and M02F4.7) (Nicholas and Hodgkin, 2004; O'Rourke et al., 2006; Alper et al., 2007). These genes were also highly regulated in response to pathogen infection (Troemel et al., 2006). Total RNA was harvested from untreated or UE-12-treated S. aureus-infected nematodes at 48 hpi. At this time point, ∼20% mortality was observed in the untreated infected worms.
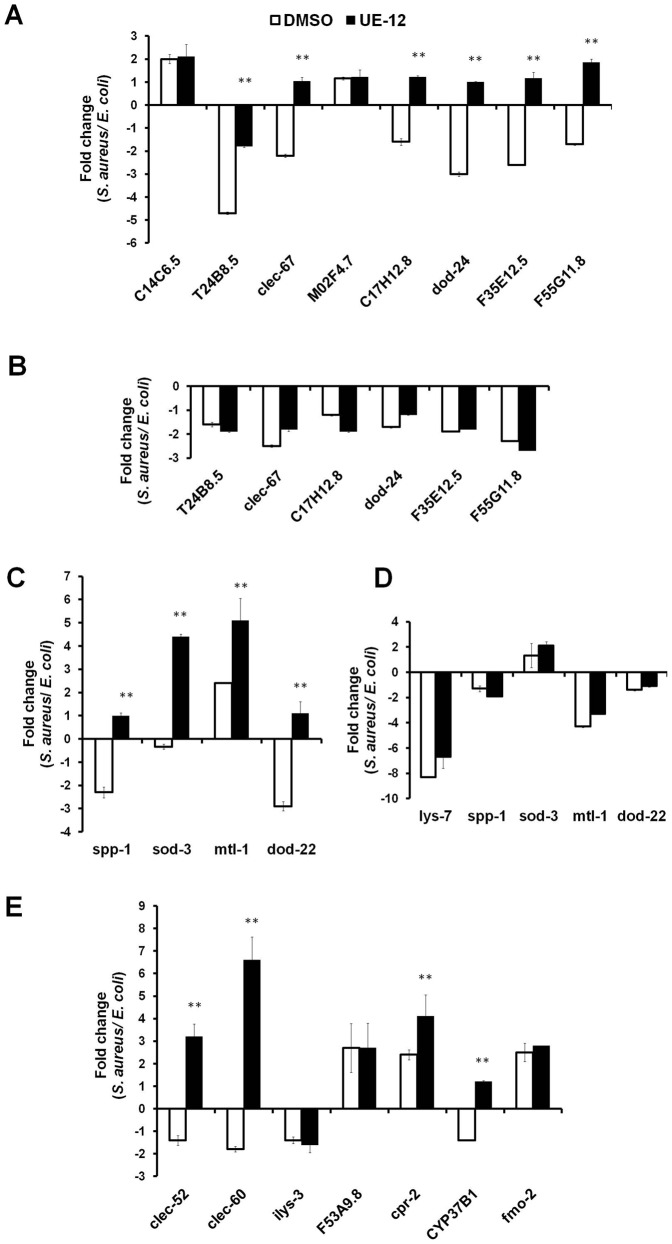
Six of the eight PMK-1-regulated genes were downregulated (∼2–5-fold) following 48 hours infection by S. aureus of worms that were not treated with UE-12 (Fig. 5A). On the other hand, in infected worms treated with 200 µg/mL UE-12, five of these 6 genes – clec-67, C17H12.8, dod-24, F35E12.5 and F55G11.8 – demonstrated a 2.8–4-fold induction in expression upon supplementation with UE-12 (p<0.05), implying that UE-12 promoted the host antimicrobial response. UE-12 also significantly reduced the suppression of T24B8.5 from ∼5-fold to ∼2-fold (p<0.05) (Fig. 5A).
Fig. 5. The expression of putative immune response genes is modulated in worms exposed to UE-12.
(A,C,E) Selected panel of immune response genes analyzed by qRT-PCR in UE-12-treated nematodes and compared to untreated animals (DMSO) following infection by S. aureus. The fold change of all genes tested is relative to the uninfected population (exposed to E. coli OP50). (A) PMK-1 regulated genes. (C) DAF-16 targets. (E) Genes that were reported to be highly regulated in S. aureus infection. (B) Six PMK-1 target genes that were found to be significantly modulated in the presence of UE-12 were tested in the pmk-1 (km25) mutant. (D) Five DAF-16 target genes that were found to be significantly modulated upon exposure to UE-12 were tested in the daf-16 (mu86) mutant. Data are the average of three replicates, each normalized to changes in three primer pairs that were found to not vary with infection. The error bars represent the standard error of the mean. **A significant difference between untreated (DMSO) and UE-12-treated worms (p<0.05).
We next seek to confirm that the effect of UE-12 is dependent on the presence of pmk-1. We infected pmk-1 (km25) mutant worms with the pathogen in the presence and absence of UE-12 and harvested the RNA for real time PCR analysis. We failed to observe any significant difference in the expression of these genes in both treated and untreated worms (Fig. 5B) further supporting the significant role played by the p38 MAP kinase signaling pathway in activating host defense responses in the presence of UE-12.
As the effect of UE-12 was partially attenuated in daf-16 (mu86) mutants, we analyzed the expression of four selected DAF-16-regulated genes to evaluate the involvement of the insulin-like signaling pathway in the UE-12 mediated protective effect. These four genes were candidate antimicrobial genes (spp-1 and dod-22) (Murphy et al., 2003; Alper et al., 2007) and stress adaptation genes (mtl-1 and sod-3) (Murphy et al., 2003). In the absence of UE-12, spp-1 and dod-22 were downregulated (2–3-fold), mtl-1 was upregulated (∼2.5-fold) whilst sod-3 was unaffected (Fig. 5C). In infected worms treated with UE-12, all four genes were significantly induced by 3.3–7.5-fold (p<0.05) (Fig. 5C), indicating that UE-12 was able to boost the expression of DAF-16-regulated genes. To confirm that the modulation of these genes require the presence of DAF-16, we profiled the expression of all four DAF-16 targets as well as lys-7 (Fig. 1H) in the daf-16 (mu86) mutant background. No significant difference was observed in the expression of these genes in both treated and untreated daf-16 mutant animals (Fig. 5D). Hence, we confirm that the effect of UE-12 is daf-16 dependent.
