Abstract
Previous ground-based experiments have shown that cranial irradiation with mission relevant (20 cGy) doses of 1 GeV/nucleon 56Fe particles leads to a significant impairment in Attentional Set Shifting (ATSET) performance, a measure of executive function, in juvenile Wistar rats. However, the use of head only radiation exposure and the biological age of the rats used in that study may not be pertinent to determine the likelihood that ATSET will be impaired in Astronauts on deep space flights. In this study we have determined the impact that whole-body exposure to 10, 15 and 20 cGy of 1 GeV/nucleon 56Fe particles had on the ability (at three months post exposure) of socially mature (retired breeder) Wistar rats to conduct the attentional set-shifting paradigm. The current study has established that whole-body exposures to 15 and 20 (but not 10) cGy of 1 GeV/nucleon 56Fe particles results in the impairment of ATSET in both juvenile and socially mature rats. However, the exact nature of the impaired ATSET performance varied depending upon the age of the rats, whether whole-body versus cranial irradiation was used and the dose of 1 GeV/u 56Fe received. Exposure of juvenile rats to 20 cGy of 1 GeV/nucleon 56Fe particles led to a decreased ability to perform intra-dimensional shifting (IDS) irrespective of whether the rats received head only or whole-body exposures. Juvenile rats that received whole-body exposure also had a reduced ability to habituate to the assay and to complete intra-dimensional shifting reversal (IDR), whereas juvenile rats that received head only exposure had a reduced ability to complete compound discrimination reversal (CDR). Socially mature rats that received whole-body exposures to 10 cGy of 1 GeV/nucleon 56Fe particles exhibited no obvious decline in set-shifting performance; however those exposed to 15 and 20 cGy had a reduced ability to perform simple discrimination (SD) and compound discrimination (CD). Exposure to 20 cGy of 1 GeV/nucleon 56Fe particles also led to a decreased performance in IDR and to ∼25% of rats failing to habituate to the task. Most of these rats started to dig for the food reward but rapidly (within 15 s) gave up digging, suggesting that they had developed appropriate procedural memories about food retrieval, but had an inability to maintain attention on the task. Our preliminary data suggests that whole-body exposure to 20 cGy of 1 GeV/nucleon 56Fe particles reduced the cholinergic (but not the GABAergic) readily releasable pool (RRP) in nerve terminals of the basal forebrain from socially-mature rats. This perturbation of the cholinergic RRP could directly lead to the loss of CDR and IDR performance, and indirectly [through the metabolic changes in the medial prefrontal cortex (mPFC)] to the loss of SD and CD performance. These findings provide the first evidence that attentional set-shifting performance in socially mature rats is impaired after whole-body exposure to mission relevant doses (15 and 20 cGy) of 1 GeV/nucleon 56Fe particles, and importantly that a dose reduction down to 10 cGy prevents that impairment. The ability to conduct Discrimination tasks (SD and CD) and reversal learning (CDR) is reduced after exposure to 15 and 20 cGy of 1 GeV/nucleon 56Fe particles, but at 20 cGy there is an additional decrement, ∼ 25% of rats are unable to maintain attention to task. These behavioral decrements are associated with a reduction in the cholinergic RRP within basal forebrain, which has been shown to play a major role in regulating the activity of the PFC.
Introduction
The proposed exploratory deep space mission to planets or asteroids will take astronauts beyond the protective effects of the Van Allen Belts, and thus astronauts will be subjected to chronic exposure to low-LET electromagnetic radiations, interspersed with periodic exposures to Galactic cosmic radiation (GCR). GCR primarily consists of high-energy protons, with a small component of high-Z and energy (HZE) particles, such as 56Fe. Even though HZE particles constitute a very small component of the total physical radiation dose, these particles constitute a large component of the biological radiation dose due to their high LET and more complex energy deposition track structure. The large footprint and complexity of the processes within an individual neuron puts these cells at high risk of being functionally impaired by HZE particles. Moreover, the fact that synchronization of neuronal activity within a neural network is required for cognitive performance, places the CNS at high risk of being impaired after exposure to HZE particles.
Ground-based rodent models suggest that 10–50 cGy of 1 GeV/nucleon 56Fe (and other ion species) results in significant impairment of hippocampal-dependent cognitive processes, such as novel object recognition (1), and spatial memory (2). Neurocognitive tasks that are dependent upon other regions of the brain, such as the striatum, are also impaired after exposure to low doses of HZE particles, such as 600 MeV/nucleon 16O (3). These data raise the possibility that neurocognitive tasks regulated by the prefrontal cortex, such as executive function, could also be impacted after exposure to mission relevant HZE doses. Executive functions can be defined as complex processes by which an individual optimizes their performance in a situation that requires the operation of a number of more basic cognitive processes (4).
We have previously established that mission relevant (<20 cGy) doses of 1 GeV/nucleon 56Fe particles leads to a significant impairment in Attentional Set-Shifting (ATSET) performance in juvenile male Wistar rats (5). ATSET is a measure of complex executive functions that enable subjects to rapidly adapt and respond to a change in the environment, and to perceive what is important for survival or completion of a task, skills that are absolutely vital to deal with a sudden emergency. The majority of irradiated rats failed to complete the Compound Discrimination Reversal (CDR or Rev1) step of the testing (5), a process that has been reported to be regulated by basal forebrain (6) and the orbital frontal cortex (7). There was also a marked reduction in the ability of the rats to conduct intra-dimensional shifting (IDS), a task believed to be regulated by the anterior and posterior cingulate cortex (8).
