Summary
Blood pressure can vary considerably during anesthesia. If blood pressure falls outside the limits of cerebrovascular autoregulation, children can become at risk of cerebral ischemic or hyperemic injury. However, the blood pressure limits of autoregulation are unclear in infants and children, and these limits can shift after brain injury. This article will review autoregulation, considerations for the hemodynamic management of children with brain injuries, and research on autoregulation monitoring techniques.
Keywords: pediatrics, blood pressure, cerebrovascular circulation, brain injuries
Introduction
When providing general anesthesia to children, anesthesiologists have limited ability to monitor one of the most important organs—the brain. Ensuring adequate cerebral perfusion pressure (CPP) is paramount to preventing cerebral ischemia or hyperemia. CPP is the difference between mean arterial blood pressure (MAP) and intracranial pressure (ICP), or between MAP and central venous pressure (CVP) if CVP exceeds the ICP. Cerebrovascular autoregulation maintains relatively constant cerebral blood flow (CBF) across changes in perfusion pressure. It functions within a specific range of blood pressures, but the limits of autoregulation are poorly defined in infants and children. Adult CPP guidelines are not useful in pediatric anesthesia because blood pressure norms vary by age (1), and anesthesia may interact with these norms. Moreover, neurologic injuries can raise ICP from mass effect or evolving secondary injury with edema. Intracranial hypertension can independently shift the limits of autoregulation (2) and increase the risk of stroke. It is therefore critical for the anesthesiologist to maintain the patient's blood pressure within a range that supports autoregulatory function.
Cerebrovascular Blood Pressure Autoregulation
Multiple mechanisms modify CBF in response to changes in cerebral metabolism, blood pressure, glucose (3), and partial pressure of carbon dioxide (4) and oxygen (3). This review will focus on the regulation of CBF with changes in blood pressure. Under normal physiologic conditions, blood vessels dilate and constrict in response to changes in perfusion pressure to maintain CBF across fluctuations in CPP. This process, known as cerebrovascular blood pressure autoregulation, is mediated by vascular reactivity to changes in perfusion pressure. When blood pressure is on the plateau between the lower and upper limits of autoregulation, CBF is held relatively constant. Along this plateau, the vascular regulation of CBF is considered “pressure-reactive” because the vasculature appropriately dilates to maintain CBF as blood pressure decreases, or constricts to constrain CBF as blood pressure increases. Under such conditions, the risks of cerebral ischemia or hyperemia are minimized.
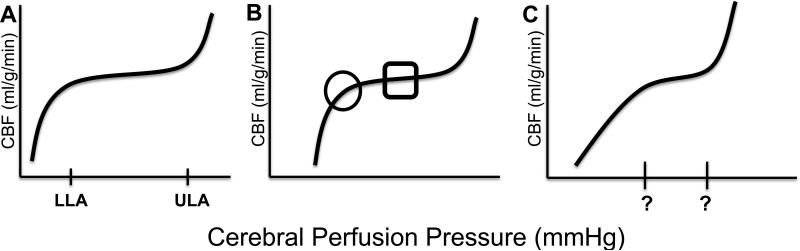
When blood pressure decreases to below the lower limit of autoregulation, the cerebral vasculature reaches its maximal vasodilatory capacity. CBF decreases with progressive hypotension, and the brain becomes vulnerable to ischemic injury. When blood pressure exceeds the upper limit of autoregulation, the cerebral vasculature cannot constrict further to constrain CBF, and the brain becomes subject to hyperemic injury. When blood pressure is either below the lower limit or above the upper limit of autoregulation, the vascular regulation of CBF is said to be “pressure-passive” (Figure 1A).
Figure 1.
The cerebrovascular autoregulation curve. (A) When cerebral perfusion pressure is on the autoregulatory plateau, which is between the lower limit of autoregulation (LLA) and upper limit of autoregulation (ULA), cerebral blood flow (CBF) is held relatively constant across changes in blood pressure through vasoreactivity. Autoregulation is functional and CBF is “pressure-reactive.” When blood pressure decreases to below the LLA or increases to above the ULA, pressure-vasoreactivity fails and CBF becomes “pressure-passive.” (B) The lower limit of autoregulation is circled. The square highlights the optimal cerebral perfusion pressure at which vasoactive responses to changes in cerebral perfusion pressure are maximal and autoregulation is most robust. Conceptually, when blood pressure is within this square, autoregulatory function is optimized. (C) After brain injury, the LLA may shift to a higher blood pressure (rightward shift), the ULA may shift to a lower blood pressure, and the autoregulatory plateau may become narrowed (62).
Traditionally, the autoregulation curve is illustrated with 1) a horizontal CBF plateau at levels of blood pressure within the range that produces pressure-reactive CBF, 2) a discrete cutoff at the lower limit of autoregulation with a decline in CBF at pressures below the lower limit of autoregulation, and 3) a cutoff at the upper limit of autoregulation with increasing CBF above that point. This representation is based on pooling CBF responses to fluctuations in CPP from multiple studies. In reality, the slope of the autoregulatory plateau for an individual is not precisely zero (5-7), and the limits of autoregulation are smooth inflections on a curve (Figures 2 and 3). When blood pressure crosses below the lower limit of autoregulation, cerebral arteries and arterioles may continue to dilate, but to a degree that is insufficient to maintain steady CBF. Likewise, when blood pressure exceeds the upper limit of autoregulation, additional cerebrovascular constriction may occur but be inadequate to maintain constant CBF. With extreme increases in arterial pressure, passive dilation of arteries can transmit pulsatile pressure to the cerebral microcirculation.
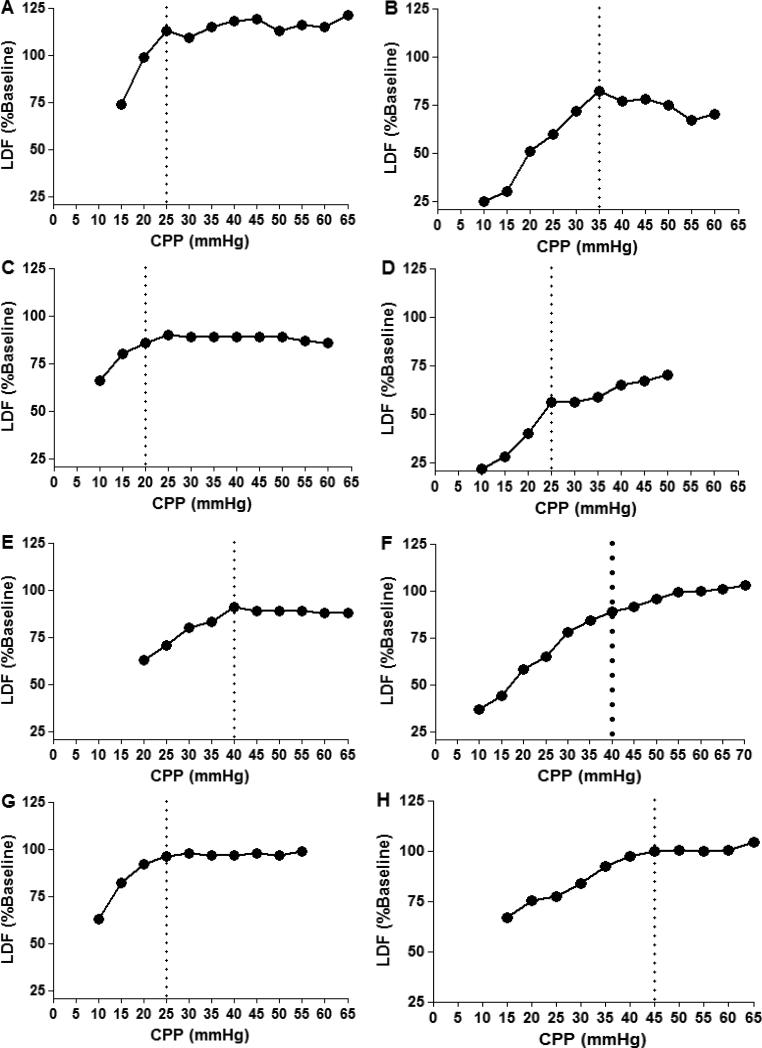
Figure 2.