A previous study reported that the expression of both lys-7 and dod-22 were co-regulated by the dbl-1/TGF-β signaling pathway (Alper et al., 2007). Therefore, we also tested the expression of these genes in UE-12-treated and untreated sma-6 (wk7) mutant worms infected by S. aureus. Similar to the profile observed in wild-type N2 worms, in a sma-6 mutant background, the suppression of lys-7 and dod-22 by S. aureus was significantly induced in the presence of UE-12 extract (supplementary material Fig. S4), suggesting that the modulation of these two genes by UE-12 did not require the presence of sma-6.
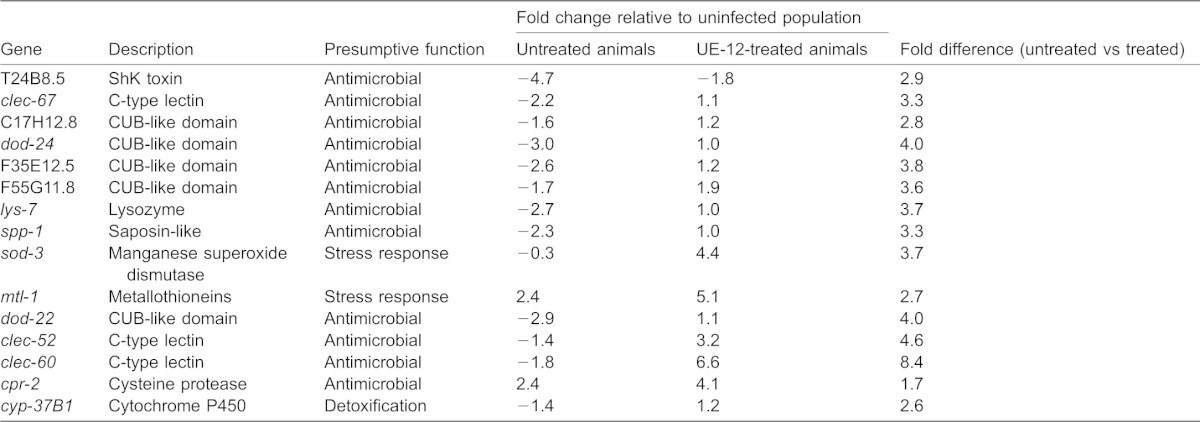
In addition, we measured transcript levels of seven genes that were strongly induced during the early phase of infection by S. aureus (4, 8 and 12 hours) (Irazoqui et al., 2010). They consist of candidate antimicrobial genes (clec-52, clec-60, ilys-3, F53A9.8 and cpr-2) and detoxification genes (cyp-37B1 and fmo-2). We found that during the late stage of infection (48 hpi), expression of clec-52, clec-60, ilys-3 and cyp-37B1 was downregulated, an effect that was reversed when infected worms were exposed to UE-12 (Fig. 5E). Specifically, the expression of clec-52, clec-60 and cyp-37B1 was induced significantly (p<0.05). Moreover, cpr-2 demonstrated a further induction (p<0.05). At this late time point, the expression of ilys-3, F53A9.8 and fmo-2 remained unchanged in treated and untreated animals (Fig. 5E). The fold difference for genes significantly modulated by UE-12 is shown in Table 1.
Table 1. C. elegans genes significantly modulated by UE-12.
We further tested if UE-12 is able to stimulate host innate immunity in the absence of infection. We measured mRNA levels of 11 immune effectors in worms grown in the presence and absence of UE-12 by qRT-PCR. Interestingly, the expression of none of these genes was significantly altered by UE-12 (supplementary material Fig. S5), implying that UE-12 does not affect the basal expression of these genes. Hence, we conclude that the effect of UE-12 is not a general stimulation of host immunity; instead, UE-12 modulates the host immune response towards pathogen infection.
In general, we noticed the suppression of host putative antimicrobial genes following 48 hours infection by S. aureus. Conversely, treatment with UE-12 resulted in a significant induction of 12/16 (75%) host antimicrobial genes tested. Thus, the protective effect of UE-12 is through the enhanced expression of host putative immune effectors upon pathogen infection, leading to the increased ability of infected nematodes to clear the bacteria and survive infection by S. aureus.
Eupatorin is the active ingredient that contributes to the anti-infective effect of UE-12
The availability of anecdotal evidence on the use of O. stamineus in folk medicine has led to substantial interest in O. stamineus and its traditional uses as indicated by the extensive volume of research devoted to this plant, including the identification of the plant extract phytochemical content. Several active chemical constituents that are prominently present in this plant are flavonoids (sinensetin and eupatorin) (Laavola et al., 2012; Yam et al., 2012) and a caffeic acid derivative (rosmarinic acid) (Olah et al., 2003; Pan et al., 2011). The chemical structures of sinensetin, eupatorin and rosmarinic acid are presented in supplementary material Fig. S6. We screened these compounds for their contribution to the anti-infective activity of UE-12. Both flavonoids sinensetin and eupatorin were able to enhance the survival of S. aureus-infected nematodes (Fig. 6A). At 96 hpi, a time point when the survival rate of untreated infected animals remained at 26%, sinensetin and eupatorin significantly increased survival to 53% and 83%, respectively (p<0.05) (Fig. 6A). Subsequently, we performed an extended time course based survival assay with a higher number of worms to validate these results and obtain the TDmean. From the time course study, we observed that eupatorin displayed equally effective curing activity as UE-12 whilst sinensetin weakly promoted nematode survival (Fig. 6B). The worms infected by S. aureus in the absence of any treatment had a TDmean of 94.4±4.5 hours. In the presence of eupatorin, the TDmean achieved 198.4±12.8 hours, which was comparable to that of UE-12 (203.5±14 hours) and significantly longer than that of untreated animals (p<0.0001). Sinensetin and rosmarinic acid only marginally extended the TDmean to 117.2±8.2 hours and 100.5±5.2 hours, respectively (p>0.0001). Moreover, similar to the lack of anti-infective effects of UE-12 on S. aureus-infected worms (Fig. 4B), eupatorin failed to protect pmk-1 (km25) mutants from infection (Fig. 6C). Together, these findings suggest that eupatorin is likely the main bioactive component in UE-12 that promotes the survival of S. aureus-infected worms.
Fig. 6. Eupatorin exerts a similar effect as UE-12 crude extract.