The impairment of ATSET performance is undesirable under any circumstances, but is likely to have a great impact upon the performance of a deep space mission because the Astronauts will, by necessity, have more autonomous control of the day to day running of the spacecraft and mission activities on deep space missions. However, because the Wistar rats used in our previous study (5) had a biological age comparable to a teenager, which is clearly not comparable to the biological age of the Astronaut population, it is unclear whether older rats will have the same sensitivity to HZE-induced ATSET impairment. Aged rats are reported to have a lower capacity to conduct extra-dimensional set shifting (EDS) than younger rats (9, 10), thus HZE exposure could result in further reductions in their ATSET. Our previous study (5) used cranial exposures to minimize radiation-induced damage to the other organs of the rats; however, Astronauts will receive whole-body exposure, and data exists that exposure of the body, either alone or in addition to the brain, can alter the level of neurocognitive deficits induced over that seen when only cranial radiation was used (11, 12).
In this study we have determined the impact that whole-body exposure to 10, 15 and 20 cGy of 1 GeV/nucleon of 56Fe particles had on the subsequent (at three months post exposure) ability of retired breeder Wistar rats to conduct the attentional set-shifting paradigm. In light of the reports that perturbation of the functionality of cholinergic (13) and GABAergic (14) neurons impacts upon various aspects of ATSET, we also established the impact that HZE exposure had on the Readily Releasable Pool (RRP) of GABA and acetylcholine in isolated nerve terminals (synaptosomes) of the basal forebrain.
Materials and Methods
Irradiation Procedure
This study was conducted in accordance with the National Research Council's “Guide for the Care and Use of Laboratory Animals (7th edition)”, at facilities of Eastern Virginia Medical School (EVMS) and Brookhaven National Laboratory (BNL), both of which are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International. All procedures where approved by the Institutional Animal Care and Use Committees (IACUC) of both EVMS and BNL.
Two cohorts of male Wistar rats (HSD:WI, Harlan Sprague-Dawley, Inc., Indianapolis, IN) were used in this study: 1. Twenty-eight juvenile rats weighing approximately 130 g and with an age range of 5–7 weeks at the time of irradiation, and 2. 114 retired breeder (socially mature) rats weighing on average 600 g and with an age range of 6–11 months at the time of irradiation. The rats were delivered directly from the supplier to BNL, where they were group housed, maintained on a 12:12 light/dark cycle and given ad libitum access to autoclaved Purina Rodent Chow 5001 and municipal water by bottle. After at least one week of acclimatization, the rats were irradiated with 1 GeV/nucleon of 56Fe exposure at the NASA Space Radiation Laboratory (NSRL).
The number of juvenile rats exposed to each dose point was: 16 juvenile rats were sham irradiated; 12 juvenile rats were exposure to 10 cGy; while the number of socially mature rats exposed to each dose point was: 46 mature rats were sham irradiated; 16 mature rats were exposed to 10 cGy; 27 mature rats were exposed to 15 cGy; and 25 rats were exposed to 20 cGy.
On the day of the experiment, the rats were placed in a well ventilated custom-made irradiation jig that was constructed of black polyacrylic plastic. Two (socially mature) or four (juvenile) rats were exposed simultaneously to the 1 GeV/nucleon 56Fe beam at a dose rate of 50 cGy/min. Dose calibration was performed as previously described (15). Control rats were placed in an identical irradiation jig that remained in the preparation room, while their counterparts were taken into the irradiation vault at NSRL. After irradiation, the rats were implanted with ID-100us RFID transponders (Trovan Ltd, United Kingdom) to facilitate identification of individual animals.
One week after irradiation, the rats were transported to EVMS, where they were group housed, maintained on a reversed 12 h light/dark cycle, and given ad libitum access to autoclaved Teklad 2018 chow and municipal water by bottle.
Attentional Set-Shifting Testing
The rats were tested for ATSET performance according to our previously published protocol (5) with a few minor refinements. Approximately 7 days before testing began the rats were single-housed and placed on food restriction. The rats received a daily allowance of approximately 6 g/day of Cheerios™ (General Mills, Minneapolis, MN), but the exact amount was varied on a daily basis to maintain an individual rat's weight at ∼85% of its weight on the first day of the testing. Testing was conducted during the dark cycle, with the first rat being tested at approximately 2 h into the 12 h dark cycle. The time at which testing was commenced was kept constant for an individual rat.
The first step of the test was to train the rats to forage for a food reward and to habituate them to the test maze and the handlers. The rats were acclimatized (for 10 min/day for 5 days) to the maze, which contained two food bowls filled with digging media and Cheerios scattered on the floor of the maze, as well as on top and buried within the digging media. Rats that passed the habituation stage were rested overnight before being tested for their Simple Discrimination (SD) ability. All other procedures were conducted as previously described (5). Rats that failed to complete a paradigm were rested overnight and re-tested the following day, rats that failed to complete the paradigm on the second attempt were excluded from further testing.