Examples of individual cerebral blood flow (CBF) autoregulation curves. CBF was measured with laser Doppler flowmetry (LDF) in 8 piglets as hypotension was slowly induced. LDF measurements were averaged in 5 mmHg bins of cerebral perfusion pressure (CPP), and the calculated lower limit of autoregulation (LLA) is demarcated with a dotted line. Variation among individual animals is observed in the LDF responses to hypotension. The left column illustrates piglets with relatively horizontal autoregulatory plateaus. The right column illustrates piglets that did not have horizontal LDF curves when CPP exceeded the LLA. Piglets in panels C, F, G, and H display smooth inflections in CBF that are often observed when blood pressure crosses below the lower limit of autoregulation. Reproduced with permission from: Larson AC, Jamrogowicz JL, Kulilkowicz E, Wang B, Yang ZJ, Shaffner DH, Koehler RC, Lee JK. Cerebrovascular autoregulation after rewarming from hypothermia in a neonatal swine model of asphyxic brain injury. J Appl Phyiol 2013; 115(10):1433-42.
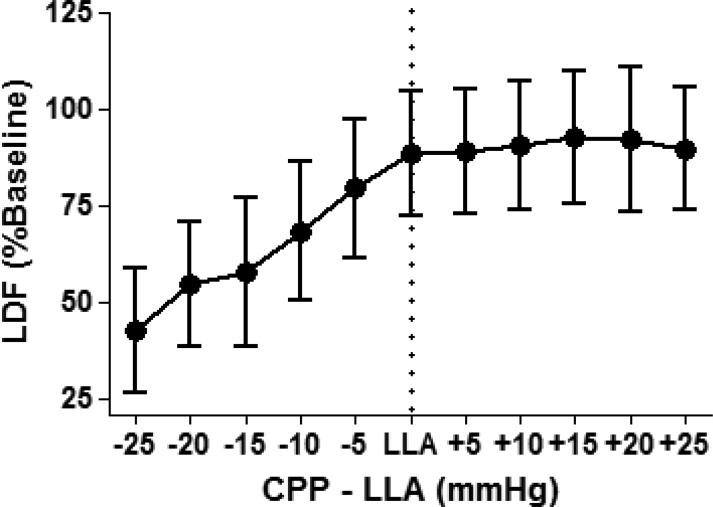
Figure 3.
Data from the 8 piglets in figure 2 were combined to generate a single cerebral blood flow (CBF) autoregulation curve. Each piglet's lower limit of autoregulation (LLA; dotted line) is centered to zero on the x-axis to permit comparison of each LLA relative to the cerebral perfusion pressure (CPP). When CBF data from multiple individuals are pooled together, the autoregulatory plateau appears horizontal, and a cut-point in CBF is obseved at the LLA. Data are displayed as means with standard deviations. LDF, laser Doppler flowmetry.
Consequently, there is an optimal CPP in the middle of the autoregulatory curve at which the percent change in arterial diameter in response to perturbations in CPP is maximal. At this optimal CPP, autoregulatory function is most robust. With deviation of CPP from this optimal point of pressure reactivity, CBF becomes increasingly pressure-passive in a graded fashion. At the extremes, vasoreactivity fails (8) and CBF becomes completely pressure-passive. To maximize the vasoreactive reserve of this homeostatic defense mechanism against decreases or increases in CPP, anesthesiologists could consider maintaining a patient's CPP near the optimal point for maximal vascular reactivity and most robust autoregulation (Figure 1B). In this article, maintenance of blood pressure within the range that provides the most robust autoregulation (maximal vasoreactivity) will be referred to as “optimizing autoregulation.”
The concept of “impaired autoregulation” commonly refers to shifts in the blood pressure limits of autoregulation or complete pressure-passivity in CBF at all blood pressures. For instance, intracranial hypertension can shift the lower limit of autoregulation to a higher blood pressure (2). Alternatively, the limits of autoregulation may remain stable but the child's blood pressure may fall to below the lower limit of autoregulation; stability in CBF could be restored by raising the blood pressure to exceed the lower limit. Identifying an individual child's lower limit of autoregulation and the blood pressure range at which autoregulation is optimized would clarify neuroprotective hemodynamic goals, even if the autoregulation curve shifts after brain injury.
Children at Risk of Permanent Neurologic Injuries
Because the blood pressure limits of autoregulation are unknown in infants and children, all pediatric patients could arguably be considered at risk of CBF dysregulation during anesthesia. Numerous conditions can disrupt autoregulation, including prematurity, brain trauma, hypoxic brain injuries, intracranial hemorrhages, vascular anomalies, congenital cardiac lesions, and cerebral inflammation. Preterm neonates regulate CBF across specific ranges of MAP (9,10). The risk of dysfunctional autoregulation in preterm neonates increases with illness severity (11), and the risk of intraventricular hemorrhage may be higher in those with longer periods of pressure-passive CBF (12).
Traumatic brain injury (TBI) is another situation in which the anesthesiologist should be cognizant of possible CBF dysregulation. Children with TBI may present emergently to the operating room for evacuation of an intracranial hematoma, decompressive craniectomy, or placement of an ICP monitor. These children are at high risk of pressure-passive CBF and stroke. The anesthesiologist must be attuned to the potential for intracranial hypertension because MAP below or within the expected limits of an uninjured child could result in a CPP that is too low if the ICP is elevated. Furthermore, elevations in ICP can independently shift the lower limit of autoregulation to a higher blood pressure (2), causing a rightward shift in the autoregulation curve for an equivalent CPP (Figure 1C). The combination of low CPP and an elevated lower limit of autoregulation exposes these children to significant risk of cerebral ischemia and stroke. When ICP monitoring is not feasible, the anesthesiologist should consider empirically raising the goal MAP to maintain CPP.