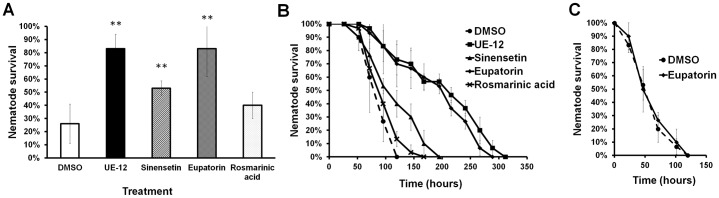
(A) S. aureus infection assay of wild-type nematodes exposed to sinensetin, eupatorin and rosmarinic acid, respectively. The graph depicts the mean percentage of survival ± SD at 96 hpi of three replicates from a representative of two individual experiments. (B) Survival of S. aureus-infected worms is significantly improved over time upon supplementation with eupatorin. In a pair-wise comparison to untreated animals (DMSO) using log-rank tests, the difference is significant (p<0.0001). (C) The effect of eupatorin is completely impaired in pmk-1 (km25) mutants. The graph shows the mean ± SD of six replicates (20 nematodes/replicate) from a representative of three independent assays. **A significant difference between untreated (DMSO) and treated worms (p<0.05).
DISCUSSION
Natural products have long been a source of therapeutics and continue to contribute significantly to the development of today's pharmaceuticals (Cragg et al., 1997). Previous studies on validation of traditional uses or on drug discovery have focused more on the antibacterial potential of medicinal plants (Cowan, 1999; Mahady et al., 2008). However, these bactericidal compounds are still capable of promoting the emergence of resistance. The ability of plant-derived natural products to boost the competence of the host immune system to defend against pathogen attack has received much less attention although this strategy may be just as important in combating infection. Taking advantage of the tractability and simplicity of C. elegans as an intact host model, we characterized the anti-infective effect of O. stamineus leaf extract (UE-12) in the context of a whole organism. As O. stamineus leaves are consumed daily by a number of different cultures and have good anecdotal evidence for treatment of infections, diabetes and hypertension (Ameer et al., 2012), the leaf extract is expected to not cause undesirable adverse effects. We provide evidence that the aqueous extract of O. stamineus leaves protect the host from infection by modulating the host innate immune response towards S. aereus. The extract modulated the host immune response via the evolutionarily conserved p38 MAP kinase pathway and insulin-like signaling pathway. Most notably, the expression of putative immune effectors regulated by PMK-1 and DAF-16 was induced in infected worms following exposure to this extract. We also present evidence that the immunomodulatory effect of this extract is most likely contributed by eupatorin, a flavonoid compound present in O. stamineus extract.
Studies to evaluate the antimicrobial properties of O. stamineus showed that the aqueous extract exhibited antibacterial action against S. aureus (Ho et al., 2010; Alshawsh et al., 2012). However, the MIC value reported was 1.56 mg/mL, which is much higher than the concentration used in our studies i.e. 200 µg/mL. Recently, Alshawsh et al. reported that O. stamineus extract has cellular immunomodulatory effects in vitro (Alshawsh et al., 2012). Specifically, O. stamineus significantly stimulated the proliferation of peripheral blood mononuclear cells in a dose-dependent manner, suggesting a role in modulating the cellular immune response. The protective role of this extract in our study, however, proposes a novel activity of targeting the innate immune response.
C. elegans has no adaptive immune system and the animal is believed to depend purely upon the secretion and action of antimicrobial molecules, including lysozymes (Schulenburg and Boehnisch, 2008), lectins (Schulenburg et al., 2008), caenopores (Roeder et al., 2010) and antibacterial factors (Kato et al., 2002). The primary route and major site of infection for S. aureus in a C. elegans model is through the intestine (Sifri et al., 2003). S. aureus accumulate and distend the worm intestinal lumen thereby killing the host. Interestingly, most of the antimicrobial effector genes modulated by UE-12 (clec-67, dod-24, lys-7, spp-1, dod-22, clec-52 and clec-60) are expressed predominantly in the intestine. Hence, the induced expression of these genes aid in the elimination of the bacteria from the host, eventually clearing the infection and resulting in increased survival of worms with low numbers of intestinal live S. aureus. A previous study has reported that lys-7 mRNA levels are upregulated at 24 hpi, followed by a decrease at 36 and 48 hpi by S. aureus strain ATCC11632 based on a liquid assay (JebaMercy et al., 2011). Nevertheless, in our study, both GFP and real time PCR data showed a homogenous suppression of lys-7 expression at 24 and 48 hpi (Fig. 1G,H). This difference could be explained by the different experimental settings such as the virulence of bacterial strain tested, bacterial culture conditions and assay medium as previously reported for other human pathogens (Tan et al., 1999; Garsin et al., 2001; Lee et al., 2011).
As the inexorable invasion by antibiotic-resistant pathogens continues to threaten the community, there is a pressing need for novel forms of therapy. Anti-infectives that target bacterial virulence or host immunity without affecting microbial cell viability offer an alternative to conventional antibiotics. Recently, through high throughput virtual screening, Khodaverdian et al. discovered a small number of compounds that inhibited the production of hemolysin and phenol-soluble modulin of MRSA without inhibiting bacterial growth (Khodaverdian et al., 2013). Interference with bacterial quorum sensing and biofilm production also appears to represent a promising intervention strategy (Wu et al., 2004; Balaban et al., 2007). At this juncture, we cannot rule out the possibility that UE-12 extract may also affect bacterial virulence as the plant extract contains numerous active constituents (Yam et al., 2012). It has been reported that plants possess compounds that can attenuate the virulence of a pathogen by inhibiting virulence factors including quorum sensing and biofilm formation (Adonizio et al., 2008; Rudrappa and Bais, 2008). The effect of UE-12 extract on S. aureus protease, hemolysin, lipase and biofilm production were accessed through several simple in vitro approaches but we did not observe any significant inhibition in production of these factors (data not shown), indicating that the extract did not disrupt the production of these virulence factors. However, we have not ruled out the possibility that these factors may be inactivated upon exposure to UE-12. The effect of UE-12 on other bacterial virulence traits is yet to be determined.