Determination of Cholinergic and GABAergic Readily Releasable Pool (RRP) in Basal Forebrain
The brains of two sham-irradiated rats and three rats exposed to 20 cGy 1 GeV/nucleon 56Fe were recovered and synaptosomes prepared, as previously described (16), from a region that encompasses the basal forebrain: ventral to the caudate putamen at around Bregma −0.3 mm. The size of the RRP, which reflects release probability (17) and synaptic strength (18), of two neurotransmitters, acetylcholine and GABA was determined using a previously described hyperosmotic sucrose-evoked protocol (16), except that 14C-choline (50–62 mCi/mmol specific activity, Perkin Elmer, Boston, MA), and 3H-GABA (70–100 Ci/mmol, Perkin Elmer) was respectively used to assess acetylcholine and GABA release.
Statistical Analysis
The nonparametric two-tailed Mann-Whitney test was used to analyze the impact that the effect of radiation had on the number of trials to reach criterion at a given stage, as there is no guarantee that the irradiated population will follow a Guassian distribution. For analysis of the categorical data (Pass/Fail), performance ability differences were analyzed using Chi-square/two-tailed Fisher's exact test.
Results
Attentional Set Shifting Performance in Juvenile Rats Exposed to 20 cGy 1 GeV/μ 56Fe Particles Whole-Body Irradiation
To determine whether whole-body irradiation (WBI) with 20 cGy 1 GeV/nucleon 56Fe particles impaired ATSET performance in a comparable manner to that observed following head-only exposures (5), 28 juvenile (16 sham irradiated and 12 irradiated) rats were screened for ATSET performance at 90 days post exposure.
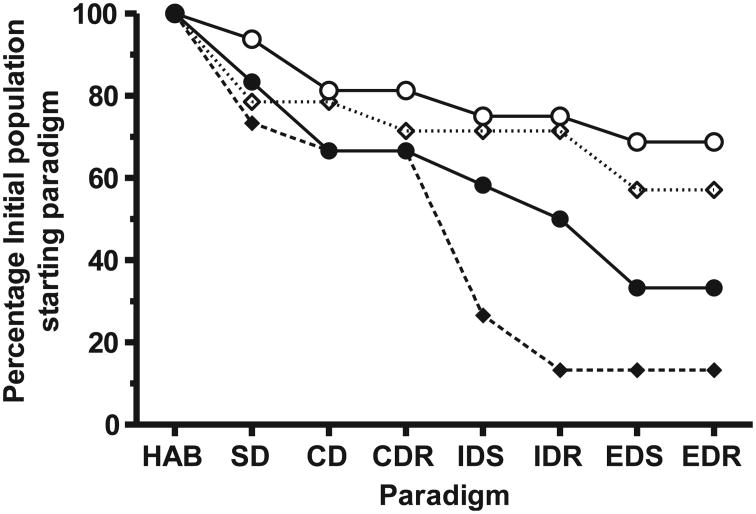
Most (15/16) of the sham-irradiated rats completed the habituation stage of the test, and proceeded to the Simple Discrimination (SD) stage of the assay, whereas 10/12 of the irradiated rats completed the habituation stage. The habituation failure rate was significantly higher (P = 0.025, two-tailed Fisher's exact test) than in the sham-irradiated rats. The rats that failed to habituate started to dig for the food reward, but rapidly (within 15 s) gave up digging. Failure to complete a stage of testing (on two consecutive days) resulted in the exclusion of that rat from further testing, thus the derived data can be viewed as a Kaplan-Meier event plot (Fig. 1). Most of the unirradiated rats [11/15 (73%)] were capable of working through all seven paradigms. Two rats failed to complete the SD step, one rat failed the CDR step and a further rat failed the Extra-Dimension Shift Reversal (EDR) stage (Fig. 1). The pattern of failures was very similar to that observed in our previous study [data from ref. (5) is presented as dotted line in Fig. 1], indicating that the use of the ketamine/xylazine anesthesia in our previous study (5) did not alter the set shifting performance of the sham-irradiated rats. While the sham-irradiated rats that completed the SD and compound discrimination (CD) steps did so after more attempts (SD: 16.33 ± 1.7; CD: 14.15 ± 1.95) than in our previous study (5) (SD: 11.64 ± 2.05; CD: 8.9 ± 1.55), this was not statistically significant (P < 0.09, Mann-Whitney test).
Fig. 1.
Ability of juvenile rats to conduct attentional set-shifting: Percentage of the initial population that started each paradigm after: whole-body exposure to 20 cGy 1 GeV/nucleon 56Fe (●) or in sham-irradiated (○) counterparts. Also shown is re-analyzed data from ref. (5) from juvenile rats that received cranial irradiation with 20 cGy 1 GeV/nucleon 56Fe (◆) or in sham-irradiated (◊) counterparts. HAB: habituation; SD: simple discrimination; CD: compound discrimination; CDR: compound discrimination reversal; IDS: intra-dimensional shifting; IDR: intra-dimensional shifting reversal; EDS: extra-dimensional shifting; EDR: extra-dimensional shifting reversal.
Only 4/12 (33%) of the rats exposed to WBI managed to complete all the paradigms, but the overall completion rate of the WBI rats was higher (33% vs. 19%) than that previously observed in rats that received cranial irradiation (data from ref. (5) is plotted as a broken line in Fig. 1), primarily due to fewer rats failing the CDR paradigm than was observed when cranial irradiation was used (5).