Whether it is optimal to raise the CPP by increasing MAP, decreasing ICP, or both, depends on the clinical scenario. Hypotension from hemorrhage warrants volume expansion. Vasodilation from anesthesia might be best treated with vasopressors. Acute intracranial hypertensive crises may respond to hyperventilation, hyperosmolar therapy, and sedatives. In swine models, raising blood pressure with norepinephrine or phenylephrine during intravenous anesthesia with fentanyl and midazolam produced stable CBF. But the same vasopressor doses during isoflurane anesthesia produced pressure-passive CBF as autoregulation failed during progressive hypertension (13). Furthermore, the effects of vasopressors on autoregulation may vary by gender after brain trauma (14,15). Additional research is needed to define optimal methods of modulating CPP to support autoregulation after TBI.
Children with mild or moderate TBI present unique challenges. ICP is generally not monitored in this population, so CPP cannot be calculated. A study of children without ICP monitors who had moderate to severe TBI and required extracranial surgery within 72 hours of injury showed no association between systemic hypotension and postoperative neurologic complications. Systemic hypotension was defined as systolic blood pressure <70+2×age or 90 mmHg, and surgeries included orthopedic, abdominal, and thoracic procedures. However, the authors did not measure CBF or oxygenation and therefore may not have detected transient perturbations in these parameters. As expected, CPP < 40 mmHg was associated with postoperative intracranial hypertension in children with ICP monitors (16).
It is reasonable to infer that intraoperative hypotension may be more detrimental to children with suspected intracranial hypertension than to neurologically normal children. However, additional studies are needed to verify this supposition. We recommend a conservative approach to these patients. If the patient may have mild or moderate TBI based on the mechanism of injury or loss of consciousness, but a computed tomography scan of the head and preoperative neurologic exam are normal, we recommend maintaining MAP near or ~10 mmHg above that measured at baseline with the patient awake. If a reliable preoperative blood pressure cannot be obtained, we suggest using the patient's age-based blood pressure norms (1) for baseline. The primary goal is to maintain adequate CPP and avoid decreases in blood pressure in case the child has undetected intracranial hypertension. Total intravenous anesthesia may be considered, as some evidence indicates that intravenous anesthetics maintain CBF autoregulation better than do volatile agents (13,17,18). The patient should be extubated as soon as possible postoperatively for serial neurologic exams. If the child exhibits preoperative neurologic changes, waxing and waning neurologic status, or hemodynamic signs of intracranial hypertension, and if time permits, we recommend obtaining a preoperative neurosurgical consult to document whether ICP monitoring is indicated.
Infants and children with hypoxic brain injuries constitute another population at high risk of disturbed autoregulation. Approximately 16,000 American children suffer a cardiac arrest each year (19), and neonatal hypoxic-ischemic encephalopathy from asphyxic birth injury affects approximately 3 in 1000 neonates (20). Cerebral edema and intracranial hypertension may evolve for days after the initial hypoxic insult. Invasive ICP monitors are typically not used in these children, leaving the anesthesiologist blind to the degree of ICP elevation. Although most research on intracranial hypertension has focused on TBI, it is known that infants with open fontanelles or sutures can experience elevated ICP (21-23). Therapeutic hypothermia may acutely decrease the lower limit of autoregulation and temporarily preserve CBF pressure-reactivity by expanding the autoregulatory plateau after hypoxia (24), although it is unclear if this effect is sustained beyond the immediate period after injury. Rewarming, which can increase ICP in severely injured patients (25), could shift the blood pressure limits of optimal autoregulatory function to higher pressures (26).
Neuroprotective hemodynamic goals during general anesthesia for children without brain injuries are poorly defined. Research on the short- and long-term neurocognitive effects of anesthesia must take into account intraoperative blood pressure management. Neonates, particularly preterm neonates, are at considerable risk of neurologic injury during anesthesia. This risk is due in part to rapid increases in normal blood pressure levels during the first week of life and a propensity toward pressure-passive cerebral blood flow with moderate decreases in blood pressure. The risk is compounded when physicians are challenged to select the correct blood pressure cuff size and modulate blood pressure with little evidence on whether fluid-based or pharmacologic interventions should be used (27). One study in healthy infants <6 months old who received sevoflurane with a caudal block suggested that the lower limit of autoregulation occurred at a MAP of 38 mmHg or when blood pressure was >20% from the preoperative baseline (28). Since little evidence is available to support or refute specific blood pressure guidelines during pediatric anesthesia, we recommend targeting the child's preoperative baseline blood pressure if it was obtained without distress and with an appropriately sized cuff. If a reliable baseline cannot be obtained, we suggest following age-based guidelines in healthy children (1). For instance, the 50th percentile for MAP in children at the 50th percentile for height is approximately 55 mmHg at 1 year of age and 67 mmHg at 5 years (1). The degree and duration of relative hypotension that can safely be tolerated remains unclear.
Optimizing Autoregulation
Given the effects of interactions between age (28), ICP (2), anesthesia (18,29), vasopressors (13), carbon dioxide (30,31), gender (14,15), temperature (24), inflammation (32,33), and brain injury on cerebral autoregulation, it is impossible to select uniform and safe blood pressure goals for all children undergoing anesthesia. Some of these factors change minute by minute, whereas others probably alter the autoregulation curve over hours to days. Techniques that can identify the lower limit of autoregulation and the MAP range with most robust autoregulation could enable anesthesiologists to individualize and target neuroprotective hemodynamic goals.
CBF can be monitored with advanced imaging techniques such as MRI (34-36) or single photon emission computed tomography (SPECT (37). However, these imaging modalities cannot be used at the patient's bedside. Traditional autoregulation metrics for continuous monitoring use invasive intracranial monitors, including partial pressure oxygen measurements from the brain parenchyma (PbO2) (38) and ICP (39,40). Trends in the PbO2 are assumed to be proportional to changes in CBF during situations of constant oxygen supply and demand. One can determine autoregulatory function by using ICP as a surrogate measure of cerebral blood volume to assess vasoreactive responses to changes in CPP (41). Laser Doppler flowmetry (42) and jugular venous bulb saturation measurements (43) have also been used in experimental settings to measure intraoperative CBF during neurosurgical procedures. In routine clinical situations, intracranial monitoring is primarily reserved for severe TBI. This practice leaves a large population of high-risk children unmonitored, including those with mild or moderate TBI, hypoxic brain injuries, congenital cardiac lesions, meningitis, or intracranial vascular malformations.
Noninvasive, direct, or surrogate measures of CBF that are most commonly used during anesthesia include transcranial Doppler (TCD) (18,28,29,44) and cerebral near-infrared spectroscopy (NIRS) (45,46). Neither modality measures deep regions of the brain. TCD measures CBF velocity within a specific vessel. One can ascertain autoregulatory function by determining whether CBF velocity remains relatively constant or fluctuates passively with changes in blood pressure (28,47). NIRS-based monitors measure oxygenated and deoxygenated tissue hemoglobin density in the frontal cortex and calculate the tissue oxyhemoglobin saturation. Dynamic changes in tissue oxyhemoglobin saturation approximate dynamic changes in cerebral venous and arterial oxyhemoglobin, thereby estimating changes in the ratio of oxygen consumption to oxygen delivery. Because oxygen delivery to the brain is the product of CBF and arterial oxygen content, changes in the NIRS-derived tissue oxyhemoglobin saturation reflect changes in CBF when hemoglobin concentration, arterial oxygen tension, and cerebral oxygen consumption are constant. Under conditions of constant oxygen supply and demand, changes in the oxygenated hemoglobin density reflect changes in CBF when blood pressure decreases to below the lower limit of autoregulation (5).