Immunomodulatory drugs targeting host immunity have been proposed as a possible alternative to conventional antibiotics (Hancock et al., 2012). This approach has been minimally employed to combat bacterial infection but is a mainstay of anti-cancer (Waldmann, 2006) and anti-viral therapy (DesJardin and Snydman, 1998; Trnková et al., 2012). A recent study by Kindrachuk et al. demonstrated that the secreted peptide from bacteria, bacteriocin, was able to augment chemokine production by human cells, leading to protection against bacterial infection in animal models (Kindrachuk et al., 2013). In the C. elegans model, a small molecule RPW-24 (Pukkila-Worley et al., 2012) and the alkaloid compound Harmane (Jakobsen et al., 2013) were reported to be able to modulate or stimulate the host innate immune response upon pathogen infection, resulting in enhanced survival of infected nematodes. Additionally, the red seaweed Chondrus crispus has recently been reported to promote the host immune responses towards Pseudomonas aeruginosa infection by inducing the expression of C. elegans innate immune genes via highly conserved pathways (Liu et al., 2013). We have shown that UE-12 activity is dependent primarily on the p38 MAP kinase and secondarily on the insulin/DAF-2 signaling pathways. As these signaling pathways are highly conserved evolutionarily, it is conceivable that findings from the nematode model could be extended to higher organisms, including humans. The immunomodulatory effect observed in this study, together with the findings in aforementioned studies, provide a scaffold upon which further approaches to treating bacterial infections by targeting the immune system are possible.
Hyperactivation or dysregulation of the immune response may contribute to life-threatening infectious and inflammatory disorders. Therefore, it is vital to study the toxic effect of a compound that confers any survival advantage to the host. The C. elegans whole animal model allows for early and direct assessment of in vivo drug efficacy, thus, eliminating compounds that are toxic to the host or with poor pharmacokinetic properties. Unlike the previously reported small molecule RPW-24 that exerted an immunostimulatory effect on the host (Pukkila-Worley et al., 2012), no observable toxic effect was detected on adult worms as well as the offspring exposed to UE-12. Furthermore, the extract did not affect the basal expression of the immune response genes under uninfected conditions. In light of this, we assume that UE-12 did not over stimulate host immunity, but rather, modulated the immune response at a level sufficient to overcome the infection whilst having no detrimental effect on the host. Thus, our findings encourage the development of the largely unexplored natural resources for immunomodulatory properties as alternative forms of treatment.
In summary, through a series of in vivo experiments involving infection of an intact host, we provide evidence that the aqueous extract of O. stamineus leaves markedly promotes the survival of S. aureus-infected nematodes by modulating the expression of immune-related genes. Of note are the candidate antimicrobial genes which are crucial for host defense during infection and furthermore, the extract did not have any notable adverse effect on the animal or its offspring. We further suggest that the flavanoid eupatorin is likely to be the active element in this extract that confers a protective advantage towards pathogen infection.
MATERIALS AND METHODS
Bacterial and nematode strains and growth conditions
S. aureus strain NCTC8325-4 was routinely propagated on Trypticase Soy (TS) agar (Pronadisa, Spain) at 37°C whilst E. coli strain OP50 was cultured on Luria Bertani (LB) media (Pronadisa, Spain) supplemented with streptomycin (100 µg/mL). The C. elegans strains used in this study were: wild-type N2 Bristol (Brenner, 1974), pmk-1 (km25) (Troemel et al., 2006), sek-1 (km4) (Troemel et al., 2006), daf-16 (mu86) (Evans et al., 2008), sma-6 (wk7) (Luo et al., 2009) and transgenic strain plys-7::GFP (Evans et al., 2008). All worm strains were obtained from the Tan Laboratory, Stanford University, USA. Growth and manipulation of C. elegans were performed as previously described (Brenner, 1974).
Preparation of extract and compounds
The preparation of UE-12 aqueous extract has been previously described (Kong et al., 2014). Briefly, Orthosiphon stamineus leaves were collected and taken through a series of stringent microbial tests as well as Atomic Absorption Spectrophotometry (AAS) to test for heavy metal presence. The leaves were then dried, ground into fine powder which was used in a water-based extraction. The microbial and trace metal element tests were repeated again on the extraction yield. The product was examined for the presence of functional groups/molecules by Fourier transform infrared spectroscopy (FTIR) and High-performance liquid chromatography (HPLC). Sinensetin (CAS no. 2306-27-6), eupatorin (CAS no. 855-96-9) and rosmarinic acid (CAS no. 20283-92-5) were purchased from ChromaDex®, Inc. (Irvine, CA). The extract and compounds were dissolved in dimethyl sulfoxide (DMSO) and filtered through a 0.2 µm membrane filter (Sartorius Stedim, Germany) followed by storage at −20°C in a desiccated storage container.
Growing worms for infection and other assays
To eliminate the confounding effects of progeny during the scoring of surviving worms, wild-type and mutant C. elegans were treated to RNAi knockdown of the pos-1 gene which resulted in worms laying unhatched eggs (Tabara et al., 1999). The dsRNA directed against pos-1 was introduced into the worms by feeding. Briefly, the pos-1 RNAi clone was cultured in 100 mL LB medium supplemented with 100 µg/mL carbenicillin and incubated overnight at 37°C. The culture was concentrated 25-fold before seeding onto Nematode Growth (NG) agar supplemented with 1 mM Isopropyl β-D-1-thiogalactopyranoside (IPTG) (Promega, USA) and 100 µg/mL carbenicillin and incubated at room temperature for 24 hours. Following synchronization by hypochlorite treatment, the eggs of wild-type N2 and mutants were plated onto pos-1 RNAi plates and allowed to grow for 45 hours at 25°C until they reached young adult stage and were ready to be used in the experiment.
Infection assay
Infection assays were performed as previously described (Kong et al., 2014). Worm M9 buffer and the overnight culture of S. aureus in TS broth with 10 µg/mL of cholesterol at a ratio of 4:1 (v/v) was dispensed into a 24-well plate prior to transferring wild-type N2 worms. In the treatment wells, UE-12 aqueous extract was added at 200 µg/mL or indicated concentrations for dose-dependent tests. To investigate the effect of UE-12 in mutant background worms, the assay was prepared as above except that wild-type N2 nematodes were replaced by the mutant strain of interest. To determine which bioactive compound in UE-12 promoted the survival of nematodes infected with S. aureus, UE-12 was substituted with 50 µM of sinensetin, eupatorin or rosmarinic acid. In control wells, the extract was replaced with 1% DMSO or S. aureus was replaced by E. coli OP50. Twenty nematodes were transferred into each well and the plate was incubated at 25°C. The total number of worms amounted to 120 in six wells representing six technical replicates. Nematode survival was monitored and scored manually every 24 hours. At least three independent experiments were performed.