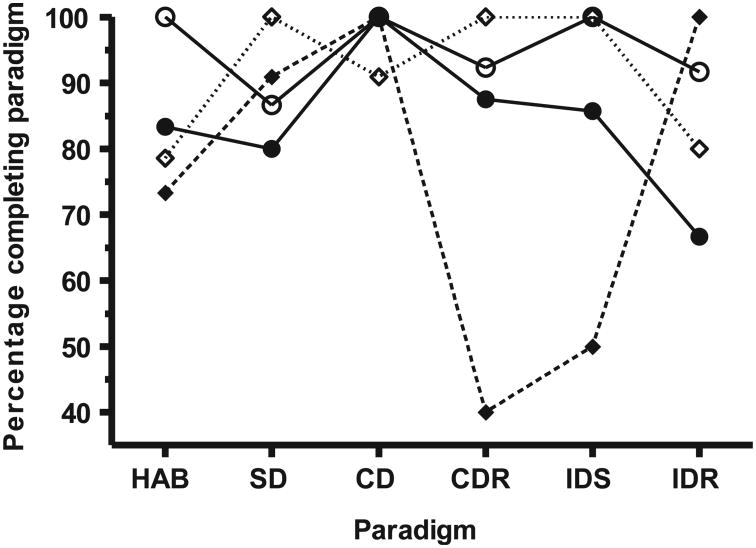
An alternate way of presenting the attentional set shifting performance data is to compare the percentage of rats that started and completed a given paradigm. Such an analysis (Fig. 2) revealed that the WBI rats had lower completion rates than the sham-irradiated rats in five of the first six paradigms, with statistically lower completion rates (χ2, two-tailed Fisher's exact test) in the habituation (P < 0.025); intra-dimensional shifting (IDS: P < 0.001) and intra-dimensional shifting reversal (IDR: P < 0.001) paradigms. There were no significant differences in the number of attempts that the sham-irradiated and irradiated rats took to pass the paradigms (completion efficiency). A similar analysis of our previous data from juvenile rats who received cranial irradiation, indicated that these rats had a significantly worse performance (P < 0.001, χ2, two-tailed Fisher's exact test) in the CDR and IDS paradigms than did the sham-irradiated rats (Fig. 2, broken line).
Fig. 2.
Paradigm-specific completion efficiency (percentage of rats that started a specific paradigm and reached criterion) of juvenile rats after: whole-body exposure to 20 cGy 1 GeV/nucleon 56Fe (●) or in sham-irradiated (○) counterparts. Also shown is re-analyzed data from ref. (5) from juvenile rats that received cranial irradiation with 20 cGy 1 GeV/nucleon 56Fe (◆) or in sham-irradiated (◊) counterparts. HAB: habituation; SD: simple discrimination; CD: compound discrimination; CDR: compound discrimination reversal; IDS: intra-dimensional shifting; IDR: intra-dimensional shifting reversal; EDS: extra-dimensional shifting; EDR: extra-dimensional shifting reversal.
Attentional Set Shifting Performance in Retired Breeder Rats Exposed to 10–20 cGy 1 GeV/nucleon 56Fe Particles Whole-Body Irradiation
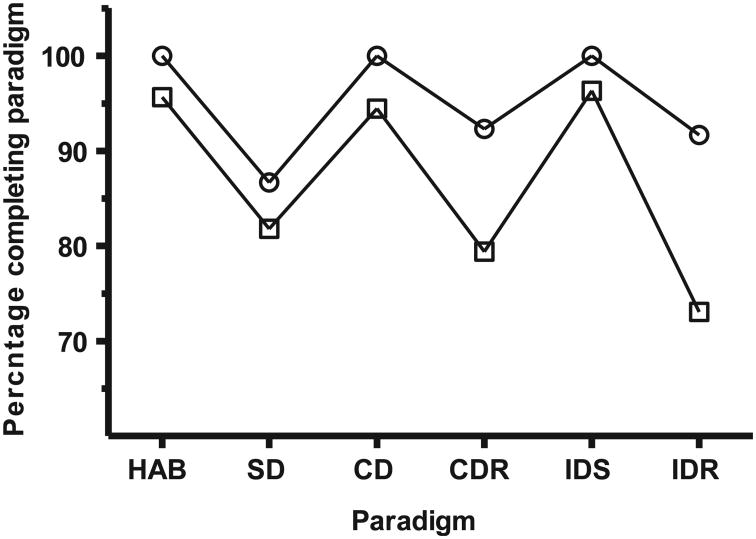
The performance of the sham-irradiated (N = 46) retired breeder rats was slightly worse than was observed with the juvenile rats, with only 62% of retired breeder rats completing all stages of the test (as opposed to 73% of the juvenile rats) (Fig. 3). Sham-irradiated retired breeder rats were significantly less capable or motivated, than their juvenile counterparts to complete the CDR (P < 0.015, χ2, two-tailed Fisher's exact test) and IDR (P < 0.006) paradigms.
Fig. 3.