Autoregulation and vasoreactivity can be monitored at the bedside by correlating measures of CBF or cerebral blood volume to spontaneous changes in blood pressure. This technique requires continuous arterial blood pressure monitoring, such as with an arterial catheter, but can be applied in real time during anesthesia and perioperatively. The overall goal is to identify an individual child's lower limit of autoregulation and the blood pressure range with optimal autoregulatory function. An individual child's blood pressure autoregulation curve can essentially be reconstructed at the bedside.
Autoregulation indices can be generated by correlating CBF to blood pressure. When CPP (or MAP if ICP monitoring is not available) is on the autoregulatory plateau, CBF remains relatively constant across changes in blood pressure because autoregulation is functional. In this scenario, surrogate or direct measures of CBF—PbO2 (38), CBF velocity from TCD (48), or oxygenated hemoglobin density from NIRS (45,49,50)—do not correlate or negatively correlate with blood pressure. The result is a near-zero or negative autoregulation index, which indicates intact autoregulation. In some individuals, a transient phenomenon of “supra-autoregulation” can be observed when the cerebral resistance vessels have robust vasoreactive responses to changes in perfusion pressure (7). In these situations, CBF exhibits a slight inverse relationship with MAP and generates a negative index. When blood pressure is below the lower limit of autoregulation and autoregulation becomes impaired, CBF correlates positively with blood pressure, yielding a positive autoregulation index (Figure 4).
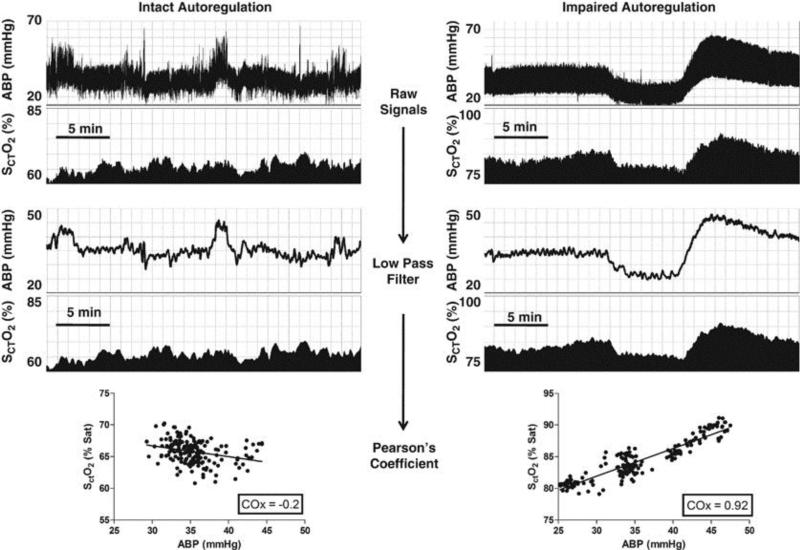
Figure 4.
Monitoring autoregulation with near-infrared spectroscopy (NIRS) in a preterm neonate. Slow, spontaneous waves in the mean arterial blood pressure (ABP) and NIRS-derived cerebral oximetry (SCTO2) were correlated to generate an index of autoregulation: the cerebral oximetry index (COx). Examples of functional and impaired autoregulation in the same neonate are shown. Raw signals of ABP and SCTO2 were collected at the bedside (top four strips). Low-pass filters removed the fast pulse and respiratory frequency waveforms. COx was generated by a correlation coefficient between ABP and SCTO2. As shown on the left (intact autoregulation), a negative COx indicates functional autoregulation. On the right (impaired autoregulation), a positive COx indicates dysfunctional autoregulation. Reproduced with permission from Macmillan Publishers Ltd: Gilmore MM, Stone BS, Shepard JA, Czosnyka M, Easley RB, Brady KM. Relationship between cerebrovascular dysautoregulation and arterial blood pressure in the premature infant. J Perintol. 2011;31(11):722-729.
Vasoreactivity, the mechanism that mediates CBF autoregulation, can be measured by changes in cerebral blood volume. As the cerebral vasculature responds to fluctuations in blood pressure through vasodilation and vasoconstriction, the cerebral blood volume and ICP change. Slow wave changes in ICP are related to changes in cerebral blood volume from autoregulatory vasodilation and vasoconstriction (41). Changes in cerebral blood volume from vasoreactivity are proportional to changes in total tissue hemoglobin density measured by NIRS.
Autoregulatory vasoreactivity can be measured with ICP (51) or NIRS (41). When vasoreactivity is functional (and autoregulation is intact), changes in blood volume do not correlate or negatively correlate with changes in blood pressure. Consequently, the autoregulation vasoreactivity index will be near-zero or negative. Impaired vasoreactivity (with impaired autoregulation) is indicated by a direct correlation between blood volume and blood pressure, which produces a positive index (Figure 5A-D).
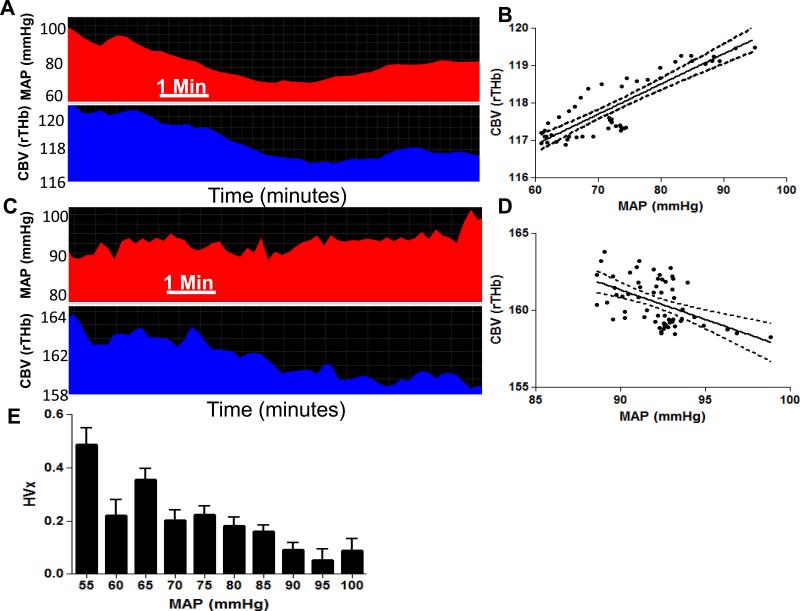
Figure 5.