Lifespan assay
The C. elegans lifespan assay was carried out in liquid medium in a 24-well plate (Yu et al., 2010). A single colony of E. coli OP50 was inoculated in 100 mL of LB broth supplemented with 500 µg/mL streptomycin and incubated overnight at 37°C. The culture was concentrated 25-fold before M9 buffer was added. UE-12 at 200 µg/mL was added into the treatment wells whilst 1% DMSO was added into the control wells. Age-synchronized young adult nematodes were transferred manually to the medium and plates were incubated at 25°C. Animals were scored daily as alive or dead by gentle prodding with a platinum wire. To eliminate any possible effect of UE-12 on E. coli OP50 that might in turn extend or shorten the C. elegans lifespan, a lifespan assay was conducted with heat-killed E. coli OP50. The concentrated culture was heat-killed at 65°C for 30 mins (Van Voorhies et al., 2005) before adding the bacteria into the liquid medium. Following heat treatment, no live cells could be detected by plating undiluted cultures on LB agar. The experiment was performed in duplicate.
Reproduction assay
The effect of UE-12 on C. elegans reproduction was assayed by picking individual L4 wild-type hermaphrodites onto NG agar supplemented with 200 µg/mL of UE-12. To provide better exposure towards the extract, the UE-12 extract was spread on the NG agar before the seeding of heat-killed E. coli OP50. The control plates were supplemented with 1% DMSO. Thereafter, gravid adult worms were moved to a fresh plate each day until reproduction ceased. The offspring on the plates were allowed to grow at 25°C and the number of progeny at the L2 and L3 stage was determined. The total number of progeny from the various plates was enumerated as the final brood size. This experiment was independently repeated twice.
Feeding rate assay
The experiment was conducted as that described previously (Dharmalingam et al., 2012) with minor modifications. Worms were prepared and exposed to S. aureus according to the infection assay protocol in the presence and absence of 200 µg/mL UE-12. At 2, 4, 8 and 10 hpi, the pharyngeal pumping rate of 5 individual worms was counted for 5 seconds under a microscope (Leica Microsystem M205 FA, Germany). A pharyngeal pump was strictly defined as a complete backward movement of the terminal bulb grinder (Keane and Avery, 2003). The average number of pharyngeal pumps in 5 worms (pumps/second) was calculated and used for statistical analysis. The experiment was repeated twice.
Colony forming units (CFU) assay
In vivo enumeration of bacterial CFU within the C. elegans gut was performed as previously described (Ooi et al., 2012) with minor modifications. Briefly, the worms were infected by S. aureus in the presence and absence of UE-12 as described above. After 4, 12, 24, 48 and 72 hours, 10–12 worms were washed with 10 µg/mL gentamicin in 25 mM Levamisole (Lev) (Sigma–Aldrich, USA) to remove bacteria present on the worm cuticle. The worms were then homogenized using a motorized pestle and lysates were serially diluted and plated on TS agar. After an overnight incubation at 37°C, colonies were counted and the CFU counts per worm were determined. To establish growth of S. aureus in liquid medium in the presence of UE-12, the liquid medium, comprising 20% S. aureus culture and 80% M9 buffer, was prepared as described above. At the indicated time points, 10 µL culture was removed from the medium, serially diluted and plated on TS agar. Three independent experiments were performed.
Visualization and scoring of transgenic C. elegans plys-7::GFP
The transgenic C. elegans strain carrying a lys-7 promoter fused to the green fluorescent protein open reading frame (ORF) was used to visualize the expression of lys-7. plys-7::GFP transgenic worms were subjected to S. aureus infection in the presence and absence of UE-12 as described in the infection assay above. A total of 20 live transgenic worms were picked and paralyzed with 5 mM Levamisole at 24 and 48 hpi. The worms were mounted on a 2% agarose gel pad for observation at 100× magnification. Fluorescence intensity was observed under a Leica DM5000B automated upright fluorescent microscope equipped with an I3 long-pass GFP filter. Fluorescence micrographs were collected using a ProgRes C10 Plus digital microscope camera (Jenoptic Laser, Jena, Germany) and Leica Application Suite software (Leica Microsystems, Wetzlar, Germany). Each image series was captured on identical settings. Each worm was given a score of 1, 2 or 3 as shown in Fig. 1D–F, indicating low, medium and high fluorescence intensity, respectively. Observations were made on transgenic worms grown on E. coli OP50 (uninfected) and on worms infected with S. aureus in the presence and absence of extract. At least three independent replicates were performed.
Total RNA isolation and quantitative RT-PCR (qRT-PCR) analysis
Animals were treated essentially as described above according to the specific experimental conditions, after which, total RNA was extracted from a synchronized population of worms (∼2500 worms) using TRIzol reagent (Invitrogen, USA) according to the manufacturer's instructions, followed by purification on RNeasy columns (Qiagen, Germany). qRT-PCR reactions were hereafter performed with DNase-treated total RNA using the iScript One-Step RT-PCR kit with SYBR green detection according to the manufacturer's instructions (BioRad Laboratories, USA) on the Bio-Rad CFX96 Touch Real-Time PCR Detection System. The system was used for amplification and quantification of the products. Specificity of amplification was confirmed by melt curve analysis after amplification. Primers for qRT-PCR were based on those previously published (Troemel et al., 2006; Alper et al., 2007; Irazoqui et al., 2008). Normalized threshold cycle values were used to calculate fold increase or decrease of RNA levels in samples from test animals as compared to controls. The Ct values were normalized to changes in three primer pairs (ama-1, pan-actin (act-1, 3, 4) and F44B9.5) that were found to not vary with infection.
Statistical analysis
For all experiments, six replicates (in six wells) per trial were carried out (with a total of 120 worms) for statistical purposes. Nematodes were classified as dead when they failed to respond to touch and no pharyngeal pumping was observed. Worms that died because of bursting vulva were censored from further analysis. Differences in survival and lifespan of C. elegans between treatment (UE-12 or its bioactive compounds) group and control group (DMSO) in both wild-type and mutant animals were assessed by the Log-rank (Mantel–Cox) significance test using StatView version 5.0.1 (SAS Institute, Inc.). The ordinal data for quantitative GFP experiments were analyzed with the Mann–Whitney U-test. For other assays, data were analyzed and compared by using the unpaired, two-tailed Student's t-test. Fold changes in the qRT-PCR analysis were also compared using Student's t-tests.