Paradigm-specific completion efficiency of sham-irradiated Retired Breeder (□) and juvenile rats (○) counterparts. HAB: habituation; SD: simple discrimination; CD: compound discrimination; CDR: compound discrimination reversal; IDS: intra-dimensional shifting; IDR: intra-dimensional shifting reversal; EDS: extra-dimensional shifting; EDR: extra-dimensional shifting reversal.
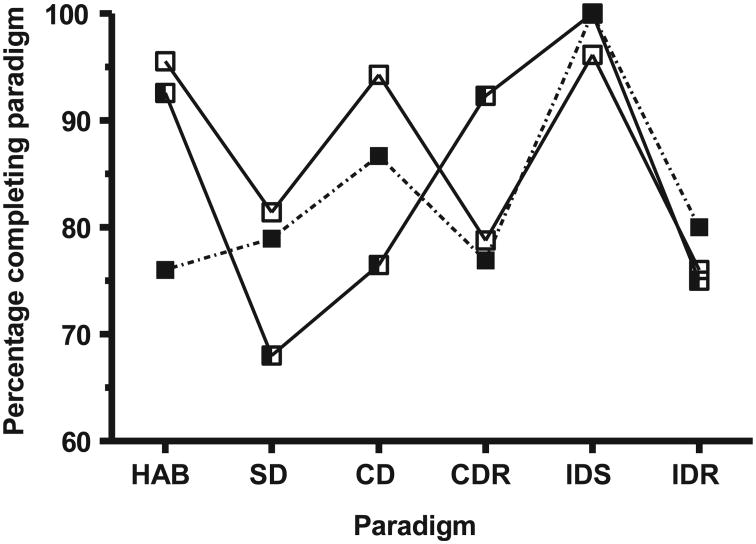
The performance of the rats exposed to 10 cGy 1 GeV/nucleon 56Fe-particle radiation was indistinguishable from that of the sham-irradiated rats (data not shown). However, rats exposed to 15 cGy 1 GeV/nucleon 56Fe, had a significantly lower completion rate in the SD (P = 0.05, χ2, two-tailed Fisher's exact test) and CD (P < 0.001) paradigms, but a significantly (P = 0.015) increased ability to complete the CDR paradigm (Fig. 4) compared to the sham-irradiated rats.
Fig. 4.
Effect of whole-body exposure to 1 GeV/nucleon 56Fe particles on the paradigm-specific completion efficiency of retired breeder rats: sham-irradiated (□); and whole-body exposure to 15 cGy (half-filled square) or 20 cGy (■) 1 GeV/nucleon 56Fe particles. HAB: habituation; SD: simple discrimination; CD: compound discrimination; CDR: compound discrimination reversal; IDS: intra-dimensional shifting; IDR: intra-dimensional shifting reversal; EDS: extra-dimensional shifting; EDR: extra-dimensional shifting reversal.
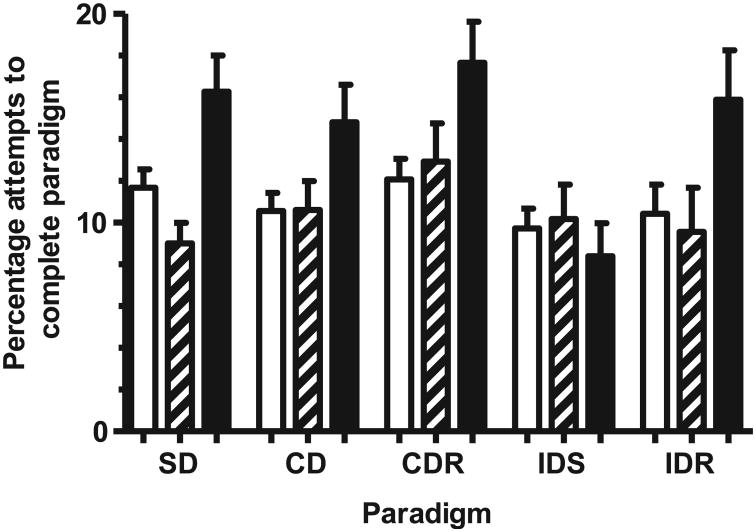
Exposure to 20 cGy of 1 GeV/nucleon 56Fe-particle radiation, resulted in 25% of rats being unable to complete the habituation phase of the assay. This was comparable to the reduction in habitation success seen in the WBI juvenile rats that were exposed to this dose (Fig. 2). As was observed in WBI juvenile rats, the majority of the rats that failed to habituate started to dig for the reward but quickly (<15 s) gave up digging. These rats showed no overt signs of anhedonia, and consumed equal amounts of 2% w/v sucrose solution and plain water (data not shown). The irradiated rats that passed the habituation paradigm had a categorical (pass/fail) performance rate comparable to that seen in the sham-irradiated retired breeders, although there was a [nonsignificant (P < 0.097), χ2, two-tailed Fisher's exact test] lower ability of these rats to complete the CD task (Fig. 4). However, further interrogation of the performance data revealed that rats exposed to 20 cGy took significantly more attempts to complete the SD (P = 0.011, Mann-Whitney test), CD (P = 0.022, Mann-Whitney test), the CDR (P = 0.02, Mann-Whitney test) and the IDR (P = 0.034, Mann-Whitney test) paradigms than did the sham-irradiated rats (Fig. 5). Rats exposed to 15 cGy had comparable performance efficiency to the sham-irradiated rats.