Monitoring autoregulatory vasoreactivity in an 11-year-old girl with moyamoya vasculopathy during anesthesia for pial synangiosis and postoperatively with near-infrared spectroscopy (NIRS). (A, B) When mean arterial blood pressure (MAP) was approximately 60–90 mmHg intraoperatively, MAP and cerebral blood volume (CBV, or the relative total hemoglobin [rTHb] measured by NIRS) were positively correlated. This positive correlation yielded a positive autoregulation index (hemoglobin volume index, HVx) value of 0.46, indicating pressure-passive vascular reactivity with impaired autoregulation. (C, D) In the same patient, when MAP was ≥90 mmHg in the postoperative period, MAP and CBV were negatively correlated. This negative correlation resulted in an HVx value of −0.26, indicating pressure-reactive vascular reactivity with functional autoregulation. (E) HVx monitoring for 8 h during and after surgery is shown. Mean values of HVx were sorted into 5-mmHg bins of MAP. HVx became higher when MAP was <90 mmHg, indicating that autoregulation was more impaired at lower blood pressures. When MAP was ≥90 mmHg, HVx decreased with improving autoregulation. Autoregulatory function was optimal at a MAP of 95 mmHg, as indicated by the most negative HVx value. Data in panels B and D are shown with linear regression lines and 95% confidence intervals. Data in panel E are shown as means ± sd. Reproduced with permission from: Lee JK, Williams M, Jennings JM, Jamrogowicz JL, Larson AC, Jordan LC, Heitmiller ES, Hogue CW, Ahn ES. Cerebrovascular autoregulation in pediatric moyamoya disease. Paediatr Anaesth 2013;23(6):547-556.
These techniques pinpoint the blood pressure at which the autoregulation index is most negative (26,45), enabling clinicians to identify the blood pressure range over which an individual child's autoregulatory function is optimized (52) (Figure 5E). A child's lower limit of autoregulation can also be identified as the blood pressure at which a positive index exceeds a defined threshold (50) (Figure 6). The limits of autoregulation measured by indices derived from NIRS, TCD, and ICP have good correlation (49,53,54).
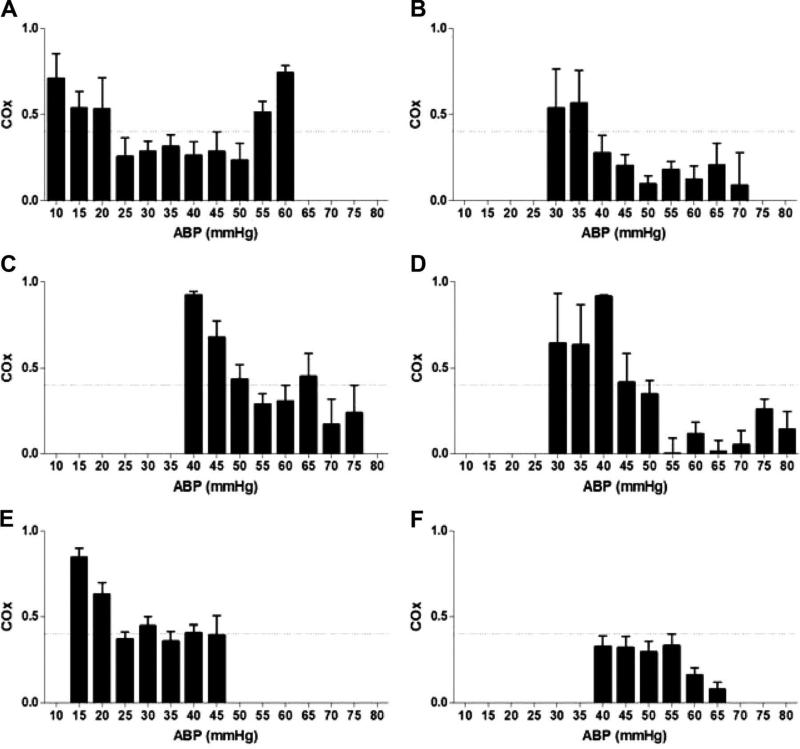
Figure 6.
The lower limit of autoregulation (LLA) can be detected during cardiopulmonary bypass in some children. The cerebral oximetry index (COx) was calculated with a correlation coefficient between mean arterial blood pressure (ABP) and regional tissue oxyhemoglobin density from cerebral near-infrared spectroscopy. For each child, the average COx is plotted versus ABP. COx ≥0.4 (horizontal dashed line) was defined as the LLA threshold in this study. Children in examples A-D had an identified LLA. (A) Sixday-old neonate with interrupted aortic arch (LLA at 20 mmHg). (B) Seven-month-old infant with ventricular septal defect (LLA at 40 mmHg). (C) Two-year-old child with ventricular septal defect (LLA at 50 mmHg). (D) Seven-year-old child with anomalous right coronary artery (LLA at 45 mmHg). Children in examples E and F did not demonstrate an LLA. (E) Four-month-old infant with truncus arteriosus. The LLA could not be determined because COx was inconsistently >0.4. (F) Fourteen-year-old adolescent with cardiomyopathy. The LLA could not be determined because COx was consistently <0.4. Reproduced with permission from: Brady KM, Mytar JO, Lee JK, Cameron DE, Vricella LA, Thompson WR, Hogue CW, Easley RB. Monitoring cerebral blood flow pressure autoregulation in pediatric patients during cardiac surgery. Stroke 2010;41(9):1957-1962.
Nevertheless, these techniques have been criticized for their reliance on spontaneous fluctuations in blood pressure to stimulate vasoreactivity. Sporadic fluctuations in blood pressure or changes in blood pressure that are incongruent with changes in CBF and blood volume introduce imprecision into the measurements. Therefore, some investigators prefer to intentionally alter blood pressure in a step-wise fashion to obtain CBF measurements during transient changes in CPP (55-57). The disadvantage of this method is that the blood pressure range with optimal autoregulation cannot be identified through continuous monitoring over a prolonged period.
Functional Autoregulation and Improved Outcomes
Observational studies suggest that better autoregulation is associated with improved neurologic outcomes. The risk of death after pediatric TBI increases as autoregulatory vasoreactivity worsens (51). Additionally, Vavilala et al. (58) reported that children with TBI and functional autoregulation had better 6-month outcomes than did children with TBI and disturbed autoregulation. In a pilot study of neonates with hypoxic-ischemic encephalopathy who received therapeutic hypothermia, neonates who spent more time at blood pressures with poorer autoregulatory function during rewarming were more likely to have moderate to severe brain injuries on MRI than were neonates whose blood pressure remained within the range of optimized autoregulation (26).
An association between functional blood pressure autoregulation and improved neurologic outcomes has also been observed in adults with subarachnoid hemorrhage (59). In a study of 299 adults with TBI, Aries et al. (40) found that blood pressures below the range with optimized autoregulation were associated with increased mortality and that blood pressures above the range with optimized autoregulation were associated increased risk of severe disability. Others have reported that during cardiopulmonary bypass in adults, greater blood pressure deviation below the lower limit of autoregulation is associated with stroke and renal injury (60,61). Whether maintaining blood pressure within the range that optimizes autoregulatory function improves neurologic outcomes cannot be determined in these observational studies. Nonetheless, there is a clear association between blood pressure autoregulation and neurologic outcomes in brain-injured patients.