Supplementary Material
Acknowledgments
We are grateful to Dr Paul Dear (LMB, Cambridge) for his comments on the manuscript.
Footnotes
Author contributions: C.K., M.-W.T. and S.N. conceived and designed the experiments. C.K. performed the experiments. C.K., M.-W.T. and S.N. analyzed the results and wrote the paper.
Competing interests: The authors have no competing interests to declare.
Funding
This work was supported by the Ministry of Science, Technology and Innovation, Malaysia (grant numbers 080502-IFN-BPH002 and 020520-SF11117). C.K. was supported by a MyBrain15 scholarship from the Ministry of Higher Education, Malaysia.
References
- Adonizio A., Leal S. M., Jr, Ausubel F. M., Mathee K. (2008). Attenuation of Pseudomonas aeruginosa virulence by medicinal plants in a Caenorhabditis elegans model system. J. Med. Microbiol. 57, 809–813 10.1099/jmm.0.47802-0 [DOI] [PubMed] [Google Scholar]
- Alper S., McBride S. J., Lackford B., Freedman J. H., Schwartz D. A. (2007). Specificity and complexity of the Caenorhabditis elegans innate immune response. Mol. Cell. Biol. 27, 5544–5553 10.1128/MCB.02070-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshawsh M. A., Abdulla M. A., Ismail S., Amin Z. A., Qader S. W., Hadi H. A., Harmal N. S. (2012). Free radical scavenging, antimicrobial and immunomodulatory activities of Orthosiphon stamineus. Molecules 17, 5385–5395 10.3390/molecules17055385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameer O. Z., Salman I. M., Asmawi M. Z., Ibraheem Z. O., Yam M. F. (2012). Orthosiphon stamineus: traditional uses, phytochemistry, pharmacology, and toxicology. J. Med. Food 15, 678–690 10.1089/jmf.2011.1973 [DOI] [PubMed] [Google Scholar]
- Archer G. L. (1998). Staphylococcus aureus: a well-armed pathogen. Clin. Infect. Dis. 26, 1179–1181 10.1086/520289 [DOI] [PubMed] [Google Scholar]
- Balaban N., Cirioni O., Giacometti A., Ghiselli R., Braunstein J. B., Silvestri C., Mocchegiani F., Saba V., Scalise G. (2007). Treatment of Staphylococcus aureus biofilm infection by the quorum-sensing inhibitor RIP. Antimicrob. Agents Chemother. 51, 2226–2229 10.1128/AAC.01097-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breger J., Fuchs B. B., Aperis G., Moy T. I., Ausubel F. M., Mylonakis E. (2007). Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathog. 3, e18 10.1371/journal.ppat.0030018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan M. M. (1999). Plant products as antimicrobial agents. Clin. Microbiol. Rev. 12, 564–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg G. M., Newman D. J., Snader K. M. (1997). Natural products in drug discovery and development. J. Nat. Prod. 60, 52–60 10.1021/np9604893 [DOI] [PubMed] [Google Scholar]
- DesJardin J. A., Snydman D. R. (1998). Antiviral immunotherapy: a review of current status. BioDrugs 9, 487–507 10.2165/00063030-199809060-00006 [DOI] [PubMed] [Google Scholar]
- Dharmalingam K., Tan B. K., Mahmud M. Z., Sedek S. A., Majid M. I., Kuah M. K., Sulaiman S. F., Ooi K. L., Khan N. A., Muhammad T. S. et al. (2012). Swietenia macrophylla extract promotes the ability of Caenorhabditis elegans to survive Pseudomonas aeruginosa infection. J. Ethnopharmacol. 139, 657–663 10.1016/j.jep.2011.12.016 [DOI] [PubMed] [Google Scholar]
- Evans E. A., Kawli T., Tan M. W. (2008). Pseudomonas aeruginosa suppresses host immunity by activating the DAF-2 insulin-like signaling pathway in Caenorhabditis elegans. PLoS Pathog. 4, e1000175 10.1371/journal.ppat.1000175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garsin D. A., Sifri C. D., Mylonakis E., Qin X., Singh K. V., Murray B. E., Calderwood S. B., Ausubel F. M. (2001). A simple model host for identifying Gram-positive virulence factors. Proc. Natl. Acad. Sci. USA 98, 10892–10897 10.1073/pnas.191378698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garsin D. A., Villanueva J. M., Begun J., Kim D. H., Sifri C. D., Calderwood S. B., Ruvkun G., Ausubel F. M. (2003). Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science 300, 1921 10.1126/science.1080147 [DOI] [PubMed] [Google Scholar]
- Grundmann H., Aanensen D. M., van den Wijngaard C. C., Spratt B. G., Harmsen D., Friedrich A. W., European Staphylococcal Reference Laboratory Working Group (2010). Geographic distribution of Staphylococcus aureus causing invasive infections in Europe: a molecular-epidemiological analysis. PLoS Med. 7, e1000215 10.1371/journal.pmed.1000215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Nijnik A., Philpott D. J. (2012). Modulating immunity as a therapy for bacterial infections. Nat. Rev. Microbiol. 10, 243–254 10.1038/nrmicro2745 [DOI] [PubMed] [Google Scholar]
- Ho C.-H., Noryati I., Sulaiman S.-F., Rosma A. (2010). In vitro antibacterial and antioxidant activities of Orthosiphon stamineus Benth. extracts against food-borne bacteria. Food Chem. 122, 1168–1172 10.1016/j.foodchem.2010.03.110 [DOI] [Google Scholar]
- Irazoqui J. E., Ng A., Xavier R. J., Ausubel F. M. (2008). Role for beta-catenin and HOX transcription factors in Caenorhabditis elegans and mammalian host epithelial-pathogen interactions. Proc. Natl. Acad. Sci. USA 105, 17469–17474 10.1073/pnas.0809527105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irazoqui J. E., Troemel E. R., Feinbaum R. L., Luhachack L. G., Cezairliyan B. O., Ausubel F. M. (2010). Distinct pathogenesis and host responses during infection of C. elegans by P. aeruginosa and S. aureus. PLoS Pathog. 6, e1000982 10.1371/journal.ppat.1000982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen H., Bojer M. S., Marinus M. G., Xu T., Struve C., Krogfelt K. A., Løbner-Olesen A. (2013). The alkaloid compound harmane increases the lifespan of Caenorhabditis elegans during bacterial infection, by modulating the nematode's innate immune response. PLoS ONE 8, e60519 10.1371/journal.pone.0060519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- JebaMercy G., Pandian S. K., Balamurugan K. (2011). Changes in Caenorhabditis elegans life span and selective innate immune genes during Staphylococcus aureus infection. Folia Microbiol. (Praha) 56, 373–380 10.1007/s12223-011-0060-y [DOI] [PubMed] [Google Scholar]