Fig. 5.
Effect of whole-body exposure to 1 GeV/nucleon 56Fe particles on the paradigm-specific performance of retired breeder rats: number of attempts required to reach criterion in sham-irradiated (open bar) and whole-body exposure to 15 cGy (hatched bar) or 20 cGy (solid bar) 1 GeV/nucleon 56Fe. Graphs show means ± SEM. HAB: habituation; SD: simple discrimination; CD: compound discrimination; CDR: compound discrimination reversal; IDS: intra-dimensional shifting; IDR: intra-dimensional shifting reversal; EDS: extra-dimensional shifting; EDR: extra-dimensional shifting reversal.
HZE-Particle Radiation-Induced Changes in the Cholinergic RRP in Basal Forebrain Synaptosomes
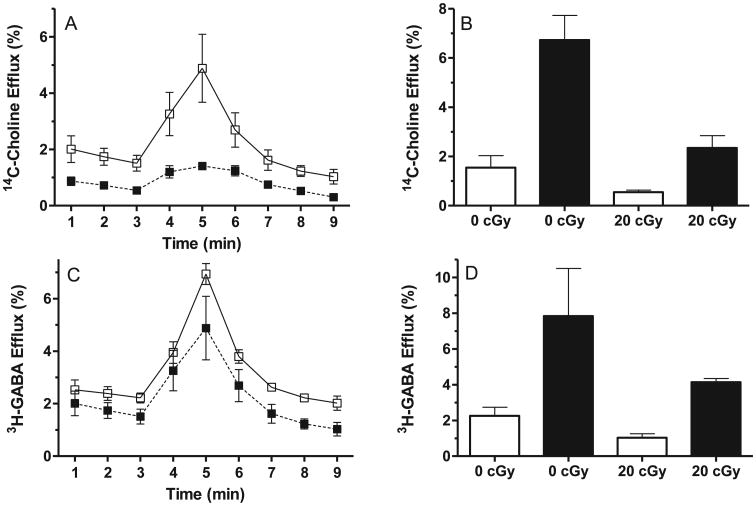
At 90 days postirradiation, there was a significant (P < 0.01, unpaired t test with Welch's correction) reduction in the cholinergic RRP in synaptosomal preparations from the basal forebrain of HZE-irradiated rats (Fig. 6A). The basal release and hyperosmotic sucrose-evoked [14C]-choline efflux from basal forebrain synaptosomes were both reduced by ∼2.8-fold (P < 0.01, unpaired t test with Welch's correction) (Fig. 6B) in the synaptosomes from the HZE-irradiated rats. There was a nonsignificant decrease in the basal release and hyperosmotic sucrose-evoked 3H-GABA (Fig. 6D) in the synaptosomes from the HZE-irradiated rats.
Fig. 6.
Effect of whole-body exposure to 20 cGy 1 GeV/nucleon 56Fe particles on the readily releasable pool (RRP) of acetylcholine and GABA. Choline (panel A) and GABA (panel C) release evoked by 30 s pulse of hypertonic sucrose of superfused synaptosomes from sham irradiated (□) or irradiated (20 cGy 1 GeV/nucleon) (■) Effects of 20 cGy 1 GeV/nucleon 56Fe particles on basal (□) and hypertonic evoked release (■) of [14C]-choline (panel B) and [3H]-GABA (panel D) from basal forebrain synaptosomes from sham-irradiated (0 cGy) and irradiated (20 cGy) rats at 90 days postirradiation. Graphs show means ± SEM.
Discussion
On a deep space mission, where astronauts will be required to work more autonomously than on previous missions, the ability to perform executive functions is likely to have a major impact upon the success of such missions. Executive function can be tested in rodents (19), using an assay that is modeled after a test of executive function in humans called the Wisconsin Card Sorting Test (20).
The current study has established that whole-body exposures to 15 and 20 (but not 10) cGy of 1 GeV/nucleon 56Fe-particles radiation results in attentional set shifting (ATSET) impairments in both juvenile and socially mature rats. However, the exact nature of the HZE-induced impairment of ATSET performance varied depending upon the age of the rats, whether whole-body versus cranial irradiation was used and the dose of 1 GeV/nucleon 56Fe received.
There were both quantitative and qualitative differences in the decrements in attentional set shifting performance observed when the same dose (20 cGy) of 1 GeV/nucleon 56Fe-particles radiation was delivered to the whole-body or just the head of juvenile rats. In our previous study (5) cranial exposure to 20 cGy of 1 GeV/nucleon 56Fe particles resulted in only 19% of rats being able to complete all paradigms, with most failures occurring at the CDR and IDS stage of testing. In contrast, rats who received whole-body exposure to 20 cGy of 1 GeV/nucleon 56Fe particles were ∼1.5-fold more (33% vs. 19%) successful in completing all stages of testing (Fig. 1), with many rats failing at the IDS and IDR paradigms. This is in contrast to what is observed with operant response, where whole-body exposures tended to result in more marked decrements in performance, than did head-only exposures (12). However, the most striking difference between the consequences of cranial versus whole-body exposures on ATSET performance in juvenile rats, was the high rate of failure to habituate to the test and the relative insensitivity of CDR performance after whole-body exposures (Fig. 2). The underlying cause of the high-habituation rate remains speculative, but the fact that most of these rats started to dig for the food reward suggests that they encoded and retrieved the procedural memories of food retrieval necessary to perform ATSET. Moreover, the nonhabituating rats showed no obvious signs of anhedonia (sucrose preference test or general appearance and activity in the cage), perhaps suggesting that the reason these rats stopped digging after 5–10 s, was because they had an inability to maintain attention on a task. While CDR performance in juvenile rats was not significantly worse after whole-body HZE irradiation, IDR performance was worse. It is generally considered that both “reversal” tasks are regulated by the basal forebrain (6) and the orbital frontal cortex (7), so it is currently unclear why only the IDR performance was impaired in these rats. IDS performance was significantly impaired under both irradiation conditions, suggesting that the functionality of the anterior and posterior cingulate cortex regions, which are vital for IDS (8), is impaired by exposure to 20 cGy of 1 GeV/nucleon 56Fe-particles radiation.