Autoregulation monitoring could eventually enable anesthesiologists to tailor hemodynamic management to individual patients as clinical situations evolve. However, it remains to be determined whether manipulating a child's blood pressure to keep it above the identified lower limit of autoregulation and/or within the range of optimized autoregulation improves neurologic outcomes. While it is tempting to consider monitoring autoregulation on an individual basis for children with mild or moderate brain injuries, these techniques remain investigative and not within standard clinical care. Multicenter and interventional studies are needed to explore the relationship between the lower limit of autoregulation, the blood pressure range with optimal autoregulation, and neurologic outcomes in children.
Conclusions
The blood pressure limits of autoregulation are poorly defined in infants and children, and as clinical conditions change with evolving brain injuries, the limits of the autoregulation curve may shift. Monitoring techniques that identify the hemodynamic range that optimizes autoregulatory function would enable anesthesiologists to individualize blood pressure management in children at risk of permanent neurologic injuries.
Acknowledgements
We are grateful to Claire Levine for her editorial assistance. We thank Dr. Raymond Koehler, Dr. Ken Brady, Dr. Donald (Hal) Shaffner, Dr. Sol Soriano, and Dr. Mary Ellen McCann for their expertise and consultation in writing this manuscript.
Sources of Funding. Dr. Lee was supported by an American Heart Scientist Development Grant. Dr. Williams was supported by NIH grant 5T32GM075774-07.
Footnotes
Disclosures. Dr. Lee previously received research funding from Covidien.
REFERENCES
- 1.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004 Aug;114(2 Suppl 4th Report):555–576. [PubMed] [Google Scholar]
- 2.Brady KM, Lee JK, Kibler KK, Easley RB, Koehler RC, Czosnyka M, et al. The lower limit of cerebral blood flow autoregulation is increased with elevated intracranial pressure. Anesth Analg. 2009 Apr;108(4):1278–1283. doi: 10.1213/ane.0b013e3181964848. [DOI] [PubMed] [Google Scholar]
- 3.Adebiyi A, McNally EM, Jaggar JH. Vasodilation induced by oxygen/glucose deprivation is attenuated in cerebral arteries of SUR2 null mice. Am J Physiol Heart Circ Physiol. 2011 Oct;301(4):H1360–8. doi: 10.1152/ajpheart.00406.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raichle ME, Posner JB, Plum F. Cerebral blood flow during and after hyperventilation. Arch Neurol. 1970;23(5):394–403. doi: 10.1001/archneur.1970.00480290014002. [DOI] [PubMed] [Google Scholar]
- 5.Larson AC, Jamrogowicz JL, Kulikowicz E, Wang B, Yang ZJ, Shaffner DH, et al. Cerebrovascular autoregulation after rewarming from hypothermia in a neonatal swine model of asphyxic brain injury. J Appl Physiol. 1985 2013 Sep 5;115(10):1433–1442. doi: 10.1152/japplphysiol.00238.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucas SJ, Tzeng YC, Galvin SD, Thomas KN, Ogoh S, Ainslie PN. Influence of changes in blood pressure on cerebral perfusion and oxygenation. Hypertension. 2010 Mar;55(3):698–705. doi: 10.1161/HYPERTENSIONAHA.109.146290. [DOI] [PubMed] [Google Scholar]
- 7.Jones SC, Radinsky CR, Furlan AJ, Chyatte D, Qu Y, Easley KA, et al. Variability in the magnitude of the cerebral blood flow response and the shape of the cerebral blood flow-pressure autoregulation curve during hypotension in normal rats [corrected]. Anesthesiology. 2002 Aug;97(2):488–496. doi: 10.1097/00000542-200208000-00028. [DOI] [PubMed] [Google Scholar]
- 8.Brady KM, Lee JK, Kibler KK, Easley RB, Koehler RC, Shaffner DH. Continuous measurement of autoregulation by spontaneous fluctuations in cerebral perfusion pressure: comparison of 3 methods. Stroke. 2008 Sep;39(9):2531–2537. doi: 10.1161/STROKEAHA.108.514877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilmore MM, Stone BS, Shepard JA, Czosnyka M, Easley RB, Brady KM. Relationship between cerebrovascular dysautoregulation and arterial blood pressure in the premature infant. J Perinatol. 2011 Mar 3;31(11):722–729. doi: 10.1038/jp.2011.17. [DOI] [PubMed] [Google Scholar]
- 10.Tyszczuk L, Meek J, Elwell C, Wyatt JS. Cerebral blood flow is independent of mean arterial blood pressure in preterm infants undergoing intensive care. Pediatrics. 1998 Aug;102(2 Pt 1):337–341. doi: 10.1542/peds.102.2.337. [DOI] [PubMed] [Google Scholar]
- 11.Wong FY, Silas R, Hew S, Samarasinghe T, Walker AM. Cerebral oxygenation is highly sensitive to blood pressure variability in sick preterm infants. PLoS One. 2012;7(8):e43165. doi: 10.1371/journal.pone.0043165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alderliesten T, Lemmers PM, Smarius JJ, van de Vosse RE, Baerts W, van Bel F. Cerebral oxygenation, extraction, and autoregulation in very preterm infants who develop peri-intraventricular hemorrhage. J Pediatr. 2013 Apr;162(4):698–704. e2. doi: 10.1016/j.jpeds.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 13.Bruins B, Kilbaugh TJ, Margulies SS, Friess SH. The anesthetic effects on vasopressor modulation of cerebral blood flow in an immature swine model. Anesth Analg. 2013 Apr;116(4):838–844. doi: 10.1213/ANE.0b013e3182860fe7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armstead WM, Kiessling JW, Kofke WA, Vavilala MS. Impaired cerebral blood flow autoregulation during posttraumatic arterial hypotension after fluid percussion brain injury is prevented by phenylephrine in female but exacerbated in male piglets by extracellular signal-related kinase mitogen-activated protein kinase upregulation. Crit Care Med. 2010 Sep;38(9):1868–1874. doi: 10.1097/CCM.0b013e3181e8ac1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstead WM, Kiessling JW, Riley J, Kofke WA, Vavilala MS. Phenylephrine infusion prevents impairment of ATP- and calcium-sensitive potassium channel-mediated cerebrovasodilation after brain injury in female, but aggravates impairment in male, piglets through modulation of ERK MAPK upregulation. J Neurotrauma. 2011 Jan;28(1):105–111. doi: 10.1089/neu.2010.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujita Y, Algarra NN, Vavilala MS, Prathep S, Prapruettham S, Sharma D. Intraoperative secondary insults during extracranial surgery in children with traumatic brain injury. Childs Nerv Syst. 2014 Jan 16; doi: 10.1007/s00381-014-2353-3. [DOI] [PubMed] [Google Scholar]