- Kato Y., Aizawa T., Hoshino H., Kawano K., Nitta K., Zhang H. (2002). abf-1 and abf-2, ASABF-type antimicrobial peptide genes in Caenorhabditis elegans. Biochem. J. 361, 221–230 10.1042/0264-6021:3610221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane J., Avery L. (2003). Mechanosensory inputs influence Caenorhabditis elegans pharyngeal activity via ivermectin sensitivity genes. Genetics 164, 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodaverdian V., Pesho M., Truitt B., Bollinger L., Patel P., Nithianantham S., Yu G., Delaney E., Jankowsky E., Shoham M. (2013). Discovery of antivirulence agents against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 57, 3645–3652 10.1128/AAC.00269-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. H., Feinbaum R., Alloing G., Emerson F. E., Garsin D. A., Inoue H., Tanaka-Hino M., Hisamoto N., Matsumoto K., Tan M. W. et al. (2002). A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 297, 623–626 10.1126/science.1073759 [DOI] [PubMed] [Google Scholar]
- Kindrachuk J., Jenssen H., Elliott M., Nijnik A., Magrangeas-Janot L., Pasupuleti M., Thorson L., Ma S., Easton D. M., Bains M. et al. (2013). Manipulation of innate immunity by a bacterial secreted peptide: lantibiotic nisin Z is selectively immunomodulatory. Innate Immun 19, 315–327 10.1177/1753425912461456 [DOI] [PubMed] [Google Scholar]
- Klevens R. M., Morrison M. A., Nadle J., Petit S., Gershman K., Ray S., Harrison L. H., Lynfield R., Dumyati G., Townes J. M. et al. Active Bacterial Core surveillance (ABCs) MRSA Investigators(2007). Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298, 1763–1771 10.1001/jama.298.15.1763 [DOI] [PubMed] [Google Scholar]
- Kong C., Yehye W. A., Abd Rahman N., Tan M. W., Nathan S. (2014). Discovery of potential anti-infectives against Staphylococcus aureus using a Caenorhabditis elegans infection model. BMC Complement. Altern. Med. 14, 4 10.1186/1472-6882-14-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laavola M., Nieminen R., Yam M. F., Sadikun A., Asmawi M. Z., Basir R., Welling J., Vapaatalo H., Korhonen R., Moilanen E. (2012). Flavonoids eupatorin and sinensetin present in Orthosiphon stamineus leaves inhibit inflammatory gene expression and STAT1 activation. Planta Med. 78, 779–786 10.1055/s-0031-1298458 [DOI] [PubMed] [Google Scholar]
- Lee S. H., Ooi S. K., Mahadi N. M., Tan M. W., Nathan S. (2011). Complete killing of Caenorhabditis elegans by Burkholderia pseudomallei is dependent on prolonged direct association with the viable pathogen. PLoS ONE 6, e16707 10.1371/journal.pone.0016707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leippe M. (1999). Antimicrobial and cytolytic polypeptides of amoeboid protozoa – effector molecules of primitive phagocytes. Dev. Comp. Immunol. 23, 267–279 10.1016/S0145-305X(99)00010-5 [DOI] [PubMed] [Google Scholar]
- Liu J., Hafting J., Critchley A. T., Banskota A. H., Prithiviraj B. (2013). Components of the cultivated red seaweed Chondrus crispus enhance the immune response of Caenorhabditis elegans to Pseudomonas aeruginosa through the pmk-1, daf-2/daf-16, and skn-1 pathways. Appl. Environ. Microbiol. 79, 7343–7350 10.1128/AEM.01927-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S., Shaw W. M., Ashraf J., Murphy C. T. (2009). TGF-beta Sma/Mab signaling mutations uncouple reproductive aging from somatic aging. PLoS Genet. 5, e1000789 10.1371/journal.pgen.1000789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahady G. B., Huang Y., Doyle B. J., Locklear T. (2008). Natural products as antibacterial agents. Studies in Natural Products Chemistry 35, 423–444 10.1016/S1572-5995(08)80011-7 [DOI] [Google Scholar]
- Mallo G. V., Kurz C. L., Couillault C., Pujol N., Granjeaud S., Kohara Y., Ewbank J. J. (2002). Inducible antibacterial defense system in C. elegans. Curr. Biol. 12, 1209–1214 10.1016/S0960-9822(02)00928-4 [DOI] [PubMed] [Google Scholar]
- Moy T. I., Ball A. R., Anklesaria Z., Casadei G., Lewis K., Ausubel F. M. (2006). Identification of novel antimicrobials using a live-animal infection model. Proc. Natl. Acad. Sci. USA 103, 10414–10419 10.1073/pnas.0604055103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C. T., McCarroll S. A., Bargmann C. I., Fraser A., Kamath R. S., Ahringer J., Li H., Kenyon C. (2003). Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424, 277–283 10.1038/nature01789 [DOI] [PubMed] [Google Scholar]
- Nicholas H. R., Hodgkin J. (2004). Responses to infection and possible recognition strategies in the innate immune system of Caenorhabditis elegans. Mol. Immunol. 41, 479–493 10.1016/j.molimm.2004.03.037 [DOI] [PubMed] [Google Scholar]
- O'Rourke D., Baban D., Demidova M., Mott R., Hodgkin J. (2006). Genomic clusters, putative pathogen recognition molecules, and antimicrobial genes are induced by infection of C. elegans with M. nematophilum. Genome Res. 16, 1005–1016 10.1101/gr.50823006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olah N. K., Radu L., Mogoşan C., Hanganu D., Gocan S. (2003). Phytochemical and pharmacological studies on Orthosiphon stamineus Benth. (Lamiaceae) hydroalcoholic extracts. J. Pharm. Biomed. Anal. 33, 117–123 10.1016/S0731-7085(03)00227-9 [DOI] [PubMed] [Google Scholar]