The reduced capability of the sham-irradiated retired breeders to complete the “reversal” (CDR and IDR) paradigms (Fig. 3) may have served to reduce the apparent impact of whole-body HZE-radiation exposure on the performance in those paradigms. Nonetheless, 20 cGy of 1 GeV/nucleon 56Fe particles induced a high (25%) failure rate to habituate to the task. Once again the rats started to dig for the food reward, but rapidly gave up and the rats did not demonstrate overt anhedonia. While the completion rate was not significantly different in any of the paradigms from that observed in the sham-irradiated rats (Fig. 4), the irradiated rats took significantly more trials to reach criteria in the SD, CD, CDR and IDR stages (Fig. 5). Exposure to 15 cGy 1 GeV/nucleon 56Fe particles resulted in significant lower completion rates in both SD and CD (Fig. 4).
In summary, exposure to 15 and 20 cGy of 1 GeV/nucleon 56Fe-particle radiation led to a decreased ability (either the ability to complete a task or the number of attempts to complete a task) to perform ATSET. However, exposure to 10 cGy of 1 GeV/nucleon 56Fe particles had no obvious impact upon set shifting performance. Performance in the SD and CD discrimination steps are impaired by 15 and 20 cGy, whereas the reversal stages (CDR and IDR) are impaired at only 20 cGy, a dose that also led to 25% of animals from even starting the testing.
The basic understanding of the mechanisms that govern executive function task performance such as ATSET are still poorly understood. Certain regions of the brain have been shown to regulate certain paradigms: SD is regulated by the mPFC (21); CD is probably regulated by perirhinal cortical region, and requires optimal dopamine D2 receptor activation in prefrontal cortex. (22); IDS is believed to be regulated by the anterior and posterior cingulate cortex (8), and “reversal” tasks are regulated by the basal forebrain (6) and the orbital frontal cortex (7). The fact that the performance of multiple (SD, CD, CDR and IDR) paradigms is impacted by exposure to 20 cGy 1 GeV/nucleon 56Fe particles could indicate that there is a universal impairment of the functionality of all regulatory brain regions. However, it is likely that sustained activation of multiple brain regions and integrity of relay stations is required to effectively associate a clue with the reward and produce cognitive flexibility (the ability to alter strategy according to changing environmental cues). The loss of synaptically released acetylcholine in the mouse forebrain leads to impaired sustained attention, an increased susceptibility to distractions and metabolic abnormalities in the PFC (13). Our preliminary data (Fig. 6) suggests that exposure to 20 cGy 1 GeV/nucleon 56Fe particles reduces the cholinergic RRP in synaptosomal preparations from the basal forebrain, which could directly lead to the loss of CDR and IDR performance, and indirectly through the metabolic changes in the PFC (13) to the loss of SD and CD performance. Within the basal forebrain, acetylcholine release may lead to activation of GABAergic neurons within the basal forebrain (23). While the GABAergic RRP was lower after exposure to 20 cGy 1 GeV/nucleon 56Fe particles, this was not as significantly reduced as was the cholinergic RRP.
These findings provide the first evidence that attentional set-shifting performance in socially mature rats is impaired after whole-body exposure to mission relevant doses (15 and 20 cGy) of 1 GeV/nucleon 56Fe particles, and importantly that a dose reduction down to 10 cGy prevents that impairment. The ability to conduct discrimination tasks (SD and CD) and reversal learning (CDR) is reduced after exposure to 15 and 20 cGy of 1 GeV/nucleon 56Fe particles, but at 20 cGy there is an additional decrement, ∼ 25% of rats are unable to maintain attention to task. These behavioral decrements are associated with a reduction in the cholinergic RRP within basal forebrain, which has been shown to play a major role in regulating the activity of the PFC. Future studies will determine if other regions of the brain exhibit changes in the RRP of acetylcholine and other neurotransmitters.
Acknowledgments
This work was funded by NASA grant support NNJ06HD89D and NNX11AC56G (RB) and by NIH research grant MH64827 (LS). The authors are indebted to Dr. Adam Rusek, for his help with the irradiation of the rats at Brookhaven National Laboratories. This study would not have been possible without his help.