- 17.Dahyot-Fizelier C, Frasca D, Debaene B. Inhaled agents in neuroanaesthesia for intracranial surgery: pro or con. Ann Fr Anesth Reanim. 2012 Oct;31(10):e229–34. doi: 10.1016/j.annfar.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Strebel S, Lam AM, Matta B, Mayberg TS, Aaslid R, Newell DW. Dynamic and static cerebral autoregulation during isoflurane, desflurane, and propofol anesthesia. Anesthesiology. 1995 Jul;83(1):66–76. doi: 10.1097/00000542-199507000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Topjian AA, Berg RA, Nadkarni VM. Pediatric cardiopulmonary resuscitation: advances in science, techniques, and outcomes. Pediatrics. 2008 Nov;122(5):1086–1098. doi: 10.1542/peds.2007-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graham EM, Ruis KA, Hartman AL, Northington FJ, Fox HE. A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am J Obstet Gynecol. 2008 Dec;199(6):587–595. doi: 10.1016/j.ajog.2008.06.094. [DOI] [PubMed] [Google Scholar]
- 21.Keenan HT, Nocera M, Bratton SL. Frequency of intracranial pressure monitoring in infants and young toddlers with traumatic brain injury. Pediatr Crit Care Med. 2005 Sep;6(5):537–541. doi: 10.1097/01.PCC.0000164638.44600.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kochanek PM, Carney N, Adelson PD, Ashwal S, Bell MJ, Bratton S, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents--second edition. Pediatr Crit Care Med. 2012 Jan;13(Suppl 1):S1–82. doi: 10.1097/PCC.0b013e31823f435c. [DOI] [PubMed] [Google Scholar]
- 23.Mehta A, Kochanek PM, Tyler-Kabara E, Adelson PD, Wisniewski SR, Berger RP, et al. Relationship of intracranial pressure and cerebral perfusion pressure with outcome in young children after severe traumatic brain injury. Dev Neurosci. 2010;32(5-6):413–419. doi: 10.1159/000316804. [DOI] [PubMed] [Google Scholar]
- 24.Lee JK, Brady KM, Mytar JO, Kibler KK, Carter EL, Hirsch KG, et al. Cerebral blood flow and cerebrovascular autoregulation in a swine model of pediatric cardiac arrest and hypothermia. Crit Care Med. 2011 Oct;39(10):2337–2345. doi: 10.1097/CCM.0b013e318223b910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iida K, Kurisu K, Arita K, Ohtani M. Hyperemia prior to acute brain swelling during rewarming of patients who have been treated with moderate hypothermia for severe head injuries. J Neurosurg. 2003 Apr;98(4):793–799. doi: 10.3171/jns.2003.98.4.0793. [DOI] [PubMed] [Google Scholar]
- 26.Howlett JA, Northington FJ, Gilmore MM, Tekes A, Huisman TAGM, Parkinson C, et al. Cerebrovascular autoregulation and neurologic injury in neonatal hypoxic-ischemic encephalopathy. Pediatr Res. 2013;74(5):525–535. doi: 10.1038/pr.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCann ME, Schouten AN. Beyond survival; influences of blood pressure, cerebral perfusion and anesthesia on neurodevelopment. Paediatr Anaesth. 2014 Jan;24(1):68–73. doi: 10.1111/pan.12310. [DOI] [PubMed] [Google Scholar]
- 28.Rhondali O, Mahr A, Simonin-Lansiaux S, De Queiroz M, Rhzioual-Berrada K, Combet S, et al. Impact of sevoflurane anesthesia on cerebral blood flow in children younger than 2 years. Paediatr Anaesth. 2013 Oct;23(10):946–951. doi: 10.1111/pan.12166. [DOI] [PubMed] [Google Scholar]
- 29.Summors AC, Gupta AK, Matta BF. Dynamic cerebral autoregulation during sevoflurane anesthesia: a comparison with isoflurane. Anesth Analg. 1999 Feb;88(2):341–345. doi: 10.1097/00000539-199902000-00022. [DOI] [PubMed] [Google Scholar]
- 30.McCulloch TJ, Boesel TW, Lam AM. The effect of hypocapnia on the autoregulation of cerebral blood flow during administration of isoflurane. Anesth Analg. 2005 May;100(5):1463–7. doi: 10.1213/01.ANE.0000148623.06596.7E. table of contents. [DOI] [PubMed] [Google Scholar]
- 31.McCulloch TJ, Visco E, Lam AM. Graded hypercapnia and cerebral autoregulation during sevoflurane or propofol anesthesia. Anesthesiology. 2000 Nov;93(5):1205–1209. doi: 10.1097/00000542-200011000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Berkowitz ID, Hayden WR, Traystman RJ, Jones MD., Jr. Haemophilus influenzae type B impairment of pial vessel autoregulation in rats. Pediatr Res. 1993 Jan;33(1):48–51. doi: 10.1203/00006450-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Pedersen M, Brandt CT, Knudsen GM, Ostergaard C, Skinhoj P, Skovsted IC, et al. The effect of S. pneumoniae bacteremia on cerebral blood flow autoregulation in rats. J Cereb Blood Flow Metab. 2008 Jan;28(1):126–134. doi: 10.1038/sj.jcbfm.9600514. [DOI] [PubMed] [Google Scholar]
- 34.Liu P, Huang H, Rollins N, Chalak LF, Jeon T, Halovanic C, et al. Quantitative assessment of global cerebral metabolic rate of oxygen (CMRO ) in neonates using MRI. NMR Biomed. 2014 Jan 7; doi: 10.1002/nbm.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krainik A, Villien M, Tropres I, Attye A, Lamalle L, Bouvier J, et al. Functional imaging of cerebral perfusion. Diagn Interv Imaging. 2013 Dec;94(12):1259–1278. doi: 10.1016/j.diii.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Horsfield MA, Jara JL, Saeed NP, Panerai RB, Robinson TG. Regional differences in dynamic cerebral autoregulation in the healthy brain assessed by magnetic resonance imaging. PLoS One. 2013 Apr 30;8(4):e62588. doi: 10.1371/journal.pone.0062588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao WG, Luo Q, Jia JB, Yu JL. Cerebral hyperperfusion syndrome after revascularization surgery in patients with moyamoya disease. Br J Neurosurg. 2013 Jun;27(3):321–325. doi: 10.3109/02688697.2012.757294. [DOI] [PubMed] [Google Scholar]
- 38.Jaeger M, Schuhmann MU, Soehle M, Meixensberger J. Continuous assessment of cerebrovascular autoregulation after traumatic brain injury using brain tissue oxygen pressure reactivity. Crit Care Med. 2006 Jun;34(6):1783–1788. doi: 10.1097/01.CCM.0000218413.51546.9E. [DOI] [PubMed] [Google Scholar]
- 39.Jaeger M, Dengl M, Meixensberger J, Schuhmann MU. Effects of cerebrovascular pressure reactivity-guided optimization of cerebral perfusion pressure on brain tissue oxygenation after traumatic brain injury. Crit Care Med. 2010 May;38(5):1343–1347. doi: 10.1097/CCM.0b013e3181d45530. [DOI] [PubMed] [Google Scholar]