- Ooi S. K., Lim T. Y., Lee S. H., Nathan S. (2012). Burkholderia pseudomallei kills Caenorhabditis elegans through virulence mechanisms distinct from intestinal lumen colonization. Virulence 3, 485–496 10.4161/viru.21808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Abd-Rashid B. A., Ismail Z., Ismail R., Mak J. W., Pook P. C., Er H. M., Ong C. E. (2011). In vitro effects of active constituents and extracts of Orthosiphon stamineus on the activities of three major human cDNA-expressed cytochrome P450 enzymes. Chem. Biol. Interact. 190, 1–8 10.1016/j.cbi.2011.01.022 [DOI] [PubMed] [Google Scholar]
- Perez F., Salata R. A., Bonomo R. A. (2008). Current and novel antibiotics against resistant Gram-positive bacteria. Infect Drug Resist 1, 27–44 10.2147/IDR.S3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukkila-Worley R., Feinbaum R., Kirienko N. V., Larkins-Ford J., Conery A. L., Ausubel F. M. (2012). Stimulation of host immune defenses by a small molecule protects C. elegans from bacterial infection. PLoS Genet. 8, e1002733 10.1371/journal.pgen.1002733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder T., Stanisak M., Gelhaus C., Bruchhaus I., Grötzinger J., Leippe M. (2010). Caenopores are antimicrobial peptides in the nematode Caenorhabditis elegans instrumental in nutrition and immunity. Dev. Comp. Immunol. 34, 203–209 10.1016/j.dci.2009.09.010 [DOI] [PubMed] [Google Scholar]
- Rudrappa T., Bais H. P. (2008). Curcumin, a known phenolic from Curcuma longa, attenuates the virulence of Pseudomonas aeruginosa PAO1 in whole plant and animal pathogenicity models. J. Agric. Food Chem. 56, 1955–1962 10.1021/jf072591j [DOI] [PubMed] [Google Scholar]
- Schulenburg H., Boehnisch C. (2008). Diversification and adaptive sequence evolution of Caenorhabditis lysozymes (Nematoda: Rhabditidae). BMC Evol. Biol. 8, 114 10.1186/1471-2148-8-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulenburg H., Hoeppner M. P., Weiner J., 3rd, Bornberg-Bauer E. (2008). Specificity of the innate immune system and diversity of C-type lectin domain (CTLD) proteins in the nematode Caenorhabditis elegans. Immunobiology 213, 237–250 10.1016/j.imbio.2007.12.004 [DOI] [PubMed] [Google Scholar]
- Sifri C. D., Begun J., Ausubel F. M., Calderwood S. B. (2003). Caenorhabditis elegans as a model host for Staphylococcus aureus pathogenesis. Infect. Immun. 71, 2208–2217 10.1128/IAI.71.4.2208-2217.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifri C. D., Begun J., Ausubel F. M. (2005). The worm has turned – microbial virulence modeled in Caenorhabditis elegans. Trends Microbiol. 13, 119–127 10.1016/j.tim.2005.01.003 [DOI] [PubMed] [Google Scholar]
- Tabara H., Hill R. J., Mello C. C., Priess J. R., Kohara Y. (1999). pos-1 encodes a cytoplasmic zinc-finger protein essential for germline specification in C. elegans. Development 126, 1–11. [DOI] [PubMed] [Google Scholar]
- Tan M. W., Mahajan-Miklos S., Ausubel F. M. (1999). Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc. Natl. Acad. Sci. USA 96, 715–720 10.1073/pnas.96.2.715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trnková K., Pastoreková S., Petrik J. (2012). Novel approaches to antiviral and anticancer immunotherapy. Acta Virol. 56, 271–282 10.4149/av_2012_04_271 [DOI] [PubMed] [Google Scholar]
- Troemel E. R., Chu S. W., Reinke V., Lee S. S., Ausubel F. M., Kim D. H. (2006). p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet. 2, e183 10.1371/journal.pgen.0020183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhies W. A., Fuchs J., Thomas S. (2005). The longevity of Caenorhabditis elegans in soil. Biol. Lett. 1, 247–249 10.1098/rsbl.2004.0278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A. (2006). Effective cancer therapy through immunomodulation. Annu. Rev. Med. 57, 65–81 10.1146/annurev.med.56.082103.104549 [DOI] [PubMed] [Google Scholar]
- Wu H., Song Z., Hentzer M., Andersen J. B., Molin S., Givskov M., Høiby N. (2004). Synthetic furanones inhibit quorum-sensing and enhance bacterial clearance in Pseudomonas aeruginosa lung infection in mice. J. Antimicrob. Chemother. 53, 1054–1061 10.1093/jac/dkh223 [DOI] [PubMed] [Google Scholar]
- Wu K., Conly J., McClure J. A., Elsayed S., Louie T., Zhang K. (2010). Caenorhabditis elegans as a host model for community-associated methicillin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 16, 245–254 10.1111/j.1469-0691.2009.02765.x [DOI] [PubMed] [Google Scholar]
- Yam M. F., Mohamed E. A., Ang L. F., Pei L., Darwis Y., Mahmud R., Asmawi M. Z., Basir R., Ahmad M. (2012). A simple isocratic HPLC method for the simultaneous determination of sinensetin, eupatorin, and 3′-hydroxy-5,6,7,4′-tetramethoxyflavone in Orthosiphon stamineus extracts. J Acupunct Meridian Stud 5, 176–182 10.1016/j.jams.2012.05.005 [DOI] [PubMed] [Google Scholar]
- Yu Y. B., Dosanjh L., Lao L., Tan M., Shim B. S., Luo Y. (2010). Cinnamomum cassia bark in two herbal formulas increases life span in Caenorhabditis elegans via insulin signaling and stress response pathways. PLoS ONE 5, e9339 10.1371/journal.pone.0009339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S. Q., Ding A. J., Li G. P., Wu G. S., Luo H. R. (2013). Drug absorption efficiency in Caenorhbditis elegans delivered by different methods. PLoS ONE 8, e56877 10.1371/journal.pone.0056877 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.