References
- 1.Haley GE, Yeiser L, Olsen RHJ, Davis MJ, Johnson LA, Raber J. Early effects of whole-body 56Fe irradiation on hippocampal function in C57BL/6J mice. Radiat Res. 2013;179:590–6. doi: 10.1667/RR2946.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Britten RA, Davis LK, Johnson AM, Keeney S, Siegel A, Sanford LD, Singletary SJ, Lonart G. Low (20 cGy) Doses of 1 GeV (56)Fe-Particle Radiation Lead to a Persistent Reduction in the Spatial Learning Ability of Rats. Radiat Res. 2012;177:146–51. doi: 10.1667/rr2637.1. [DOI] [PubMed] [Google Scholar]
- 3.Rabin BM, Shukitt-Hale B, Joseph JA, Carrrihill KL, Carey AN, Cheng V. Operant responding following exposure to HZE particles and its relationship to particle energy and linear energy transfer. Adv Space Res. 2011;48:370–7. [Google Scholar]
- 4.Baddeley AD. Exploring the central executive. Q J Exp Psychol. 1996;49A:5–28. 4. [Google Scholar]
- 5.Lonart G, Parris B, Johnson AM, Miles S, Sanford LD, Singletary SJ, Britten RA. Executive Function in Rats is Impaired by Low (20 cGy) Doses of 1 GeV 56Fe Particles. Radiat Res. 2012;178:289–94. doi: 10.1667/rr2862.1. [DOI] [PubMed] [Google Scholar]
- 6.Tait DS, Brown VJ. Lesions of the basal forebrain impair reversal learning but not shifting of attentional set in rats. Behav Brain Res. 2008;187:100–8. doi: 10.1016/j.bbr.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 7.McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Ng CW, Noblejas MI, Rodefer JS, Smith CB, Poremba A. Double dissociation of attentional resources: prefrontal versus cingulate cortices. J Neurosci. 2007;27:12123–31. doi: 10.1523/JNEUROSCI.2745-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicolle MM, Baxter MG. Glutamate receptor binding in the frontal cortex and dorsal striatum of aged rats with impaired attentional set-shifting. Eur J Neurosci. 2003;18:3335–42. doi: 10.1111/j.1460-9568.2003.03077.x. [DOI] [PubMed] [Google Scholar]
- 10.Barense MD, Fox MT, Baxter MG. Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats. Learn Mem. 2002;9:191–201. doi: 10.1101/lm.48602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabin BM, Hunt WA, Lee J. Effects of dose and of partial body ionizing radiation on taste aversion learning in rats with lesions of the area postrema. Physiol Behav. 1984;32:119–22. doi: 10.1016/0031-9384(84)90081-7. [DOI] [PubMed] [Google Scholar]
- 12.Rabin BM, Shukitt-Hale B, Carrihill-Knoll K, Gomes SM. Comparison of the effects of partial- or whole-body exposures to 16O particles on cognitive performance in rats. Radiat Res. 181:251–257. doi: 10.1667/RR13469.1. [DOI] [PubMed] [Google Scholar]
- 13.Kolisnyk B, Al-Onaizi MA, Hirata PH, Guzman MS, Nikolova S, Barbash S, Soreq H, Bartha R, Prado MA, Prado VF. Forebrain deletion of the vesicular acetylcholine transporter results in deficits in executive function, metabolic, and RNA splicing abnormalities in the prefrontal cortex. J Neurosci. 2013;33:14908–20. doi: 10.1523/JNEUROSCI.1933-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bissonette GB, Lande MD, Martins GJ, Powell EM. Versatility of the mouse reversal/set-shifting test: effects of topiramate and sex. Physiol Behav. 2012;107:781–6. doi: 10.1016/j.physbeh.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vlkolinsky R, Krucker T, Smith AL, Lamp TC, Nelson GA, Obenaus A. Effects of lipopolysaccharide on 56Fe particle radiation-induced impairment of synaptic plasticity in the mouse hippocampus. Radiat Res. 2007;168:462–70. doi: 10.1667/RR1038.1. [DOI] [PubMed] [Google Scholar]
- 16.Machida M, Lonart G, Britten RA. Low (60 cGy) doses of 56Fe HZE radiation lead to a persistent reduction in the glutamatergic readily releasable pool in rat hippocampal synaptosomes. Radiat Res. 2010;174:619–23. doi: 10.1667/RR1988.1. [DOI] [PubMed] [Google Scholar]
- 17.Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16:1197–207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- 18.Stevens CF. Presynaptic function. Curr Opin Neurobiol. 2004;14:341–5. doi: 10.1016/j.conb.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berg EA. A simple objective technique for measuring flexibility in thinking. J Gen Psychol. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- 21.Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J Neurosci. 2008;28:11124–30. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glickstein SB, Desteno DA, Hof PR, Schmauss C. Mice lacking dopamine D2 and D3 receptors exhibit differential activation of prefrontal cortical neurons during tasks requiring attention. Cereb Cortex. 2005;15:1016–24. doi: 10.1093/cercor/bhh202. [DOI] [PubMed] [Google Scholar]
- 23.Yang C, McKenna JT, Zant JC, Winston S, Basheer R, Brown RE. Cholinergic neurons excite cortically projecting basal forebrain GABAergic neurons. J Neurosci. 2014;34:2832–44. doi: 10.1523/JNEUROSCI.3235-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]