- 40.Aries MJ, Czosnyka M, Budohoski KP, Steiner LA, Lavinio A, Kolias AG, et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med. 2012 May 22;40(8):2456–2463. doi: 10.1097/CCM.0b013e3182514eb6. [DOI] [PubMed] [Google Scholar]
- 41.Lee JK, Kibler KK, Benni PB, Easley RB, Czosnyka M, Smielewski P, et al. Cerebrovascular reactivity measured by near-infrared spectroscopy. Stroke. 2009 May;40(5):1820–1826. doi: 10.1161/STROKEAHA.108.536094. [DOI] [PubMed] [Google Scholar]
- 42.Kawamata T, Kawashima A, Yamaguchi K, Hori T, Okada Y. Usefulness of intraoperative laser Doppler flowmetry and thermography to predict a risk of postoperative hyperperfusion after superficial temporal artery-middle cerebral artery bypass for moyamoya disease. Neurosurg Rev. 2011 Jul;34(3):355–62. doi: 10.1007/s10143-011-0331-8. discussion 362. [DOI] [PubMed] [Google Scholar]
- 43.Oshima H, Katayama Y, Hirayama T. Intracerebral steal phenomenon associated with global hyperemia in moyamoya disease during revascularization surgery. J Neurosurg. 2000 Jun;92(6):949–954. doi: 10.3171/jns.2000.92.6.0949. [DOI] [PubMed] [Google Scholar]
- 44.Siriussawakul A, Sharma D, Sookplung P, Armstead W, Vavilala MS. Gender differences in cerebrovascular reactivity to carbon dioxide during sevoflurane anesthesia in children: preliminary findings. Paediatr Anaesth. 2011 Feb;21(2):141–147. doi: 10.1111/j.1460-9592.2010.03498.x. [DOI] [PubMed] [Google Scholar]
- 45.Lee JK, Williams M, Jennings JM, Jamrogowicz JL, Larson AC, Jordan LC, et al. Cerebrovascular autoregulation in pediatric moyamoya disease. Paediatr Anaesth. 2013 Jun;23(6):547–556. doi: 10.1111/pan.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raux O, Sola C, Macq C, Dadure C. Cerebral near-infrared spectroscopy (NIRS) in paediatric anaesthesia. Ann Fr Anesth Reanim. 2013 Jan;32(1):e49–53. doi: 10.1016/j.annfar.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 47.Joshi B, Ono M, Brown C, Brady K, Easley RB, Yenokyan G, et al. Predicting the limits of cerebral autoregulation during cardiopulmonary bypass. Anesth Analg. 2012 Mar;114(3):503–510. doi: 10.1213/ANE.0b013e31823d292a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joshi B, Brady K, Lee J, Easley B, Panigrahi R, Smielewski P, et al. Impaired autoregulation of cerebral blood flow during rewarming from hypothermic cardiopulmonary bypass and its potential association with stroke. Anesth Analg. 2010 Feb 1;110(2):321–328. doi: 10.1213/ANE.0b013e3181c6fd12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brady K, Joshi B, Zweifel C, Smielewski P, Czosnyka M, Easley RB, et al. Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke. 2010 Sep;41(9):1951–1956. doi: 10.1161/STROKEAHA.109.575159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brady KM, Mytar JO, Lee JK, Cameron DE, Vricella LA, Thompson WR, et al. Monitoring cerebral blood flow pressure autoregulation in pediatric patients during cardiac surgery. Stroke. 2010 Sep;41(9):1957–1962. doi: 10.1161/STROKEAHA.109.575167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brady KM, Shaffner DH, Lee JK, Easley RB, Smielewski P, Czosnyka M, et al. Continuous monitoring of cerebrovascular pressure reactivity after traumatic brain injury in children. Pediatrics. 2009 Dec;124(6):e1205–12. doi: 10.1542/peds.2009-0550. [DOI] [PubMed] [Google Scholar]
- 52.Lazaridis C, Smielewski P, Steiner LA, Brady KM, Hutchinson P, Pickard JD, et al. Optimal cerebral perfusion pressure: are we ready for it? Neurol Res. 2013 Mar;35(2):138–148. doi: 10.1179/1743132812Y.0000000150. [DOI] [PubMed] [Google Scholar]
- 53.Easley RB, Kibler KK, Brady KM, Joshi B, Ono M, Brown C, et al. Continuous cerebrovascular reactivity monitoring and autoregulation monitoring identify similar lower limits of autoregulation in patients undergoing cardiopulmonary bypass. Neurol Res. 2013;35(4):344–354. doi: 10.1179/1743132812Y.0000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zweifel C, Castellani G, Czosnyka M, Helmy A, Manktelow A, Carrera E, et al. Noninvasive Monitoring of Cerebrovascular Reactivity with Near Infrared Spectroscopy in Head-Injured Patients. J Neurotrauma. 2010 Nov;27(11):1951–1958. doi: 10.1089/neu.2010.1388. [DOI] [PubMed] [Google Scholar]
- 55.Liu J, Zhu YS, Hill C, Armstrong K, Tarumi T, Hodics T, et al. Cerebral autoregulation of blood velocity and volumetric flow during steady-state changes in arterial pressure. Hypertension. 2013 Nov;62(5):973–979. doi: 10.1161/HYPERTENSIONAHA.113.01867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang R, Behbehani K, Levine BD. Dynamic pressure-flow relationship of the cerebral circulation during acute increase in arterial pressure. J Physiol. 2009 Jun 1;587(Pt 11):2567–2577. doi: 10.1113/jphysiol.2008.168302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gong XP, Li Y, Jiang WJ, Wang Y. Impaired dynamic cerebral autoregulation in middle cerebral artery stenosis. Neurol Res. 2006 Jan;28(1):76–81. doi: 10.1179/016164106X91915. [DOI] [PubMed] [Google Scholar]
- 58.Vavilala MS, Tontisirin N, Udomphorn Y, Armstead W, Zimmerman JJ, Chesnut R, et al. Hemispheric differences in cerebral autoregulation in children with moderate and severe traumatic brain injury. Neurocrit Care. 2008;9(1):45–54. doi: 10.1007/s12028-007-9036-9. [DOI] [PubMed] [Google Scholar]
- 59.Jaeger M, Soehle M, Schuhmann MU, Meixensberger J. Clinical significance of impaired cerebrovascular autoregulation after severe aneurysmal subarachnoid hemorrhage. Stroke. 2012 Aug;43(8):2097–2101. doi: 10.1161/STROKEAHA.112.659888. [DOI] [PubMed] [Google Scholar]
- 60.Ono M, Brady K, Easley RB, Brown C, Kraut M, Gottesman RF, et al. Duration and magnitude of blood pressure below cerebral autoregulation threshold during cardiopulmonary bypass is associated with major morbidity and operative mortality. J Thorac Cardiovasc Surg. 2013 Sep 26; doi: 10.1016/j.jtcvs.2013.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ono M, Arnaoutakis GJ, Fine DM, Brady K, Easley RB, Zheng Y, et al. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Crit Care Med. 2013 Feb;41(2):464–471. doi: 10.1097/CCM.0b013e31826ab3a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rangel-Castilla L, Gasco J, Nauta HJ, Okonkwo DO, Robertson CS. Cerebral pressure autoregulation in traumatic brain injury. Neurosurg Focus. 2008 Oct;25(4):E7. doi: 10.3171/FOC.2008.25.10.E7. [DOI] [PubMed] [Google Scholar]